Abstract
Objective:
Tea catechins (TCCs) have gained significant attention owing to their health effects. However, evidence is limited regarding the benefit of TCC and essential amino acids (EAAs) ingestion plus that of TCC ingestion after resistance exercise (RE) among older individuals with sarcopenia. We aimed to evaluate whether a 24-week nutritional program involving EAA and TCC supplementation after RE improved skeletal muscle mass (SMM) among older adults with sarcopenia.
Methods:
We conducted an open-label, pilot, randomized controlled trial among older adults with sarcopenia at the Harima Care Center or community in Hyogo, Japan. Participants were allocated to RE (n = 18), RE with EAA supplementation (RE + EAA, n = 18), or RE with EAA and TCC supplementation (RE + EAA + TCC, n = 18) groups. Sarcopenia was defined using the Asian Working Group for Sarcopenia 2019 criteria. A 24-week resistance exercise program was carried out twice weekly, with an intake of 3,000 mg and 540 mg of EAA and TCC supplements, respectively. SMM was the primary outcome parameter.
Results:
The mean adherence rate to exercise and supplementation intake over the 24-week intervention period was 86.8% in the RE + EAA + TCC group, 86.4% in the RE + EAA group, and 85.4% in the RE group. A significant group-by-time interaction was identified for SMM (p = 0.010). The pre- to post-intervention increase in SMM was significantly higher in the RE + EAA + TCC group than in the RE group (p = 0.010).
Conclusions:
These results suggest that supplementation with EAA and TCC after RE, compared to RE only, improves SMM in older people with sarcopenia. To the best of our knowledge, our study is the first pilot randomized controlled trial to evaluate the effect of TCC supplementation on SMM in older people with sarcopenia.
Supplemental data for this article is available online at http://dx.doi.org/10.1080/07315724.2022.2025546
Introduction
Sarcopenia is defined as a condition that results in low skeletal muscle mass (SMM) combined with reduced muscle strength and/or physical performance (Citation1). Therefore, sarcopenia occurs due to a progressive age-related decline in SMM and performance, low physical activity, malnutrition, and disease, which ultimately increase the risk of adverse events, such as incidental fall and disability (Citation1). Exercise can help maintain and increase SMM and strength in older adults. However, previous intervention studies did not present clear evidence regarding the effectiveness of exercise and/or nutrition interventions in treating sarcopenia, as defined by the Asian Working Group for Sarcopenia (AWGS) criteria (Citation2).
Skeletal muscles metabolize proteins in response to exercise (Citation3). In particular, resistance exercise (RE) increases the synthesis of muscle proteins (MP) for up to 24 h after exercise (Citation4). MP synthesis peaks immediately after exercise and reduces over time. Therefore, the intake of essential amino acids (EAAs) immediately after RE is important for MP accumulation. Reportedly, EAA intake stimulates MP synthesis by activating the mammalian target of the rapamycin (mTORC) signaling pathway, which is associated with MP anabolism (Citation5). Since the synthesis of MP peaks after RE, after which a gradual, time-dependent decrease occurs, ingesting EAA supplement after exercise may be important in optimizing the accumulation of MP (Citation6).
Recently, among the different forms of nutraceuticals, tea catechins (TCCs), such as those present in green teas, have gained significant attention owing to their health benefits (Citation7). A previous study reported the effects of green TCCs on skeletal muscle through the maintenance of a strong balance between protein synthesis and breakdown, along with boosting the synthesis of mitochondrial energy metabolism. Moreover, in vitro and in vivo (animal) studies have shown favorable muscle homeostasis and mitigation of muscle atrophy with age, owing to green TCC ingestion (Citation8). However, a previous randomized controlled trial, conducted among an Asian population of older adults with sarcopenia, did not clearly identify any improvements in the participants’ SMM upon the administration of TCC supplements (Citation2). In addition, because there was no report concerning optimal treatment, dosage, combination with other nutrients, timing, and frequency of TCC intake to increase the SMM in older people with sarcopenia, there was a need to conduct a pilot intervention study. Therefore, whether such nutritional supplement interventions are beneficial for improving SMM in older people with sarcopenia is unknown. In the present study, we hypothesized that individuals ingesting both EAA and TCC supplements after RE would have higher SMM than those who only performed RE or those who ingested EAA alone after performing RE. Therefore, this study aimed to evaluate the effectiveness of a 24-week nutritional intervention program involving EAA and TCC supplements intake after RE to improve SMM among older individuals with sarcopenia, using a randomized controlled design.
Materials and methods
Participants
This study was approved by the Medical Ethics Committee of Tokushima University Hospital (approval number: 3777–2; clinical registry trial number: UMIN 000040024). The contents of the study and potential risks were explained to the participants (both verbally and in written form) according to the Helsinki Declaration. Written informed consent was obtained from all participants enrolled in this study. The CONSORT checklist is shown in . Participants were recruited from June to April 2020. Based on the AWGS 2019 criteria (Citation2), older people aged ≥65 years (n = 114) were screened for sarcopenia at the Harima Care Center or community in Hyogo, Japan. The AWGS cutoff values were as follows: low hand-grip strength (males: <28.0 kg, females: <18.0 kg) or slow gait speed (males and females: <1.0 m/sec), and low SMM index (SMM index, males: <7.0 kg/m2, females: <5.7 kg/m2). SMM index was calculated by dividing the upper- and lower-limb SMM by height squared. Individuals who refused participation (n = 12), did not have sarcopenia (n = 40), and/or had undertaken nutritional therapies for conditions, such as type 2 diabetes (n = 7) or chronic kidney disease (n = 1) within 1-year pre-intervention were excluded. The inclusion criteria were as follows: nonparticipation in RE therapy with the intake of enriched EAA or TCC supplements within 1 year of the commencement of the study and not suffering from heart and respiratory diseases. Finally, 54 participants with sarcopenia were randomly divided into three intervention groups: RE group (n = 18), RE + EAA group (n = 18), and RE + EAA + TCC group (n = 18). Eligible subjects were randomly allocated in a 1:1:1 manner to either the RE, RE + EAA, or RE + EAA + TCC groups (9 block size 6) using randomization envelopes with three different randomization codes stratified by sex. The randomization sequence was computer-generated by a statistician who was blinded to the details of assignment and was not otherwise involved in the study. This open-label and exploratory randomized controlled trial used a parallel-group comparison. Participants in the RE or RE + EAA groups did not receive EAA and/or TCC placebo supplements.
Intervention design
Participants in the RE + EAA + TCC group ingested EAA and TCC supplements after the RE program. The effects observed in the RE + EAA + TCC group were evaluated against those in the RE group, wherein participants completed the same RE program but without EAA and TCC intake, and those in the RE + EAA group, wherein participants ingested the same amount of EAA after RE but without TCC intake. The intervention lasted 24 weeks, with participants completing their assigned protocols twice weekly. This intervention study was conducted from July to December 2020. All participants were instructed to record the days supplementation and exercise were carried out in a book.
Exercises
The exercise program session included a 20-min warming up exercise and 40-min RE (Citation9, Citation10). The RE program included exercises using a resistance elastic band and bodyweight resistance exercises. Elastic band REs (Thera-Band-, The Hygenic Corporation, USA) included lower and upper skeletal muscle exercises. The RE load was modified using a standardized method (rating of perceived exertion: somewhat hard) over the 24-week program. Bodyweight REs of the lower skeletal muscle included leg extensions exercise, and sitting and standing exercise ().
EAA and TCC supplement
The EAA supplement (Amino Aile, Ajinomoto Co., Inc., Japan) was provided to participants in the RE + EAA and RE + EAA + TCC groups. The EAA supplement powder containing 17.6 kcal of energy and 3,000 mg of EAAs (containing leucine 1,200 mg, valine 330 mg, isoleucine 320 mg) per serving was consumed with mineral water. The TCC supplement powder (Healthya, Kao Co., Inc., Japan) was provided to the participants in the RE + EAA + TCC group. The TCC supplement containing 19 kcal of energy and 540 mg of TCCs per serving was consumed with mineral water (Supplementary Table S3).
Food and nutrition management
The total amounts of energy and protein consumed by participants in all groups were controlled to reach at least 30.0 kcal/kg ideal body weight (IBW)/day and 1.2 g/kg IBW/day, respectively, during the intervention period (Citation11). Food and nutrition management was led by a registered dietitian. A survey on the participants’ nutrition and diet was conducted before the intervention and was based on the Dietary Reference Intakes for Japanese (Citation12). The detailed nutritional and food management program are presented in Supplementary Table S3.
Outcome measures
The following outcomes were measured pre- and post-intervention by blinded research staff during each designated visit of a given participant. SMM was the primary outcome parameter. Grip strength, knee extension strength, gait speed, and health-related quality-of-life (QOL) were the secondary outcome parameters.
Body composition and SMM
Body mass index (BMI) and SMM were measured using a body composition analyzer (Bioelectrical impedance analyzer, In Body Japan, Japan) and multi-frequency bioelectrical impedance analysis (BIA), respectively. BIA was performed in the morning after a 10-h overnight fast. Calf circumference was measured using a measure tape.
Muscle strength and physical performance
We measured the hand-grip strength for each hand using maximum isometric grip strength (Digital Grip Strength Tester 5401; Takei Scientific Instruments, Japan) in a standing position; the value of the highest grip strength was used as the participant’s muscle strength. A hand-held dynamometer (μTas F1; Anima, Japan) was used to evaluate the maximum isometric knee extension strength, which represents lower extremity muscle strength (Citation13). The participants sat on a bench, and the force sensor was firmly fixed on the distal end of the tibia to a rigid bar using a belt. Maximal isometric knee extension strength was defined as the highest value of the three trials. To identify the usual gait speed, participants were instructed to walk 10 m at their normal speed, and the time taken to walk 4 m (3–7 m) was measured (Citation2).
Health-related QOL
The health-related QOL of the investigated participants was calculated using the 36-item Short-Form Health Questionnaire Survey (SF-36) (Citation14), a widely used QOL survey. In this survey, a summary score of physical components (physical QOL) and that of mental components (mental QOL) were calculated.
Nutritional intake and nutritional status
We conducted a nutritional survey to document the total daily energy and protein intake, adjusted by IBW/day. The IBW was calculated by multiplying an ideal BMI of 22.0 kg/m2 with a person’s actual height (m) squared. Nutrient and food intake were calculated using the fourth edition of the Standard Tables of Food Composition in Japan and a nutrition calculation database (Version 8 Excel eiyou, Kenpaku, Japan) (Citation12).
The nutritional survey included a food weighing method, in which food intake was documented for five consecutive days pre-, during (at 12 weeks), and post-intervention. Furthermore, in the case of missing responses, we conducted individual interviews with participants regarding their food intake status. The total energy and protein intake data were collected pre-, during, and post-intervention. Nutritional status was determined using the Mini Nutritional Assessment®-Short Form (MNA-SF). An MNA-SF score lower than 12 was suggestive of a risk or possibility of malnutrition (Citation15). The MNA-SF scores were collected before the intervention.
Physical activity
All participants recorded their daily activities for three consecutive days before the commencement of the program. We used this information to calculate each participant’s regular physical activity level (Citation12). We conducted individual interviews in the case of missing information.
Statistical analyses
All data were checked for normality using the Shapiro–Wilk-test, and were represented as median (25 and 75 percentiles). We evaluated between-group differences in the distribution of pre-intervention clinical characteristics, using an independent group one-way repeated-measures analysis of variance test or a chi-squared-test. Within-group differences in primary and secondary outcome measures (SMM, grip strength, knee extension strength, gait speed, physical QOL, and mental QOL) pre- and post-intervention were evaluated using the Wilcoxon-signed-rank-test. Two-way repeated-measures analysis of variance was used to evaluate the effect of the intervention on the primary and secondary outcome measures, and the total energy and protein intake between groups. The rate of change (Δ) in measured primary and secondary outcomes, pre- to post-intervention, was compared using the one-way repeated-measures analysis of variance test. The calculations were based on an effect size (the change in the SMM from pre- to post-intervention period) of 0.80, α level of 0.05, and power (1 − β) of 80%, giving a total of 15 participants required per group. Considering a drop-out rate of 15%, we recruited 18 older people with sarcopenia per group. All statistical analyses were conducted using SPSS software (SPSS version 25, IBM, Japan), and the level of significance was set at p < 0.05.
Results
Eight participants did not complete the intervention after randomization, as they withdrew their consent (RE group, lost to follow-up [n = 1] or lacked motivation [n = 2]; RE + EAA group, were hospitalized [n = 1] and lost to follow-up [n = 2]; RE + EAA + TCC, lacked motivation [n = 2]; ). The final analysis of the RE, RE + EAA, and RE + EAA + TCC groups comprised 15, 15, and 16 participants, respectively. The mean adherence rate to exercise and supplementation intake over the 24-week intervention period was 86.8% in the RE + EAA + TCC group, 86.4% in the RE + EAA group, and 85.4% in the RE group. The pre-intervention clinical characteristics of the participants are presented in . There were no differences in SMM, grip strength, knee extension strength, gait speed, physical QOL, mental QOL, total energy intake, and total protein intake between the groups during the pre-intervention period.
Figure 1. A flow chart showing the study participants.
We randomly allocated participants with sarcopenia to one of the following three groups: completing the exercise intervention alone (RE group), completing the exercise intervention followed by amino acid ingestion (RE + EAA group), and completing the exercise intervention followed by amino acid and tea catechin supplementation (RE + EAA + TCC group)
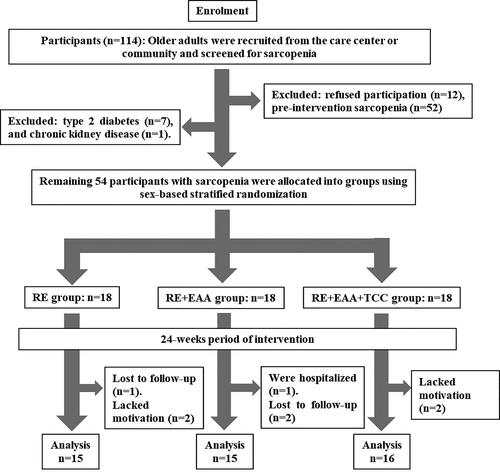
Table 1. Pre-intervention clinical characteristics of participants.
The pre- to post-intervention changes in primary and secondary outcomes are shown in . We identified a significant group-by-time interaction for SMM (p = 0.010). After the 24-week intervention period, the RE + EAA + TCC group showed an increase in SMM, knee extension strength, gait speed, and physical QOL value (SMM: p < 0.01, knee extension strength, gait speed, and physical QOL (p < 0.05). The difference (%Δ) in SMM was significantly greater in the RE + EAA + TCC group than in the RE group (p = 0.010). However, the %Δ SMM did not significantly differ between the RE + EAA and RE groups (p = 0.748).
Table 2. Pre- and post-intervention comparison of primary and secondary outcome.
Details regarding the pre- to 12 weeks, and 24 weeks intervention changes in total energy, protein, and food intake are shown in Supplementary Table S4. At 12 and 24 weeks of the intervention, there were no differences in total energy, protein, food intake among the groups. There was no significant interaction (group-by-time) between total energy, protein, and food intake.
Discussion
The pre- to post-intervention increase in SMM was significantly higher in the RE + EAA + TCC group than in the RE group. However, EAA supplementation after RE did not show additional benefit to the SMM over exclusively performing RE, suggesting that RE could be followed by both EAA and TCC supplementation in older adults with sarcopenia to possibly improve their SMM.
Previous randomized controlled trials did not investigate the benefit of exercise with the combination of EAA and TCC supplementation on older adults with sarcopenia. To the best of our knowledge, our study is the first pilot randomized controlled trial to evaluate the combined effects of both supplements (EAA + TCC) on SMM in older people diagnosed with sarcopenia using the AWGS 2019 criteria (Citation2).
Among older adults, RE is a standard treatment for increasing SMM. In our study, combined intake of EAA and TCC improved the SMM of participants in the RE + EAA + TCC group. However, intake of EAA supplementation alone after RE did not show additional benefit to the SMM over exclusively performing RE, suggesting that RE should be followed by EAA and TCC supplementation in older adults with sarcopenia to improve their SMM. At pre-, 12, and 24 weeks of intervention, total energy and protein intake were not different among the groups. We observed no significant group-by-time interaction between total energy and protein intake. Therefore, the changes in SMM values possibly reflect the combined effects of EAA and TCC supplements.
In this study, the RE + EAA + TCC group showed improvements in SMM post-intervention. This outcome was supported by the results of a randomized controlled trial, in which exercise combined with EAA and TCC intake improved SMM than exercise alone in older people. Previous reports revealed that the risk of recurrent falls and functional limitations were greater in older people with sarcopenia and low SMM than in those without these conditions (Citation16). Since low SMM is associated with incidental falls in older people, EAA and TCC supplementation after RE may help prevent falls and functional limitations by improving low SMM in adults with sarcopenia.
The PROT-AGE study suggested that the intake of EAAs is effective for improving SMM in older people (Citation11). In particular, leucine intake stimulates MP synthesis by activating the mTOR signaling pathway, which is associated with protein anabolism through the regulation of mRNA translation (Citation4). To maximize MP synthesis in older people, ingesting 3000 mg of EAAs containing leucine 1200 mg is recommended per serving. A recent report confirmed that MP synthesis and muscle mass increased in older people who consumed 3000 mg of EAA (Citation17, Citation18). Moreover, there is evidence of progressive age-associated attenuation of sensitivity to leucine for high MP synthesis activity in skeletal muscles. A previous report revealed that MP synthesis in older people peaked approximately 1–3 h after RE and 1–2 h after ingestion of an EAA (Citation3–5). Although we did not identify the benefits of RE + EAA compared with only RE regarding the increase in SMM and strength, the physiological mechanisms by which a single dose of ingested EAA following RE influences MP synthesis, requires further clarification. In this study, the frequency of EAA intake being low (twice a week) may have caused a decrease in SMM.
Green tea beverages sold in Japan contain approximately 80 mg of TCC per serving (120 mL). In this study, the RE + EAA + TCC group participants ingested 540 mg of highly concentrated TCCs. The results from previous studies on humans revealed that TCC (450–500 mg) supplementation could decrease the levels of inflammatory markers, such as those due to exercise-induced oxidative stress and creatine kinase, which are associated with muscle damage, (Citation19, Citation20). Interestingly, supplementation of 540–570 mg resulted in improved physical and aerobic capacity in adults (Citation21, Citation22). However, the functionality of highly concentrated TCCs has attracted attention as a component that inhibits MP breakdown (Citation7). A previous study suggested that oxidative stress reduced upon supplementation with green TCCs after eccentric exercise (Citation23), while green TCC supplementation alone reduced the loss of soleus muscle force during hindlimb suspension in mice (Citation24). Catechins suppress the expression of ubiquitin ligase in muscle atrophy-related genes (Citation25). Catechins also enhance phosphorylation of Akt, which translocate the transcription factor FoxO3a out of the nucleus, inhibits breakdown of SMM, and attenuates the apoptotic pathway associated with the progression of muscle atrophy (Citation26). While the intake of EAA with high amount of leucine after RE enhances MP synthesis, the intake of TCCs inhibits MP breakdown. This suggests that the positive enhancement of the dynamic equilibrium of MP accumulation may contribute to the improvement of SMM in older people with sarcopenia. Although this was an interventional study in humans, it is possible that the combined intake of EAA and TCCs after RE may enhance MP synthesis, inhibit MP breakdown, and increase MP accumulation in skeletal muscles. Therefore, the introduction of nutritional interventions adjusted to the dynamics of MP metabolism after RE has important implications for the treatment of low SMM and sarcopenia.
A previous study showed the superiority of exercise in improving muscle strength and gait speed in middle-aged to older people. However, there was no difference in effects on muscle strength and gait speed between exercise combined with nutrition and exercise alone (Citation27, Citation28). In this study, as all groups received exercise intervention, there was no significant difference in the improvement of muscle strength, physical performance, and QOL between the RE + EAA + TCC and RE groups.
Our study had several limitations. First, the randomized control trial design was that of an open-label study, and we did not use EAA or TCC placebo supplements. Second, the number of subjects in this study was small because it was a pilot study. Moreover, the sex difference in the RE + EAA + TCC group was unknown. Third, in this study, there was no significant difference in the improvement of grip strength, knee extensor strength, and QOL between the RE + EAA + TCC and RE groups. This study included patients who ingested EAA and TCC twice a week. In the future, increasing the frequency of EAA and TCC intake (>twice a week) may improve grip strength, knee extensor strength, and QOL in older people with sarcopenia. Finally, although we estimated SMM using BIA measurements, a strong positive correlation between dual-energy X-ray absorptiometry- and BIA-based measurements has been reported previously, confirming the validity of BIA measurements of SMM among older individuals (Citation29). The AWGS 2019 criteria allow for the assessment of SMM using the BIA method. However, the body composition assessment using BIA may be suboptimal in intervention trials. Therefore, dual X-ray absorptiometry may be the best method for assessing body composition.
Conclusions
The pre- to post-intervention increase in SMM was significantly higher in the RE + EAA + TCC group than in the RE group. In conclusion, our findings suggest that RE could be followed by both EAA and TCC supplementation in older adults with sarcopenia to possibly improve their SMM. To the best of our knowledge, our study is the first pilot randomized controlled trial to evaluate the combined effects of both supplements (EAA + TCC) on SMM in older people diagnosed with sarcopenia using the AWGS 2019 criteria.
Acknowledgments
We thank the participants of the study.
Disclosure statement
Hiroyasu Mori received Grants from the Foundation for Dietary Scientific Research. More payments of grant were made to Tokushima University. Yasunobu Tokuda declares no conflict of interest.
Data availability statement
All data sets generated during and/or analyzed during the current study are not publicly available. However, date sets are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, Chou MY, Chen LY, Hsu PS, Krairit K, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. 2014;15(2):95–101. doi:10.1016/j.jamda.2013.11.025.
- Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, Jang HC, Kang L, Kim M, Kim S, et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 2020;21(3):300–7. doi:10.1016/j.jamda.2019.12.012.
- Shad BJ, Thompson JL, Breen L. Does the muscle protein synthetic response to exercise and amino acid‐based nutrition diminish with advancing age? A systematic review. Am J Physiol Endocrinol Metab. 2014;311(5):803–17. doi:10.1152/ajpendo.00213.2016.
- Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol Endocrinol Metab. 2006;291(2):381–7. doi:10.1152/ajpendo.00488.2005.
- Paddon-Jones D, Short KR, Campbell WW, Volpi E, Wolfe RR. Role of dietary protein in the sarcopenia of aging. Am J Clin Nutr. 2008;87(5):1562S–1566. doi: 10.1093/ajcn/87.5.1562S.
- Jordan LY, Melanson EL, Melby CL, Hickey MS, Miller BF. Nitrogen balance in older individuals in energy balance depends on timing of protein intake. J Gerontol A Biol Sci Med Sci. 2010;65A (10):1068–76. doi:10.1093/gerona/glq123.
- Luk HY, Appell C, Chyu MC, Chen CH, Wang CY, Yang RS, Shen CL. Impacts of green tea on joint and skeletal muscle health: prospects of translational nutrition. Antioxidants (Basel). 2020;9(11):1050. doi:10.3390/antiox9111050.
- Wang L, Wang Z, Yang K, Shu G, Wang S, Gao P, Zhu X, Xi Q, Zhang Y, Jiang Q. Epigallocatechin gallate reduces slow-twitch muscle fiber formation and mitochondrial biosynthesis in C2C12 cells by repressing AMPK activity and PGC-1α expression. J Agric Food Chem. 2016;64(34):6517–23. doi:10.1021/acs.jafc.6b02193.
- Mori H, Tokuda Y. Effect of increased daily intake of protein, combined with a program of resistance exercises, on the muscle mass and physical function of community‐dwelling elderly women. J Aging Res Lifestyle. 2016;6:1–61. doi:10.14283/jarcp.2016.124.
- Mori H, Tokuda Y. Effect of whey protein supplementation after resistance exercise on the muscle mass and physical function of healthy older women: a randomized controlled trial. Geriatr Gerontol Int. 2018;18(9):1398–404. doi:10.1111/ggi.13499.
- Bauer J, Biolo G, Cederholm T, Cesari M, Cruz-Jentoft AJ, Morley JE, Phillips S, Sieber C, Stehle P, Teta P, et al. Evidence‐based recommendations for optimal dietary protein intake in older people: a position paper from the PROT‐AGE Study Group. J Am Med Dir Assoc. 2013;14(8):542–59. doi:10.1016/j.jamda.2013.05.021.
- National Institute of Health and Nutrition. Dietary reference intakes for Japanese -2015-: the summary report from the scientific committee of “dietary reference intakes for Japanese”. Ministry of Health, Labour and Welfare, Toyama Building Official Web Site; 2015. https://www.mhlw.go.jp/stf/shingi/0000041824.html. Published March 1, 2016. Accessed July 21, 2021. (in Japanese)
- Katoh M, Isozaki K. Reliability of isometric knee extension muscle strength measurements of healthy elderly subjects made with a hand-held dynamometer and a belt. J Phys Ther Sci. 2014;26(12):1855–9. doi:10.1589/jpts.26.1855.
- Rizzoli R, Reginster JY, Arnal JF, Bautmans I, Beaudart C, Bischoff-Ferrari H, Biver E, Boonen S, Brandi ML, Chines A, et al. Quality of life in sarcopenia and frailty. Calcif Tissue Int. 2013;93(2):101–20. doi:10.1007/s00223-013-9758-y.
- Rubenstein LZ, Harker JO, Salvà A, Guigoz Y, Vellas B. Screening for undernutrition in geriatric practice: developing the Short-Form Mini Nutritional Assessment (MNA-SF). J Geront A Biol Sci Med Sci. 2001;56(6):M366–372. doi:10.1093/gerona/56.6.M366.
- Landi F, Liperoti R, Russo A, Giovannini S, Tosato M, Capoluongo E, Bernabei R, Onder G. Sarcopenia as a risk factor for falls in elderly individuals: results from the ilSIRENTE study. Clin Nutr. 2012;31(5):652–8. doi:10.1016/j.clnu.2012.02.007.
- Bukhari SS, Phillips BE, Wilkinson DJ, Limb MC, Rankin D, Mitchell WK, Kobayashi H, Greenhaff PL, Smith K, Atherton PJ. Intake of low-dose leucine-rich essential amino acids stimulates muscle anabolism equivalently to bolus whey protein in older women at rest and after exercise. Am J Physiol Endocrinol Metab. 2015;308(12):E1056–1065. doi:10.1152/ajpendo.00481.2014.
- Yoshimura Y, Bise T, Shimazu S, Tanoue M, Tomioka Y, Araki M, Nishino T, Kuzuhara A, Takatsuki F. Effects of a leucine-enriched amino acid supplement on muscle mass, muscle strength, and physical function in post-stroke patients with sarcopenia: a randomized controlled trial. Nutrition. 2019;58:1–6. doi:10.1016/j.nut.2018.05.028.
- Hadi A, Pourmasoumi M, Kafeshani M, Karimian J, Maracy MR, Entezari MH. The effect of green tea and sour tea (Hibiscus sabdariffa L.) supplementation on oxidative stress and muscle damage in athletes. J Diet Suppl. 2017;14(3):346–57. doi:10.1080/19390211.2016.1237400.
- Silva WD, Machado AS, Souza MA, Mello-Carpes PB, Carpes FP. Effect of green tea extract supplementation on exercise-induced delayed onset muscle soreness and muscular damage. Physiol Behav. 2018;194:77–82. doi:10.1016/j.physbeh.2018.05.006.
- Kim H, Suzuki T, Saito K, Yoshida H, Kojima N, Kim M, Sudo M, Yamashiro Y, Tokimitsu I. Effects of exercise and tea catechins on muscle mass, strength and walking ability in community-dwelling elderly Japanese sarcopenic women: a randomized controlled trial. Geriatr Gerontol Int. 2013;13(2):458–65. doi:10.1111/j.1447-0594.2012.00923.x.
- Ota N, Soga S, Shimotoyodome A. Daily consumption of tea catechins improves aerobic capacity in healthy male adults: a randomized double-blind, placebo-controlled, crossover trial. Biosci Biotechnol Biochem. 2016;80(12):2412–7. doi:10.1080/09168451.2016.1224638.
- Haramizu S, Ota N, Hase T, Murase T. Catechins attenuate eccentric exercise-induced inflammation and loss of force production in muscle in senescence-accelerated mice. J Appl Physiol (1985). 2011;111(6):1654–63. doi:10.1152/japplphysiol.01434.2010.
- Ota N, Soga S, Haramizu S, Yokoi Y, Hase T, Murase T. Tea catechins prevent contractile dysfunction in unloaded murine soleus muscle: a pilot study. Nutrition. 2011;27(9):955–9. doi:10.1016/j.nut.2010.10.008.
- Alway SE, Bennett BT, Wilson JC, Edens NK, Pereira SL. Epigallocatechin-3-gallate improves plantaris muscle recovery after disuse in aged rats. Exp Gerontol. 2014;50:82–94. doi:10.1016/j.exger.2013.11.011.
- Wang H, Lai YJ, Chan YL, Li TL, Wu CJ. Epigallocatechin-3-gallate effectively attenuates skeletal muscle atrophy caused by cancer cachexia. Cancer Lett. 2011;305(1):40–9. doi:10.1016/j.canlet.2011.02.023.
- Hanach NI, McCullough F, Avery A. The impact of dairy protein intake on muscle mass, muscle strength, and physical performance in middle-aged to older adults with or without existing sarcopenia: a systematic review and meta-analysis. Adv Nutr. 2019;10(1):59–69. doi:10.1093/advances/nmy065.
- Wu PW, Huang KS, Chen KM, Chou CP, Tu YK. Exercise, nutrition, and combined exercise and nutrition in older adults with sarcopenia: a systematic review and network meta-analysis. Maturitas. 2021;145:38–48. doi:10.1016/j.maturitas.2020.12.009.
- Ling CH, de Craen AJ, Slagboom PE, Gunn DA, Stokkel MP, Westendorp RG, Maier AB. Accuracy of direct segmental multi-frequency bioimpedance analysis in the assessment of total body and segmental body composition in middle-aged adult population. Clin Nutr. 2011;30(5):610–5. doi:10.1016/j.clnu.2011.04.001.
