Abstract
Introduction: Reduced circulating levels of 25(OH)VD are associated with an increased incidence of chronic lung diseases. Alpha-1-antitrypsin (AAT) is needed to maintain healthy lung function.
Objective: This study examined the hypothesis that circulating levels of AAT are lower in adult type 2 diabetic patients and that a positive association exists between circulating AAT levels and 25(OH)VD levels in these patients.
Methods: Fasting blood was obtained after written informed consent from type 2 diabetic patients (n = 80) and normal siblings or volunteers (n = 22) attending clinics at LSUHSC according to the protocol approved by the Institutional Review Board for Human studies. Plasma AAT and 25(OH)VD levels were determined using ELISA kits. HbA1c levels and chemistry profiles were analyzed at the clinical laboratory of LSUHSC hospital.
Results: ATT and 25(OH)VD levels were significantly lower in type 2 diabetic patients compared with those of age-matched healthy controls. There was a significant positive correlation between 25(OH)VD and ATT deficiency. AAT levels showed significant positive correlation with HDL cholesterol levels in type 2 diabetic patients. There was no correlation between AAT levels and those of HbA1c or with the duration of diabetes of T2D patients.
Conclusions: These results suggest that 25(OH)VD deficiency may predispose type 2 diabetic patients to AAT deficiency. Whether reduced levels of circulating AAT indeed contribute to the increased risk for lung dysfunction in subjects with type 2 diabetes needs further investigation.
Introduction
Numerous studies have documented the association of 25-hydroxyvitamin D (25(OH)VD) deficiency with various adverse health events [Citation1–4]. Vitamin D (VD) has an anti-inflammatory function. The incidence of VD deficiency is increased in subjects with type 2 diabetes (T2D) [Citation4–6]. Alpha-1-antitrypsin deficiency (AATD) is characterized by low serum levels of alpha-1-antitrypsin (AAT), causing disease involving lung, liver, skin, and other various organs [Citation7–9]. The primary function of AAT is to inhibit neutrophil elastase and prevent elastin degradation in the lungs. AAT deficiency and excess elastin degradation impair the recoiling of elastin and make breathing difficult, as observed in chronic obstructive pulmonary disease (COPD) [Citation7–9].
Low levels of serum 25(OH)VD have been associated with subclinical interstitial lung disease and COPD [Citation10–13]. Although both 25(OH)VD insufficiency and AATD are extensively linked to decreased lung function, no study has investigated the relationship between 25(OH)VD and AAT levels. This study examined plasma levels of AAT in the adult with T2D patients and investigated whether any association exists between circulating AAT levels and 25(OH)VD levels in adult withT2D patients.
Research design and methods
Enrollment of adults with T2D and healthy control subjects
Informed written consent was obtained from all patients according to the protocol approved by the Louisiana State University Health Sciences Center (LSUHSC) Institutional Review Board (IRB). All patients included in this study were adults with type 2 diabetes attending the medicine/diabetic clinic at the LSUHSC hospital. After obtaining their written informed consent, they were advised to return to have blood drawn after overnight fasting. Volunteers for healthy controls were enrolled from among siblings of patients or workers at LSUHSC. All healthy control subjects invited to participate came to the clinic after overnight fasting and providing informed written consent, at which time blood was drawn. is a flow chart that shows the total number of the invited participant with T2D followed by the number of participants who signed informed consent and finally the number of participants who participated in sample collection for tests, including screening.
Inclusion/exclusion criteria
Patients were excluded if they had any history of cardiovascular disease, sickle cell disease, treatment with insulin, or metabolic disorders, including uncontrolled hypertension, hypothyroidism, or hyperthyroidism. Additional exclusion criteria included significant hepatic dysfunction, defined as any underlying chronic liver disease or elevated liver enzymes by greater than 1.5 times the upper limit of normal, or renal dysfunction, defined as serum creatinine greater than 1.5 mg/dL. Breastfeeding or pregnant women, and subjects taking any supplemental vitamins or herbal products were not enrolled in this study. The same inclusion/exclusion criteria were used for healthy controls
Blood collection and handling
After overnight fast blood was drawn from all participants into precooled tubes kept in an ice bucket: 2 mL in a tube without anticoagulant (for the chemistry profile), and in tubes with EDTA, 2 mL each for HbA1c and CBC, and 8 mL for the isolation of plasma. EDTA-blood was centrifuged, and the clear plasma saved in several aliquots for various assays. The plasma was stored at −80 °C. CBC, HbA1c, and a blood chemistry profile for Ca2+, total protein, creatinine, BUN, AST, ALT ALP, HLD-c, and pregnancy tests were done at the clinical laboratory of LSUHSC-Shreveport. Information on age, height, body weight, and any medication use were collected from each subject at the time of enrollment.
Following blood collection, the serum tubes for the chemistry profile and the EDTA tubes for HbA1C and complete blood counts were promptly delivered to the LSUHSC clinical laboratory. Additional tubes of EDTA-blood were brought to the research laboratory. Clear plasma was separated via centrifugation at 3000 rpm (1500× g) for 15 minutes. All plasma samples were stored at −80 °C for use in the analyses of the biochemical parameters.
Clinical chemistry profile and 25(OH)VD and alpha-1 antitrypsin assays
Blood tests were analyzed in the LSUHSC clinical laboratory. Tests included HbA1c, complete blood count, blood glucose, calcium, and other parameters, including HDL cholesterol (HDL-C). Frozen aliquots that were stored at −80 °C and not previously thawed were used for all assays. Measurements were made in duplicate using standards provided in the kit by the manufacturer and included in the assay at the same time. 25(OH)VD and CRP levels in the plasma were determined with the sandwich ELISA method using commercially available kits from Fisher Thermo Scientific Co. (Rockford, IL). AAT was assayed using an ELISA kit from Abcam (Catalogue # ab108798). All appropriate controls and standards as specified by the manufacturer’s kit were used. Control samples were analyzed each time to check any variation from plate to plate on different days of analysis. The inter-assay variability was less than 4%.
All chemicals were purchased from Sigma Chemical Co. (St. Louis, MO) unless mentioned otherwise. Differences in values between diabetic patients and healthy subjects were analyzed using a Mann-Whitney Rank Sum Test, and correlations were calculated statistically using multiple regression analyses using Sigma Stat. A p-value of less than 0.05 for a statistical test was considered significant.
Results
lists the ages, BMI, duration of diabetes, glucose, HbA1c, and other laboratory parameters of diabetic patients and control subjects. There were no differences in their mean ages in both groups. BMI and levels of glucose, HbA1c, and triglycerides were significantly higher. In contrast, levels of calcium, HDL-C, and 25(OH)VD were lower in diabetic patients compared with those of age-matched control subjects.
Table 1. Ages, sex, BMI, chemistry profile, and 25(OH)VD levels in T2D patients and normal controls.
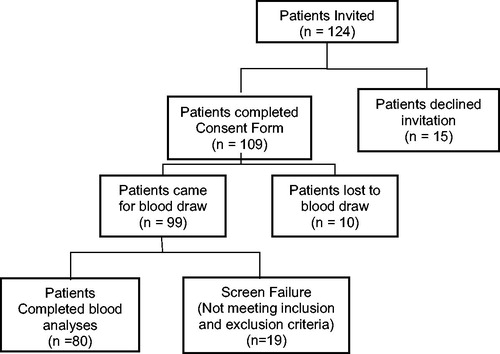
shows that AAT levels were significantly lower in a participant with T2D patients compared to those of control subjects, whether AAT was expressed per volume or normalized per gram of protein. AAT values were normalized with protein because AAT is an antitrypsin protein, which can influence the total protein level. illustrates a modest but significant correlation of AAT levels with those of 25(OH)VD (r = 0.30, p < 0.05) and HDL-C (r = 0.28, p < 0.05) in the blood of diabetic patients. shows that no association exists between the levels of plasma AAT and the level of glycemic control (HbA1c) or with the duration of diabetes. HbA1c did show association (−0.27, p < 0.02) with 25(OH)VD levels in diabetic patients. There was not a significant association between blood levels of ATT with that of 25(OH)VD and HDL-cholesterol in normal subjects.
Figure 2. Plasma alpha-1-antitrypsin levels in type 2 diabetic patients (T2D, n = 80) and age-matched normal subjects (N, n = 22). Note the significant decrease in AAT levels in T2D patients.
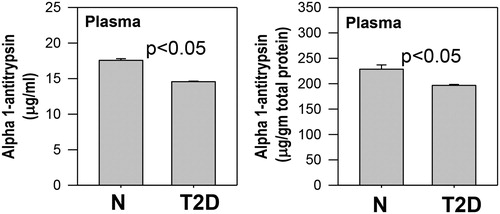
Figure 3. Relationship between plasma alpha-1-antitrypsin levels and 25(OH)VD and HDL-C levels in T2D patients. Correlations were calculated using BMI as an additional variable. Plasma alpha-1-antitrypsin shows an association with levels of 25(OH)VD and HDL-C in T2D patients.
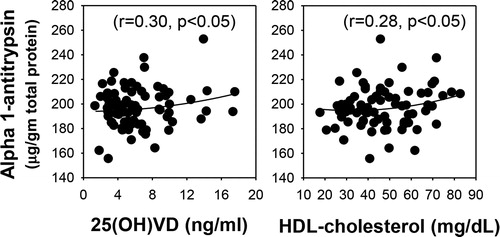
Figure 4. Relationship of plasma alpha-1-antitrypsin levels with those of HbA1c and with the duration of diabetes in T2D patients. Plasma alpha-1-antitrypsin does not show any association with the levels of HbA1c or the duration of diabetes in T2D patients.
shows whether taking different hypoglycemic drugs, such as biguanides, alone or in combination with a sulfonylurea, had any influence on HDL-C or 25(OH)VD, which in turn may have affected reduced AAT levels. This shows there was no difference in levels of HDL-C, vitamin D, or AAT among those taking only biguanides or taking biguanides in combination with sulfonylurea. This suggests that the intake of different diabetes medications does not have any effect on AAT levels. As expected, interestingly, the HbA1c levels were altered in patients taking only biguanides versus those taking a combination of biguanides and sulfonylurea in combination.
Table 2. Effect of taking biguanides only or a combination of biguanides and sulfonylurea drugs on levels of HDL-C,25(OH)VD, and alpha-1-antitrypsin in T2D patients.
Discussion
Circulating 25(OH)VD is considered a comprehensive and stable metabolite and can be used to diagnose 25(OH)VD deficiencies and monitor VD consumption [Citation1, Citation2]. Deficient 25(OH)VD levels are associated with impaired lung function and chronic lung diseases [Citation10–12, Citation14]. Diabetic patients have increased vascular inflammation and a higher incidence of COPD [Citation14]. Alpha-1-antitrypsin (AAT) is a protease inhibitor produced mainly by hepatocytes [Citation7–9]. Its deficiency, AATD, is characterized by a point mutation that leads to misfolding of the alpha-1-antitrypsin protein [Citation7]. The liver is the main site of AAT synthesis, so when the misfolded proteins accumulate in the endoplasmic reticulum of the hepatocytes, proteotoxic consequences can occur, including fibrosis, cirrhosis, and carcinogenesis [Citation7–9]. Vitamin D deficiency has been shown to result in significantly lower AAT expression in the lungs and emphysema in mice exposed to cigarette smoke [Citation15]. However, there is no previous study that determined ATT levels in type 2 diabetes or examined whether there is an association between ATT deficiency and 25(OH)VD deficiency in T2D patients.
This study shows that circulating levels of AAT were lower in T2D patients and that a modest but significant positive correlation exists between levels of AAT and those of 25(OH)VD. It also showed that glycemic control, as determined by HbA1c, did not have any correlation with plasma AAT levels. This lack of association may be because vitamin D levels were similar in both groups of higher A1c and lower A1c, as shown in . This suggests that glycemic control does not have a direct effect on AAT levels in patients and may be related to Vitamin D level. Data from a previous study show that a decline in the decreased forced air expiratory capacity of lungs (FEV1 and FVC) could be related to osteoporotic manifestations, including rib fractures and kyphosis [Citation16]. Circulating AAT is glycated within about a week in hyperglycemic individuals; as a result, AAT becomes inactive [Citation17]. Type 1 diabetic subjects have lower plasma AAT levels than controls [Citation18]. It has been hypothesized that the heightened risk of diabetes is related to systemic inflammation and that COPD patients with diabetes tend to have worse outcomes than COPD patients without diabetes [Citation14]. This suggests that the effects of 25(OH)VD deficiency on impaired calcium homeostasis and AAT could contribute to the increased incidence of COPD in diabetes.
HDL-C has been explicitly linked to lung function in several ways. It has been shown to stimulate surfactant secretion from type II alveolar cells [Citation19]. The anti-inflammatory properties of HDL-C are demonstrated by its ability to inhibit TNF-α-induced expression of some inflammatory markers, including ICAM-1 and VCAM-1 [Citation20]. It is known that HDL can bind and carry AAT [Citation21]. Patients with AAT deficiency are often treated with intravenous AAT. Moreno et al. sought better vectorization of AAT to allow for smaller doses to be administered with greater efficacy [Citation21]. They found HDL to be a competent vector for AAT; intravenous HDL enriched with AAT decreased pulmonary inflammation caused by elastase activity, both of which have been associated with alveolar damage [Citation21]. These anti-inflammatory effects were not shown with the administration of HDL alone or purified AAT [Citation21]. While it has been determined that HDL and AAT are in some way related, no previous study has examined the relationship between blood levels of AAT and HDL-C. Our study demonstrates a significant correlation between the blood levels of HDL-C and AAT. The mechanism of the increased bioavailability of AAT in conjunction with HDL is not well understood. Additionally, the relationship between HDL and pulmonary disease is unclear.
Nevertheless, the relationship is essential because of the many clinical implications of HDL and AAT as a unit, including the possibility of using HDL as a vector for AAT augmentation therapy, as indicated by Moreno’s research. AAT has also been successfully tested in various autoimmune disease animal models [Citation22, Citation23]. ATT synthesis by the CD4+ T cells is required in mediating the vitamin D (1, 25(OH)2D3) controlled immune regulatory system [Citation24].
C-reactive protein (CRP) is an inflammatory biomarker. Unlike a previous report [Citation25], blood levels of CRP did not show any significant association with 25(OH)VD or even AAT levels in healthy subjects or diabetic patients. The relationship between AAT and 25(OH)VD was also seen in healthy subjects in this study. This study does not rule out the possibility that improvement in the HDL and AAT levels is mediated by the anti-oxidative effects of 25(OH)VD in reducing oxidative stress [Citation6].
This study included the participants with T2D who are taking either biguanide alone or in combination with sulfonylurea. It does not cover the vast majority of patients with type 2 DM who now days have been taking other anti-diabetes medicine, including GLP1 agonist, a DPP4 inhibitor, SGLT2 inhibitor, and insulin. So, we do not know the effect of these medicines on the ATT level. This study did not compare the participants who are taking biguanide either in combination with other drugs or alone with the participants who are not taking biguanide but taking different medicine for diabetes. Is that low ATT level related to biguanide use or related to disease process? This question remains to be answered. AAT is post-translationally modified by glycosylation through the addition of N-glycosidically linked oligosaccharides, and glycated-AAT can be identified on diagnostic isoelectric focusing gels or LC-MS/MS [Citation26, Citation27]. The present study measured total AAT using the ELISA kit. This ELISA kit does not differentiate between glycated and non-glycated AAT. Future studies are needed to determine whether the 25(OH)VD and HDL-cholesterol levels have an association with both non-glycated AAT and glycated AAT in diabetic patients. This study did not collect data on the pulmonary function of the enrolled patients. Future studies are also needed whether levels of AAT indeed are positively associated with pulmonary functions in type 2 diabetic patients.
In conclusion, low levels of AAT positively associated with lower 25(OH)VD and HDL-C levels in type 2 diabetes. The reduced level of circulating AAT may compromise lung function and contribute to a higher incidence of COPD associated with diabetes. Previous studies demonstrate a relationship between dairy intake and adherence to better dietary approaches and improved lung density and functions [Citation28, Citation29]. Whether vitamin D supplementation can potentially improve the circulating levels of HDL and AAT and have a positive influence on lung function is not known and needs further investigation.
Acknowledgments
The authors are grateful to Nurse Coordinator John Rowell, RN, for the outstanding help in conducting this study. The authors thank Ms. Georgia Morgan for the excellent editing of this manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Herrick KA, Storandt RJ, Afful J, Pfeiffer CM, Schleicher RL, Gahche JJ, Potischman N. Vitamin D status in the United States, 2011-2014. Am J Clin Nutr. 2019;110(1):150–7. doi:10.1093/ajcn/nqz037.
- Calvo MS. Monitoring vitamin d status and intake in the US population: Essential to understanding the role of vitamin D in health. Am J Clin Nutr. 2019;110(1):6–7. doi:10.1093/ajcn/nqz069.
- Pantovic A, Zec M, Zekovic M, Obrenovic R, Stankovic S, Glibetic M. Vitamin D is inversely related to obesity: Cross-sectional study in a small cohort of Serbian adults. J Am Coll Nutr. 2019;38(5):405–14. doi:10.1080/07315724.2018.1538828.
- Tabesh M, Azadbakht L, Faghihimani E, Tabesh M, Esmaillzadeh A. Effects of calcium plus vitamin D supplementation on anthropometric measurements and blood pressure in vitamin D insufficient people with type 2 diabetes: A randomized controlled clinical trial. J Am Coll Nutr. 2015;34(4):281–9. doi:10.1080/07315724.2014.905761.
- Jain SK, Micinski D, Huning L, Kahlon G, Bass PF, Levine SN. Vitamin D and l-cysteine levels correlate positively with GSH and negatively with insulin resistance levels in the blood of type 2 diabetic patients. Eur J Clin Nutr. 2014;68(10):1148–53. doi:10.1038/ejcn.2014.114.
- Jain SK, Parsanathan R, Achari AE, Kanikarla-Marie P, Bocchini JA. Jr. Glutathione stimulates vitamin D regulatory and glucose-metabolism genes, lowers oxidative stress and inflammation, and increases 25-hydroxy-vitamin D levels in blood: A novel approach to treat 25-hydroxyvitamin d deficiency. Antioxid Redox Signal. 2018;29(17):1792–807. doi:10.1089/ars.2017.7462.
- Brode SK, Ling SC, Chapman KR. Alpha-1 antitrypsin deficiency: A commonly overlooked cause of lung disease. CMAJ. 2012;184(12):1365–71. doi:10.1503/cmaj.111749.
- Greulich T, Vogelmeier CF. Alpha-1-antitrypsin deficiency: Increasing awareness and improving diagnosis. Ther Adv Respir Dis. 2016;10(1):72–84. doi:10.1177/1753465815602162.
- Greulich T, Nell C, Hohmann D, Grebe M, Janciauskiene S, Koczulla AR, Vogelmeier CF. The prevalence of diagnosed alpha1-antitrypsin deficiency and its comorbidities: Results from a large population-based database. Eur Respir J. 2017;49(1):1600154. doi:10.1183/13993003.00154-2016.
- Janssens W, Decramer M, Mathieu C, Korf H. Vitamin D and chronic obstructive pulmonary disease: Hype or reality? Lancet Respir Med. 2013;1(10):804–12. doi:10.1016/S2213-2600(13)70102-4.
- Gilbert CR, Arum SM, Smith CM. Vitamin D deficiency and chronic lung disease. Can Respir J. 2009;16(3):75–80. doi:10.1155/2009/829130.
- Zendedel A, Gholami M, Anbari K, Ghanadi K, Bachari EC, Azargon A. Effects of vitamin D intake on FEV1 and COPD exacerbation: A randomized clinical trial study. Glob J Health Sci. 2015;7(4):243–8. doi:10.5539/gjhs.v7n4p243.
- Kim SM, Zhao D, Podolanczuk AJ, Lutsey PL, Guallar E, Kawut SM, Barr RG, de Boer IH, Kestenbaum BR, Lederer DJ, et al. Serum 25-hydroxyvitamin D concentrations are associated with computed tomography markers of subclinical interstitial lung disease among community-dwelling adults in the multi-ethnic study of atherosclerosis (MESA). J Nutr. 2018;148(7):1126–34. doi:10.1093/jn/nxy066.
- Putcha N, Drummond MB, Wise RA, Hansel NN. Comorbidities and chronic obstructive pulmonary disease: Prevalence, influence on outcomes, and management. Semin Respir Crit Care Med. 2015;36(4):575–91. doi:10.1055/s-0035-1556063.
- Crane-Godreau MA, Black CC, Giustini AJ, Dechen T, Ryu J, Jukosky JA, Lee HK, Bessette K, Ratcliffe NR, Hoopes PJ, et al. Modeling the influence of vitamin D deficiency on cigarette smoke-induced emphysema. Front Physiol. 2013;4:132. doi:10.3389/fphys.2013.00132.
- Herr C, Greulich T, Koczulla RA, Meyer S, Zakharkina T, Branscheidt M, Eschmann R, Bals R. The role of vitamin D in pulmonary disease: COPD, asthma, infection, and cancer. Respir Res. 2011;12:31. doi:10.1186/1465-9921-12-31.
- Lisowska-Myjak B, Pachecka J, Kaczyńska B, Miszkurka G, Kądziela K. Serum protease inhibitor concentrations and total antitrypsin activity in diabetic and non-diabetic children during adolescence. Acta Diabetol. 2006;43(4):88–92. doi:10.1007/s00592-006-0220-8.
- Sandstrom CS, Ohlsson B, Melander O, Westin U, Mahadeva R, Janciauskiene S. An association between type 2 diabetes and alpha-antitrypsin deficiency. Diabet Med. 2008;25(11):1370–3. doi:10.1111/j.1464-5491.2008.02584.x.
- Pian MS, Dobbs LG. Lipoprotein-stimulated surfactant secretion in alveolar type II cells: Mediation by heterotrimeric g proteins. Am J Physiol. 1997;273(3 Pt 1):L634–639. doi:10.1152/ajplung.1997.273.3.L634.
- Tran-Dinh A, Diallo D, Delbosc S, Varela-Perez LM, Dang QB, Lapergue B, Burillo E, Michel JB, Levoye A, Martin-Ventura JL, Meilhac O. HDL and endothelial protection. Br J Pharmacol. 2013;169(3):493–511. doi:10.1111/bph.12174.
- Moreno J-A, Ortega-Gomez A, Rubio-Navarro A, Louedec L, Ho-Tin-Noé B, Caligiuri G, Nicoletti A, Levoye A, Plantier L, Meilhac O. High-density lipoproteins potentiate alpha1-antitrypsin therapy in elastase-induced pulmonary emphysema. Am J Respir Cell Mol Biol. 2014;51(4):536–49. doi:10.1165/rcmb.2013-0103OC.
- Janciauskiene S, Welte T. Well-known and less well-known functions of alpha-1 antitrypsin. Its role in chronic obstructive pulmonary disease and other disease developments. Annals ATS. 2016;13(Supplement_4):S280–S288. doi:10.1513/AnnalsATS.201507-468KV.
- Kim M, Cai Q, Oh Y. Therapeutic potential of alpha-1 antitrypsin in human disease. Ann Pediatr Endocrinol Metab. 2018;23(3):131–5. doi:10.6065/apem.2018.23.3.131.
- Dimeloe S, Rice LV, Chen H, Cheadle C, Raynes J, Pfeffer P, Lavender P, Richards DF, Nyon MP, McDonnell JM, et al. Vitamin D (1,25(oh)2d3) induces alpha-1-antitrypsin synthesis by CD4(+) t cells, which is required for 1,25(oh)2d3-driven il-10. J Steroid Biochem Mol Biol. 2019;189:1–9. doi:10.1016/j.jsbmb.2019.01.014.
- Devaraj S, Yun JM, Duncan-Staley CR, Jialal I. Low vitamin D levels correlate with the proinflammatory state in type 1 diabetic subjects with and without microvascular complications. Am J Clin Pathol. 2011;135(3):429–33. doi:10.1309/AJCPJGZQX42BIAXL.
- McCarthy C, Dunlea DM, Saldova R, Henry M, Meleady P, McElvaney OJ, Marsh B, Rudd PM, Reeves EP, McElvaney NG. Glycosylation repurposes alpha-1 antitrypsin for resolution of community-acquired pneumonia. Am J Respir Crit Care Med. 2018;197(10):1346–9. doi:10.1164/rccm.201709-1954LE.
- Yin H, An M, So PK, Wong MY, Lubman DM, Yao Z. The analysis of alpha-1-antitrypsin glycosylation with direct LC-MS/MS. Electrophoresis. 2018;39(18):2351–61. doi:10.1002/elps.201700426.
- Jiang R, Jacobs DR, He K, Hoffman E, Hankinson J, Nettleton JA, Barr RG. Associations of dairy intake with CT lung density and lung function. J Am Coll Nutr. 2010;29(5):494–502. doi:10.1080/07315724.2010.10719886.
- Ardestani ME, Onvani S, Esmailzadeh A, Feizi A, Azadbakht L. Adherence to dietary approaches to stop hypertension (dash) dietary pattern in relation to chronic obstructive pulmonary disease (COPD): A case-control study. J Am Coll Nutr. 2017;36(7):549–55. doi:10.1080/07315724.2017.1326858.