Abstract
Blossom blight of alfalfa, caused by Botrytis cinerea and Sclerotinia sclerotiorum, can be an important constraint to alfalfa seed production on the Canadian Prairies. Botrytis cinerea is the predominant pathogen in rain-fed production areas across the northern Prairie region. This study assessed the optimum conditions for infection of alfalfa florets by B. cinerea, and susceptibility in alfalfa based on cultivar and flower colour/orientation. The disease reaction of the cultivars was assessed in detached and intact inflorescences inoculated under controlled conditions and in field trials. The optimum temperature for infection was 20°C, with a minimum of 12 h of surface wetness. Increased duration of surface wetness generally increased infection, except at 30°C, where continued exposure to high temperature reduced infection. There were small but consistent differences in flower infection among cultivars, which were consistent across the testing protocols (detached inflorescence, whole-plant and field). Upward-facing inflorescences had a lower incidence of infection than downward-facing inflorescences. Also, purple florets were slightly less susceptible relative to white/yellow florets, but in only one of the three cultivars assessed. Knowledge of infection requirements from this study can be used to improve management of blossom blight, but the differences among cultivars were generally too small to have an important impact on disease management in the field.
Résumé
La brûlure de la fleur de la luzerne, causée par Botrytis cinerea et Sclerotinia sclerotiorum, peut être un obstacle de taille à la production de semence de luzerne sur les Prairies canadiennes. Botrytis cinerea est le principal agent pathogène dans les régions productrices non irriguées du nord des Prairies. Cette étude évalue les conditions optimales relatives à l’infection des fleurons par B. cinerea ainsi que la sensibilité de la luzerne en se basant sur les cultivars et la couleur de même que sur l’orientation des fleurs. La réaction des cultivars à la maladie a été évaluée chez des inflorescences détachées et intactes inoculées dans le cadre d’essais dans des conditions contrôlées et au champ. La température optimale favorisant l’infection était 20°C pour une humidité de surface d’au moins 12 heures. Le prolongement de la durée de l’humidité de surface accroît généralement le taux d’infection, sauf à 30°C, température à laquelle l’exposition continue à une température élevée a réduit l’infection. Il y avait de faibles, mais constantes différences quant à l’infection des fleurs parmi les cultivars, qui étaient constantes dans tous les protocoles d’essais (inflorescence détachée, plante entière et champ). Les inflorescences tournées vers le haut affichaient une plus faible incidence de l’infection que celles tournées vers le bas. De plus, les fleurons pourpres étaient légèrement moins sensibles que les jaunes ou les blancs, mais seulement chez un des trois cultivars évalués. L’information tirée de cette étude concernant les critères relatifs à l’infection peut être utilisée pour améliorer la gestion de la brûlure de la fleur, mais les différences entre les cultivars étaient généralement trop faibles pour avoir une influence notable sur la gestion de la maladie au champ.
Introduction
Alfalfa (Medicago sativa L.) is a large acreage crop in Canada, and seed for the national market is largely produced on the Canadian Prairies. In addition, Canada exports about 14 700 t of alfalfa seed annually, with a value of US$48 million (WITS Citation2020). Blossom blight is an important constraint to alfalfa seed production on the Canadian Prairies, with outbreaks in one or more centres of production almost every year (Gossen and Howard Citation2021). It was identified for the first time on the Canadian Prairies in 1993 (Gossen et al. Citation1994). Outbreaks can develop quickly if weather conditions are conducive for disease development, resulting in yield reductions of up to 50% (Gossen Citation1997; Gossen et al. Citation1997; Reich et al. Citation2017).
Blossom blight is caused by two pathogens, Botrytis cinerea Pers. and Sclerotinia sclerotiorum (Lib.) de Bary (Gossen et al. Citation1994; Holley et al. Citation1996; Huang et al. Citation2000). Both pathogens are favoured by cool, wet conditions, but it is unusual to find both pathogens at high levels in the same field. Botrytis cinerea is often the dominant pathogen in the blossom blight complex in northern Alberta and across Saskatchewan and Manitoba (Gossen and Howard Citation2021). Sclerotinia sclerotiorum requires a prolonged period of soil wetness to stimulate germination of sclerotia and trigger release of air-borne ascospores. Botrytis cinerea reproduces quickly via conidia, which can produce multiple cycles of infection.
When B. cinerea infects an alfalfa floret under optimum conditions, the mycelium from one floret can infect adjacent florets in the raceme (Verhoeff Citation1980) and quickly colonize the entire raceme to produce a large number of conidia. Under cool, wet conditions, the infected raceme becomes covered with grey mycelium. However, when conditions are less conducive to mycelial growth, the pathogen spreads more slowly and infected florets often abscise prematurely without setting seed (Gossen and Howard Citation2021).
The basic requirement for infection is the availability of surface wetness for a minimum period, which is a function of precipitation, temperature and relative humidity (Jarvis Citation1980). The details differ among hosts and tissues (Nelson Citation1951; Bulger et al. Citation1987; Thomas et al. Citation1988; Wilcox and Seem Citation1994; Zhang and Sutton Citation1994; Sosa-Alvarez et al. Citation1995; Sirjusingh and Sutton Citation1996), but optimum conditions for infection of flowers are generally around 20°C and 6–12 h of surface wetness. For example, infection of geranium flowers required a surface wetness period ≥ 8 h at 15°C but ≥ 4 h at temperatures above 21°C (Sirjusingh and Sutton Citation1996). Infection of old grape flowers required only 2 h at 20°C (Ciliberti et al. Citation2015).
No strong source of gene-for-gene resistance to B. cinerea has been identified in any crop, but transposable elements in B. cinerea are involved in the production of small RNAs that silence the expression of host defence genes (Elad et al. Citation1995; Mengiste et al. Citation2003). Structural traits such as cuticle structure and thickness, cutin content, cell wall thickness and papillae formation are also associated with reduced susceptibility to infection by B. cinerea (Rijkenberg et al. Citation1980; Marois et al. Citation1986; Rewal and Grewal Citation1989; Hammer and Evensen Citation1994; Smith Citation1998).
Other traits involved in the susceptibility to infection by B. cinerea include canopy architecture, flower colour, timing of flowering and even the amount of pollen in flowers. An open canopy dries more quickly than a dense canopy (Michailides Citation1991; Vail and Marois Citation1991; Bond et al. Citation1994). Compact inflorescences develop more severe disease than open inflorescences in grape (Gubler et al. Citation1987; Vail and Marois Citation1991) and other hosts. In some host species, the anthocyanin pigments in purple flowers have antioxidant activity that reduced infection (Tamura and Yamagami Citation1994; Hipskind et al. Citation1996; Ruberto et al. Citation1997). Also, early flowering may result in disease escape (Bond et al. Citation1994).
In this study, the interaction of temperature and surface wetness duration on infection of alfalfa flowers was examined under controlled environmental conditions to identify the conditions required for infection of alfalfa florets. This information was used to assess the susceptibility of locally adapted alfalfa cultivars to blossom blight caused by B. cinerea in detached-raceme, whole-plant and field studies. The effects of floret orientation and colour on infection incidence were also evaluated in a greenhouse study. The objectives were to determine whether there were differences in susceptibility to infection by B. cinerea among adapted alfalfa cultivars and to assess the influence of raceme architecture and floret colour. The studies were conducted more than two decades ago and the specific cultivars assessed have largely been replaced with new lines. However, blossom blight continues to be an important constraint to alfalfa seed production across the region (Gossen and Howard Citation2021) so studies of the factors that affect infection and disease reaction are still relevant.
Materials and methods
Inoculum production
Two isolates of B. cinerea were utilized in the studies. Isolate Nov9703, collected from chickpea near Saskatoon, Saskatchewan, was used for the surface wetness × temperature study because of its consistently high sporulation. Nov9603, isolated from an alfalfa floret near Brooks, Alberta, was used for the studies on cultivar reaction.
Conidia were produced on potato-dextrose agar (PDA) under continuous cool-white fluorescent lamps at room temperature. Spores were harvested from actively growing colonies by pouring small aliquots of sterile deionized water plus surfactant (0.1 mL Triton X-100 per 100 mL) on to the colony, scraping the mycelium gently with a small sterile spatula to release the spores, collecting the spore suspension and filtering it through four layers of sterile cheesecloth. The spore concentration was estimated using a haemacytometer and diluted to 1 × 105 spores mL−1. The inoculum suspension was applied by spraying each raceme to run-off with a fine mist of a fresh spore suspension applied using a thumb-pump hand atomizer in the controlled environment and greenhouse studies and using a hand-pump backpack sprayer with a single flat fan nozzle to produce a fine, uniform spray mist in the field trial.
Host plants
For the assessment of surface wetness and temperature requirements for infection, seed of alfalfa cv. Vernal was planted in plastic rootrainers (2 × 2.5 × 10.5 cm) containing a soil-less mix and maintained at about 20°C under a 14-h photoperiod in a greenhouse. The seedlings were transferred to plastic pots (20 × 15.5 cm high) at 4 weeks after seeding. At the mid-bloom stage, all the unopened flower buds were removed and discarded, and newly opened florets were selected for the study. This procedure ensured that all the florets assessed were the same age.
In the cultivar assessments, 12 alfalfa cultivars representing the range of winter hardiness available to Canadian producers were evaluated for their susceptibility to infection by B. cinerea. Five cultivars were characterized as extremely winter hardy, based on performance on the Canadian Prairies: ‘AC Nordica’ (Goplen and Gossen Citation1994), ‘Algonquin’, ‘Beaver’, ‘Heinrichs’ and ‘Vernal’. The other seven cultivars were popular cultivars grown primarily in eastern Canada, characterized as winter hardy: ‘Apica’, ‘Apollo II’, ‘DK 135ʹ, ‘Iroquois’, ‘OAC Minto’, ‘Oneida-VR’ and ‘Saranac’ (Melton et al. Citation1988). Plants were produced and florets selected for study as described previously.
Temperature × wetness duration study
The experiment was laid out in a split-plot design with four replicates. The main-plot treatments were incubation temperatures (10°C, 15°C, 20°C, 25°C and 30°C) assigned at random to an incubator in each replication. The exception was the 30°C treatment, which was always assigned to a small oven because growth cabinets often failed to maintain this high temperature. The subplot treatments were surface wetness duration (0, 4, 8, 12, 16, 20, 24, 48 h).
Detached racemes with unopened flower buds removed were inoculated and immediately placed into small moist chambers (8.5 cm diameter × 2.7 cm high) with three racemes per chamber. The chambers were constructed by sealing the bottom halves of two 9-cm-diameter plastic Petri dishes together with stretchy plastic film to prevent moisture loss. Each chamber contained a layer of moist filter paper on the bottom, covered by a layer of sterile plastic mesh to prevent contact between the filter paper and the contents of the chamber. The moist chambers were then placed into incubators set at 10°C, 15°C, 20°C, 25°C and 30°C. The racemes were maintained in the dark to minimize the temperature variance associated with light in the chamber. After 0, 4, 8, 12, 16, 20, 24 and 48 h, two chambers (six racemes) were removed from each incubator. Five florets were then removed from each raceme, surface-sterilized with 70% ethanol for 30 s, then 0.6% NaOCl for 90 s, and incubated on PDA for 10–14 days. The incidence of floret infection by B. cinerea was estimated via microscopic examination for the presence of sporulation of B. cinerea. The entire protocol was repeated four times to produce four replicates.
Cultivar reaction – detached-raceme assessments
Ten racemes from two plants per cultivar were picked and trimmed to remove unopened flower buds, inoculated by spraying the flower clusters to run-off with a spore suspension (1 × 105 spores mL−1) using an atomizer, and immediately placed in small moist chambers as described previously. The racemes were incubated at room temperature in the dark. After 12 h or 24 h, four florets were removed from each raceme. A total of 40 florets for each cultivar × time combination was surface-sterilized and incubated, and infection was assessed as described previously. The protocol was repeated four times, to produce four replications.
Cultivar reaction, whole-plant assessments
Vigorously flowering plants were inoculated by spraying the racemes to run-off with a spore suspension (1 × 105 spores mL−1) as described previously. To ensure that all the florets were at the same stage of development, all the unopened flower buds on each plant were trimmed off immediately before inoculation. Each plant was then covered with a clear plastic bag and incubated for 48 h in an incubator set at 20°C and 14-h photoperiod. The four youngest florets were removed from each of 10 racemes per cultivar, surface-sterilized and plated onto PDA, and then infection was assessed as described previously. The youngest flowers available were selected, rather than the oldest flowers (which are most susceptible to infection), to provide samples of uniform age and development for the assessment. The experiment was repeated four times, using new plants for each repetition.
Cultivar reaction, field trial
In the field, plants of each cultivar were grown in 1-m2 micro-plots in a randomized complete block design with five replications. The plots were established in 1996 by transplanting nine seedlings per plot. Some of the plants died in the winter of 1997 and were replaced with 2-year-old plants in the spring of 1998. To ensure that all the florets were at the same stage of development, all the unopened flower buds on each plant were trimmed off immediately before inoculation. Flowering plants were inoculated with a spore suspension (1 × 105 spores per mL) produced as described previously using a hand-pump backpack sprayer in the early evening of 21 August 1998, 1 day after heavy rain. The inoculated plants were mist-irrigated for 3 min at 9 pm on the following 2 days to maintain high levels of humidity overnight. On the fourth day, the two youngest flowers that had been open at the time of inoculation from each of 20 racemes per cultivar were collected from each plot, surfaced-sterilized and assessed as described previously.
Flower orientation and colour
Greenhouse-grown plants of cv. Vernal were trimmed, inoculated and covered with individual plastic bags as described previously. Ten upward-facing and 10 downward-facing racemes were collected after 48 h of incubation, and four young florets were collected from each raceme, surface sterilized and assessed for infection incidence. The protocol was conducted three times to produce three replicates.
In a second study, greenhouse-grown plants of cvs. ‘AC Nordica’, ‘Apollo II’ and ‘Iroquois’ were selected based on their flower colour, with two plants selected per cultivar that had predominantly purple florets and two plants with predominantly white or yellow florets. Ten racemes of each colour (purple vs. yellow/white) per cultivar were collected, trimmed, inoculated, incubated and assessed per replicate as described previously. The study was arranged in a randomized complete block design with four replicates, using new plants for each repetition.
Statistical analysis
Analysis of variance of infection incidence was performed using SAS software (PROC ANOVA, SAS Citation1985). The effect of surface wetness duration was treated as a split-plot-in-time. Means were separated, where appropriate, using Duncan’s Multiple Range Test at P ≤ 0.05. Cultivar susceptibility was assessed based on Kendall’s coefficient of rank correlation (τ). Kendall’s coefficient of concordance (W) was used to assess the association among the assessments (detached raceme, whole-plant, field). The significance level for τ was estimated using a t-test, and for W was estimated using a chi-square test. Differences are significant at P ≤ 0.05 unless otherwise stated.
Results
Temperature, wetness duration and floral infection
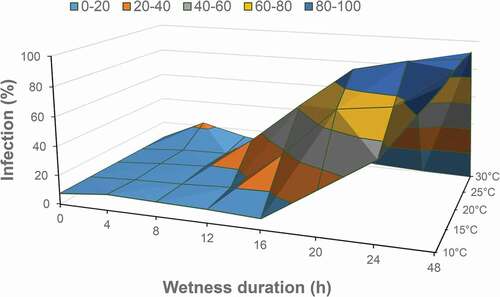
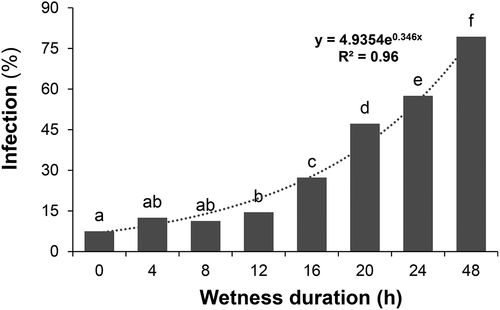
The effects of temperature, wetness duration and their interaction (P ≤ 0.001) all affected the infection of alfalfa flowers by B. cinerea in analysis of variance. The highest incidence of infection occurred at 20°C and the lowest at 30°C (). Incidence at 15°C was higher than at 25°C or 10°C. After 12 h of surface wetness, infection incidence had increased over the background as wetness duration increased (). Incidence had increased sharply after 12 h at 20°C, 16 h at 15°C, and 20 h at 10°C and 25°C (). Infection at 30°C was highest at 4 h of wetness but very low at 8–48 h of wetness. The optimum temperature for infection was between 15°C and 20°C, with a minimum of 12–16 h of surface wetness. This study supports the pattern of flower infection observed in other host species (Nelson Citation1951; Bulger et al. Citation1987; Thomas et al. Citation1988; Wilcox and Seem Citation1994; Zhang and Sutton Citation1994; Sosa-Alvarez et al. Citation1995; Sirjusingh and Sutton Citation1996), so was not repeated.
Fig. 1 Effect of temperature on infection of alfalfa florets by Botrytis cinerea in detached racemes under controlled conditions. Columns topped with the same letter do not differ based on Duncan’s Multiple Range Test at P < 0.05. The dotted line represents the best fit based on polynomial regression analysis
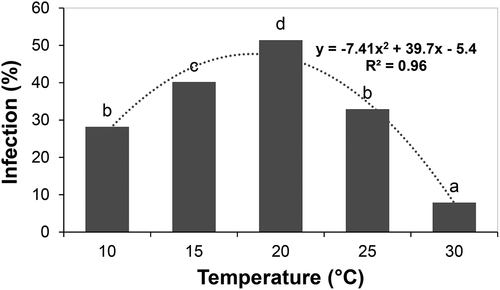
Fig. 2 Effect of surface wetness duration infection of alfalfa florets by Botrytis cinerea in detached racemes under controlled conditions. Columns topped with the same letter do not differ based on Duncan’s Multiple Range Test at P ≤ 0.05. The dotted line represents the best fit based on exponential regression analysis
Cultivar reaction
In the detached-floret tests after 12 h of incubation, the incidence of infection among cultivars ranged from 12% to 33% (). One group of cultivars (‘OAC Minto’, ‘Beaver’ and ‘DK 135ʹ) had a lower incidence of infection (mean 12%) than the most heavily infected cultivars (‘Apollo II’, ‘Heinrichs’ and ‘AC Nordica’; mean 30%). In addition, ‘Iroquois’ had a lower incidence of infection (17%) than the most heavily infected cultivar, ‘Apollo II’ (33%).
Table 1. Comparison of the incidence of floral infection by Botrytis cinerea in selected alfalfa cultivars in controlled environment studies of detached racemes at 12 and 24 h after inoculation, greenhouse studies of attached racemes, and in a field trial at Saskatoon, Saskatchewan
After 24 h of incubation, the incidence of infection had increased substantially (). Cultivar ‘DK 135ʹ still had a lower incidence of infection (54%) than the other cultivars, and incidence in ‘Iroquois’ was still slightly lower (68%) than the most heavily infected group (cvs. ‘Algonquin’, ‘Heinrichs’, ‘Apollo II’ and ‘Vernal’; mean 90%), and was slightly lower in ‘OAC Minto’ than ‘Vernal’ (72% vs. 92%). However, the rank order of the cultivars was not correlated between the two sampling intervals (r = 0.41).
In the whole-plant assessment, there were consistent differences among cultivars in the incidence of flower infection by B. cinerea. Infection incidence was lower in cvs. ‘OAC Minto’, ‘Iroquois’ and ‘DK 135ʹ than in cvs. ‘Apica’, ‘Algonquin’, ‘Heinrichs’, ‘AC Nordica’ and ‘Apollo II’ (mean 37% vs. 73%, ). ‘Saranac’, ‘Vernal’ and ‘Oneida-VR’ had an intermediate incidence of infection, lower than ‘AC Nordica’ and ‘Apollo II’ (mean 47% vs. 77%), but not different from cvs. ‘Apica’, ‘Algonquin’ and ‘Heinrichs’.
In the field trial, the incidence of flower infection was lower than under controlled conditions (). The incidence of flower infection in ‘DK 135ʹ (3%) and ‘OAC Minto’ (4%) was lower than in ‘Heinrichs’ (22%) and ‘Apollo II’ (22%). Also, ‘DK 135ʹ (3%) had a lower incidence of infection than ‘Algonquin’ (20%).
The rank order of cultivars based on infection incidence was strongly correlated (P ≤ 0.01) between the whole-plant test and the detached-raceme test at 12 h (τ = 0.78), but not with the detached-raceme test at 24 h (P ≤ 0.01) (τ = 0.32). The rank of cultivars in the field test was correlated with each of the other assessments: τ = 0.69 (P ≤ 0.01) in the detached-raceme test at 12 h, τ = 0.61 at 24 h (P ≤ 0.01) and τ = 0.78 (P ≤ 0.01) with the whole-plant inoculation test. The overall similarity among the tests was assessed using Kendall’s coefficient of concordance (W). The value of W was 0.84 (P ≤ 0.001), which indicated a strong similarity in cultivar response across the assessments.
Flower orientation and colour
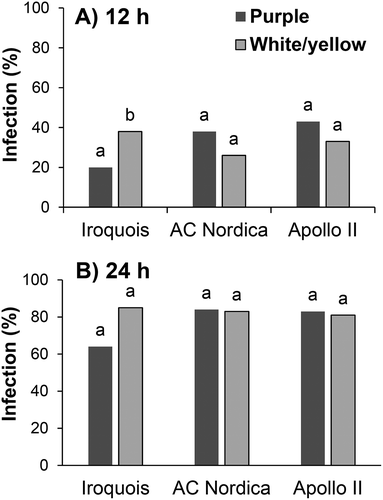
Upward-facing flowers had a lower (P ≤ 0.001) incidence of infection compared with downward-facing flowers (16% vs. 86%) in whole-plant assessments. The effect of flower colour on infection frequency was not consistent among the three cultivars. Purple flowers had a lower incidence of infection than white flowers in ‘Iroquois’ (20% vs. 38% at 12 h; 64% vs. 85% at 24 h) but there were no differences in incidence between purple and white/yellow flowers in ‘Apollo II’ or between purple and white/yellow flowers in ‘AC Nordica’ ().
Discussion
In this study, the optimal temperature for floral infection by B. cinerea on alfalfa was about 20°C, with a minimum of 8–12 h of surface wetness. Increased duration of surface wetness generally resulted in increased incidence of infection. This finding is consistent with assessments of infection in flowers of other hosts (Jarvis Citation1980; Verhoeff Citation1980), except that the wetness duration is slightly longer than in some other crops (Bulger et al. Citation1987). For example, infection of geranium flowers required only 4–6 h of wetness at 21°C (Sirjusingh and Sutton Citation1996).
This difference may be due, at least in part, to the age of the flowers being tested. Very young grape inflorescence required a longer period (6–12 h) of wetness at 20°C for infection than older flowers, which required as little as 3 h of wetness (Ciliberti et al. Citation2015). This indicates that infection of newly opened alfalfa florets has a similar requirement for wetness and temperature to that of young grape flowers. Older flowers in geranium and macadamia are also more susceptible to infection than younger flowers (Harrison Citation1984; Sirjusingh and Sutton Citation1996).
None of the cultivars assessed in this study demonstrated strong resistance to infection by B. cinerea. There were, however, consistent differences in susceptibility among the cultivars and there was a strong positive correlation for cultivar reactions among the four protocols assessed. For example, ‘OAC Minto’ and ‘DK 135ʹ were generally less susceptible than cvs. ‘Apollo II’, ‘Heinrichs’ and ‘AC Nordica’ in all four assessment protocols. The only exception was the detached-floret assessment at 24 h, where infection levels were consistently high.
The incidence of infection was generally lower in field trials than in other assessments. Surface wetness on the flowers may not have persisted long enough to establish a high level of infection, despite humid conditions and periodic mist irrigation. The specific cultivars assessed in this study have largely been replaced with new lines since the studies were conducted, but it appears likely that the cultivars that have replaced them also differ slightly in susceptibility to infection.
Variation in susceptibility can be the cumulative result of many traits, including flower architecture and colour. In the current study, the incidence of infection by B. cinerea was slightly lower in purple than white florets in cv. ‘Iroquois’, but there was no difference in ‘AC Nordica’ or ‘Apollo II’, both of which were characterized as highly susceptible based on the assessments of cultivar reaction. Previously, flower colour in bean was associated with a small difference in infection of flowers by ascospores of S. sclerotiorum, but no differences associated with flower colour were observed in alfalfa (Olivier et al. Citation2008).
Infection was substantially lower in upward-facing compared with downward-facing florets. This difference may have been associated with a difference in wetness duration on the flower surfaces. After 48 h of incubation, the surface of upward-facing flowers appeared to be dry, but the downward-facing flowers were still wet, based on visual observation. Other factors that might influence infection incidence, such as floret density, or cuticle thickness and composition, were not assessed.
Understanding the relationship between surface wetness and floral infection could have potential applications for managing blossom blight in alfalfa. Wetness duration and associated increase in susceptibility to B. cinerea may be affected by several factors in the field, including wind speed (Thomas et al. Citation1988; Hammer and Evensen Citation1994), plant density (English et al. Citation1989) and canopy architecture (Michailides Citation1991; Vail and Marois Citation1991). The current study demonstrated that surface wetness periods of > 8 h promote floral infection by B. cinerea. Therefore, selecting a field with good air movement and maintaining an open crop canopy for seed production will reduce the risk of severe blossom blight. Infection occurs rapidly, so application of fungicide before a rain event may suppress infection and slow down the establishment of infection during favourable conditions. Also, overhead irrigation during flowering should be avoided.
This study demonstrated that a detached-flower test at 12 h provides a simple and practical assessment of susceptibility to flower infection by B. cinerea, which was highly correlated with other methods that required more time and other resources. The detached-flower test at 24 h did not provide as good an assessment as at 12 h. The whole-plant test in the greenhouse was also easy, inexpensive and strongly correlated with the results from field trials. Field tests for blossom blight in rain-fed production can be problematic because their success is highly dependent on the weather in the absence of irrigation to provide favourable surface wetness conditions for infection.
This study found consistent differences in susceptibility to infection by B. cinerea among cultivars. Also, differences in flower architecture had a strong influence on infection incidence. However, the observation that differences in cultivar reactions were much less pronounced after 24 h than 12 h was a cause for concern. It indicated that differences among cultivars were likely to be quickly overcome under conditions conducive to disease development, and so were unlikely to have a substantial impact on levels of blossom blight in the field.
Acknowledgements
Thanks to K. Anderson, K. Bassendowski, M. Wigness and B. Wong for technical assistance.
Additional information
Funding
References
- Bond DA, Jellis GJ, Rowland GG, Leguen J, Robertson JD, Khalil SA, Lijuan L. 1994. Present status and future strategy in breeding faba beans (Vicia faba L.) for resistance to biotic and abiotic stresses. Euphytica. 73:151–166. doi:https://doi.org/10.1007/BF00027191
- Bulger MA, Ellis MA, Madden LV. 1987. Influence of temperature and wetness duration on infection of strawberry flowers by Botrytis cinerea and disease incidence of fruit originating from infected flowers. Phytopathology. 77:1225–1230. doi:https://doi.org/10.1094/Phyto-77-1225
- Ciliberti N, Fermaud M, Languasco L, Rossi V. 2015. Influence of fungal strain, temperature, and wetness duration on infection of grapevine inflorescences and young berry clusters by Botrytis cinerea. Phytopathology. 105:325–333. doi:https://doi.org/10.1094/PHYTO-05-14-0152-R
- Elad Y, Gullino ML, Shtienberg D, Aloi C. 1995. Managing Botrytis cinerea on tomatoes in greenhouse in the Mediterranean. Crop Prot. 14:105–109. doi:https://doi.org/10.1016/0261-2194(95)92863-I
- English JT, Thamos CS, Marois JJ, Gubler WD. 1989. Microclimates of grape canopies associated with leaf removal and control of botrytis bunch rot. Phytopathology. 79:395–401. doi:https://doi.org/10.1094/Phyto-79-395
- Goplen BP, Gossen BD. 1994. AC Nordica alfalfa. Can J Plant Sci. 74:145–147. doi:https://doi.org/10.4141/cjps94-030
- Gossen BD. 1997. Blossom blight, a new constraint to alfalfa seed production in western Canada. In: Chloupek O, Simon U, editors. Proceedings of the 12th Eucarpia Meeting of the Medicago Group. Brno (Czech Republic): EUCARPIA; July, 2–5, 1996; p. 111–113.
- Gossen BD, Howard RJ. 2021. Distribution of blossom blight in alfalfa seed production on the Canadian Prairies. Can J Plant Pathol. 42:xxx–xxx. (In press). doi:https://doi.org/10.1080/07060661.2020.1825015
- Gossen BD, Lan Z, Harrison LM, Holley JD, Smith SR. 1997. Survey of blossom blight of alfalfa on the Canadian prairies in 1996. Can Plant Dis Surv. 76:87–88.
- Gossen BD, Smith SR, Platford RG. 1994. Botrytis cinerea blossom blight of alfalfa on the Canadian Prairies. Plant Dis. 78:1218. doi:https://doi.org/10.1094/PD-78-1218D
- Gubler WD, Marois JJ, Bledsoe AM, Bettiga LJ. 1987. Control of Botrytis bunch rot of grape with canopy management. Plant Dis. 71:599–601. doi:https://doi.org/10.1094/PD-71-0599
- Hammer PE, Evensen KB. 1994. Differences between rose cultivars in susceptibility to infection by Botrytis cinerea. Phytopathology. 84:1305–1312. doi:https://doi.org/10.1094/Phyto-84-1305
- Harrison JG. 1984. Effects of environmental factors on sporulation of Botrytis fabae. Trans Br Mycol Soc. 83:295–298. doi:https://doi.org/10.1016/S0007-1536(84)80150-3
- Hipskind J, Wood K, Nicholson RL. 1996. Localized stimulation of anthocyanin accumulation and delineation of pathogen ingress in maize genetically resistant to Bipolaris maydis race O. Physiol Mol Plant Pathol. 49:247–256. doi:https://doi.org/10.1006/pmpp.1996.0052
- Holley JD, Linowski R, Gossen BD, Harrison LM. 1996. Sclerotinia sclerotiorum involved in blossom blight of alfalfa. Can J Plant Pathol. 18:489. (Abstr.).
- Huang HC, Acharya SN, Erickson RS. 2000. Etiology of alfalfa blossom blight caused by Sclerotinia sclerotiorum and Botrytis cinerea. Plant Prot Bull. 9:11–16.
- Jarvis WR. 1980. Epidemiology. In: Coley-Smith JR, Verhoeff K, Jarvis WR, editors. The biology of Botrytis. London: Academic Press; p. 219–251.
- Marois JJ, Nelson JK, Morrison JC, Lile LS, Bledsoe AM. 1986. The influence of berry contact within grape cluster on the development of Botrytis cinerea and epicuticular wax. Am J Enol Vitic. 37:293–296.
- Melton B, Moutary JB, Bouton JH. 1988. Geographic adaptation and cultivar selection. In: Hanson AA, Bames DK, Hill RR Jr., editors. Alfalfa and Alfalfa improvement. Madison (WI): American Society of Agronomy; p. 595–620.
- Mengiste T, Chen X, Salmeron J, Dietrich R. 2003. The Botrytis Susceptible1 gene encodes an R2R3MYB transcription factor protein that is required for biotic and abiotic stress responses in Arabidopsis. Plant Cell. 15:2551–2565. doi:https://doi.org/10.1105/tpc.014167
- Michailides T. 1991. Susceptibility of pistachio male cultivars to botrytis blossom blight and shoot blight caused by Botrytis cinerea. Plant Dis. 75:410–415. doi:https://doi.org/10.1094/PD-75-0410
- Nelson KE. 1951. Factors influencing the infection of table grapes by Botrytis cinerea (Pres.). Phytopathology. 41:319–326.
- Olivier C, Gossen BD, Séguin-Swartz G. 2008. Impact of flower age and colour on infection of bean and alfalfa by Sclerotinia sclerotiorum. Can J Plant Pathol. 30:58–65. doi:https://doi.org/10.1080/07060660809507496
- Reich J, Chatterton S, Johnson D. 2017. Temporal dynamics of Botrytis cinerea and Sclerotinia sclerotiorum in seed alfalfa fields in southern Alberta, Canada. Plant Dis. 101:331–343. doi:https://doi.org/10.1094/PDIS-04-16-0492-RE
- Rewal N, Grewal JS. 1989. Differential response of chickpea to grey mold. Ind Phytopathol. 42:265–268.
- Rijkenberg FHJ, Leeuw GTND, Verhoeff K. 1980. Light and electron microscopy studies on the infection of tomato fruits by Botrytis cinerea. Can J Bot. 58:1394–1404. doi:https://doi.org/10.1139/b80-170
- Ruberto G, Renda A, Piattelli M, Rapisarda P, Starrantino A. 1997. Essential oil of two new pigmented citrus hybrids, Citrus clementina x Citrus sinensis. J Agri Food Chem. 45:467–471. doi:https://doi.org/10.1021/jf960109j
- SAS Institute, Inc. 1985. SAS user’s guide: statistics. Version 5 ed. Cary (NC): SAS Institute, Inc.; p. 956
- Sirjusingh C, Sutton JC. 1996. Effects of wetness duration and temperature on infection of geranium by Botrytis cinerea. Plant Dis. 80:160–165. doi:https://doi.org/10.1094/PD-80-0160
- Smith BJ. 1998. Botrytis blossom blight of southern blueberries: cultivar susceptibility and effect of chemical treatments. Plant Dis. 82:924–927. doi:https://doi.org/10.1094/PDIS.1998.82.8.924
- Sosa-Alvarez M, Madden LV, Ellis MA. 1995. Effects of temperature and wetness duration on sporulation of Botrytis cinerea on strawberry leaf residues. Plant Dis. 79:609–615. doi:https://doi.org/10.1094/PD-79-0609
- Tamura H, Yamagami A. 1994. Antioxidative activity of monoacylated anthocyanins isolated from muscat Bailey A grape. J Agric Food Chem. 42:1612–1615. doi:https://doi.org/10.1021/jf00044a005
- Thomas CS, Marois JJ, English JT. 1988. The effects of wind speed, temperature, and relative humidity on development of aerial mycelium and conidia of Botrytis cinerea on grape. Phytopathology. 78:260–265. doi:https://doi.org/10.1094/Phyto-78-260
- Vail ME, Marois JJ. 1991. Grape cluster architecture and the susceptibility of berries to Botrytis cinerea. Phytopathology. 81:188–191. doi:https://doi.org/10.1094/Phyto-81-188
- Verhoeff K. 1980. The infection process and host-pathogen interactions. In: Coley-Smith JR, Verhoeff K, Jarvis WR, editors. The biology of Botrytis. London: Academic Press; p. 153–180.
- Wilcox WF, Seem RC. 1994. Relationship between strawberry gray mold incidence, environmental variables, and fungicide applications during different periods of fruiting season. Phytopathology. 84:264–270. doi:https://doi.org/10.1094/Phyto-84-264
- WITS. 2020. World integrated trade solution, Canada Seed; lucerne (alfalfa) seed, of a kind used for sowing exports by country in 2019. [accessed 2021 Jan 21]. https://wits.worldbank.org/trade/comtrade/en/country/CAN/year/2019/tradeflow/Exports/partner/ALL/product/120921.
- Zhang PG, Sutton JC. 1994. Effects of wetness duration, temperature, and light on infection of black spruce seedlings by Botrytis cinerea. Can J For Res. 24:707–713. doi:https://doi.org/10.1139/x94-094