Abstract
Species of Pestalotiopsis occur commonly as plant pathogens. Accordingly, the aim of this study was to identify the causal agent of a shrub disease in northeastern and northern Tunisian forests. Field surveys showed a progressive dieback of branches, twig blight and trunk cankers on Pistacia lentiscus and Quercus coccifera. Pestalotiopsis spp. were the main fungi consistently isolated from these shrub species. Morphological identification and phylogenetic analysis of the internal transcribed spacer (ITS) region of the nuclear ribosomal DNA and partial sequence of the translation elongation factor 1-alpha gene (tef1-α) identified the isolated fungi as Pestalotiopsis biciliata. This identification was confirmed by PCR analysis and Sanger sequencing, and demonstrated further using Koch’s postulates. To the best of our knowledge, this is the first report of P. biciliata associated with branch dieback and stem canker on Quercus coccifera and Pistacia lentiscus in Tunisia.
Résumé
Les espèces de Pestalotiopsis sont souvent des agents phytopathogènes. En conséquence, le but de cette étude était d’identifier l’agent causal d’une maladie des arbrisseaux des forêts du nord-est et du nord de la Tunisie. Des examens sur le terrain ont indiqué le dépérissement graduel des branches, la brûlure des rameaux et la formation de chancres sur les troncs de Pistacia lentiscus et de Quercus coccifera. Pestalotiopsis spp. étaient les principaux champignons systématiquement isolés de ces espèces d’arbrisseaux. L’identification morphologique et l’analyse phylogénétique de la région de l’espaceur transcrit interne (ITS) de l’ADN ribosomique nucléaire et la séquence partielle du gène 1-alpha (tef1-α) du facteur d’élongation de la transcription ont permis d’identifier les champignons isolés en tant que Pestalotiopsis biciliata. Cette identification a été confirmée par PCR ainsi que par séquençage de Sanger et d’autre part démontrée en y appliquant les postulats de Koch. Pour autant qu’on le sache, il s’agit du premier rapport de P. biciliata associé au dépérissement des branches et au chancre des tiges chez Quercus coccifera et Pistacia lentiscus en Tunisie.
Introduction
The species-rich genus Pestalotiopsis Steyaert includes a large number of plant pathogens as well as endophytes (Maharachchikumbura et al. Citation2011; Satini et al. Citation2013; Zhang et al. Citation2013). To date, 299 names of species of Pestalotiopsis are listed in the Citation2016 (status March 2016), the global fungal nomenclature database. The genus Pestalotiopsis Steyaer is the anamorph of Pestalosphaeria belonging to the family Amphisphaeriaceae (Sutton Citation1980). Barr (Citation1975) studied the teleomorph-anamorph connections in Pestalotiopsis and described the genus Pestalosphaeria, as the teleomorph of Pestalotiopsis. Furthermore, Pestalosphaeria (teleomorph) has been observed in only a few species. This is mainly because it is difficult to develop and induce even under laboratory conditions (Judith-Hertz Citation2016). The Pestalotiopsis genus is a heterogeneous group of coelomycetous fungi that are differentiated primarily by their conidial characteristics such as size, septation, pigmentation, and the presence or absence of appendages (CABI Bioscience database 2001). It is characterized by fusiform conidia having mostly four-septate and pigmented median cells, with two to four apical appendages arising as tubular extensions from the apical cell and a centric basal appendage formed within compact acervuli (Jeewon et al. Citation2002). Hu et al. (Citation2007) found that conidial characters such as conidial length, median cell length, conidial width and colour of median cells were stable characters within Pestalotiopsis, but that the length of the apical and basal appendages was variable. Pestalotiopsis species are ubiquitous in distribution, occurring on a wide range of plants, and may cause a serious decline phenomenon (Das et al. Citation2010). They often occur as endophytes (Liu et al. Citation2006; Watanabe et al. Citation2010), or as pathogens causing economic losses (Maharachchikumbura et al. Citation2011). Indeed, numerous Pestalotiopsis species are regarded primarily as opportunistic pathogens that affect stressed plants. They have been consistently associated with a variety of plant diseases, including canker lesions, shoot dieback, stem blights, leaf spots and leaf blotches (Tagne and Mathur Citation2001; Espinoza et al. Citation2008; Zhang et al. Citation2012; Maharachchikumbura et al. Citation2013a, Citation2013b; Morales-Rodríguez et al. Citation2019). Pestalotiopsis spp. have been reported on hazelnut in Turkey, Iran and Serbia (Karakaya Citation2001; Arzanlou et al. Citation2012; Tanja et al. Citation2017). Furthermore, P. chamaeropis has been shown to be associated with shoot blight and stem necrosis of Erica arborea in Tunisia (Hlaiem et al. Citation2018).
The genus Pestalotiopsis has gained considerable attention from the scientific community in recent years, not only because of its role as a plant pathogen, but also as a commonly isolated endophyte which has been shown to produce a wide range of secondary diverse metabolites (Li and Strobel Citation2001; Yasuda et al. Citation2003; Xu et al. Citation2010).
During surveys of the Rimel and Henchir Kort forests of Tunisia, a widespread withering affecting Pistacia lentiscus L. and Quercus coccifera L., respectively, was noted. In fact, the presence of shoot blight, branch cankers and light brown necrosis on twigs, as well as black acervuli on infected tissues, was observed on many individuals. These symptoms, along with the morphological characteristics of the fungi isolated from diseased tissues, indicated that Pestalotiopsis species are the causal agents of P. lentiscus and Q. coccifera diseases.
Until now, data regarding the identity of Pestalotiopsis species in North Africa and in Tunisia have been limited. Thus, the objectives of this work were to (i) characterize and cluster isolates of Pestalotiopsis associated with twig dieback symptoms on shrub species and (ii) confirm their aggressiveness.
Material and methods
Sample collection
Samples were obtained from symptomatic branches of declining Q. coccifera and P. lentiscus in Northeastern (Henchir Kort forest, Cap-Bon) and Northern (Rimel forest, Bizerte) Tunisia, respectively. From each shrub species, 10 shoots showing necrosis were collected and carried to the laboratory for fungal isolation.
Fungal isolation and morphological characterization
Fragments (3 × 3 mm) were taken from the margins between the necrotic and healthy tissues of the branches. Samples were then surface-disinfected in 70% ethanol for 2 min and rinsed three times in sterilized water before being placed in Petri dishes containing potato dextrose agar (PDA) containing streptomycin sulphate (0.05 g/l), and incubated in darkness at 25°C for 3 days (Franceschini et al. Citation2005). Cultures were purified by the hyphal tip technique and incubated under the same conditions described above. Pestalotiopsis species were identified based on morphological traits of 10-day-old cultures and conidial morphology, shape and size according to the key of Maharachchikumbura et al. (Citation2014).
DNA extraction, PCR amplification and sequencing
Based on morphological characterization, two representative isolates of Pestalotiopsis spp. were selected for molecular identification. Total DNA from fresh mycelium grown on PDA was extracted using an innuPREP Plant DNA Kit (Analytik Jena AG, Germany) according to the manufacturer’s instructions. The ITS rDNA region and fragments of a nuclear protein coding gene (tef1-α) were amplified using the universal primer pairs ITS1/ITS4 (White et al. Citation1990) and EF1-728F/ EF2 (Carbone and Kohn Citation1999). Amplification conditions for the ITS rDNA region and tef1-α were followed Crous et al. (Citation2013). Amplified products were sent for Sanger sequencing (Laboratory of the Interdepartmental Center for Biotechnology Services of Agricultural, Chemical and Industrial Interest-CIBIACI, Italy). New sequences were edited with FinchTV v1.4.0 (Geospiza, Inc., Seattle, Washington, USA; (http://www.geospiza.com/finchtv) and compared with those deposited in GenBank through BLAST searches (http://www. ncbi.nih.gov/) (). The DNA sequence alignment was performed using MUSCLE 3.8 (https://www.ebi.ac.uk/Tools/msa/muscle/). ITS and tef1-α sequences were deposited in the National Center for Biotechnology Information (NCBI) as GenBank accession (https://www.ncbi.nlm.nih.gov/) under the accession numbers indicated in .
Table 1. Pestalotiopsis isolates included in the phylogenetic analysis (*).
Table 2. Identity of the isolates, GenBank accession numbers of the sequences, spores size and necrosis length.
Phylogenetic analysis
The ITS and tef1-α sequences obtained in this study were combined and then supplemented with further sequences of Pestalotiopsis spp. retrieved from GenBank () based on BLAST searches and the literature (Maharachchikumbura et al. Citation2014). Sequences of all isolates were aligned with ClustalX v. 1.83 (Thompson et al. Citation1997). Phylogenetic trees were generated using the Neighbour-Joining (NJ) method (Saitou and Nei Citation1987; Tamura et al. Citation2004) with MEGA v. 6 (Tamura et al. Citation2013).
Pathogenicity assay
Pathogenicity assay was conducted according to the methodology of González et al. (Citation2012) by inoculating two Pestalotiopsis isolates on healthy excised branches (n = 10) collected from their host species and measuring about 30 cm [Q. coccifera (n = 5; isolate TN.20) and P. lentsicus (n = 5; isolate TN.109)]. Briefly, after removal of the leaves, branches were surface-disinfected with ethanol (70%) and wounded with a sterilized scalpel. For each isolate, a mycelial plug (6 mm−diam.) was taken from the margin of an actively growing, 7-day-old colony and placed on the wounds of the branches. The inoculation point was covered with cotton wool soaked in sterile water and wrapped in Parafilm. The inoculated branches were then placed in a beaker containing 200 mL of sterile water, whereas the bottom and the top ends of each cane were sealed with a synthetic grafting resin to prevent drying and contamination and enclosed in a transparent plastic bag. Ten control branches for each species [Q. coccifera (n = 5) and P. lentsicus (n = 5)] were inoculated with sterile PDA plugs. All inoculated branches were kept at room temperature (20–26°C) in the laboratory and monitored for necrosis development over 30 days. The pathogen was re-isolated at the end of the experiment.
Data analysis
Average lesion lengths caused by the two Pestalotiopsis species were reported as the mean ± standard error of mean (MSE). The results were evaluated statistically by analysis of variance (ANOVA) with SPSS v.20.
Results
Morphological characterization
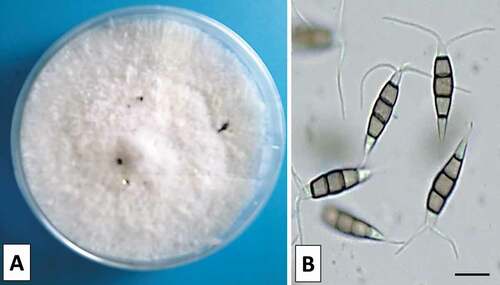
A collection of 38 isolates was obtained from symptomatic branches of Q. coccifera in Henchir Kort and P. lentiscus in Rimel (). Colony colour was whitish with moderately dense mycelium on PDA and reaching 80 mm in diameter after 7 days at 25°C. Black globulose acervular conidiomata on the surface of the mycelium were produced after about 10 days (). Conidiophores indistinct reduced to conidiogenous cells discrete, filiform, clavate or subcylindrical and hyaline with immersed to semi-immersed conidiomata. Conidia were fusoid, ellipsoid, straight to slightly curved, 4-septate. There were three median cells doliiform dark brown concolourous and septa darker than the rest of the cell. Both apical and basal cells were hyaline, the apical cell with 2 or 3 tubular appendages (mostly 3) and the basal cell with two tubular appendages with a truncate base measured 21 to 29.5 × 5 to 7.5 μm in size (). These morphological characteristics were congruous with the description of Pestalotiopsis species (Espinoza et al. Citation2008; Maharachchikumbura et al. Citation2014).
Molecular identification and phylogenetic analysis
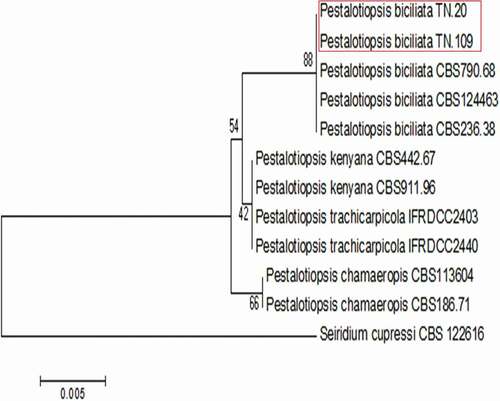
The two representative Pestalotiopsis isolates (TN.20) and (TN.109) collected from Q. coccifera and P. lentiscus, respectively, showed 100% identity at all sequenced sites with Pestalotiopsis biciliata (CBS 124463). These isolates were clustered unambiguously with P. biciliata strains CBS 124463, CBS 236.38 and CBS 790.68 used in the phylogenetic analysis. The combined ITS and tef1-α dataset of Pestalotiopsis spp. included 12 taxa (11 in-group and 1 out-group) and contained 788 characters (including gaps). Seiridium cupressi CBS 122616 was included as the out-group ().
Pathogenicity assay
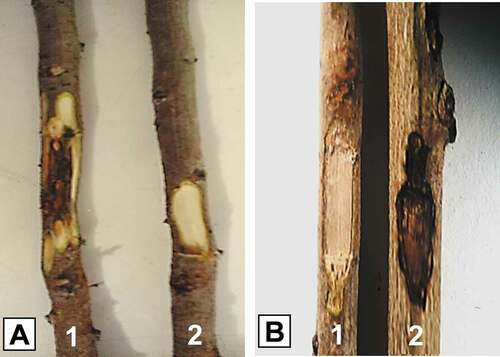
Thirty days after inoculation, necrosis developed extending from the inoculation points, confirming the virulence of the two Pestalotiopsis isolates (TN20 and TN.109) on the shrub species (Q. coccifera and P. lentiscus) ( a2,b2). Stem lesions measured respectively 5.00 ±0.00 cm and 4.66 ± 0.19 cm (). Statistical analysis of variance (ANOVA) showed no significant difference in pathogenicity between the two isolates (F (1,8) = 1.854, P = 0.577). Disease symptoms were absent on control twigs and without fungi development ( a1,b1). Both pathogenic isolates were successfully re-isolated from the margins of the necrotic lesions of each inoculated excised branch. Their identity was confirmed based on morphological and cultural characters.
Discussion
During surveys in Tunisia, disease symptoms, including shoot dieback, branch cankers and vascular discoloration of the wood were observed on P. lentiscus and Q. coccifera. Similar symptoms were reported previously on grapevines and found to be associated with Pestalotiopsis spp. (Maharachchikumbura et al. Citation2011). McQuilken and Hopkins (Citation2004) reported that Pestalotiopsis isolates were not host-specific and induced typical symptoms and signs, including browning of the foliage, stems and roots, and the presence of black acervuli, on various species of ericaceous plants, herbaceous and woody hosts such as Eucalyptus spp. (Morales-Rodríguez et al. Citation2019) and grapevine (Úrbez-Torres et al. Citation2009). Moreover, Pestalotiopsis spp. are responsible for a number of plant diseases (Keith et al. Citation2006; Espinoza et al. Citation2008; Chen et al. Citation2013).
Considering both the morphological and molecular data, we identified Pestalotiopsis biciliata as the causal agent of brown vascular necrosis on Q. coccifera in Kenchir Kort and P. lentiscus in Rimel. Furthermore, P. biciliata was isolated from dry needles of Taxus baccata L. in the Netherlands and in the UK (Liu et al. Citation2019), from Paeonia sp. in Italy and from Platanus hispanica in Slovakia (Maharachchikumbura et al. Citation2014). The fungus was referred as the causal agent of fruit rot on withered grapes and as a foliar pathogen of Eucalyptus spp. in Italy (Lorenzini and Zapparoli Citation2018; Morales-Rodríguez et al. Citation2019). It also was reported as a foliar pathogen of Ceratonia siliqua in Algeria (Louanchi et al. Citation2021). P. biciliata was also associated with grapevine trunk diseases in France (Maharachchikumbura et al. Citation2017) and as pathogen of stone pine orchard in Portugal (Silva et al. Citation2020). Recently, P. biciliata has been recorded to cause dieback and branch necrosis on Pinus pinea in Tunisia (Hlaiem et al. Citation2021).
The pathogenicity assay confirmed the virulence of P. biciliata on wounded branches of Q. coccifera and P. lentiscus in this study, with the fungus-causing symptoms similar to those observed in the field. In contrast, control branches remained asymptomatic. A previous report by Hopkins and McQuilken (Citation2000) indicated that wounding prior to inoculation was necessary for disease development. Similarly, studies conducted with other Pestalotiopsis species have also found that nonwounded samples remained symptomless (Rivera and Wright Citation2000).
Our paper provides valuable information for further study of P. biciliata, reported here as a new causal agent of shoot dieback and stem blight of Q. coccifera and P. lentiscus in the world and specifically in Tunisia. Given the high value of these evergreen shrubs, with high economic and medicinal interests, considerable attention should be given to the serious decline phenomena that have occurred in these regions. Pestalotiopsis species may represent a threat to the health status of forests in the Mediterranean basin and in Tunisia.
Acknowledgements
The authors thank Dr. Mohamed Rabeh HAJLAOUI (Laboratory of Applied Biotechnology in Agriculture, National Agricultural Research Institute of Tunisia) and Dr. Hanen ZAIER (Laboratory of the Improvement and Protection of the Genetic Resources of the olive tree, Olive tree institute) for their help during the course of the molecular work.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Arzanlou M, Torbati M, Khodaei S, Bakhshi M. 2012. Contribution to the knowledge of pestalotioid fungi of Iran. Mycosphere. 3(5):871–878:. https://doi.org/https://doi.org/10.5943/mycosphere/3/5/12
- Barr ME. 1975. Pestalosphaeria, a new genus in the Amphisphaeriaceae. Mycologia 67:187–194.
- Carbone I, Kohn LM. 1999. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia. 91(3):553–556. doi:https://doi.org/10.2307/3761358
- Chen F, Lu L, Wang D, Wang Y, Ni H, Du Z. 2013. Biological characterization and genetic diversity analysis of two species of Pestalotiopsis causing twig dieback of Myrica rubra. Eur J For Pathol. 136:737–747. doi:https://doi.org/10.1007/s10658-013-0203-x
- Crous PW, Wingfield MJ, Guarro J, Cheewangkoon R, Van der Bank M, Swart WJ, Stchigel AM, Cano-Lira JF, Roux J, Madrid H, et al. 2013. Fungal planet description sheets: 154–213. Persoonia. 31:188–296. doi:https://doi.org/10.3767/003158513X675925
- Das R, Chutia M, Das K, Jha DK. 2010. Factors affecting sporulation of Pestalotiopsis disseminata causing grey blight disease of Persea bombycina Kost., the primary food plant of muga silkworm. Crop Prot. 29:963–968. doi:https://doi.org/10.1016/j.cropro.2010.05.012
- Espinoza JG, Briceno EX, Keith LM, Latorre BA. 2008. Canker and twig dieback of blueberry caused by Pestalotiopsis spp. and a Truncatella sp. in Chile. Plant Dis. 92:1407. doi:https://doi.org/10.1094/PDIS-92-10-1407
- Franceschini A, Linaldeddu BT, Marras F. 2005. Occurrence and distribution of fungal endophytes in declining cork oak forests in Sardinia (Italy). IOBC/WPRS Bull. 28:67–74.
- González P, Alaniz S, Montelongo MJ, Rauduviniche L, Rebellato J, Silvera-Pérez E, Mondino P. 2012. First report of Pestalotiopsis clavispora causing dieback on blueberry in Uruguay. Plant Dis. 96(6):914. doi:https://doi.org/10.1094/PDIS-12-11-1070-PD
- Hlaiem S, Yangui I, Della Rocca G, Barberini S, Danti R, Ben Jamâa ML. 2021. First report of Pestalotiopsis biciliata associated with twig canker and dieback of Pinus pinea in Tunisia. J Plant Pathol. https://rdcu.be/cy6Vd.
- Hlaiem S, Zouaoui-Boutiti M, Ben Jemâa ML, Della Rocca G, Barberini S, Danti R. 2018. Identification and pathogenicity of Pestalotiopsis chamaeropis, causal agent of white heather (Erica arborea) dieback, and in vitro biocontrol with the antagonist Trichoderma sp. Tunisian J Plant Prot. 13(si):49–60.
- Hopkins KE, McQuilken MP. 2000. Characteristics of Pestalotiopsis associated with hardy ornamental plants in the UK. Eur J Plant Pathol. 106:77–85. doi:https://doi.org/10.1023/A:1008776611306
- Hu H, Jeewon R, Zhou D, Zhou T, Hyde KD. 2007. Phylogenetic diversity of endophytic Pestalotiopsis species in Pinus armandii and Ribes spp.: evidence from rDNA and β-tubulin gene phylogenies. Fungal Divers. 24:1–22.
- Index Fungorum. 2016. http://www.indexfungorum.org/names/names.asp
- Jeewon R, Liew ECY, Hyde KD. 2002. Phylogenetic relationships of Pestalotiopsis and allied genera inferred from ribosomal DNA sequences and morphological characters. Mol Phylogenet Evol. 25:378–392. doi:https://doi.org/10.1016/S1055-7903(02)00422-0
- Judith-Hertz C. 2016. Systematics and species delimitation in Pestalotia and Pestalotiopsis S.L. (Amphisphaeriales, Ascomycota) [Thesis]. Darmstadt, Frankfurt am Main; p. 203.
- Karakaya A. 2001. First report of infection of kiwifruit by Pestalotiopsis sp. in Turkey. Plant Dis. 85:1028. doi:https://doi.org/10.1094/PDIS.2001.85.9.1028C
- Keith LM, Velásquez ME, Zee FT. 2006. Identification and characterization of Pestalotiopsis spp. causing scab diseases of guava, Psidium guajava, in Hawaii. Plant Dis. 90:16–23. doi:https://doi.org/10.1094/PD-90-0016
- Li JY, Strobel GA. 2001. Jesterone and hydroxy-jesterone antioomycet-cyclohexenenone epoxides from the endophytic fungus Pestalotiopsis jesteri. Phytochemistry. 57:261–265. doi:https://doi.org/10.1016/S0031-9422(01)00021-8
- Liu F, Bonthond G, Groenewald JZ, Cai L, Crous PW. 2019. Sporocadaceae, a family of coelomycetous fungi with appendage-bearing conidia. Stud Mycol. 92:287–415. [CrossRef] [PubMed]. doi:https://doi.org/10.1016/j.simyco.2018.11.001
- Liu AR, Wu XP, Xu T, Guo LD, Wei JG. 2006. Notes on endophytic Pestalotiopsis from Hainan, China. Mycosystema. 25:389–397.
- Lorenzini M, Zapparoli G. 2018. Identification of Pestalotiopsis bicilita, Diplodia seriata and Diaporthe eres causing fruit rot in withered grapes in Italy. Eur J Plant Pathol. 151:1089–1093. [CrossRef]. doi:https://doi.org/10.1007/s10658-017-1416-1
- Louanchi M, Zazoua M, Hammad M, Alem M, Kerkoud M, Keddad A, Bouznad Z. 2021. First report of necrotic leaf spot on Ceratonia siliqua caused by Pestalotiopsis biciliata. J Plant Pathol. 103(3):1081. doi:https://doi.org/10.1007/s42161-021-00887-1
- Maharachchikumbura SSN, Chukeatirote E, Guo LD, Crous PW, McKenzie EHC, Hyde KD. 2013b. Pestalotiopsis species associated with Camellia sinensis (tea). Mycotaxon. 123:47–61. doi:https://doi.org/10.5248/123.47
- Maharachchikumbura SSN, Guo LD, Chukeatirote E, Bahkali AH, Hyde KD. 2011. Pestalotiopsis–morphology, phylogeny, biochemistry and diversity. Fungal Divers. 50:167–187. doi:https://doi.org/10.1007/s13225-011-0125-x
- Maharachchikumbura SSN, Guo LD, Chukeatirote E, McKenzie EHC, Hyde KD. 2013a. A destructive new disease of Syzygium samarangense in Thailand caused by the new species Pestalotiopsis samarangensis. Trop Plant Pathol. 38(3):227–235. doi:https://doi.org/10.1590/S1982-56762013005000002
- Maharachchikumbura SSN, Hyde K, Groenewald JZ, Xu J, Crous PW. 2014. Pestalotiopsis revisited. Stud Mycol. 79:12186. doi:https://doi.org/10.1016/j.simyco.2014.09.005
- Maharachchikumbura SSN, Larignon P, Hyde K, Al-Sadi A, Liu ZY. 2017. Characterization of Neopestalotiopsis, Pestalotiopsis and Truncatella species associated with grapevine trunk diseases in France. Phytopathol Mediterr. 55:380–390. [ CrossRef].
- McQuilken MP, Hopkins K. 2004. Biology and integrated control of Pestalotiopsis on container-grown ericaceous crops. Pest Manage Sci. 60:135–142. doi:https://doi.org/10.1002/ps.792
- Morales-Rodríguez C, Dalla Valle M, Aleandri M, Vannini A. 2019. Pestalotiopsis biciliata, a new leaf pathogen of Eucalyptus spp. recorded in Italy. For Pathol. 49:e12492. Eutypa armeniacae. Plant Dis Rep. 62:254–258. doi:https://doi.org/10.1111/efp.12492
- Rivera MC, Wright ER. 2000. First report of azalea petal blight caused by Pestalotiopsis guepini in Argentina. Plant Dis. 84:100. doi:https://doi.org/10.1094/PDIS.2000.84.1.100C
- Saitou N, Nei M. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 4:406–425. doi:https://doi.org/10.1093/oxfordjournals.molbev.a040454
- Satini A, Ghelardini L, Pace De C, Desprez-Loustaundelier A, Cech T, Chira D, Diamandis S, Gaitniekis T, Hantula J, Holdenrieder O, et al. 2013. Biogeographical patterns and determinants of invasion by forest pathogens in Europe. New Phytol. 197:238–250. doi:https://doi.org/10.1111/j.1469-8137.2012.04364.x
- Silva AC, Diogo E, Henriques J, Paula Ramos A, Sandoval-Denis M, Crous PW, Braganç H. 2020. Pestalotiopsis pini sp. nov., an emerging pathogen on stone pine (Pinus pinea L.). Forests. 11:805. doi:https://doi.org/10.3390/f11080805
- Sutton BC. 1980. The Coelomycetes. Fungi imperfecti with pycnidia, acervuli and stromata. Kew (UK): Commonwealth Mycological Institute; p. 696.
- Tagne A, Mathur SB. 2001. First report of chlorotic spot of maize caused by Pestalotiopsis neglecta. Plant Pathol. 50:791. doi:https://doi.org/10.1046/j.1365-3059.2001.00614.x
- Tamura K, Nei M, Kumar S. 2004. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc Natl Acad Sci USA. 101(30):11030–11035. doi:https://doi.org/10.1073/pnas.0404206101
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 30(12):2725–2729. doi:https://doi.org/10.1093/molbev/mst197
- Tanja V, Darko J, Vesna K, Aleksandar L, Andjelković S, Sanja Z, Svetlana P. 2017. Morphological description and molecular detection of Pestalotiopsis sp. on hazelnut in Serbia. Span J Agric Res. 15(3):e10SC02, 5. doi:https://doi.org/10.5424/sjar/2017153-11297
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. 1997. The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acid Res. 25(24):4876–4882. doi:https://doi.org/10.1093/nar/25.24.4876
- Úrbez-Torres JR, Adams P, Kamas J, Gubler WD. 2009. Identification, incidence, and pathogenicity of fungal species associated with grapevine dieback in Texas. Am J Enol Vitic. 60:497–507.
- Watanabe K, Motohashi K, and Ono Y. 2010. Description of Pestalotiopsis pallidotheae: a new species from Japan. Mycoscience 51:182–188.
- White TJ, Bruns T, Lee S, Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols. A guide to methods and applications. San Diego (CA): Academic Press; p. 315–322.
- Xu J, Ebada SS, Proksch P. 2010. Pestalotiopsis a highly creative genus: chemistry and bioactivity of secondary metabolites. Fungal Divers. 44(1):15–31. doi:https://doi.org/10.1007/s13225-010-0055-z
- Yasuda F, Kobayashi T, Watanabe H, Izawa H. 2003. Addition of Pestalotiopsis spp. to leaf spot pathogens of Japanese persimmon. J Gen Plant Pathol. 69:29–32. doi:https://doi.org/10.1007/s10327-002-0011-1
- Zhang Y, Crous PW, Schoch CL, Hyde KD. 2012. Pleosporales. Fungal Divers. 53:1–2211414. doi:https://doi.org/10.1007/s13225-011-0117-x
- Zhang YM, Maharachchikumbura SSN, Tian G, Hyde KD. 2013. Pestalotiopsis species on ornamental plants in Yunnan Province, China. Sydowia. 65:113–128.