ABSTRACT
Objectives
To measure home-based older adults’ attentional biases (AB) using webcam-based eye-tracking (WBET) and examine internal consistency.
Methods
Twelve participants with and without cognitive impairment completed online self-report anxiety and depression screens, and a 96-trial dot-probe task with eye-gaze tracking. For each trial, participants fixated on a cross, free-viewed sad-neutral, sad-angry, sad-happy, angry-neutral, angry-happy, and happy-neutral facial expression pairings, and then fixated on a dot. In emotional-neutral pairings, the time spent looking (dwell-time) at neutral was averaged and subtracted from the emotional average to indicate biases “away from” (negative score) and “toward” (positive score) each emotional face. Internal consistency was estimated for dwell-times and bias scores using Cronbach’s alpha and Spearman – Brown corrected split-half coefficients.
Results
The full-cohort and a comorbid anxious and depressed sub-group (n = 6) displayed AB away from sad faces, and toward angry and happy faces, with happy-face AB being more pronounced. AB indices demonstrated low reliability except sub-group happy-face indices. Happy-face AB demonstrated the highest reliability.
Conclusions
AB measures were in-line with lab-based eye-tracking literature, providing some support for WBET-based measurement.
Clinical Implications
Establishing the feasibility of WBET-based measures is a step toward an objective home-based clinical tool. Literature-based suggestions are provided to improve reliability.
Introduction
By the year 2050, it is expected that 131.5 million people worldwide will be living with dementia (Barbarino, Citation2017). Alzheimer’s disease (AD), which accounts for 60–70% of all cases, is often accompanied by neuropsychiatric symptoms such as anxiety and depression (Botto et al., Citation2022). Anxiety and depression can increase the rate of cognitive decline, reduces quality of life (Breitve et al., Citation2016; Gonfrier et al., Citation2012; Spalletta et al., Citation2012), and are associated with higher rates of conversion from mild cognitive impairment (MCI) to AD (Gallagher et al., Citation2011). Given these implications, it is important to effectively detect and monitor symptoms, and evaluate treatment. Self-report measures are widely used. However, difficulties can arise from their use for older adults with and without cognitive impairment such as scale accessibility (e.g., wording, or the need for verbal or written responses), a reluctance to report and endorse negative emotions or items, and the respondent’s level of awareness (Balsamo, Cataldi, Carlucci, & Fairfield, Citation2018; Balsamo, Cataldi, Carlucci, Padulo, et al., Citation2018). Moreover, age-/cognitive status-appropriate measures employing dichotomous scale formats reveal less information (i.e., incremental change) (Kolanowski et al., Citation2019). Therefore, additional non-verbal objective assessment methods may be useful for clinicians, particularly when self-report is not possible (Kolanowski et al., Citation2019).
The usefulness of eye gaze data
Eye-movement data could provide an objective measure of disease status and symptom progression monitoring (Anderson & MacAskill, Citation2013). People with AD (pwAD) have been differentiated from people with other types of dementias and cognitively healthy (HC) participants by their eye movements, and alterations can correlate with disease severity (see Molitor et al., Citation2015 for a detailed review). The types of visual stimuli that an individual looks toward or away from, i.e., their attentional bias (AB), can also correlate with health outcomes, and anxiety and depression severity (De Raedt & Koster, Citation2010; MacLeod & Clarke, Citation2015). Looking longer at positive stimuli, as demonstrated by some cognitively healthy older adults (Demeyer et al., Citation2017), has been linked to experiencing a positive mood and greater life satisfaction (Sanchez & Vazquez, Citation2014). Positive biases in relation to recall have also been demonstrated by non-depressed older adults with MCI (Callahan et al., Citation2016). Negative AB (looking longer at negative stimuli) may be demonstrated by depressed or clinically anxious older adults, and pwAD in relation to recall and emotion discrimination (Armstrong & Olatunji, Citation2012; Cabrera et al., Citation2020; Maria & Juan, Citation2017). Negative AB can represent a trait depression vulnerability marker (Harmer et al., Citation2009). Therefore, eye movement and AB may have the potential to help clinicians identify at-risk individuals.
Anxiety and depression often co-occur in dementia (Ryu et al., Citation2005) and comorbidity can modulate AB. In the few dot-probe response-time/button-press studies examining comorbidity in younger individuals, findings are mixed showing that clinically comorbid individuals may show no bias or a bias toward sad faces (Hankin et al., Citation2010; Kishimoto et al., Citation2021; LeMoult & Joormann, Citation2012), show a bias away from (Kishimoto et al., Citation2021; LeMoult & Joormann, Citation2012) or toward angry faces (Hankin et al., Citation2010; Kishimoto et al., Citation2021), or show a bias toward or away from happy faces depending on their clinical history (i.e., current versus lifetime symptoms respectively) (Hankin et al., Citation2010). Understanding an individual’s attentional bias, especially using a more direct measure such as eye-tracking (Arditte & Joormann, Citation2014), could inform treatment.
Webcam-based eye-tracking of attentional biases
Webcam-based eye-tracking (WBET) research has increased, and comparable results to those obtained by other eye-tracking devices have been produced (Bott et al., Citation2020; Semmelmann & Weigelt, Citation2018). WBET removes the need for travel, expensive equipment and eye-tracking specialists, and potentially enables regular and longitudinal assessment of AB. WBET could therefore facilitate timely intervention as negative AB can prospectively predict higher depressive symptom scores (Beevers et al., Citation2011), and enable clinicians to objectively monitor intervention effects as changes in positive AB can occur after antidepressant treatment (Zhang et al., Citation2020). Changes in AB may be seen before subjective mood improvements and can correlate with successful anti-depressant treatment (Harmer et al., Citation2009).
WBET measures of attentional biases could benefit pwAD. Internet-based dot-probe paradigms (MacLeod et al., Citation2002) include a response-time/button-press task which involves several complex cognitive processes (Gratton et al., Citation2018). A response-time/button-press element is unnecessary using eye-tracking, and therefore cognitive load can be reduced for pwAD (Bourgin et al., Citation2018). However, AB would need to be examined in a large number of pwAD (Kruijt et al., Citation2019), and unfortunately, many studies recruit a low number of participants with dementia (Mooldijk et al., Citation2021). WBET could enable more pwAD to access eye-tracking studies thereby facilitating statistically powered findings. A recent study (Greenaway et al., Citation2021) examined WBET (i.e., set-up/time, calibration failures, and related issues) with older adults living with and without AD. The authors found that WBET is feasible when assistance is provided, particularly when positioning the face and the eyes. Utilizing this information, we measured the AB of older adults living with and without AD using WBET, and examined the reliability of WBET-measured AB.
Methods
Participants
We recruited 22 participants to the study. Full eye-tracking datasets were obtained from 12 participants (AD = 6, MCI = 3, HC = 3; 4 females, 8 males) aged 60 to 79 yrs old (see for descriptive data). Participant TICS scores fell within cognitively non-impaired (n = 5), ambiguously impaired (n = 6), and mildly impaired (n = 1) ranges. Four participants were classified as being non-anxious/non-depressed (MCI = 2; HC = 2), one as anxious (pwAD), one as depressed (pwAD), and six as having comorbid anxiety and depression (pwAD = 4; MCI = 1; HC = 1). Five participants with cognitive impairment (pwAD = 3, MCI = 2) were taking cognitive medication (Donepezil) and one participant (pwAD group) was taking anti-depressant medication. All participants with cognitive impairment (pwAD and MCI) were required to have a carer or representative provide written or verbal confirmation of the participant’s ability to provide informed consent. All participants provided written or verbal consent before the start of the study.
Table 1. Descriptive analyses of age, cognitive, mood, and bias data.
Procedure
Participants received preparation notes, a link to JISC Online surveys (https://www.onlinesurveys.ac.uk/) to complete a self-report questionnaire assesing their levels of anxiety and depression, and a Microsoft Teams meeting link via e-mail. Participants joined the Microsoft Teams meeting for the eye-tracking session, and shared their laptop screens with the researcher throughout the session for eye-tracking set-up support (e.g., lighting and positioning), and conditions monitoring (e.g., noise or interruptions during trials). During the session, the Telephone Interview for Cognitive Status (TICS) (Brandt et al., Citation1988) was conducted and a link was emailed to the participant to access Gorilla (Anwyl-Irvine et al., Citation2020), the web-based eye-tracking platform used in the study, to complete an attentional bias measure task. Participating took up to 1.5 hours in total. The participants could have assistance from another person to navigate the study’s technical requirements (e.g., accessing Microsoft Teams without software download). The study was reviewed in accordance with the procedures of the University of Reading’s Research Ethics Committee and received a favorable ethical opinion for conduct (UREC 19/71).
Measures
Anxiety
The 7-item Generalized Anxiety Disorder Scale (GAD-7) (Löwe et al., Citation2008) was used to screen for anxiety symptoms. A score of 0 (not at all) to 3 (nearly every day) is assigned for each item giving a total of between 0 and 21. Scores of 5, 10 and 15 represent the lower cutoff point for mild, moderate, and severe anxiety, respectively. It has high internal consistency (α = 0.89) (Löwe et al., Citation2008).
Depression
The 9-item Patient Health Questionnaire 9 (PHQ-9) (Kroenke & Spitzer, Citation2002) was used to screen for depressive symptoms. The suicidal ideation item was removed due to ethical concerns. The removal of this item does not affect the interpretation of final scores (Kroenke & Spitzer, Citation2002). A score of 0 (not at all) to 3 (nearly every day) is assigned for each item giving a total of between 0 and 24. Scores of 5, 10, 15, and 20 represent the lower cutoff points for mild, moderate, moderately severe, and severe depression respectively. A score of ≥ 10 has a specificity and sensitivity of 88% for major depression disorder (MDD) (Kroenke & Spitzer, Citation2002).
Cognitive status
The 11-item Telephone Interview for Cognitive Status (TICS), developed as a screen for dementia, was used to assess the cognitive domains of memory, orientation, attention, and language. Scores range from 0 to 41 with scores ≤ 30 indicating cognitive impairment. The TICS has a discriminative ability (those with and without dementia) comparable to the Mini Mental State Exam (Folstein et al., Citation1975; Seo et al., Citation2011).
Eye tracking
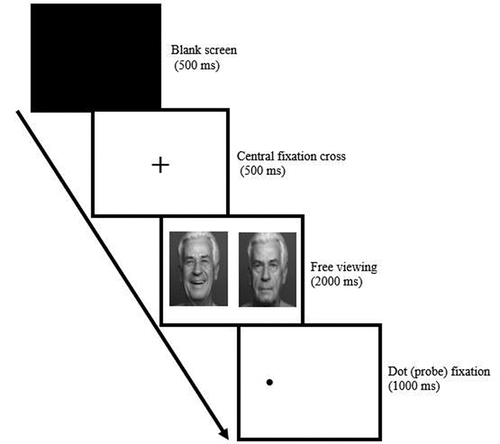
Positioning the face/eyes. Participants were instructed to sit directly in front of their webcam, to only move their eyes (rather than their head or body) to look at the different parts of the screen, and to remain still and blink as little as possible. Participants viewed their video feed which was presented in the top left corner of their screens. A black box outline was overlaid in the center of the video feed, and a green face-mesh, which detects the user’s face, was also displayed in the video feed. Using their video feed as a guide, participants were asked to align themselves such that their faces appeared in the middle of the box. Participants were told the box outline must turn green, and the green face outline must match their features (face-mesh) () to enable a start button (see Greenaway et al., Citation2021 for detailed face-meshing information). Glasses were only removed (where possible) if a participant’s eyes were not face-meshing correctly due to lens reflection. The start button changed color to a deeper shade of red when enabled, and once clicked, the participants advanced to a calibration and validation phase.
Figure 1. a). (left) showing successful face-meshing, and 1b). (right) illustrating the dot locations.
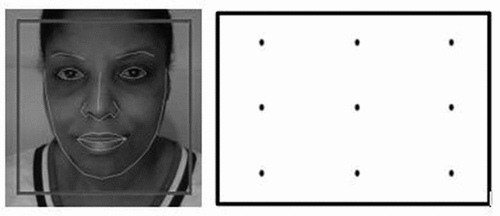
Calibration and validation. Within the calibration phase, a 50 × 50-pixel red dot appeared consecutively in each of 9 fixed locations in a random order. The 9 locations were arranged in a 3 × 3 grid spanning the screen’s height and width () (see Greenaway et al., Citation2021; Semmelmann & Weigelt, Citation2018 for detailed calibration and validation phase descriptions). The participants were instructed to look at the dot as quickly as possible and fixate on it until it disappeared. The validation phase was identical to the calibration phase, except the dot was green.
Attentional bias measure
A modified dot-probe task (MacLeod et al., Citation2002) was used to measure AB. Each trial began with a blank screen for 500 ms. A fixation cross then appeared in the center of the screen for a fixation of 500 ms. Two faces from the same actor were then presented for 2000 ms, to the left and right of where the fixation cross had been located. The faces, selected from the FACES database (Ebner et al., Citation2010), were presented in sad-angry, sad-happy, sad-neutral, angry-happy, angry-neutral and, happy-neutral facial emotion pairings. Once the faces had disappeared, a black dot appeared in the center of one of the face’s previous location for 1000 ms (). Participants were instructed to look at the cross and the dot quickly and fixate on them, and to naturally view the facial stimuli when presented. A total of 96 trials were shown randomly. Each facial emotion type was presented 48 times by a total of 24 actors. Each actor was presented four times. The trials were counterbalanced for actor gender, and the side of the screen the facial emotion type and dot appeared on.
Data analysis
Descriptive and reliabililty analyses were performed with the Statistical Package for the Social Sciences, version 25 (IBM Corp, Citation2017). Significance testing and correlational analyses of attentional bias and mood measures were not conducted due to sample size.
Cognitive status
Participants who scored between 33–41 points on the TICS were classed as cognitively non-impaired, between 26–32 points as ambiguously impaired, between 21–25 points as mildly impaired, and ≤ 20 as moderately to severely impaired (Chappelle et al., Citation2022).
Symptom status
Participants who scored < 5 on both the GAD-7 and the PHQ-9 scales were classified as non-anxious/non-depressed, ≥5 on the GAD-7 and < 5 on the PHQ-9 scales as anxious, and < 5 on the GAD-7 and ≥ 5 on the PHQ-9 scales as depressed. Participants who scored ≥ 5 on both the GAD-7 and PHQ-9 were classified as comorbid (anxious and depressed).
Attentional biases
The time spent looking at (dwell time in ms) each half of the screen per trial, per participant, was selected from the metrics provided by the eye-tracking platform. The dwell time was summed and averaged for each expression type in sad-neutral, angry-neutral, and happy-neutral pairings. The findings relating to emotional-emotional pairings will be presented elsewhere. Bias scores were calculated (the emotional expression average minus the corresponding neutral expression average). Scores below zero represented a bias away from the emotional expressions and those above zero, a bias toward the emotional expressions (Duque & Vázquez, Citation2015; Lazarov et al., Citation2018).
Reliability
Internal consistency was estimated for sad, angry, and happy face dwell-times and bias scores from emotional-neutral trials using Cronbach’s alpha (CA) and the Spearman – Brown corrected split-half (S-BCS-H) reliability coefficients (Sears et al., Citation2019; Waechter et al., Citation2014). Split-half reliability was based on the first half (i.e., trials 1 to 8) and second half (i.e., trials 9 to 16) of the trial dwell-times and bias scores.
Results
Participant characteristics
Due to the small sample size, group comparisons (e.g., by cognitive or mood status) were not conducted. Analyses were conducted for the full cohort, a comorbid sub-group as anxiety and depression symptoms often co-occur and can persist for older adults (Almeida et al., Citation2012; Braam et al., Citation2014), and individual participant bias scores. The average cognitive status scores for the full cohort and for the comorbid sub-group fell within the ambigously impaired range and, on average, participants in the full cohort were also comorbid anxious and depressed (see for descriptive data). Eight of 12 participants were classified as having mild to severe anxiety and/or depression, three of whom had scores indicative of comorbid generalized anxiety disorder (GAD) and major depressive disorder (MDD) (see for participant data).
Table 2. Individual participants’ symptom status, mood, and bias data.
Attentional biases
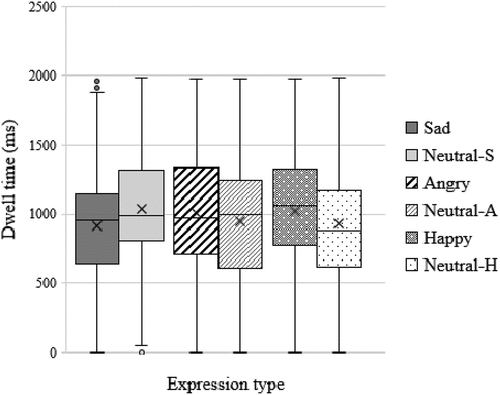
The full-cohort and comorbid sub-group, on average, displayed a bias away from sad faces, and toward angry and happy faces, with the AB toward happy faces being more pronounced (see . for bias scores, and . for dwell-time averages). While individual participants displayed differences in terms of bias direction and/or magnitude to each of the emotional faces, the pronounced full-cohort and comorbid sub-group average bias toward happy faces relative to sad and faces was mainly driven by participants with anxiety and/or depression (see .). Individual participants with potential comorbid GAD and MDD displayed a bias toward sad faces, and both away and toward angry and happy faces.
Figure 3. Showing the full cohort’s (N = 12) average dwell-time data for emotional-neutral pairings. A hyphen followed by S, A, or H denotes the corresponding neutral face data for sad, angry, and happy pairings respectively. Dwell-time data were non-normally distributed.
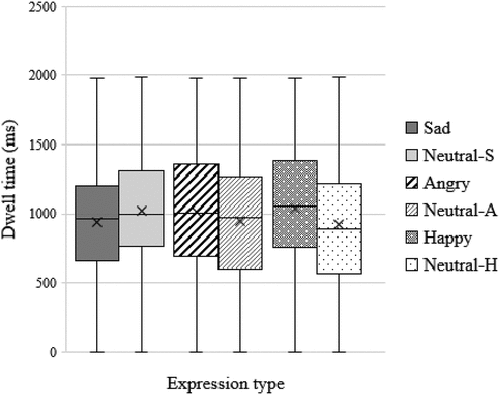
Figure 4. Showing the comorbid (anxious and depressed) groups’s (n = 6) average dwell-time data for emotional-neutral pairings. A hyphen followed by S, A, or H denotes the corresponding neutral face data for sad, angry, and happy pairings respectively. Dwell-time data were non-normally distributed except for angry, happy, and neutral-H face data.
Reliability
For the full cohort, dwell-time CA scores (sad = −.48, angry = −.49, happy = −.49) and S-BCS-H estimates (sad = −.61, angry = .40, happy = .59) demonstrated low reliability. Bias score CA scores (sad = −.33, angry = −.44, happy = −.33) and S-BCS-H estimates (sad = −.42, angry = .40, happy = .62) also demonstrated low reliability (see for reliability scores and estimates).
Table 3. Reliability analyses.
For the comorbid sub-group, dwell-time CA scores (sad = .06, angry = −1.12, happy = .12), and S-BCS-H estimates for sad and angry faces (sad = .45, angry = .54) demonstrated low reliability, whereas S-BCS-H estimate for happy faces (.87) demonstrated good reliability. Bias score CA scores (sad = .21, angry = −1.16, happy = .21) and S-BCS-H estimates for sad and angry faces (sad = .56, angry = .55) demonstrated low reliability, whereas S-BCS-H estimate for happy faces (.87) demonstrated good reliability (see for reliability scores and estimates).
Discussion
To our knowledge, this is the first study to measure the attentional biases (AB) of older adults living with and without Alzheimer’s disease using webcam-based eye-tracking (WBET). Our study lays the foundation for further research exploring the potential of WBET-measured AB by providing preliminary data to inform future study design. Our study findings also contribute to the sparse older adult AB literature. We found that, on average, the full cohort and comorbid (anxious and depressed) sub-group displayed a relatively positive AB – looking at happy faces longer than sad and angry faces. While healthy older adults are thought to demonstrate a “positivity effect” (i.e., preferentially attending to positive over negative material) (Carstensen & Mikels, Citation2005), it was predominantly the anxious and/or depressed participants within the current study who displayed prominent, relatively positive AB, with biases toward happy faces possibly indicating increased emotional regulation strategy use (Demeyer et al., Citation2017). However, our findings should be interpreted with caution given the small sample size and further research is required to directly explore how older adults’ AB relate to emotional regulation/dysregulation.
Although our findings for participants with mood scores indicative of comorbid GAD and MDD within the current study are in-line with previous studies (Hankin et al., Citation2010; Kishimoto et al., Citation2021; LeMoult & Joormann, Citation2012), the literature presents mixed results, and is therefore likely to support most findings. As demonstrated by our individual participant AB data, ABs are complex and personalized (Zvielli et al., Citation2014) in that no two biases are the same in terms of direction and/or magnitude. Still, AB profiles were observable using WBET. The non-anxious/non-depressed participants displaying prominent AB toward angry faces relative to sad and happy faces (HC1 and MCI1) could represent individuals at risk. Biases toward angry faces can occur prior to the onset of anxiety and in individuals who have experienced past depression and are at risk of recurrence (Barry et al., Citation2015; Woody et al., Citation2016). WBET could potentially be used to monitor change in such individuals.
Though it is unclear what level of reliability is appropriate for eye-tracking measures (Waechter et al., Citation2014), in-line with lab-based eye-tracking studies analyzing stimuli presentation durations between 0–2000 ms with younger adults (Price et al., Citation2015; Sears et al., Citation2019; Waechter et al., Citation2014), WBET-measured AB generally demonstrated low dwell-time and bias score reliabilities. However, AB indices at short stimuli presentation duration tend to demonstrate low reliability whereas longer presentation durations demonstrate higher reliability (Lazarov et al., Citation2016; Sears et al., Citation2019) (see Skinner et al., Citation2018 for higher reliability in regard to word-based threat stimuli). Similarly to Sears et al. (Citation2019), WBET AB reliability (i.e., Spearman-Brown corrected split-half estimates) was higher for happy faces than angry and sad faces.
Limitations and future studies
While we hope that our study prompts further (WBET) research examining (1) AB, anxiety, and depression in older adults which is lacking (Baruch et al., Citation2021; Cabrera et al., Citation2020), and (2) the general clinical utility of WBET, our study design (i.e., stimuli number and presentation duration) and sample size did not allow for a fuller investigation of internal consistency, and test-retest reliability was not examined. Future WBET measured AB studies may consider incorporating a large number of trials containing the contrast under focus (e.g., threat-neutral), longer stimuli presentation times (e.g., 3.5 to 8 seconds), and also conduct analyses relating to the entire stimuli time-course (e.g., total dwell-time) as these study parameters have exhibited moderate to excellent internal consistency, and adequate to high test–retest reliability of AB measures collected 30 minutes, one week, or 6 months apart (Blanco et al., Citation2019; Lazarov et al., Citation2016; Molloy & Anderson, Citation2020; Rodebaugh et al., Citation2016; Sears et al., Citation2019; Skinner et al., Citation2018; Waechter et al., Citation2014). However, there is still a need to establish which parameters (e.g., stimuli type [words, natural scenes, or faces], stimuli categories [dysphoric, threat, pleasant, social, illness], and contrasts [emotional-neutral, or emotional-emotional]), are more relevant and reliable in late-life depression and/or anxiety, and to investigate the reliability of AB indices using multiple test-retest points across time in depression and/or anxiety (Sears et al., Citation2019; Skinner et al., Citation2018).
Other limitations of our study are that visuospatial disturbances, and emotion recognition capability were not assessed. Older adults with and without cognitive impairment may exhibit emotional recognition difficulties, to differing extents for different emotion types (Weiss et al., Citation2008). While longer looking may be associated with correct and incorrect recognition responses (Low et al., Citation2022), recognition errors occurring within attentional bias measure trials could potentially impact relative bias scores (e.g., if neutral faces were mistaken for sad faces) and therefore should be assessed. Neuropsychiatric symptom history was also not assessed but could have influenced our findings (Hankin et al., Citation2010). Future larger studies should allow for subgroup analysis (e.g., neuropsychiatric symptom history and symptom status), and assess visuospatial and emotion recognition capabilities. Future studies should also directly compare WBET data against data obtained from other eye-trackers.
Clinical Implications
Older adults’ AB are measurable via WBET and may demonstrate similar reliability to lab-based studies
With careful measurement design, WBET may provide clinicians with an additional objective tool for screening, monitoring, and evaluating treatment response either virtually or with patients in their homes
Supplemental Material
Download MS Excel (34.8 KB)Acknowledgments
This project has been funded by a University of Reading Strategic PhD Studentship and by the School of Biological Sciences. We would like to thank the Sir Richard Stapley Educational Trust, Hilda Martindale Educational Trust, and the Funds for Women Graduates Educational Charity for their non-project student awards.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/07317115.2023.2240783
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article’s supplementary materials.
Additional information
Funding
References
- Almeida, O. P., Draper, B., Pirkis, J., Snowdon, J., Lautenschlager, N. T., Byrne, G., Sim, M., Stocks, N., Kerse, N., Flicker, L., & Pfaff, J. J. (2012). Anxiety, depression, and comorbid anxiety and depression: Risk factors and outcome over two years. International Psychogeriatrics, 24(10), 1622–1632. https://doi.org/10.1017/S104161021200107X
- Anderson, T. J., & MacAskill, M. R. (2013). Eye movements in patients with neurodegenerative disorders. Nature Reviews Neurology, 9(2), 74–85. https://doi.org/10.1038/nrneurol.2012.273
- Anwyl-Irvine, A., Massonnié, J., Flitton, A., Kirkham, N., & Evershed, J. (2020). Gorilla in our Midst: An online behavioral experiment builder. Behavior Research Methods, 52(1), 388–407. https://doi.org/10.3758/s13428-019-01237-x
- Arditte, K. A., & Joormann, J. (2014). Rumination moderates the effects of cognitive bias modification of attention. Cognitive Therapy and Research, 38(2), 189–199. https://doi.org/10.1007/s10608-013-9581-9
- Armstrong, T., & Olatunji, B. O. (2012). Eye tracking of attention in the affective disorders: A meta-analytic review and synthesis. Clinical Psychology Review, 32(8), 704–723. https://doi.org/10.1016/j.cpr.2012.09.004
- Balsamo, M., Cataldi, F., Carlucci, L., & Fairfield, B. (2018). Assessment of anxiety in older adults: A review of self-report measures. Clinical Interventions in Aging, 13, 573–593. https://doi.org/10.2147/CIA.S114100_
- Balsamo, M., Cataldi, F., Carlucci, L., Padulo, C., & Fairfield, B. (2018). Assessment of late-life depression via self-report measures: A review. Clinical Interventions in Aging, 13, 2021–2044. https://doi.org/10.2147/CIA.S114100
- Barbarino, P. (2017). Global dementia policy overview. Alzheimer’s & Dementia, 13(7), 1451–P1451. https://doi.org/10.1016/j.jalz.2017.07.760
- Barry, T. J., Vervliet, B., & Hermans, D. (2015). An integrative review of attention biases and their contribution to treatment for anxiety disorders. Frontiers in Psychology, 6, 968–968. https://doi.org/10.3389/fpsyg.2015.00968
- Baruch, N., Behrman, S., Wilkinson, P., Bajorek, T., Murphy, S. E., & Browning, M. (2021). Negative bias in interpretation and facial expression recognition in late life depression: A case control study. International Journal of Geriatric Psychiatry, 36(9), 1450–1459. https://doi.org/10.1002/gps.5557
- Beevers, C. G., Lee, H. J., Wells, T. T., Ellis, A. J., & Telch, M. J. (2011). Eye gaze bias for emotion stimuli prospectively predicts PTSD and depression symptoms among soldiers deployed in Iraq. American Journal of Psychiatry, 168(July), 735–741. https://doi.org/10.1176/appi.ajp.2011.10091309
- Blanco, I., Poyato, N., Nieto, I., Boemo, T., Pascual, T., Roca, P., & Vazquez, C. (2019). Attentional biases in dysphoria when happy and sad faces are simultaneously presented. Journal of Behavior Therapy and Experimental Psychiatry, 65, 101499. https://doi.org/10.1016/j.jbtep.2019.101499
- Bott, N. T., Madero, E. N., Glenn, J. M., Lange, A. R., Anderson, J. J., Newton, D. O., Brennan, A. H., Buffalo, E. A., Rentz, D. M., & Zola, S. M. (2020). Device-embedded cameras for eye tracking-based cognitive assessment: Implications for teleneuropsychology. Telemedicine Journal and E-Health, 26(4), 477–481. https://doi.org/10.1089/tmj.2019.0039
- Botto, R., Callai, N., Cermelli, A., Causarano, L., & Rainero, I. (2022). Anxiety and depression in Alzheimer’s disease: A systematic review of pathogenetic mechanisms and relation to cognitive decline. Neurological Sciences, 43(7), 4107–4124. https://doi.org/10.1007/s10072-022-06068-x
- Bourgin, J., Guyader, N., Chauvin, A., Juphard, A., Sauvée, M., Moreaud, O., Silvert, L., Hot, P., & Kumfor, F. (2018). Early emotional attention is impacted in alzheimer’s Disease: An eye-tracking study. Journal of Alzheimer’s Disease, 63(4), 1445–1458. https://doi.org/10.3233/JAD-180170
- Braam, A. W., Copeland, J. R. M., Delespaul, P. A. E. G., Beekman, A. T. F., Como, A., Dewey, M., Fichter, M., Holwerda, T. J., Lawlor, B. A., Lobo, A., Magnússon, H., Prince, M. J., Reischies, F., Wilson, K. C., & Skoog, I. (2014). Depression, subthreshold depression and comorbid anxiety symptoms in older Europeans: Results from the EURODEP concerted action. Journal of Affective Disorders, 155(1), 266–272. https://doi.org/10.1016/j.jad.2013.11.011
- Brandt, J., Spencer, M., & Folstein, M. (1988). The telephone interview for cognitive status. Neuropsychiatry, Neuropsychology, and Behavioral Neurology, 1(2), 111–117. https://doi.org/10.1037/t28542-000
- Breitve, M. H., Hynninen, M. J., Brønnick, K., Chwiszczuk, L. J., Auestad, B. H., Aarsland, D., & Rongve, A. (2016). A longitudinal study of anxiety and cognitive decline in dementia with Lewy bodies and Alzheimer’s disease. Alzheimer’s Research & Therapy, 8(3). https://doi.org/10.1186/s13195-016-0171-4
- Cabrera, I., Brugos, D., & Montorio, I. (2020). Attentional biases in older adults with generalized anxiety disorder. Journal of Anxiety Disorders, 71, 102207. https://doi.org/10.1016/j.janxdis.2020.102207
- Callahan, B. L., Simard, M., Mouiha, A., Rousseau, F., Laforce, R., Hudon, C., & Conde Sala, J. L. (2016). Impact of depressive symptoms on memory for emotional words in mild cognitive impairment and late-life depression. Journal of Alzheimer’s Disease, 52(2), 451–462. https://doi.org/10.3233/JAD-150585
- Carstensen, L. L., & Mikels, J. A. (2005). At the intersection of emotion and cognitiom: Aging and the positivity effect. Current Directions in Psychological Science, 14(3), 117–121. https://doi.org/10.1111/j.0963-7214.2005.00348.x
- Chappelle, S. D., Gigliotti, C., Leger, G. C., Peavy, G. M., Jacobs, D. M., Banks, S. J., Little, E. A., Galasko, D. R., & Salmon, D. P. (2022). Comparison of the telephone‐montreal cognitive assessment (T‐MoCA) and telephone interview for cognitive status (TICS) as remote screening tests for early alzheimer’s disease. Alzheimer’s & Dementia, 18(S7), 1–10. https://doi.org/10.1002/alz.065917
- Demeyer, I., Sanchez, A., & De Raedt, R. (2017). Older adults’ attentional deployment: Differential gaze patterns for different negative mood states. Journal of Behavior Therapy and Experimental Psychiatry, 55, 49–56. https://doi.org/10.1016/j.jbtep.2016.11.012
- De Raedt, R., & Koster, E. H. W. (2010). Understanding vulnerability for depression from a cognitive neuroscience perspective: A reappraisal of attentional factors and a new conceptual framework. Cognitive, Affective, & Behavioral Neuroscience, 10(1), 50–70. https://doi.org/10.3758/CABN.10.1.50
- Duque, A., & Vázquez, C. (2015). Double attention bias for positive and negative emotional faces in clinical depression: Evidence from an eye-tracking study. Journal of Behavior Therapy and Experimental Psychiatry, 46, 107–114. https://doi.org/10.1016/j.jbtep.2014.09.005
- Ebner, N. C., Riediger, M., & Lindenberger, U. (2010). FACES-a database of facial expressions in young, middle-aged, and older women and men: Development and validation. Behavior Research Methods, 42(1), 351–362. https://doi.org/10.3758/BRM.42.1.351
- Folstein, M. F., Folstein, S. E., & McHugh, P. R. (1975). “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12(3), 189–198. https://doi.org/10.1016/0022-3956(75)90026-6
- Gallagher, D., Coen, R., Kilroy, D., Belinski, K., Bruce, I., Coakley, D., Walsh, B., Cunningham, C., & Lawlor, B. A. (2011). Anxiety and behavioural disturbance as markers of prodromal Alzheimer’s disease in patients with mild cognitive impairment. International Journal of Geriatric Psychiatry, 26(2), 166–172. https://doi.org/10.1002/gps.2509
- Gonfrier, S., Andrieu, S., Renaud, D., Vellas, B., & Robert, P. (2012). Course of neuropsychiatric symptoms during a 4-year follow up in the REAL-FR cohort. Journal of Nutrition, Health and Aging, 16(2), 134–137. https://doi.org/10.1007/s12603-011-0147-9
- Gratton, G., Cooper, P., Fabiani, M., Carter, C. S., & Karayanidis, F. (2018). Dynamics of cognitive control: Theoretical bases, paradigms, and a view for the future. Psychophysiology, 55(3), 1–29. https://doi.org/10.1111/psyp.13016
- Greenaway, A. M., Nasuto, S., Ho, A., & Hwang, F. (2021). Is home-based webcam eye-tracking with older adults living with and without Alzheimer’s disease feasible? The 23rd International ACM SIGACCESS Conference on Computers and Accessibility (ASSETS ’21), 1–3. https://doi.org/10.1145/3441852.3476565
- Hankin, B. L., Gibb, B. E., Abela, J. R. Z., & Flory, K. (2010). Selective attention to affective stimuli and clinical depression among youths: Role of anxiety and specificity of emotion. Journal of Abnormal Psychology, 119(3), 491–501. https://doi.org/10.1037/a0019609
- Harmer, C. J., Goodwin, G. M., & Cowen, P. J. (2009). Why do antidepressants take so long to work? A cognitive neuropsychological model of antidepressant drug action. British Journal of Psychiatry, 195(2), 102–108. https://doi.org/10.1192/bjp.bp.108.051193
- IBM Corp. (2017) . IBM SPSS statistics software for windows (No. 25).
- Kishimoto, T., Wen, X., Li, M., Zhang, R. Y., Yao, N., Huang, Y., & Qian, M. (2021). Vigilance-avoidance toward negative faces in social anxiety with and without comorbid depression. Frontiers in Psychiatry, 12, 636961. https://doi.org/10.3389/fpsyt.2021.636961
- Kolanowski, A., Gilmore-Bykovskyi, A., Hill, N., Massimo, L., & Mogle, J. (2019). Measurement challenges in research with individuals with cognitive impairment. Research in Gerontological Nursing, 12(1), 7–15. https://doi.org/10.3928/19404921-20181212-06
- Kroenke, K., & Spitzer, R. L. (2002). The PHQ-9: A new depression diagnostic and everity measure. Psychiatric Annals, 32(9), 509–515. https://doi.org/10.3928/0048-5713-20020901-06
- Kruijt, A. W., Parsons, S., & Fox, E. (2019). A meta-analysis of bias at baseline in RCTs of attention bias modification: No evidence for dot-probe bias towards threat in clinical anxiety and PTSD. Journal of Abnormal Psychology, 128(6), 563–573. https://doi.org/10.1037/abn0000406
- Lazarov, A., Abend, R., & Bar-Haim, Y. (2016). Social anxiety is related to increased dwell time on socially threatening faces. Journal of Affective Disorders, 193, 282–288. https://doi.org/10.1016/j.jad.2016.01.007
- Lazarov, A., Ben-Zion, Z., Shamai, D., Pine, D. S., & Bar-Haim, Y. (2018). Free viewing of sad and happy faces in depression: A potential target for attention bias modification. Journal of Affective Disorders, 238, 94–100. https://doi.org/10.1016/j.jad.2018.05.047
- LeMoult, J., & Joormann, J. (2012). Attention and memory biases in social anxiety disorder: The role of comorbid depression. Cognitive Therapy and Research, 36(1), 47–57. https://doi.org/10.1007/s10608-010-9322-2
- Löwe, B., Decker, O., Müller, S., Brähler, E., Schellberg, D., Herzog, W., & Herzberg, P. Y. (2008). Validation and standardization of the generalized anxiety disorder screener (GAD-7) in the general population. Medical Care, 46(3), 266–274. https://doi.org/10.1097/MLR.0b013e318160d093
- Low, A. C. Y., Oh, V. Y. S., Tong, E. M. W., Scarf, D., & Ruffman, T. (2022). Older adults have difficulty decoding emotions from the eyes, whereas easterners have difficulty decoding emotion from the mouth. Scientific Reports, 12(1), 7408–7408. https://doi.org/10.1038/s41598-022-11381-8
- MacLeod, C., & Clarke, P. J. F. (2015). The attentional bias modification approach to anxiety intervention. Clinical Psychological Science, 3(1), 58–78. https://doi.org/10.1177/2167702614560749
- MacLeod, C., Rutherford, E., Campbell, L., Ebsworthy, G., & Holker, L. (2002). Selective attention and emotional vulnerability: Assessing the causal basis of their association through the experimental manipulation of attentional bias. Journal of Abnormal Psychology, 111(1), 107–123. https://doi.org/10.1037/0021-843X.111.1.107
- Maria, G.-G., & Juan, G.-G. (2017). Negative bias in the perception and memory of emotional information in Alzheimer disease. Journal of Geriatric Psychiatry and Neurology, 30(3), 131–139. https://doi.org/10.1177/0891988716686833
- Molitor, R. J., Ko, P. C., & Ally, B. A. (2015). Eye movements in Alzheimer’s disease. Journal of Alzheimer’s Disease, 44(1), 1–12. https://doi.org/10.3233/JAD-141173
- Molloy, A., & Anderson, P. L. (2020). Evaluating the reliability of attention bias and attention bias variability measures in the dot-probe task among people with social anxiety disorder. Psychological Assessment, 32(9), 883–888. https://doi.org/10.1037/pas0000912
- Mooldijk, S. S., Licher, S., & Wolters, F. J. (2021). Characterizing demographic, racial, and geographic diversity in dementia research: A systematic review. Jama Neurology, 78(10), 1255–1261. https://doi.org/10.1001/jamaneurol.2021.2943
- Price, R. B., Kuckertz, J. M., Siegle, G. J., Ladouceur, C. D., Silk, J. S., Ryan, N. D., Dahl, R. E., & Amir, N. (2015). Empirical recommendations for improving the stability of the dot-probe task in clinical research. Psychological Assessment, 27(2), 365–376. https://doi.org/10.1037/pas0000036
- Rodebaugh, T. L., Scullin, R. B., Langer, J. K., Dixon, D. J., Huppert, J. D., Bernstein, A., Zvielli, A., & Lenze, E. J. (2016). Unreliability as a threat to understanding psychopathology: The cautionary tale of attentional bias. Journal of Abnormal Psychology, 125(6), 840–851. https://doi.org/10.1037/abn0000184
- Ryu, S.-H., Katona, C., Rive, B., & Livingston, G. (2005). Persistence of and changes in neuropsychiatric symptoms in Alzheimer disease over 6 months: The LASER-AD study. The American Journal of Geriatric Psychiatry, 13(11), 976–983. https://doi.org/10.1097/00019442-200511000-00008
- Sanchez, A., & Vazquez, C. (2014). Looking at the eyes of happiness: Positive emotions mediate the influence of life satisfaction on attention to happy faces. The Journal of Positive Psychology, 9(5), 435–448. https://doi.org/10.1080/17439760.2014.910827
- Sears, C., Quigley, L., Fernandez, A., Newman, K., & Dobson, K. (2019). The reliability of attentional biases for emotional images measured using a free-viewing eye-tracking paradigm. Behavior Research Methods, 51(6), 2748–2760. https://doi.org/10.3758/s13428-018-1147-z
- Semmelmann, K., & Weigelt, S. (2018). Online webcam-based eye tracking in cognitive science: A first look. Behavior Research Methods, 50(2), 451–465. https://doi.org/10.3758/s13428-017-0913-7
- Seo, E. H., Lee, D. Y., Kim, S. G., Kim, K. W., Kim, D. H., Kim, B. J., Kim, M. D., Kim, S. Y., Kim, Y. H., Kim, J. L., Kim, J. W., Moon, S. W., Park, J. H., Ryu, S. H., Yoon, J. C., Lee, N. J., Lee, C. U., Jhoo, J. H., Choo, L. H., & Woo, J. I. (2011). Validity of the telephone interview for cognitive status (TICS) and modified TICS (TICSm) for mild cognitive impairment (MCI) and dementia screening. Archives of Gerontology and Geriatrics, 52(1), e26–e30. https://doi.org/10.1016/j.archger.2010.04.008
- Skinner, I. W., Hübscher, M., Moseley, G. L., Lee, H., Wand, B. M., Traeger, A. C., Gustin, S. M., & McAuley, J. H. (2018). The reliability of eye tracking to assess attentional bias to threatening words in healthy individuals. Behavior Research Methods, 50(5), 1778–1792. https://doi.org/10.3758/s13428-017-0946-y
- Spalletta, G., Caltagirone, C., Girardi, P., Gianni, W., Casini, A. R., & Palmer, K. (2012). The role of persistent and incident major depression on rate of cognitive deterioration in newly diagnosed Alzheimer’s disease patients. Psychiatry Research, 198(2), 263–268. https://doi.org/10.1016/j.psychres.2011.11.018
- Waechter, S., Nelson, A. L., Wright, C., Hyatt, A., & Oakman, J. (2014). Measuring attentional bias to threat: Reliability of dot probe and eye movement indices. Cognitive Therapy and Research, 38(3), 313–333. https://doi.org/10.1007/s10608-013-9588-2
- Weiss, E. M., Kohler, C. G., Vonbank, J., Stadelmann, E., Kemmler, G., Hinterhuber, H., & Marksteiner, J. (2008). Impairment in emotion recognition abilities in patients with mild cognitive impairment, early and moderate Alzheimer disease compared with healthy comparison subjects. The American Journal of Geriatric Psychiatry, 16(12), 974–980. https://doi.org/10.1097/JGP.0b013e318186bd53
- Woody, M. L., Owens, M., Burkhouse, K. L., & Gibb, B. E. (2016). Selective attention toward angry faces and risk for major depressive disorder in women: Converging evidence from retrospective and prospective analyses. Clinical Psychological Science, 4(2), 206–215. https://doi.org/10.1177/2167702615581580
- Zhang, L., Yu, F., Hu, Q., Qiao, Y., Xuan, R., Ji, G., Zhu, C., Cai, C., & Wang, K. (2020). Effects of SSRI antidepressants on attentional bias toward emotional scenes in first-episode depressive patients: Evidence from an eye-tracking study. Psychiatry Investigation, 17(9), 871–879. https://doi.org/10.30773/pi.2019.0345
- Zvielli, A., Bernstein, A., Koster, E. H. W., & Voracek, M. (2014). Dynamics of attentional bias to threat in anxious adults: Bias towards and/or away? PLoS ONE, 9(8), e104025–e104025. https://doi.org/10.1371/journal.pone.0104025