Abstract
Biocontrol, an alternative to chemical control against plant pathogens, may also improve plant health and enhance fruit yield. Tomato production in open fields or greenhouses is constrained by the pathogens Fusarium oxysporum f. sp. lycopersici race 3 (Fol R3) and F. oxysporum f. sp. radicis-lycopersici (Forl). In this work, we studied the biocontrol effect of the antagonistic bacteria Acinetobacter calcoaceticus AcDB3, Bacillus thuringiensis BtMB9, B. subtilis BsTA16, and B. amyloliquefaciens BaMA26 in field trials with four tomato hybrids over two consecutive growing seasons (2019–2020 and 2020–2021). The effect of these bacteria on plant growth was also evaluated. The presence of F. oxysporum in field soil and/or infected plants was confirmed microbiologically. All four bacterial strains significantly suppressed the severity of Fusarium crown and root rot of tomato (FCRRT) and Fusarium wilt symptoms, as well as increased tomato yield under field conditions. Among the four strains, B. subtilis BsTA16 showed the highest reduction in symptoms of Fusarium wilt (68%) and FCRRT (74%). To the best of our knowledge, this is the first report of biological control agents (BCAs) exerting antagonistic activity against both FCRRT caused by Forl and Fusarium wilt caused by Fol in tomato under field conditions.
Résumé
La lutte biologique, une solution de rechange à la lutte chimique contre les agents pathogènes, peut aussi contribuer à améliorer la santé des plantes et le rendement en fruits. La production de tomates en plein champ ou en serre est limitée par les agents pathogènes Fusarium oxysporum f. sp. lycopersici race 3 (Fol R3) et F. oxysporum f. sp. radicis-lycopersici (Forl). Dans le cadre de ces travaux, nous avons étudié, dans un contexte de lutte biologique, l’action des bactéries antagonistes Acinetobacter calcoaceticus AcDB3, Bacillus thuringiensis BtMB9, B. subtilis BsTA16 et B. amyloliquefaciens BaMA26 dans des essais menés en champ avec quatre hybrides de tomate au cours de deux saisons de croissance consécutives (2019-2020 et 2020-2021). L’effet de ces bactéries sur la croissance des plants a également été évalué. La présence de F. oxysporum dans le sol ou sur les plants infectés a été confirmée microbiologiquement. Les quatre souches de bactéries ont significativement supprimé la gravité des symptômes de la pourriture de la couronne et des racines de la tomate causée par le fusarium (PCRTF) et du flétrissement fusarien, tout en accroissant le rendement des tomates dans des conditions naturelles. Parmi les quatre souches, B. subtilis BsTA16 a affiché la plus forte réduction des symptômes du flétrissement fusarien (68%) et de la PCRTF (74%). À notre connaissance, il s’agit du premier rapport traitant d’agents de lutte biologique qui exercent une activité antagoniste contre la PCRTF causée par Forl et le flétrissement fusarien causé par Fol chez la tomate dans des conditions naturelles.
Introduction
Globally, tomato (Solanum lycopersicum L.) is one of the most important horticultural crops cultivated in greenhouses and fields (Atherton and Rudich Citation2013). In 2019, Mexico was ranked ninth worldwide in tomato production, with an annual harvest of 4.58 million tons (FAOSTAT Citation2019). Fusarium oxysporum Schlectend.: Fr. f. sp. lycopersici (Sacc.) W.C. Snyder and H.N. Hansen (Fol) and f. sp. radicis-lycopersici Jarvis and Shoemaker (Forl) are the causal agents of two devastating diseases of this crop, Fusarium wilt and Fusarium crown and root rot of tomato (FCRRT) (Shanmugam and Kanoujia Citation2011). Fusarium wilt caused by Fol R3 is a diverse phytopathogenic fungus due to its specificity and virulence (Vega-Gutiérrez et al. Citation2019). Forl, another forma specialis of F. oxysporum, causes FCRRT, a disease that frequently threatens both greenhouse and open-field tomato production (Manzo et al. Citation2016). Indeed, tomato seedlings can be infected by either Fol R3 or Forl in greenhouses and open fields (Baysal et al. Citation2008).
Recently, it was shown that Fusarium wilt caused by Fol can reduce tomato production under open-field conditions in India by up to 45% (Elanchezhiyan et al. Citation2018). Similarly, FCRRT disease can damage tomato production under open field conditions by up to 50% in Mexico (Apodaca-Sánchez et al. Citation2002), and by 90% in Turkey (Çolak and Biçici Citation2013).
High doses of fungicides used for F. oxysporum control in tomato are not only expensive, but also have negative effects on ecosystems as well as animal and human health (Hu et al. Citation2015). In addition, chemical fungicides may adversely affect the soil microbiota, which is important to agriculture and the environment (Singh et al. Citation2020). One environmentally friendly approach for plant pathogen management is biological control. Biocontrol uses microorganisms (i.e. bacteria and fungi) that are capable of inhibiting or suppressing pathogen populations (Wisniewski et al. Citation2016; Chow et al. Citation2018; San Millan et al. Citation2021), making it a suitable alternative for controlling phytopathogenic fungi. Some microorganisms that control fungal plant pathogens are also able to promote plant growth (Zouari et al. Citation2020). In recent years, the demand for biocontrol has increased among growers, as it is an environmentally friendly alternative to chemical fungicides (Barratt et al. Citation2018). Biological control agents (BCAs) may have a better chance of establishment and effective pathogen control if they are native to the soil, in comparison with exotic microorganisms. Indeed, native microorganisms are already adapted to the local climate and edaphic conditions as well as to the soil microbiota (Ali et al. Citation2018). For this reason, native bacterial strains can be a better choice for the development of BCAs.
Plant rhizospheric bacteria can suppress plant pathogens as well as promote plant growth. Bacillus and Acinetobacter species can inhibit a large variety of pathogens in diverse ecological niches, as they are widespread in soil, water, and the rhizosphere (Pignatelli et al. Citation2009; Connor et al. Citation2010; Durán et al. Citation2018). From a biotechnological standpoint, the bacteria in these two genera have been well identified as biocontrol agents against different phytopathogens, due to their direct antagonistic mechanisms employing chitinase, protease and glucanase activities and siderophore production, which can be used to control pathogen growth (Figueroa-López et al. Citation2016; Khalil et al. Citation2021). In addition, they can exhibit plant growth-promoting traits such as phosphate solubilization or indole-3-acetic acid (IAA) production (Farokh et al. Citation2011; Iyer et al. Citation2017; Shah et al. Citation2020). Plant disease control and enhancement of plant growth depend on a stable rhizosphere community, with multiple modes of action and a wide range of environmental conditions (Larkin and Fravel Citation1998). Finally, recent work has demonstrated that biofilm formation is an advantageous trait for BCAs such as bacteria, since it helps them attach to the plant root surface (Pandin et al. Citation2019).
The present study aimed to evaluate four bacteria (Acinetobacter calcoaceticus (Beijerinck 1911) Baumann et al. 1968, AcDB3; Bacillus thuringiensis Berliner, 1915, BtMB9; Bacillus subtilis (Ehrenberg 1835) Cohn, 1872, BsTA16; and Bacillus amyloliquefaciens (ex Fukumoto 1943) Priest et al. 1987 emend. Wang et al. 2008, BaMA26) as antagonists of Fol R3 and Forl in naturally infested tomato fields, and to measure their plant growth-promotion effects, in order to select the most efficient bacterium or combination of bacteria.
Materials and methods
Microorganisms
Three rhizospheric Bacillus strains (B. thuringiensis BtMB9, B. subtilis BsTA16, and B. amyloliquefaciens BaMA26) and the A. calcoaceticus strain AcDB3 were used in field trials. The rhizospheric bacteria were obtained from Datura sp., maize, and tomato. The bacteria selection criteria, strain IDs and growth conditions were previously described in Khalil et al. (Citation2021). The bacterial strains were preserved at −70°C in Luria Bertani (LB, Sigma, No. Cat. L3022, USA) broth supplemented with 15% glycerol.
Inoculum preparation
Cryopreserved bacterial strains (AcDB3, BtMB9, BsTA16 and BaMA26) were grown on LB agar (LBA) medium for 24 h. A single colony was transferred to 5 mL of LB broth medium and incubated at 30°C for 24 h at 200 rpm on an orbital shaker (VWR, Mexico) to obtain the pre-inoculum bacterial suspension. Next, 1 mL of bacterial suspension was transferred to 100 mL of LB and incubated at 30°C for 9 h at 200 rpm on an orbital shaker until the exponential growth phase, in order to collect bacteria for inoculum. Bacteria were diluted in LB medium to the optical density (OD) corresponding to the desired CFU mL−1 concentration (2 x 108 CFU mL−1).
Detection of soil type and composition
Soil samples were collected from each experimental field and brought into the laboratory for further analysis on the same day. The soil was collected at a depth of 25–30 cm. Briefly, three soil samples were collected from each experimental site in the field, mixed thoroughly to make a composite sample, and dried in a hot air oven at 80°C for 24 h. The soil was ground further and very fine particles were collected through a mesh; particles greater than 2 mm in diameter were discarded. Different amounts of soil were used for laboratory analyses (soil texture analysis: 100 g; organic content: 0.5 g; inorganic content: 5 g; pH: 10 g; and electrical conductivity: 5 g). Soil texture (Bouyoucos Citation1962), composition organic (Walkley and Black Citation1934), and inorganic particles: phosphorus (Olsen et al. Citation1954), potassium, calcium, magnesium, sodium (ammonium acetate method), nitrate (automated colorimetry method), pH (electrometric method) and electrical conductivity (saturation paste method) were measured in the laboratory of Plant Nutrition at the Department of Agricultural Biotechnology, CIIDIR-Sinaloa, IPN, following the official Mexican standard (Proyecto de Norma Oficial Mexicana PROY-NOM-021-RECNAT-2000) (). After obtaining the soil particle composition (percentage clay, sand, and silt), the soil texture was detected using the triangle texture diagram from the USDA (Gee and Or Citation2002) ().
Table 1. Soil composition of four different fields.
Table 2. Geographic and agronomic information for the experimental fields from Sinaloa, Mexico.
Field trials
Two different field trials were performed in two consecutive autumn-winter agronomic cycles, in 2018–2019 (trial I) and 2019–2020 (trial II). Each trial was conducted in two different fields in the municipality of Guasave, Sinaloa, Mexico (). The experiments were conducted at the experimental field station (CIIDIR-Sinaloa), as well as in three different field locations of Agrícola Doña Alicia. Crop management during the two trials was monitored by the tomato farmers, except for the bacterial inoculum application. Antagonism against disease caused by Fol R3 and Forl was evaluated in both field trials (field trials I and II), as well as plant growth-promotion effects (stem thickness and total fruit weight). The following fertilizer composition was applied to the soils of the three fields (: Sites B-D) during tomato cultivation: phosphonitrate, 12 kg per ha; phosphoric acid, 4 L per ha; potassium nitrate, 15 kg per ha; potassium sulphate, 6 kg per ha; calcium nitrate, 18 kg per ha; magnesium sulphate, 18 kg per ha; boron, 600 g per ha; and micronutrients, 2 kg per ha. These ingredients were mixed with water in a large tank and applied to the soil through dripping lines in 3-h sessions, three times per week. The agrochemicals were applied to plants, when needed, by a spraying machine (). Irrigation was performed three times per week for 3 h. The treatments were arranged in a randomized complete block design, with three blocks and 30 plants (replicates) per block for both field trials (field trials I and II).
Table 3. Tomato plant transplant and harvest dates, including agricultural management information, for the field trials conducted at four sites in two consecutive growing seasons. All tomato hybrids were susceptible to Fol race 3 and Forl and resistant to Fol races 0–2, except hybrid SV8579TE. The hybrid SV8579TE was resistant to all races of Fol. Tomato fruits were collected at different times while the producer harvested. The disease incidence and severity of infected plants were calculated by the end of the cycle (hybrid Tisey: 119 days; DRD 8551: 180 days; SV8579TE: 209 days; and hybrid Katya: 117 days).
Field trial I (2018–2019 season)
The first field experiment was arranged in two different field locations (Sites A and B; ). The experimental field was divided into three blocks of three rows, and each row was 10 m long and contained 30 plants. The experimental plots were set in the middle of the field, and a total of nine rows were arranged in each block (Lizárraga-Sánchez et al. Citation2015). The plants were transplanted 30 cm apart from each other, with a distance of 1.5 m between rows. Two indeterminate tomato hybrids (‘Tisey’ and ‘DRD 8551’, Seminis, USA) resistant to Fol races 1 and 2, but susceptible to Fol R3 and Forl, were used. The fields were not inoculated, but were kept under natural field infestation conditions of Fol races (0–3) and Forl. The two sites were selected based on previous observations (Mr. Christian Robles Herrera, pers. Comm.) of > 50% Fusarium wilt and FCRRT incidence at these sites in the previous growth cycle (2018–2019). Tomato seedlings (12 cm in length) were transplanted manually into the field according to the producer’s practices. For each treatment, 90 plants were selected, and a 5-mL bacterial suspension (2 × 108 CFU mL−1) was added to each tomato root system in a germination tray 2 days before transplanting. The bacterial isolates AcDB3, BtMB9, BsTA16 and BaMA26 were tested individually and in combination. According to previous greenhouse selection assays (Khalil et al. Citation2021), BsTA16 and BaMA26 are effective control agents against Fol R3, while AcDB3 and BtMB9 are effective against Forl. A total of nine treatments were included, in addition to a negative control. Applications consisted of four single treatments (AcDB3, BtMB9, BsTA16 and BaMA26) and four combined treatments (AcDB3 + BsTA16, AcDB3 + BaMA26, BtMB9 + BsTA16, and BtMB9 + BaMA26). Forty-seven days after transplanting, the second bacterial inoculation (2 x 108 CFU mL−1; ~12 mL per plant) was applied to the soil surrounding the plant roots using a backpack sprayer 1 h before the start of irrigation. Thirty plants per treatment were selected from the middle of each row (10 plants per block) for data collection. Mature tomatoes were harvested five times, and the total weight of fruits on each plant was recorded.
Field trial II (2019-2020 season)
A second field experiment was conducted in two different field locations (Sites C and D, ) during the 2019–2020 growing season. Similar single and combined bacterial treatments (nine treatments with a negative control) as used in field trial I were performed in this trial to confirm the results of trial I under natural Fol R3 and Forl field infestation conditions. In addition, similar field arrangements were employed as those described for field trial I. For Site C, the determinate hybrid ‘Katya’ (Hazera, USA; resistant to Fol races 0–2, and susceptible to Fol R3) was used; for Site D, the indeterminate tomato hybrid ‘SV8579TE’ (Seminis, USA; resistant to Fol races 0–3) was used. A similar pattern of bacterial inoculation was performed, with a second bacterial inoculation 47 days after field transplant. Finally, similar irrigation management was used as described in the first field trial. Agrochemical usage information is provided for both experimental field trials in .
Fruit yield and stem diameter measurements
Thirty plants per treatment (three rows of 10 plants each) were randomly selected from the centre of each plot for data collection. The time the first tomatoes were harvested varied depending on the hybrid. Tomato fruits were collected each week at harvest time. The ripened tomatoes (colour breaking and red) were harvested a total of five times (Supplementary Fig. S1E), and the total weight (kg) of fruits per plant was measured. Plant stem thickness (~10 cm aboveground) was measured with a Vernier caliper (Series 530 – Standard Model, Truper, Mexico).
Disease rating
The incidence of Fol R3 and Forl was assessed by visually estimating the presence or absence of disease symptoms for each of the 30 sampled plants (10 plants per block). For each hybrid, the disease severity of Fol R3 and Forl was evaluated at different times after transplanting (‘Tisey’: 119 days; ‘DRD 8551’: 180 days; ‘SV8579TE’: 209 days; and ‘Katya’: 117 days). For disease rating (Fusarium wilt and FCRRT), the plants were carefully removed by hand, and the stem and crown were dissected longitudinally with a knife to observe for the presence of brown necrosis. For both diseases, different severity scales with modifications (Rowe Citation1980) were used to estimate the disease severity on each plant. Fusarium wilt was determined by the presence of brown necrosis in the stem and FCRRT in the crown. Each plant was rated on a scale from 0 to 4 as follows: 0 = no internal browning, 1 = 25% (slight internal browning), 2 = 50% (moderate internal browning), 3 = 75% (moderate to severe internal browning), and 4 = 100% (the plant is almost dead). The presence of the disease agents from the diseased tissues in both field trials was confirmed microbiologically (data not shown).
Statistical analysis
These data were subjected to the Shapiro-Wilk test of normality, and ANOVA was performed once the normality of data was confirmed. Tukey’s test was used for post-hoc analysis at α = 0.05 (Driscoll Citation1996). The response indexes related to the control treatment were calculated for all evaluated variables and at each experimental site. These indexes were used to reduce the number of variables and to determine those with the greatest contribution to the total variability, by means of a principal component analysis (Pearson Citation1901). A significant contribution of principal component variables was observed when the value of its unitary contribution was higher than 0.8 (80%) (Shabala and Munns Citation2017). Principal components were presented in a biplot graphic. From the principal component variables, a hierarchical conglomerate analysis of complete linkage was performed, based on a Euclidean distance matrix (Rohlf and Fisher Citation1968), in order to group the variables. The distance values from the control treatment and between each formed group were determined. Finally, the correlation matrix between variables was obtained as well as the partial correlations between variables from Sites A-B, A-C and B-C (Mertler and Reinhart Citation2016). The analysis was performed using Statistics v. 8.1 and the Statistica professional statistical package v. 8.4 (StatSoft, Tulsa, OK).
Results
Soil type and composition
The percentage of organic matter in all fields was between 1.20% and 1.74%. All measured nutrients had values in the moderate range. The electrical conductivity in all fields was between 0.15 and 1.5 mS cm−1. The soil pH was in the neutral range (7–7.5) in the four fields (). A silty clay loam soil type was detected in Sites A and D, while Sites B and C contained loam and clay loam soil ().
Trial I (2018-2019 season)
Two indeterminate tomato hybrids (‘DRD 8551’ and ‘Tisey’) resistant to Fol R1 and R2 and susceptible to Fol R3 and Forl were used in trial I. In Agrícola Doña Alicia, where hybrid ‘DRD 8551’ was used, all four bacterial strains significantly decreased (P ≤ 0.05) the severity of Fusarium wilt and FCRRT when used alone or combined under natural field-occurring inoculum conditions of Fol R3 and Forl (; Supplementary Fig. S1A-D). The incidences of Fusarium wilt and FCRRT were 60% and 57% in non-inoculated plants, respectively. By comparison, the incidence of Fusarium wilt and FCRRT was 27–43% and 47–53%, respectively, in bacteria-inoculated plants, with some treatments significantly (P ≤ 0.05) lower than the control. BsTA16 was the most effective, as it decreased Fusarium wilt and FCRRT severity by 72% and 68%, respectively (). In the ‘Tisey’ hybrid at the CIIDIR-Sinaloa experimental field, the bacterial strains BtMB9 and BaMA26 did not decrease the incidence or severity of Fusarium wilt (alone or combined), and they only reduced FCRRT severity when applied alone ().
Table 4. Trial I. Plant growth-promotion and biocontrol effect of four antagonistic bacterial strains (AcDB3, BtMB9, BsTA16 and BaMA26) in two different tomato hybrids (DRD 8551 and Tisey) against natural Fol R3 and Forl inoculum and infection conditions in two different fields, during the 2018–2019 growing season. The two experimental fields are Site A: Agrícola Doña Alicia, and Site B: the CIIDIR-Sinaloa experimental field station.
Plants of the hybrid ‘DRD 8551’ showed increased stem diameter when the bacteria were inoculated individually, but not when used in combination, as compared with non-inoculated plants (). All four bacterial strains (AcDB3, BtMB9, BsTA16 and BaMA26), whether single or combined, significantly increased (P ≤ 0.05) the total fruit yield as compared with the untreated controls (). ‘Tisey’ plants treated with BtMB9 or BaMA26 (single or combined) increased their stem diameter significantly as compared with control plants (). Isolate BtMA26 significantly (P ≤ 0.05) promoted total fruit yield when either administered alone or combined with BmMB9, in comparison with untreated control tomato plants. Isolate BmMB9 did not promote fruit yield when used alone ().
Trial II (2019-2020 season)
For the second trial at Site C, the indeterminate hybrid ‘SV8579TE’ was used. It is resistant to Fol R3 and susceptible to Forl. The determinate hybrid ‘Katya’, which is susceptible to Fol R3 and Forl, was used at Site D.
The four bacteria, either single or combined, significantly (P ≤ 0.05) decreased Fusarium wilt and FCRRT severity on ‘SV8579TE’ and ‘Katya’ in comparison with the non-inoculated controls (; Supplementary Fig. S1.A-D). The incidence of Fusarium wilt and FCRRT was 60% and 57%, respectively, in non-inoculated ‘SV8579TE’ hybrid plants, and 70% and 77% in non-inoculated ‘Katya’ hybrid plants. By comparison, the Fusarium wilt and FCRRT incidences were 17–23% and 27–47% in bacteria-inoculated ‘SV8579TE’ hybrid plants; and 33–57% and 40–57% in bacteria-inoculated ‘Katya’ hybrid plants, respectively, with some treatments significantly (P ≤ 0.05) lower than the control. Fusarium wilt incidence was significantly (P ≤ 0.05) decreased by all bacterial treatments in hybrid ‘SV8579TE’. Individual inoculation with bacterial strains BtMB9, BsTA16 and BaMA26 significantly increased the plant stem diameter in ‘SV8579TE’ and ‘Katya’ compared with the non-treated plants; AcDB3 did not increase this parameter in the hybrid ‘SV8579TE’. All bacterial combinations increased stem diameter in ‘SV8579TE’ compared with the control plants, whereas in ‘Katya’, only the combinations BtMB9 + BsTA16 and BtMB9 + BaMA26 increased stem diameter as compared with the non-inoculated controls (). All four bacterial strains increased total fruit yield in hybrids ‘SV8579TE’ and ‘Katya’, either when used alone or in combination ().
Table 5. Trial II. Plant growth-promotion and biocontrol effect of four antagonistic bacterial strains (AcDB3, BtMB9, BsTA16 and BaMA26) in two different tomato hybrids (SV8579TE and Katya) against natural Fol R3 and Forl inoculum and infection conditions in two different fields, during the 2019–2020 growing season. The two experimental fields are Site C: Agrícola Doña Alicia (Batamote), and Site D: Agrícola Doña Alicia (Guayparime).
PCA and cluster analysis
Principal component analysis indicated that 74.47% of the total variability among treatments could be explained by the first two components (). In the first component, 13 variables were accumulated, while only two variables accumulated in the second component; these two variables contributed 13% (Site C-FCRRT and Site C-SFW) to the total variability. The variables Site A-SD (stem diameter), Site A-SFW (severity of Fusarium wilt), and Site D-SD did not classify as principal components ( and Supplementary Table S1). None of the four bacteria contributed to increased plant stem diameter in any of the four tomato hybrids when applied alone or in combination.
Fig. 1 Biplot of principal component analysis. Factor loadings (unrotated) extraction: principal components (marked loadings are > 0.700000). SA = Site A: Agrícola Doña Alicia (Batamote); SC = Site C: Agrícola Doña Alicia (Batamote); SD = Site D: Agrícola Doña Alicia (Guayparime); FW = fruit weight; SD = stem diameter; IFW = incidence of Fusarium wilt; IFCRRT = incidence of Fusarium crown and root rot of tomato; SFW = severity of Fusarium wilt; SFCRRT = severity of Fusarium crown and root rot of tomato.
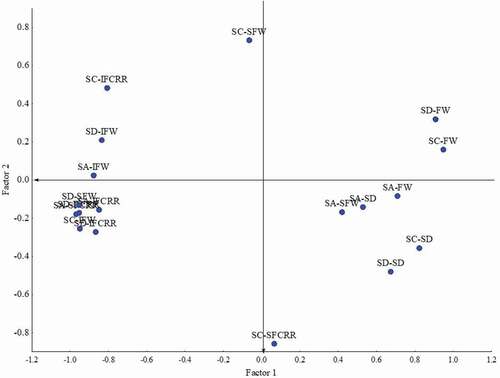
Cluster analysis indicated the presence of two groups (Supplementary Fig. S2). The first group contained the two treatments (BtMB9 + BaMA26 and BsTA16) most effective against Fusarium wilt and FCRRT. Inoculation with bacterial strain BsTA16 resulted in the lowest disease incidence and severity of FCRRT in two indeterminate tomato hybrids (‘DRD 8551’ and ‘SV8579TE’). Furthermore, it increased tomato fruit yield in these hybrids and the indeterminate tomato hybrid ‘Katya’, in addition to significantly decreasing the disease incidence and severity of Fusarium wilt.
The second group was formed by the six remaining treatments: BmMB9 + BsTA16; AcDB3 + BtMA26; AcDB3 + BsA16; BtMA26; BmMB9; and AcDB3.
Discussion
Biological control agents (BCAs) are broadly used in the control of soilborne plant pathogens to minimize the use of harmful agrochemicals, making them ideal for sustainable agricultural practices (Kamali et al. Citation2019). These factors make the search for environmentally friendly, inexpensive, sustainable alternatives for the management of Fusarium wilt and FCRRT a good choice for tomato growers (Nguyen et al. Citation2019). Field experiments are often considered the final step for selecting potential BCAs. The advancement of biological control against fungal phytopathogens is aimed at finding ideal antagonistic bacterial strains with different modes of action. Nevertheless, the effects of antagonistic bacteria may depend on their establishment in a specific geographic area (Gómez et al. Citation2016), since direct (e.g. the bacteria use their antagonistic potential, including plant growth promotion) and indirect mechanisms (such as interaction with other members of the soil microbiota) may allow the bacterial strain to exert or impede its biological control (Winding et al. Citation2004; Jangir et al. Citation2019).
This study focused on Fusarium wilt and FCRRT, two major limiting factors in northern Mexican tomato fields (Apodaca-Sánchez et al. Citation2002). We investigated the efficacy of native BCAs against Fol R3 and Forl. Four bacterial strains (AcDB3, BtMB9, BsTA16, and BaMA26) previously selected in greenhouse bioassays against Fol R3 and Forl (Khalil et al. Citation2021) were evaluated in field trials during the 2018–2019 and 2019–2020 seasons. The presence of these two pathogens was previously reported in different tomato-producing regions of Mexico (Hernández-Martínez et al. Citation2014), including Sinaloa (Mendívil-Trujillo Citation2013).
Bacillus bacteria are well known for their plant growth and biocontrol effects in different crops (Moradi et al. Citation2012). In the present study, the four bacterial strains (AcDB3, BtMB9, BsTA16, and BaMA26) (Khalil et al. Citation2021) were found to enhance the tomato yield as well as improve the disease symptomatology of Fusarium wilt and FCRRT under natural Fol R3 and Forl inoculum and infection conditions, as compared with plants that were not inoculated with any bacteria (Supplementary Fig. S1). These effects were observed in two consecutive growing seasons (2018–2019 and 2019–2020) in four different fields with four different tomato hybrids (three indeterminate and one determinate) under natural Fol R3 and Forl inoculum and infection conditions. The results demonstrate that all four bacterial strains were able to control both Fol R3 and Forl under field conditions. These findings are in agreement with Guo et al. (Citation2004), who tested rhizobacteria against Fusarium wilt and found good control in the fields. They are also consistent with Moradi et al. (Citation2012), who reported that Bacillus species decrease the incidence and severity of Fusarium wilt under natural field conditions and increase total chickpea yields. Previous reports have shown that Bacillus cereus and B. subtilis decrease FCRRT severity and increase tomato growth under controlled environments (Baysal et al. Citation2008; Kamou et al. Citation2015), in agreement with our findings. Moreover, Xue et al. (Citation2009) reported that Acinetobacter species decrease the severity of Ralstonia wilt of tomato under natural field conditions and increase the total yield of tomato, again in agreement with our results.
Based on PCA and cluster analysis, the two best treatments for disease control and fruit yield promotion in this study were the Bacillus strain BsTA16 when applied alone, and a combination of the Bacillus strains BmMB9 and BaMA26. This is in agreement with recent work by Kariuki et al. (Citation2020), who reported that Bacillus strains increase tomato production, as well as decrease bacterial wilt disease caused by Ralstonia solanacearum in tomato under natural field conditions. Prabhukarthikeyan et al. (Citation2014) previously demonstrated that Bacillus subtilis and Beauveria bassiana reduced Fusarium wilt and fruit borer diseases when applied in combination in tomato. Domenech et al. (Citation2006) also concluded that the combined application of BCAs is more effective than a single application.
The results of this study indicate that the development of the plant stem diameter depends on the tomato hybrid as well as the antagonistic bacterium and whether it is used alone or in combination. In hybrid ‘DRD 8551’ (Site A), individual application of all four bacterial strains increased plant stem diameter; however, stem diameter did not increase when strains were used in combination. The single application of AcDB3 did not promote plant stem diameter in the hybrid ‘SV8579TE’ (Site C), or in the hybrid ‘Katya’ (Site D), when combined with BsTA16 and BaMA26. We can infer from these results that stem diameter is not an important variable for evaluating the growth-promoting effect or disease effect when these treatments are applied to tomato. Lizárraga-Sánchez et al. (Citation2015) reported that the biological control agent Bacillus cereus sensu lato strain B25 did not promote plant stem diameter, although it did significantly increase maize grain production. Their work, like ours, thus suggests that plant stalk/stem diameter is not a useful variable for analysis.
All four bacterial strains reduced disease severity significantly in the ‘DRD 8551’, ‘SV8579T’ and ‘Katya’ tomato hybrids, but not in the ‘Tisey’ hybrid. Acinetobacter calcoaceticus AcDB3 and various Bacillus strains (B. thuringiensis BtMB9, B. subtilis BsTA16, and B. amyloliquefaciens BaMA26) tested in this work decreased the severity of Fusarium wilt and FCRRT under greenhouse bioassays (Khalil et al. Citation2021). However, they were unable to protect against both pathogens in vitro and in greenhouse tests (BaMA26 and BsTA16 showed antagonistic effects against Fol R3, while BtMB9 and AcDB3 showed antagonistic effects against Forl) (Khalil et al. Citation2021). Since all previous work with these bacteria was performed under sterile substrate conditions, it is possible that under natural conditions the interactions between these bacteria and the soil microbiota could be either detrimental or beneficial to their ability to protect against fungal pathogens, resulting in either the loss (De Corato Citation2020) or broadening of their disease control potential (Topalović et al. Citation2020). Amaresan et al. (Citation2019) reported that Bacillus spp. enhanced tomato growth and promoted fruit yield under natural field conditions, in addition to protecting against damping-off (caused by Pythium sp., Sclerotium rolfsii and Fusarium sp.), fruit rot (caused by Colletotrichum capsici), and bacterial wilt (caused by Ralstonia solanacearum). Our results suggest that the bacterial antagonistic and plant growth-promoting effects may depend on the specific microbiota presence in the soil. Under natural field conditions, soil may contain a large quantity of beneficial microbiota that can stimulate BCAs to counteract phytopathogens, as well as plant growth-promoting microbiota (Zuluaga et al. Citation2021). In our greenhouse bioassays, BCAs were added in sterile conditions, where the bacteria may have displayed antagonistic effects against a single specific pathogen (Fol R3 or Forl). However, under natural soil conditions, other beneficial soil microbiota could influence BCAs to inhibit growth of both Fol R3 and Forl, as well as stimulate fruit production.
To the best of our knowledge, this is the first study to report that BCAs (AcDB3, BtMB9, BsTA16 and BaMA26) exert plant growth-promoting effects and antagonism against Fusarium wilt and FCRRT in commercial tomato fields naturally infested with Fol and Forl. We also report for the first time that A. calcoaceticus strain AcDB3 displays antagonism against Forl in tomato under natural field conditions. In conclusion, our field studies demonstrated that all four rhizospheric bacterial strains (A. calcoaceticus AcDB3, B. thuringiensis BtMB9, B. subtilis BsTA16, and B. amyloliquefaciens BaMA26), when applied alone and in combination, reduced FCRRT severity caused by Forl and Fusarium wilt severity caused by Fol under natural inoculum conditions, as well as increased fruit yield. The single application of BsTA16 and the combined application of BtMB9 + BaMA26 showed the highest efficacy of control against both FCRRT and Fusarium wilt. Based on these results, a formulation of BsTA16 and BtMB9 + BaMA26 could be prepared to control these diseases and induce plant growth-promotion, which would reduce the need for agrochemicals in the management of this disease.
Supplementary_Table_1.docx
Download MS Word (16 KB)Supplementary_Fig_2.docx
Download MS Word (25.3 KB)Supplementary_Fig_1.docx
Download MS Word (8.3 MB)Acknowledgements
We thank Dr. Brandon Loveall from Improvence editing services for the English proofreading of the manuscript. M.M.R. Khalil acknowledges the Consejo Nacional de Ciencia y Tecnología (CONACyT) of Mexico, and the BEIFI program from Instituto Politécnico Nacional (IPN), for the Ph.D. fellowships. We also thank Eng. Manuel Othón Cruz Burgos and Agrícola Doña Alicia for allowing us to work in their fields and for all their assistance in the field work.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online here: https://doi.org/10.1080/07060661.2022.2087104.
Additional information
Funding
References
- Ali RS, Poll C, Kandeler E. 2018. Dynamics of soil respiration and microbial communities: interactive controls of temperature and substrate quality. Soil Biol Biochem. 127:60–70. doi:10.1016/j.soilbio.2018.09.010
- Amaresan N, Jayakumar V, Kumar K, Thajuddin N. 2019. Biocontrol and plant growth-promoting ability of plant-associated bacteria from tomato (Lycopersicum esculentum) under field condition. Microb Pathog. 136:103713. doi:10.1016/j.micpath.2019.103713
- Apodaca-Sánchez MA, Zavaleta-Mejía E, Osada KS, García-Espinoza R. 2002. Frecuencia de campos infestados con Fusarium oxysporum f. sp. radicis-lycopersici en Sinaloa, México, y su control. Rev Mex Fitopatol. 20:1–7.
- Atherton JG, Rudich J. 2013. The tomato crop: a scientific basis for improvement. Roberts EH, Series editor. London, UK: Springer-Dordrecht. Series editor. doi:10.1007/978-94-009-3137-4.
- Barratt BIP, Moran VC, Bigler F, van Lenteren JC. 2018. The status of biological control and recommendations for improving uptake for the future. BioControl. 63:155–167. doi:10.1007/s10526-017-9831-y
- Baysal Ö, Çalışkan M, Yeşilova Ö. 2008. An inhibitory effect of a new Bacillus subtilis strain (EU07) against Fusarium oxysporum f. sp. radicis-lycopersici. Physiol Mol Plant Pathol. 73:25–32. doi:10.1016/j.pmpp.2008.11.002
- Bouyoucos GJ. 1962. Hydrometer method improved for making particle size analysis of soils. Agron J. 54:464–465. doi:10.2134/agronj1962.00021962005400050028x
- Chow YY, Rahman S, Ting ASY. 2018. Interaction dynamics between endophytic biocontrol agents and pathogen in the host plant studied via quantitative real-time polymerase chain reaction (qPCR) approach. Biol Control. 125:44–49. doi:10.1016/j.biocontrol.2018.06.010
- Çolak A, Biçici M. 2013. PCR detection of Fusarium oxysporum f.sp. radicis-lycopersici and races of F. oxysporum f.sp. lycopersici of tomato in protected tomato growing areas of Eastern Mediterranean Region of Turkey. Turk J Agric For. 37:457–467. doi:10.3906/tar-1203-71
- Connor N, Sikorski J, Rooney AP, Kopac S, Koeppel AF, Burger A, Cole SG, Perry EB, Krizanc D, Field NC, et al. 2010. Ecology of speciation in the genus Bacillus. Appl Environ Microbiol. 76:1349–1358. doi:10.1128/AEM.01988-09
- De Corato U. 2020. Soil microbiota manipulation and its role in suppressing soil-borne plant pathogens in organic farming systems under the light of microbiome-assisted strategies. Chem Biol Technol Agric. 7:17. doi:10.1186/s40538-020-00183-7
- Domenech J, Reddy MS, Kloepper JW, Ramos B, Gutierrez-Mañero J. 2006. Combined application of the biological product LS213 with Bacillus, Pseudomonas or Chryseobacterium for growth promotion and biological control of soil-borne diseases in pepper and tomato. Biocontrol. 51:245–258. doi:10.1007/s10526-005-2940-z
- Driscoll WC. 1996. Robustness of the ANOVA and Tukey-Kramer statistical tests. Comput Ind Eng. 31:265–268. doi:10.1016/0360-8352(96)00127-1
- Durán P, Viscardi S, Acuña JJ, Cornejo P, Azcón R, de la Luz Mora M. 2018. Endophytic selenobacteria and arbuscular mycorrhizal fungus for selenium biofortification and Gaeumannomyces graminis biocontrol. J Soil Sci Plant Nutr. 18(4):1021–1035. doi:10.4067/S0718-95162018005002902.
- Elanchezhiyan K, Keerthana U, Nagendran K, Prabhukarthikeyan SR, Prabakar K, Raguchander T, Karthikeyan G. 2018. Multifaceted benefits of Bacillus amyloliquefaciens strain FBZ24 in the management of wilt disease in tomato caused by Fusarium oxysporum f. sp. lycopersici. Physiol Mol Plant Pathol. 103:92–101. doi:10.1016/j.pmpp.2018.05.008
- FAOSTAT - Food and Agriculture Organization Corporate Statistical Database, United Nations. 2019. http://www.fao.org/faostat/en/#home Accessed 2021 Mar 10.
- Farokh RZ, Sachdev D, Kazemi-Pour N, Engineer A, Pardesi KR, Zinjarde S, Dhakephalkar PK, Chopade BA. 2011. Characterization of plant-growth-promoting traits of Acinetobacter species isolated from rhizosphere of Pennisetum glaucum. J Microbiol Biotechnol. 21(6):556–566. doi:10.4014/jmb.1012.12006.
- Figueroa-López AM, Cordero-Ramírez JD, Martínez-Álvarez JC, López-Meyer M, Lizárraga-Sánchez GJ, Félix-Gastélum R, Maldonado-Mendoza IE. 2016. Rhizospheric bacteria of maize with potential for biocontrol of Fusarium verticillioides. SpringerPlus. 5:1–12. doi:10.1186/s40064-016-1780-x
- Gee GW, Or D. 2002. Particle Size Analysis. In: Dane JH, Topp GC, editors. Methods of soil analysis, Part 4, Physical methods, Soils science society of America, Book Series No. 5, Madison, WI, USA. p. 255–293.
- Gómez P, Paterson S, De Meester L, Liu X, Lenzi L, Sharma MD, McElroy K, Buckling A. 2016. Local adaptation of a bacterium is as important as its presence in structuring a natural microbial community. Nat Commun. 7:12453. doi:10.1038/ncomms12453
- Guo JH, Qi HY, Guo YH, Ge HL, Gong LY, Zhang LX, Sun PH. 2004. Biocontrol of tomato wilt by plant growth-promoting rhizobacteria. Biol Control. 29:66–72. doi:10.1016/S1049-9644(03)00124-5
- Hernández-Martínez R, López-Benítez A, Borrego-Escalante F, Espinoza-Velázquez J, Sánchez-Aspeytia D, Maldonado-Mendoza IE, López-Ochoa LA. 2014. Races of Fusarium oxysporium f.sp. lycopersici in tomato farmlands in San Luis Potosí. Rev Mex Cienc Agríc. 5(7):1169–1178.
- Hu R, Huang X, Huang J, Li Y, Zhang C, Yin Y, Chen Z, Jin Y, Cai J, Cui F. 2015. Long- and short-term health effects of pesticide exposure: a cohort study from China. PLOS ONE. 10:e0128766. doi:10.1371/journal.pone.0128766
- Iyer B, Rajput MS, Rajkumar S. 2017. Effect of succinate on phosphate solubilization in nitrogen fixing bacteria harbouring chickpea and their effect on plant growth. Microbiol Res. 202:43–50. doi:10.1016/j.micres.2017.05.005
- Jangir M, Sharma S, Sharma S. 2019. Target and non-target effects of dual inoculation of biocontrol agents against Fusarium wilt in Solanum lycopersicum. Biol Control. 138:104069. doi:10.1016/j.biocontrol.2019.104069
- Kamali M, Ahmadi J, Naeimi S, Guo D. 2019. Characterization of Bacillus isolates from the rhizosphere of tomato suppressing Fusarium wilt disease. Acta Phytopathol Entomol Hung. 54(1):53–68. doi:10.1556/038.54.2019.006.
- Kamou NN, Karasali H, Menexes G, Kasiotis KM, Bon MC, Papadakis EN, Tzelepis GD, Lotos L, Lagopodi AL. 2015. Isolation screening and characterisation of local beneficial rhizobacteria based upon their ability to suppress the growth of Fusarium oxysporum f. sp. radicis-lycopersici and tomato foot and root rot. Biocontrol Sci Technol. 25(8):928–949. doi:10.1080/09583157.2015.1020762.
- Kariuki CK, Mutitu EW, Muiru WM. 2020. Effect of Bacillus and Trichoderma species in the management of the bacterial wilt of tomato (Lycopersicum esculentum) in the field. Egypt J Biol Pest Control. 30:109. doi:10.1186/s41938-020-00310-4
- Khalil MMR, Fierro-Coronado RA, Peñuelas-Rubio O, Villa-Lerma AG, Plascencia-Jatomea R, Félix-Gastélum R, Maldonado-Mendoza IE. 2021. Novel native tomato rhizospheric bacteria as potential biocontrol agents of Fusarium oxysporum ff. spp. lycopersici race 3 and radicis-lycopersici. Saudi J Biol Sci. 28:7460–7471. doi:10.1016/j.sjbs.2021.08.043
- Larkin RP, Fravel DR. 1998. Efficacy of various fungal and bacterial biocontrol organisms for control of Fusarium wilt of tomato. Plant Dis. 82(9):1022–1028. doi:10.1094/pdis.1998.82.9.1022.
- Lizárraga-Sánchez GJ, Leyva-Madrigal KY, Sánchez-Peña P, Quiroz-Figueroa FR, Maldonado-Mendoza IE. 2015. Bacillus cereus sensu lato strain B25 controls maize stalk and ear rot in Sinaloa, Mexico. Field Crops Res. 176:11–21. doi:10.1016/j.fcr.2015.02.015
- Manzo D, Ferriello F, Puopolo G, Zoina A, Esposito D, Tardella L, Ferrarini A, Ercolano MR. 2016. Fusarium oxysporum f.sp. radicis-lycopersici induces distinct transcriptome reprogramming in resistant and susceptible isogenic tomato lines. BMC Plant Biol. 16:53. doi:10.1186/s12870-016-0740-5
- Mendívil-Trujillo HR, 2013. Identificación molecular de razas fisiológicas de Fusarium oxysporum f. sp. lycopersici en el estado de Sinaloa, México. M. Sc. Thesis dissertation. México: Universidad autónoma de Sinaloa.
- Mertler CA, Reinhart RV. 2016. Advanced and multivariate statistical methods: practical application and interpretation. New York: Routledge. doi:10.4324/9781315266978.
- Moradi H, Bahramnejad B, Amini J, Siosemardeh A, Haji-Allahverdipoor K. 2012. Suppression of chickpea (Cicer arietinum L.) Fusarium wilt by Bacillus subtilis and Trichoderma harzianum. Plant Omics J. 5(2):68–74. https://www.pomics.com/bahramnejad_5_2_2012_68_74.pdf
- Nguyen DT, Hieu NC, Hung NV, Thao HTB, Keswani C, Van Toan P, Hoat TX. 2019. Biological control of Fusarium root rot of Indian mulberry (Morinda officinalis How.) with consortia of agriculturally important microorganisms in Viet Nam. Chem Biol Technol Agric. 6:1–11. doi:10.1186/s40538-019-0168-x
- Olsen S, Cole C, Watanabe F, Dean L 1954. Estimation of available phosphorus in soils by extraction with sodium bicarbonate. USDA Circular Nr 939, Washington (D.C): US Gov. Print. Office.
- Pandin C, Darsonval M, Mayeur C, Le Coq D, Aymerich S, Briandeta R. 2019. Biofilm formation and synthesis of antimicrobial compounds by the biocontrol agent Bacillus velezensis QST713 in an Agaricus bisporus compost micromodel. Appl Environ Microbiol. 85(12):e00327–19. doi:10.1128/AEM.00327-19.
- Pearson K. 1901. Notes on regression and inheritance in the case of two parents. Proc R Soc Lond. 58:240–242.
- Pignatelli M, Moya A, Tamames J. 2009. EnvDB, a database for describing the environmental distribution of prokaryotic taxa. Environ Microbiol Rep. 1(3):191–197. doi:10.1111/j.1758-2229.2009.00030.x.
- Prabhukarthikeyan R, Saravanakumar D, Raguchander T. 2014. Combination of endophytic Bacillus and Beauveria for the management of Fusarium wilt and fruit borer in tomato. Pest Manag Sci. 70:1742–1750. doi:10.1002/ps.3719
- PROYECTO de Norma Oficial Mexicana PROY NOM-021-RECNAT-2000. Que establece las especificaciones de fertilidad, salinidad y clasificación de suelos. Estudios, muestreo y análisis. https://biblioteca.semarnat.gob.mx/janium/Documentos/Ciga/libros2009/DO2280n.pdf.
- Rohlf FJ, Fisher DR. 1968. Tests for hierarchical structure in random data sets. Syst Biol. 17(4):407–412. doi:10.1093/sysbio/17.4.407.
- Rowe RC. 1980. Comparative pathogenicity and host ranges of Fusarium oxysporum isolates causing crown and root rot of greenhouse and field-grown tomatoes in North America and Japan. Phytopathology. 70(12):1143–1148. doi:10.1094/Phyto-70-1143.
- San Millan AF, Larraya L, Farran I, Ancin M, Veramendi J. 2021. Successful biocontrol of major postharvest and soil-borne plant pathogenic fungi by antagonistic yeasts. Biol Control. 160:104683. doi:10.1016/j.biocontrol.2021.104683
- Shabala S, Munns R. 2017. Salinity stress: physiological constraints and adaptive mechanisms. In: Shabala S, editor. Plant Stress Physiology. 2nd ed. Wallingford: CABI; p. 24–63. doi:10.1079/9781780647296.0024
- Shah R, Amaresan N, Patel P, Jinal HN, Krishnamurthy R. 2020. Isolation and characterization of Bacillus spp. endowed with multifarious plant growth-promoting traits and their potential effect on tomato (Lycopersicon esculentum) seedlings. Arab J Sci Eng. 45:4579–4587. doi:10.1007/s13369-020-04543-1
- Shanmugam V, Kanoujia N. 2011. Biological management of vascular wilt of tomato caused by Fusarium oxysporum f.sp. lycospersici by plant growth-promoting rhizobacterial mixture. Biol Control. 57:85–93. doi:10.1016/j.biocontrol.2011.02.001
- Singh D, Singh SK, Modi A, Sing PK, Zhimo Y, Kumar A. 2020. Impacts of agrochemicals on soil microbiology and food quality. Agrochemicals detection, treatment and remediation: pesticides and chemical fertilizers. 1st ed. Cambridge, MA: Butterworth-Heinemann. p. 101–116. doi:10.1016/B978-0-08-103017-2.00004-0.
- Topalović O, Hussain M, Heuer H. 2020. Plants and associated soil microbiota cooperatively suppress plant-parasitic nematodes. Front Microbiol. 11:313. doi:10.3389/fmicb.2020.00313
- Vega-Gutiérrez TA, López-Urquídez AL, Allende-Molar R, Amarillas-Bueno LB, Romero-Gómez SJ, López-Orona CA. 2019. Aggressiveness and molecular characterization of Fusarium spp. associated with foot rot and wilt in Tomato in Sinaloa, Mexico. Biotech. 9:276. 3. doi:10.1007/s13205-019-1808-3
- Walkley A, Black IA. 1934. An examination of Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci. 37:29–37. doi:10.1097/00010694-193401000-00003
- Winding A, Binnerup SJ, Pritchard H. 2004. Non-target effects of bacterial biological control agents suppressing root pathogenic fungi. FEMS Microbiol Ecol. 47:129–141. doi:10.1016/S0168-6496(03)00261-7
- Wisniewski M, Droby S, Norelli J, Liu J, Schena L. 2016. Alternative management technologies for postharvest disease control: the journey from simplicity to complexity. Postharvest Biol Technol. 122:3–10. doi:10.1016/j.postharvbio.2016.05.012
- Xue QY, Chen Y, Li SM, Chen LF, Ding GC, Guo DW, Guo JH. 2009. Evaluation of the strains of Acinetobacter and Enterobacter as potential biocontrol agents against Ralstonia wilt of tomato. Biol Control. 48:252–258. doi:10.1016/j.biocontrol.2008.11.004
- Zouari I, Masmoudi F, Medhioub K, Tounsi S, Trigui M. 2020. Biocontrol and plant growth-promoting potentiality of bacteria isolated from compost extract. Antonie van Leeuwenhoek. 113:2107–2122. doi:10.1007/s10482-020-01481-8
- Zuluaga MYA, Milani KML, Miras-Moreno B, Lucini L, Valentinuzzi F, Mimmo T, Pii Y, Cesco S, Rodrigues EP, de Oliveira ALM. 2021. Inoculation with plant growth-promoting bacteria alters the rhizosphere functioning of tomato plants. Appl Soil Ecol. 158:103784. doi:10.1016/j.apsoil.2020.103784
