ABSTRACT
Objective: The objective of this study was to investigate the effect of a broad-spectrum wellness beverage (Zeal Wellness [ZW]) on standardized measures of mood states, including overall feelings of vitality, in healthy, moderately stressed adults.
Methods: A randomized, double-blind, placebo-controlled clinical trial was conducted among 99 eligible participants prescreened for moderate stress. Participants were randomized to one of four groups and received ZW once daily (1-dose-ZW; 14 g), ZW twice daily (2-dose-ZW; 28 g), placebo once daily (1-dose-placebo), or placebo twice daily (2-dose-placebo) for 4 weeks. A stress/vitality questionnaire assessed stress and the Profile of Moods (POMS) Questionnaire assessed vigor via mental/physical energy and global mood state. Safety was assessed by clinical chemistry, liver, kidney function, and anthropometric measures and adverse event reporting.
Results: Participants receiving 2-dose-ZW reported a 6.6% decrease in scores on POMS-Total Mood Disturbance (TMD; p < 0.05) and a 6.8% decrease in the anger-hostility mood state (p < 0.022) compared to the combined placebo group at day 29. The 2-dose-ZW provided a 12.8% greater improvement in POMS-TMD scores when compared to participants receiving 1-dose-ZW after 28 days of supplementation (p = 0.014). Within groups, there was a 22.4% and a 9.6% decrease in POMS-TMD scores in participants with 2-dose-ZW and 1-dose-ZW, respectively. In addition, participants receiving 2-dose-ZW showed significant improvements (p = 0.001) in the POMS t-score iceberg profile, which represented a shift to a more healthy profile.
Conclusion: These data show that daily supplementation with 2-dose-ZW significantly decreased POMS-TMD scores and anger-hostility mood state and shifted the POMS iceberg profile to a healthy profile compared to the combined placebo, reflecting the functional benefit of rice-bran-fruit-vegetable extracts based beverage on health.
Abbreviations
| ANCOVA | = | analysis of covariance |
| ICH | = | International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use |
| ITT | = | intent-to-treat |
| POMS-TMD | = | Profile of Mood States–Total Mood Disturbance |
| ZW | = | Zeal Wellness |
Introduction
Inferior mood states and stress affects well-being and results in a cascade of events that have physiological effects, particularly influencing mental and digestive health, leading to a poorer quality of life Citation(1,2). Although stress can arise from many factors common in daily life, anxiety and depression are the most frequently diagnosed disorders Citation(3–5). Stress is a common 21st-century condition associated with increases in urbanization, industrialization, and Westernization. Burdensome workloads were cited as primary stressors, with 62% of individuals citing “work” as their main source of stress, with finance, time constraints, and family and personal matters following suit. The uncertain nature of these dynamic life areas poses problems that are difficult to readily address, thus contributing to unresolved stress. Long-term activation of the stress response has been linked to a wide range of health challenges, including hypertension, hypercholesterolemia, and hyperglycemia Citation(5–7).
In 21st-century North America, there is little doubt that stress is a pervasive aspect of modern life and can have serious physiological consequences. Pharmaceutical drugs such as benzodiazepines and selective serotonin reuptake inhibitors are regularly prescribed for anxiety, stress, and depression but are often associated with serious side effects, a fact that has led to the demand for nutraceutical alternatives to combat the effects of stress while supporting the body's natural ability to respond to stress. Supplements utilizing fish oil, Ginkgo biloba, and ginseng, as well as several vitamins and minerals, have routinely been shown to positively impact mental health and to modulate oxidative stress during exercise Citation(8,9). Given the wide range of factors that influence stress, as well as the multiple indications through which stress can manifest itself, a combinatory strategy that uses a carefully selected nutraceutical formulation—providing multiple vitamins, minerals, amino acids, and naturally sourced ingredients—may represent a valid course of action.
Natural health products, including “amylase-enzyme-modified” stabilized rice bran, Bacopa extract, ashwagandha, and specific amino acids (e.g., glycine, and tyrosine), have been shown to have a positive effect on overall health, including cognitive health. “Amylase-enzyme-modified” stabilized rice bran is a source of complex and simple carbohydrates, tocopherols and tocotrienols, gamma oryzanol, plant sterols, and polyphenols that have been shown to positively influence cholesterol, blood sugar, inflammation, and immune function Citation(10). Bacopa monnieri is a nootropic herb that in clinical trials improved several memory-related outcomes in healthy older participants Citation(11–13). In clinical trials involving stressed individuals, ashwagandha supplementation improved cognitive performance and reduced cortisol levels Citation(14,15). In intermediary metabolism, amino acids can act as neurotransmitters in the brain and thus can have substantial effects on measures of cognitive function Citation(16–18). Indeed, in a published human clinical trial, supplementation with the amino acid glycine reduced negative symptoms associated with schizophrenia Citation(16). Several plant concentrates and extracts plus vitamins, minerals, and amino acids may act on neuroendocrine pathways associated with mood and stress to bring about favorable changes, hence functioning as a nutritional food that optimizes overall health. Indeed, influences of dietary factors on neuronal function and synaptic plasticity have revealed some of the vital mechanisms that are responsible for the action of diet on brain health and mental function and have been extensively reviewed Citation(19).
The aim of this study was to investigate the efficacy of two different dosages of a proprietary wellness beverage, Zeal Wellness (ZW), on mood state, stress, and vitality in healthy, moderately stressed adults. The focus of this study was on the Profile of Mood States (POMS)–Total Mood Disturbance (TMD) score, a self-reported assessment of mood that was used to measure mood states of participants and that has been shown to be effective in providing a comprehensive assessment of mood by measuring indicators of potential mood disturbance Citation(20). The key objective of this study was to investigate how 28 days of ZW supplementation affected various standardized measures of mood states, including overall feelings of vitality in a moderately stressed but otherwise healthy adult population.
Materials and methods
Study design
This study was reviewed by the institutional review board (IRB Services, Aurora, Ontario) and unconditional approval was granted on November 17, 2015. This trial was conducted according to the ethical principles that have their origin in the Declaration of Helsinki (2008) and the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) Harmonised Tripartite Guideline for Good Clinical Practice. The ICH is uniquely positioned to foster bringing together regulatory authorities and the pharmaceutical industry to discuss scientific and technical aspects of drug registration, which governments can then transpose into regulations for clinical trials involving human participants. All participants gave written informed consent prior to any study-related procedures.
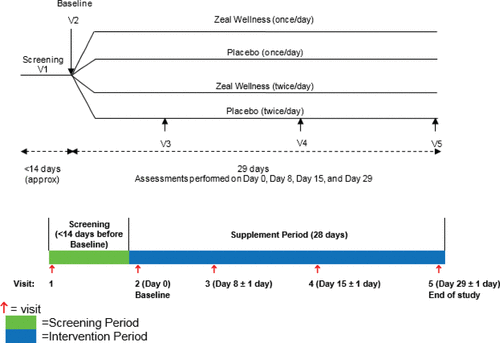
This was a randomized double-blind, placebo-controlled, four-arm parallel study conducted at the KGK Science Clinical Trial Center in Orlando, Florida, USA, between December 2015 and June 2016. The duration of the study was 4 weeks, with four in-clinic visits at baseline, day 8, day 15, and day 29 (). At screening, volunteers completed the Mini Neurological Assessment and the stress/vitality questionnaire to determine eligibility. At baseline, eligible volunteers were randomized into four groups of 25 participants receiving either the investigational product once daily (1-dose-ZW; 14 g), the investigational product twice daily (2-dose-ZW; 28 g), placebo once daily (1-dose-placebo), or placebo twice daily (2-dose-placebo). At all visits, participants' diaries were reviewed for concomitant therapies, adverse events, and rescue medication use; the stress/vitality questionnaire was administered; and anthropometric measures were assessed. The POMS questionnaire was administered at baseline and on days 8, 15, and 29. Safety parameters were assessed at screening and the end of study (day 29). Compliance with the study instructions was assessed on days 8, 15, and 29.
Participants
Ninety-nine participants were randomized into the study after having their eligibility assessed for “moderate stress” using the stress/vitality questionnaire. screening. For the purposes of this study, moderate stress was defined as a score of 4 to 8 on question 3 of the stress/vitality questionnaire. The stress/vitality questionnaire supports the POMS questionnaire used in the study for its measure of the vitality subdomain. The study participants met the following inclusion criteria: male or female aged 18 to 65 years (female participants of childbearing potential agreed to use a medically approved method of birth control and have a negative urine pregnancy test result), body mass index 18.5 to 29.9 kg/m2, self-reported moderate stress (as determined using the stress/vitality questionnaire), status of “healthy” as determined by laboratory results and medical history, agreement to comply with study procedures, and provision of voluntary, written, informed consent to participate in the study. Exclusion criteria included, but was not limited to, psychiatric disorders as determined by the Mini International Neuropsychiatric Interview for neurological status; use of products known to have central nervous system activity within 6 weeks of enrollment; inability to complete questionnaires and give written informed consent; low stress levels; and any other condition that, in the investigators' opinion, may have affected the participant's ability to complete the study or its measures or may have posed significant risk to the participant. No protocol amendments were made for this study.
Intervention
The investigational beverage, ZW, is a proprietary product of Zurvita (Houston, Texas, USA), with active ingredients that includes “amylase-enzyme-modified” stabilized rice bran and rice germ, fructooligosaccharide from chicory root, crystalline fructose, citric acid, guarana seed powder with natural caffeine, ionic mineral powder and fulvic minerals, stevia, aloe vera powder, Moringa olifeira, gotu kola, maca, beta glucan, lychee, alfalfa leaf, broccoli (sprout) extract, cranberry, milk thistle extract, Bacopa extract, ashwagandha, green tea extract, wild blueberry, turmeric extract, red ginseng, yerba mate, kudzu, fennel, goji berry, acai berry, noni juice powder, Chlorella, grape seed extract, L-arginine, glycine, lysine, ornithine, natural flavors and colors, potassium citrate, retinyl palmitate (vitamin A palmitate), ascorbic acid (vitamin C), thiamin (vitamin B1), riboflavin (vitamin B2), niacin (vitamin B3), pyridoxine hydrochloride (vitamin B6), methylcobalamin (vitamin B12), biotin, and pantothenic acid (vitamin B5). A dose of ZW consisted of 14 g of powder dissolved in approximately 250 mL of cold water. Participants randomized to the once-daily dose of ZW arm were instructed to take the ZW serving with breakfast each morning starting the day following randomization. Participants randomized to the twice-daily dose of ZW arm were instructed to take one serving of ZW with breakfast and the second midafternoon (approximately 3 pm) starting the day following randomization. The placebo product was matched to the investigational supplement and contained similar excipients to ensure blinding. Participants randomized to the once-daily dose groups were instructed to take the study product with breakfast each morning. The participants randomized to the twice-daily dose groups were instructed to take one serving of the study product with breakfast and the second midafternoon.
Assessments
The stress/vitality questionnaire was administered at screening, baseline, and days 8, 15, and 29. The POMS questionnaire was administered at baseline and days 8, 15, and 29, and the product perception questionnaire was administered at days 8, 15, and 29. Laboratory parameters (complete blood counts and levels of electrolytes [sodium, potassium, chloride], creatinine, hemoglobin A1c [only at screening], aspartate aminotransferase, alanine aminotransferase, gamma-glutamyltransferase, and bilirubin) for safety were assessed at screening and end of study using established experimental protocols for the laboratory assessment.
The POMS index was used to measure mood states, with its validity well established as its methodology has been used in more than 2900 studies Citation(21). POMS is a self-reported assessment of mood that is adaptable to capturing transient and fluctuating feelings or relatively enduring affect states and contributes to a comprehensive assessment by providing indications of potential mood disturbance. This measure is flexibly employed at different stages of clinical or applied intervention. Baseline TMD and the six other mood state scores reflect an individual's current mood and may be used to inform treatment plans. Responses on the POMS assessment questionnaire are combined to produce a TMD score and scores on six mood clusters: anger-hostility, confusion-bewilderment, depression-dejection, fatigue-inertia, tension-anxiety, and vigor-activity. Higher TMD and negative mood state scores and lower scores on the positive mood state of vigor-activity may signal negative emotional changes. An extension of the POMS, the POMS iceberg profile, was used for assessing active/healthy individuals. The profiles of healthy individuals show a peak for the positive mood state, vigor-activity, with values for tension-anxiety, depression-dejection, anger-hostility, fatigue-inertia, and confusion contributing to the trough values of the profile, a concept that has been applied to assess physical activity and mood among healthy individuals.
The stress/vitality questionnaire was adapted from a combination of Statistics Canada's General Social Survey questionnaire and Bishop and Yardley's Wellness Belief Scale Citation(22) to better serve the needs of assessing a dietary supplement.
Statistical analysis
Between-group comparisons were made using the Student's t test or Fisher's exact test. A separate analysis of covariance (ANCOVA) model based on POMS-TMD was run to determine the effect of taking two doses per day. Results from the intent-to-treat (ITT) analysis are presented. To test the effect of taking two doses per day, a separate ANCOVA model based on the POMS-TMD was run with an additional dichotomous covariate; based on this adjustment, it was found that taking two doses per day did not significantly affect the POMS-TMD when treatment groups were already included in the model. A separate analysis with a combined placebo group versus the two doses of ZW was then carried out. Probabilities ≤ 0.05 were considered statistically significant. In the study populations, the ITT population consisted of all participants who received either product and for whom any postrandomization efficacy information was available. The per protocol population consisted of all participants who did not have any major protocol violations and completed all study visits and procedures connected with measurement of the primary variable. The safety population consisted of all participants who received any amount of either product and for whom any postrandomization safety information was available. All statistical analysis was completed using the R Statistical Software Package Version 3.2.2 (R Core Team, 2015) for Microsoft Windows.
Results
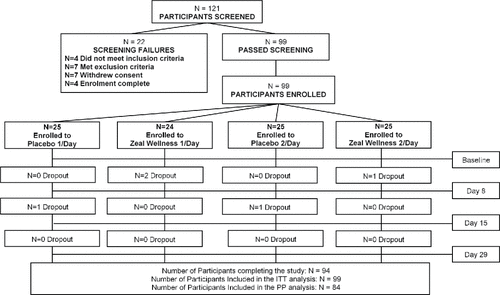
Participants were well matched for age and body mass index and met the required stress level for enrollment (). The stress levels of participants, based on the stress/vitality questionnaire, ranged from 4 to 8 confirming that they were moderately stressed and met the inclusion criteria for the study. A total of 121 participants were screened and 99 eligible participants were enrolled in the study, with 25 participants in each study group except for the 1-dose-ZW group, which had 24 (). Ninety-nine participants were analyzed in the ITT analysis.
Table 1. Demographics and characteristics of all randomized participants at screening (numbers and percentages; mean values with their standard errors).
Figure 2. Disposition of study participants. A total of 121 potential participants were screened and 99 eligible participants were enrolled in the study (with 25 participants in each study group, except for 1-dose Zeal Wellness, which had 24). Ninety-four participants completed the study. Fifteen participants were removed from the per protocol (PP) analysis; nine participants had missing Profile of Mood States, two participants were noncompliant, one participant had low compliance, two participants withdrew from the study because of an adverse event, and one participant requested to be removed from the study. ITT = intent-to-treat.
This study tested two doses of ZW against two respective doses of placebo. In order to test the effect of taking two doses of placebo, a sensitivity analysis was performed. As the statistical covariate was found to be not significant, the two placebo groups were combined to gain additional power through reduction in variation by increasing participant number. Analysis with a combined placebo group versus ZW provided for better expression of between-group differences in this clinical study.
Compliance
Unused study product was returned to the study site at each visit and was used to determine compliance with the dosing regimen; dose compliance was estimated to be approximately 99.8% across all groups.
POMS assessment
The POMS questionnaire was used to measure TMD. The TMD scale combines six mood clusters consisting of five negative mood states (anger-hostility, confusion-bewilderment, depression-dejection, fatigue-inertia, and tension-anxiety) and one positive mood state (vigor-activity). A decrease in total mood state disturbance or negative mood states is indicative of an improvement in mood.
Both placebo groups had a placebo effect of 16% in POMS-TMD. Data analysis showed that there was no dose effect contributing to the placebo effect. Therefore, a statistical model was run with an additional covariate accounting for taking two products versus taking one product. This covariate was found to be not significant and therefore the two placebo groups (combined placebo) were combined to gain additional power.
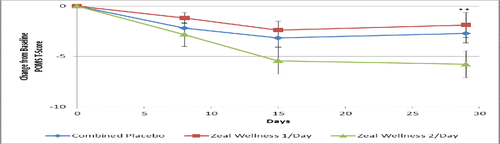
Participants receiving 2-dose-ZW reported a 6.6% decrease in POMS-TMD versus combined placebo (p < 0.05), a 6.8% decrease in the mood state of anger-hostility versus combined placebo (p < 0.022), and reduced fatigue-inertia over 1-dose-ZW (p = 0.034), with a trend toward a decrease in depression-dejection and an increase in vigor-activity after a 28-day supplementation. These results were achieved despite a 16% placebo effect.
Within groups, there was a 22.4% decrease in POMS-TMD with 2-dose-ZW and a 9.6% decrease in POMS-TMD in participants receiving 1-dose-ZW (). The most prominent increase of 21.2% in vigor-activity was reported with 2-dose-ZW after the 28-day supplementation, while the combined placebo only showed an improvement of 11.1% in vigor-activity. In the per protocol analyses, participants receiving 2-dose-ZW reported a 7.7% decrease in POMS-TMD versus combined placebo (p < 0.05) and an 8.6% decrease in the mood state of anger-hostility versus combined placebo (p < 0.022; ). The per protocol population showed a 12.8% decrease in POMS-TMD with 2-dose-ZW compared to 1-dose-ZW (p = 0.047) and increased vigor-activity by 19.4% (p = 0.014) over 1-dose-ZW, with a trend to decreased depression-dejection and decreased fatigue-inertia after a 28-day supplementation.
Figure 3. Total mood disturbance score results at days 0, 8, 15, and 29 for participants receiving supplementation with Zeal Wellness 1/day, Zeal Wellness 2/day, and placebo (N = 99). POMS = Profile of Mood States.
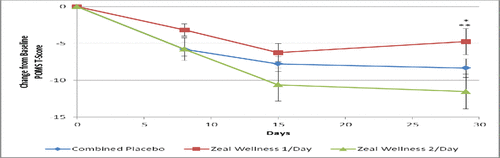
Figure 4. Anger-hostility score results at days 0, 8, 15, and 29 for participants receiving supplementation with Zeal Wellness 1/day, Zeal Wellness 2/day, and placebo (N = 99). POMS = Profile of Mood States.
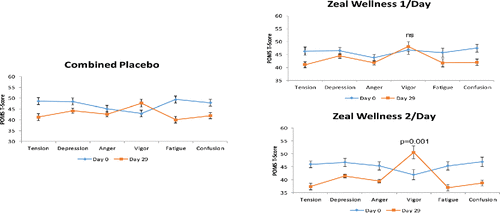
The POMS iceberg profile was applied to POMS t-scores of healthy, moderately stressed participants in this study. Average baseline profiles showed that participants in all groups had a profile that was inverse to the expected normal profile, with a depressed peak in vigor-activity. After the 28-day supplementation, the average iceberg profiles shifted for all groups, with the positive mood state vigor-activity showing peak values (). The participants in the 2-dose-ZW group were found to achieve a healthy profile, with a significant peak in the positive mood state of vigor-activity. In the 2-dose-ZW group, there was an increase of 21.2% in vigor-activity (p = 0.001) and a decrease of 18% in tension-anxiety (p < 0.001), 11.1% in depression-dejection (p = 0.003), 12.8% in anger-hostility (p < 0.001), 17.7% in fatigue-inertia (p < 0.001), and 17.3% in confusion-bewilderment (p < 0.001), eliciting healthy profiles in moderately stressed participants.
Safety
The analysis of safety markers including hematology, clinical chemistry, vital signs, and anthropometric measures showed no difference among groups after 28 days of supplementation and were all within their respective clinical reference ranges. There was a significant increase in mean systolic (p = 0.008) and diastolic (p = 0.005) blood pressure in the 2-dose-ZW group at day 15 compared to baseline recordings. In participants in the 1-dose-ZW group, mean systolic blood pressure significantly increased (p = 0.014) at day 8 compared to baseline. There was a significant increase in mean heart rate in the 2-dose-ZW group at day 8 (p = 0.001), in the 1-dose-ZW group at day 15 (p = 0.018), and in the 1-dose-placebo group at day 15 (p = 0.005) compared to baseline recordings. However, these changes were within the normal range and were not of clinical significance for this population. There were a total of 51 adverse events reported by 31 participants. Of these, 23 adverse events were reported by 16 participants receiving placebo, 21 were reported by 11 participants receiving 1-dose-ZW, and 7 were reported by 4 participants receiving 2-dose-ZW. There were 4 adverse events that were assessed as possibly related to the placebo product (e.g., headache, increase in heart rate, diarrhea), 10 adverse events possibly related to 1-dose-ZW (e.g., nausea, poor quality of sleep, decreased appetite, dyspepsia, nasal pharyngitis, energy increase), and 4 adverse events possibly related to 2-dose-ZW (e.g., hunger, chest and abdominal discomfort).
Discussion
Given that dietary supplements may fill a void by providing necessary nutrients for optimizing health, enhancing micronutrient and vitamin levels may be a more pragmatic approach to address stress and improve psychosocial mood than pharmacological interventions with their associated negative side effects. There is continued belief among consumers that functional food supplements may act synergistically to meet some or all the requirements necessary to overcome the gradual descent to ill health brought about by aging, poor diet, and sedentary lifestyles, providing alternatives to standard management practices that are designed to treat symptoms. Further, uniquely formulated products may help to establish a nutritional foundation that may otherwise be absent when individuals rely on self-selected diets without the support of comprehensive foundational nutrition products. Indeed, the primary nutritional guideline in virtually all widely accepted recommendations for healthy eating is to eat a wide variety of foods. The extent of the ingredients contained in the wellness beverage studied here provides both a wide variety of nutritional sources along with a high level of nutrient density per calorie consumed.
The current single-center, randomized, double-blind, placebo-controlled, four-arm parallel study investigated the efficacy of ZW on mood state, stress, and vitality in healthy and moderately stressed adults over a 28-day supplementation period.
After 28 days of supplementation, 14 g of ZW taken twice daily (28 g) resulted in a significant 6.6% decrease in POMS-TMD score, with an associated decrease of 6.8% in the mood state of anger-hostility. Two-dose ZW outperformed the single dose of ZW (14 g) by 12.8% on POMS-TMD. This reduction was associated with a significant increase in the positive mood state of vigor-activity of 18.4% and a decrease in the negative mood states of fatigue-inertia and depression-dejection. Improvements were not reported at day 8 or 15, suggesting that a longer supplementation period was required. It is noteworthy that despite a 16% placebo effect and a small sample size, there was a 6.6% improvement in POMS-TMD over combined placebo in this study.
These results were supported by the POMS iceberg profiles that showed optimal peaks of vigor-activity and a decrease in negative mood clusters. Baseline profiles of participants were inverse to the expected normal profile, with a depressed peak in the vigor-activity subscale. However, after the 28-day supplementation, POMS iceberg profiles shifted for all groups, with the positive mood state vigor-activity showing peak values. The greatest impact was seen in participants who consumed 28 g of ZW, which elicited more robust and healthy POMS iceberg profiles with a peak in the positive mood state of vigor-activity. In the 2-dose-ZW group, there was a significant increase of 21.2% in vigor-activity and a decrease of 18% in tension-anxiety, 11.1% in depression-dejection, 12.8% in anger-hostility, 17.7% in fatigue-inertia, and 17.3% in confusion-bewilderment, eliciting healthy profiles in moderately stressed participants ().
The rejuvenation of mood states, particularly anxiety and depression, in the present clinical study corroborates earlier investigations into the anxiolytic and antidepressive functions of fruit and vegetable/plant extracts Citation(23–30)
The active constituents of maca (Lepidium meyenii)—alkaloids, isothiocyanates, and glucosinolates—also reduced depression (6%) and anxiety (10%) over placebo in postmenopausal women Citation(23).The main active constituent in gotu kola (Centella asiatica), asiaticoside, has been shown to exert an anxiolytic activity in healthy participants Citation(25) and reduces stress, depression, and anxiety by 26% in individuals with generalized anxiety disorder Citation(26). Turmeric extract containing curcumin, a polyphenol with antioxidant activity, reduced depression Citation(27). Chlorella vulgaris, a unicellular green microalga with antioxidant properties, was effective against depression and anxiety Citation(28). A combination of L-lysine and L-arginine decreased trait anxiety by 10.9% compared to 1.2% in the placebo group and increased state anxiety by 0.8% compared to an increase of 8.7% in the placebo group Citation(29). Glycowithanolides, the active ingredient present in Withania somnifera (ashwagandha) reduced anxiety by 88.2% Citation(30). It can be argued that the ingredients in the ZW formulation worked in tandem to improve mood states in moderately stressed populations, an outcome that will rely on future directed research to define. Of interest, all the studies reported placebo effects ranging from 1% to 50%, which indicated that the 16% placebo effect seen in the current study is within reported values for this group of investigational products.
ZW was found to significantly improve TMD and several parameters of mood state by 7% in healthy individuals with moderate stress. Compared to a pharmacological approach in mitigating anxiety, Vilazodone and 5-HT1 A receptor partial agonist, reduced anxiety by 7% Citation(31), suggesting a potential future role in alleviating anxiety. Regarding safety, ZW was found to be safe and tolerable in this population, and there were no differences in the incidence of adverse events reported among the study groups and placebo groups. This strengthens evidence for its use in healthy populations to address moderate stress.
The participants who consumed 28 g of ZW reported an approximately 7% decrease in POMS-TMD scores and mood state anger-hostility. This study demonstrated significant dose-dependent efficacy of the investigational product. It was found that 28 g of ZW outperformed 14 g of ZW in decreasing POMS-TMD scores by 12.8% and fatigue-inertia by 9%, thereby improving the POMS iceberg profile to a healthier state. Although this study had a 16% placebo effect, statistically significant improvements in mood states were achieved with ZW compared to the combined placebo group. The study supports a comprehensive improvement in mood and stress that was anticipated due to the blend of nutrients and vitamins, the effects on psychosomatic well-being of which had been scientifically validated previously.
From the results of this study, it is possible to suggest that supplementation with ZW addressed a nonspecific nutrient deficiency in the population studied. Assessing participant vitamin and mineral levels prior to the start of the study may have provided valuable information for application in future studies on functional beverages to be extended to populations such as the elderly, vegans, and smokers. A longer supplementation period should be considered not only to allow for a better separation between the placebo and investigational product effects but also because the beneficial effect on mood and stress gained significance only at day 29. It is evident that the POMS iceberg profiles indicated a dramatic difference between baseline and end of study for ZW compared to placebo. This effect would perhaps be better expressed if the study is optimized for length of supplementation period.
In summary, ZW significantly improved mood states after 28 days of supplementation in moderately stressed but otherwise healthy individuals. An interesting observation was that 87% of participants were not previous dietary supplement users, suggesting that dietary supplementation with ZW may have bridged the gap between micronutrient deficiency and emotional health. This concept certainly warrants further research.
Study limitations
In light of the results obtained from the present study, the following recommendations should be considered for future studies in a similar vein to assess the biological efficacy of functional beverages. Future studies should consider determining the status of vitamins and other nutrients in participants to compare the effect of the beverage with presupplementation/baseline vitamin and nutrient values. Determining salivary or serum cortisol levels as an adjunct and complementary marker of mood states and an increase in the sample size to allow for better expression of between-group differences in the parameters that displayed trends toward significance would be of value.
Disclosures
The study was supported by a grant from Zurvita Holdings Inc., Houston, Texas, USA. Okezie I. Aruoma and Bernie Landes are members of the Scientific Advisory Board for Zurvita. Malkanthi Evans, Joseph Antony, and Najla Guthrie are not employees of Zurvita and are employees of KGK Science Inc., which conducted the clinical trial on the wellness beverage Zeal Wellness.
Acknowledgments
The authors thank all participants of this study for their diligence to the protocol. The authors are grateful to Professor Keith A. Wesnes, Wesnes Cognition Ltd, UK, for comments on the statistical interpretation of the study data.
Additional information
Funding
References
- Gur TL , Bailey MT . Effects of stress on commensal microbes and immune system activity. Advances in Exp Med Biol. 2016;874:289–300.
- Delpech JC , Madore C , Nadjar A , Joffre C , Wohleb ES , Laye S . Microglia in neuronal plasticity: Influence of stress. Neuropharmacology. 2015;96:19–28. doi:10.1016/j.neuropharm.2014.12.034.
- Fricchione G . Generalized anxiety disorder. N Engl J Med. 2004;351:675–682. doi:10.1056/NEJMcp022342.
- Eaton SB , Konner M . Paleolithic nutrition. A consideration of its nature and current implications. N Engl J Med. 1985;312:283–289. doi:10.1056/NEJM198501313120505.
- Steptoe A , Kivimaki M . Stress and cardiovascular disease. Nature Reviews in Cardiology. 2012;9:360–370. doi:10.1038/nrcardio.2012.45.
- Schnorr SL , Bachner HA . Integrative therapies in anxiety treatment with special emphasis on the gut microbiome. Yale Journal of Biology and Medicine. 2016;89:397–422.
- Tiller JWG . Depression and anxiety. Med J Aust 2013;199(Suppl 6):S28–S31. doi:10.5694/mja12.10628.
- Laditka JN , Laditka SB , Tait EM , Tsulukidze MM . Use of dietary supplements for cognitive health: results of a national survey of adults in the United States. Am J Alzheimer's Dis Dement 2012. 2012;27:55–64.
- Munoz ME , Galan AI , Palacios E , Diez MA , Muguerza B , Cobaleda C , Calvo JI , Aruoma OI , Sanchez-Garcia I , Jiminez R . Effect of an antioxidant functional food beverage on exercise-induced oxidative stress: A long-term and large-scale clinical intervention study. Toxicology. 2010;278:101–111. doi:10.1016/j.tox.2009.10.015.
- Qureshi AA , Sami SA , Salser WA , Khan FA . Dose-dependent suppression of serum cholesterol by tocotrienol-rich fraction (TRF25) of rice bran in hypercholesterolemic humans. Atherosclerosis. 2002;161:199–207. doi:10.1016/S0021-9150(01)00619-0.
- Calabrese C , Gregory WL , Leo M , Kraemer D , Bone K , Oken B . Effects of a standardized Bacopa monnieri extract on cognitive performance, anxiety, and depression in the elderly: a randomized, double-blind, placebo-controlled trial. J Altern Comp Med. 2008;14:707–713. doi:10.1089/acm.2008.0018.
- Morgan A , Stevens J . Does Bacopa monnieri improve memory performance in older persons? Results of a randomized, placebo-controlled, double-blind trial. J Altern Comp Med. 2010;16:753–759. doi:10.1089/acm.2009.0342.
- Stough C , Downey LA , Lloyd J , Silber B , Redman S , Hutchison C , Wesnes K , Nathan PJ . Examining the nootropic effects of a special extract of Bacopa monniera on human cognitive functioning: 90-day double-blind placebo-controlled randomized trial. Phytother Res. 2008;22:1629–1634. doi:10.1002/ptr.2537.
- Chandrasekhar K , Kapoor J , Anishetty S . A prospective, randomized double-blind, placebo-controlled study of safety and efficacy of a high-concentration full-spectrum extract of ashwagandha root in reducing stress and anxiety in adults. Ind J Psych Med. 2012;34:255–262. doi:10.4103/0253-7176.106022.
- Cooley K , Szczurko O , Perri D , Mills EJ , Bernhardt B , Zhou Q , Seely D . Naturopathic care for anxiety: a randomized controlled trial ISRCTN78958974. PLoS One. 2009;4:e6628.
- Heresco-Levy U , Ermilov M , Lichtenberg P , Bar G , Javitt DC . High-dose glycine added to olanzapine and risperidone for the treatment of schizophrenia. Biol Psychiat. 2004;55:165–171. doi:10.1016/S0006-3223(03)00707-8.
- Lakhan SE , Vieira KF . Nutritional and herbal supplements for anxiety and anxiety-related disorders: systematic review. Nutri J. 2010;9:42. doi:10.1186/1475-2891-9-42.
- Aruoma OI , Jen SSM , Watt HR , George J , Gentleman SM , Anderson PJB , Jen LS . Acute and chronic effects of intravitreally injected amyloid beta on the neurotransmitter system in the retina. Toxicology. 2009;256:92–100. doi:10.1016/j.tox.2008.11.007.
- Gome-Pinilla F . Brain foods: the effect of nutrients on brain function. Nature Rev Neurosci. 2008;9:568–578. doi:10.1038/nrn2421.
- Terry PC , Lane AM , Fogarty GJ . Construct validity of the profile of mood state -adolescent for use with adults. Psych Sport Exer. 2003;4:125–139. doi:10.1016/S1469-0292(01)00035-8.
- McNair DM , Heuchert JWP , Shilony E . Profile of Mood States manual: bibliography 1964–2002. New York ( NY ): Multi-Health Systems Inc.; 2003.
- Bishop F , Yardley L . The development and initial validation of a new measure of lay definitions of health: the wellness beliefs scale. J Psych Health. 2010;25:271–287. doi:10.1080/08870440802609980.
- Stojanovska L , Law C , Lai B , Chung T , Nelson K , Day S , Apostolopoulos V , Haines C . Maca reduces blood pressure and depression, in a pilot study in postmenopausal women. Climacteric. 2015;18:69–78. doi:10.3109/13697137.2014.929649.
- Terauchi M , Horiguchi N , Kajiyama A , Akiyoshi M , Owa Y , Kato K , Kubota T . Effects of grape seed proanthocyanidin extract on menopausal symptoms, body composition, and cardiovascular parameters in middle-aged women: a randomized, double-blind, placebo-controlled pilot study. Menopause. 2014;21:990–996. doi:10.1097/GME.0000000000000200.
- Bradwejn J , Zhou Y , Koszycki D , Shlik J . A double-blind, placebo-controlled study on the effects of gotu kola (Centella asiatica) on acoustic startle response in healthy subjects. J Clin Psychopharmacol. 2000;20:680–684. doi:10.1097/00004714-200012000-00015.
- Jana U , Sur TK , Maity LN , Debnath PK , Bhattacharyya D . A clinical study on the management of generalized anxiety disorder with Centella asiatica . Nepal Med Coll J. 2010;12:8–11.
- Lopresti AL , Maes M , Maker GL , Hood SD , Drummond PD . Curcumin for the treatment of major depression: a randomised, double-blind, placebo controlled study. J Affect Disord. 2014;167:368–375. doi:10.1016/j.jad.2014.06.001.
- Panahi Y , Badeli R , Karami GR , Badeli Z , Sahebkar A . A randomized controlled trial of 6-week Chlorella vulgaris supplementation in patients with major depressive disorder. Complement Ther Med. 2015;23:598–602. doi:10.1016/j.ctim.2015.06.010.
- Smriga M , Ando T , Akutsu M , Furukawa Y , Miwa K , Morinaga Y . Oral treatment with L-lysine and L-arginine reduces anxiety and basal cortisol levels in healthy humans. Biomed Res. 2007;28:85–90. doi:10.2220/biomedres.28.85.
- Andrade C , Aswath A , Chaturvedi SK , Srinivasa M , Raguram R . A double-blind, placebo-controlled evaluation of the anxiolytic efficacy of an ethanolic extract of Withania somnifera . Indian J Psychiatry. 2000;42:295–301.
- Gommoll C , Durgam S , Mathews M , Forero G , Nunez R , Tang X , Thase ME . A double-blind, randomized, placebo-controlled, fixed-dose phase III study of vilazodone in patients with generalized anxiety disorder. Depress Anxiety. 2015;32:451–459. doi:10.1002/da.22365.