Abstract
Apomixis in angiosperms is asexual reproduction from seed. Its importance to angiospermous evolution and biodiversity has been difficult to assess mainly because of insufficient taxonomic documentation. Thus, we assembled literature reporting apomixis occurrences among angiosperms and transferred the information to an internet database (http://www.apomixis.uni-goettingen.de). We then searched for correlations between apomixis occurrences and well-established measures of taxonomic diversity and biogeography. Apomixis was found to be taxonomically widespread with no clear tendency to specific groups and to occur with sexuality at all taxonomic levels. Adventitious embryony was the most frequent form (148 genera) followed by apospory (110) and diplospory (68). All three forms are phylogenetically scattered, but this scattering is strongly associated with measures of biodiversity. Across apomictic-containing orders and families, numbers of apomict-containing genera were positively correlated with total numbers of genera. In general, apomict-containing orders, families, and subfamilies of Asteraceae, Poaceae, and Orchidaceae were larger, i.e., they possessed more families or genera, than non-apomict-containing orders, families or subfamilies. Furthermore, many apomict-containing genera were found to be highly cosmopolitan. In this respect, 62% occupy multiple geographic zones. Numbers of genera containing sporophytic or gametophytic apomicts decreased from the tropics to the arctic, a trend that parallels general biodiversity. While angiosperms appear to be predisposed to shift from sex to apomixis, there is also evidence of reversions to sexuality. Such reversions may result from genetic or epigenetic destabilization events accompanying hybridization, polyploidy, or other cytogenetic alterations. Because of increased within-plant genetic and genomic heterogeneity, range expansions and diversifications at the species and genus levels may occur more rapidly upon reversion to sexuality. The significantly-enriched representations of apomicts among highly diverse and geographically-extensive taxa, from genera to orders, support this conclusion.
I. INTRODUCTION
Apomixis in angiosperms is reproduction via asexually formed seed (Asker and Jerling, Citation1992). By excluding vegetative propagation per definition, apomixis is development of an embryo from an unreduced and unfertilized egg cell (gametophytic apomixis) or from a somatic cell of the ovule (sporophytic apomixis). Apomixis thus combines the benefits of seed dispersal with those of asexual reproduction (Mogie, Citation1992). The cellular mechanisms of apomixis have been studied for over 100 years and have recently been reviewed (Ozias-Akins, Citation2006; Tucker and Koltunow, Citation2009; Rodriguez-Leal and Vielle-Calzada, Citation2012). In brief, gametophytic apomixis involves development of an unreduced embryo sac (apomeiosis) and formation of an embryo from the unreduced egg, within the embryo sac, without fertilization (parthenogenesis). The unreduced embryo sac can develop from a somatic cell of the nucellus, which replaces the megaspore (apospory). Alternatively, the megaspore mother cell may undergo a restitutional meiosis or mitotic-like division, resulting in an unreduced functional spore (diplospory). In gametophytic apomicts, endosperm formation may require fertilization of polar nuclei (pseudogamous apomixis) or it may develop independently (autonomous apomixis). Adventitious embryos of sporophytic apomicts form from nucellar or integumentary cells, and their formation usually occurs in parallel with the formation of sexual embryos. Both sexual and adventitious embryony require endosperm, the formation of which usually involves fertilization of the polar nuclei. Species exhibiting adventitious embryony often produce multiple embryos per seed. Hence, it is considered a form of polyembryony, and it is widespread among tropical plants (Naumova, Citation1992). Polyembryony, however, can also arise from sexual pathways, e.g. from rare cases of functional twin megasporocytes (MMC) or by fertilization of a synergid that has assumed egg-like properties.
Gametophytic apomicts, except in a few cases, are polyploid, and they are often related to species that exhibit other developmental abnormalities such as polyspory (Carman, Citation1997). Gametophytic and sporophytic apomixis are heritable, but they are generally expressed facultatively, in individual plants, along with sex (Ozias-Akins and van Dijk, Citation2007). Confusion arising from facultativeness and the existence of multiple apomixis pathways has hampered the gathering of reliable quantitative data for relating apomixis to various evolutionary and biogeographical phenomena.
Apomixis and sexuality are not exclusive traits, as almost all apomictic plants exhibit facultative sexuality. In aposporous plants, the sexual process in ovules generally aborts during meiosis or early embryo sac formation, and it is replaced by aposporous embryo sac formation, which occurs more or less simultaneously. In some ovules, a reduced embryo sac forms, and these may produce recombinant 1n embryos by haploid or polyhaploid parthenogenesis or recombinant 2n embryos following fertilization. In other ovules, a reduced embryo sac and one or more aposporous embryo sacs may form in parallel, but the apomictic pathway is often more successful (e.g., Hojsgaard et al., Citation2013). Nevertheless, polyembryony is common in aposporous plants, and occasionally an individual ovule will contain sexually and aposporously-produced embryos. In the case of diplospory, facultative sexuality occurs at lower or higher frequencies due to completion of a normal sexual meiosis and embryo sac formation followed either by parthenogenesis and the formation of a recombinant 1n haploid (or polyhaploid) embryo or by fertilization and the formation of a recombinant 2n embryo (Aliyu et al., Citation2010).
In natural populations of apomicts, genetic studies using character compatibility analysis have confirmed the regular appearance of recombinant genotypes and ploidy variants (Bashaw et al., Citation1992; Van der Hulst et al., Citation2000; Paun et al., Citation2006; Aliyu et al., Citation2010; Paule et al., Citation2011; Cosendai et al., Citation2013). Such variations suggest that the reproductive elements of sex and apomixis, i.e., meiosis, syngamy, apomeiosis and parthenogenesis, involve different genetic and epigenetic control mechanisms that can be uncoupled. This phenomenon is well documented experimentally (Ogawa et al., Citation2013). Thus, apomeiosis followed by syngamy leads to an increase in ploidy (2n+n; BIII offspring), while meiosis followed by parthenogenesis leads to a reduction in ploidy (1n+0; haploid or polyhaploid offspring). Such cases of partial apomixis and partial sex occur regularly in progeny test populations (Bicknell and Koltunow, Citation2004) and natural populations (Cosendai and Hörandl, Citation2010; Paule et al., Citation2011). The point of interest here is that sexual processes and various combinations of sexual and apomictic processes occur infrequently to regularly in most apomictic species.
Apomixis was first described in Antennaria by Juel in 1898 (Nogler, Citation2006). By 1941, apomixis had been reported in 44 genera from 23 families (Stebbins, Citation1941). Gustafsson's seminal treatment of apomixis in angiosperms (Gustafsson, Citation1946; Citation1947a; Citationb) listed 73 agamospermous genera from 25 families (excluding viviparous species), and it provided comprehensive descriptions of developmental pathways, evolutionary origins and ecological preferences. Asker and Jerling (Citation1992) recognized 108 genera in which apomixis occurs in at least one species (referred to herein as apomict-containing genera), and they listed an additional 26 questionable records. Naumova (Citation1992) recognized 116 genera with sporophytic apomixis, and Carman (Citation1997) listed 222 genera that contain sporophytic or gametophytic apomicts.
The phylogenetic distributions of apomicts and their evolutionary and biogeographical relevance remain unclear. The doomed view of Darlington (Darlington, Citation1939), Stebbins (Stebbins, Citation1950) and Grant (Grant, Citation1981), that apomixis is an evolutionary dead end, was based on the assumption that loss of genotype heterogeneity in populations would result in loss of potential to adapt to environmental change. Thus, agamic lineages were thought to be doomed for extinction. This view, of a static, closed system, has been revised by more recent theoretical papers (Carman, Citation1997; Whitton et al., Citation2008; Hörandl and Hojsgaard, Citation2012), which are based on empirical observations that agamic complexes harbor considerable genetic variability (Grant, Citation1981; Hörandl and Paun, Citation2007) and represent dynamic and flexible systems.
Three large families, Poaceae, Asteraceae, and Rosaceae, contain a majority of the known apomict-containing genera, and this has led to speculations that a developmental predisposition may exist in these families or that apomixis is more likely to emerge in large families (Richards, Citation1997; Ozias-Akins and van Dijk, Citation2007). It has also been postulated that a rapid spread of apomixis in major clades could account for the observed pattern (van Dijk and Vijverberg, Citation2005). Recent phylogenetic reconstructions of the major clades (orders) of angiosperms (Hörandl and Hojsgaard, Citation2012) support the view of a broadly scattered distribution of apomixis over the entire phylogeny of angiosperms coupled with rapid spread in large families. However, correlation analyses have not previously been conducted between apomixis occurrences and general measures of biodiversity.
Facultative apomixis in perennials provides long-term reproductive stability for the colonization of large areas. Such long-term stability may also provide time for multiple genetic and genomic variants to form and be tested, e.g., progeny with unique genetic recombinations and/or genomic translocations, inversions, aneuploidy, etc. Some of these could be sexual revertants with normal Polygonum-type embryo sac formation or with developmentally-unusual embryo sac formation, e.g., polyspory. After reversion to obligate sexuality, such genetically enriched and recombined lineages may diversify more rapidly than their obligately-sexual ancestors (Carman, Citation1997; Hörandl and Hojsgaard, Citation2012). Hence, the breadth of distribution and richness of genera and species in families containing apomicts could reasonably increase as a direct consequence of apomixis. For this review, we have updated the apomixis occurrence literature and have related it to biodiversity at various taxonomic levels. Such relationships have not previously been extensively characterized.
A deeper interest in biogeographical and ecological aspects of apomixis emerged in the last decades of the twentieth century. Consequently, it was found that some apomicts have larger distributions in higher latitudes than their sexual relatives and have abundantly populated previously glaciated areas (Bierzychudek, Citation1985; Kearney, Citation2005; Hörandl, Citation2006; Hörandl et al., Citation2008). The term “geographical parthenogenesis” is used to describe this phenomenon, but it has been studied extensively in only a few genera (Hörandl et al., Citation2008). For most apomictic taxa, information on distribution patterns has not previously been assembled or remains too scarce to allow for generalizations. In tropical apomicts, biogeographical patterns of apomictic plants are largely unexplored, and records of polyembryony without detailed information on apomictic biotypes have blurred the patterns (Carman, Citation1997). Superior colonizing ability and polyploidy can make apomicts formidable invaders (Richards, Citation2003), as has occurred on all continents (Chapman et al., Citation2003; Brock, Citation2004; Rambuda and Johnson, Citation2004; Hao et al., Citation2011). But generalizing these observations to the majority of apomicts is premature. The necessary biogeographical surveys simply have not been conducted.
Herein we provide a comprehensive survey of the literature that describes the occurrence and biogeography of apomict-containing genera, and we discuss hypotheses relating phylogenies and biogeographical patterns to biodiversity at multiple taxonomic levels. Specifically, we tested whether angiosperms in general are predisposed to apomixis or whether a predisposition statistically favors certain families. Further, we examined frequencies of the three major forms of apomixis (apospory, diplospory, adventitious embryony) and related these to putative taxonomic and biogeographical preferences. We also tested for positive correlations between apomixis frequencies and biodiversity across apomict and non-apomict-containing sister clades among orders and families. Such correlations are expected if highly-successful apomixis-to-sex reversals occur. Finally, we report frequencies of apomict-containing genera that occupy multiple geographic zones and discuss hypotheses for interpreting the observed patterns. The database constructed for this review is publically available (http://www.apomixis.uni-goettingen.de) and includes lists of apomict-containing genera, types of apomixis found, and original references.
II. ASSESSMENT OF OCCURRENCES
A. Data Collection and Database Construction
We updated the occurrence of apomixis in angiosperms as represented in Carman (Citation1997) by searching the Web of Knowledge (Thomson Reuters (http://www.webofknowledge.com/), Scientific Commons (St. Gallen, Institute for Media and Communications Management, http://en.scientificcommons.org/), Redalyc (Universidad Autónoma del Estado de México, http://www.redalyc.org/home.oa, version 2.0© 2012), Ingenta Connect (http://www.ingentaconnect.com/), and The Reference in Scientific Document Supply (French National Center for Scientific Research, CNRS, http://www.refdoc.fr/). Articles not in the public domain were accessed through the Library of the University of Goettingen, the Library of the University of Vienna, or the Merrill Library, Utah State University. Literature was searched through December, 2012. Records were maintained at the genus level. For records originating before 1997, only the first documentation of apomixis per genus is reported, and only records with sufficient reliability were accepted. Criteria for acceptance included verification by microscopic investigations of embryo sac and/or embryo development, flow cytometric seed screening (Matzk et al., Citation2000), molecular progeny tests, or well documented isolation and emasculation experiments. Preliminary records based on indirect observation (e.g., clonality in wild populations, lack of pollen tubes on stigmas, etc.) were included as uncertain, but these were excluded from statistical investigations. Records of polyembryony without proof of adventitious embryony are reported separately. We did not include reports of occasional haploid parthenogenesis in sexual plants. Records for Asteraceae rejected by Noyes (Citation2007) were included in the uncertain category to encourage detailed re-investigation. We consider all angiospermous apomicts to be facultative, with the possible exception of Alchemilla (Asker and Jerling, Citation1992), which means both sexually and apomictically-produced seeds can form on the same plant. Since apomixis as a reproductive character state is not exclusive, we refrained from standard phylogenetic tests of diversification, e.g., Johnson et al. (Citation2011). All records were transferred to the taxonomic system of families and orders as in APG III (Bremer et al., Citation2009), and records published since 1997 were plotted by year of publication.
The references reported in Carman (Citation1997) were re-examined and traced back to the original literature, and only original documents are included in our database. Types of apomixis expressed are also reported. Records were imported into Oracle®, and the online query application was generated using Oracle Application Express (APEX).
B. Data Analyses
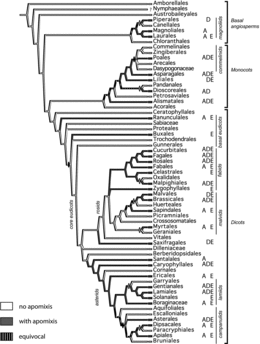
Frequencies of apomict-containing families and genera were compared across orders. In modern systematics, these taxonomic units represent almost exclusively clades, i.e., they are phylogenetic groups reflecting common diversification processes and representing hierarchically-nested entities. We refrained from species-level taxonomy because of (i) scarcity of information concerning apomixis at the species level, and (ii) difficulties in defining species in agamic complexes where reticulate evolution and other speciation processes are occurring (Grant, Citation1981; Hörandl, Citation1998). To provide an overview, we mapped records onto the phylogenetic tree of the APG III classification as in Hörandl and Hojsgaard (Citation2012), and we added information concerning types of apomixis (). More detailed phylogenetic analyses on diversification rates (as in Johnson et al., Citation2011) are difficult to conduct because of facultativeness, which exists at the population and individual levels. Sex and facultative apomixis simply are not exclusive binary character states. Hence, they cannot be correlated with speciation and extinction rates on a phylogenetic tree.
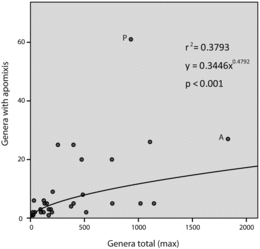
To test the hypothesis that apomixis is causal to diversity (Hörandl and Hojsgaard, Citation2012), we selected apomict and non-apomict-containing sister clades in the phylogenetic tree (). In cases of polytomies (see ), every combination among branches was evaluated. A Shapiro-Wilk test for normality was applied to numbers of genera and families, and similarities in medians were tested using a non-parametric one-tailed Wilcoxon Matched-Pairs Signed-Rank Test where differences in scores were assumed to be distributed symmetrically around their median. Numbers of genera per family were as in Heywood (2007) except for minor adjustments to accommodate the APG III system. A Q–Q plot comparing numbers of apomict to non-apomict-containing genera per order suggested the data are not distributed normally (X ∼ N(0,1)). The hypothesis that apomixis occurs preferentially in certain orders was tested by non-parametric Spearman's rank correlation (rs). Numbers of apomict-containing genera per order were plotted against total numbers of genera per order for all 63 angiospermous orders. Regression models were evaluated, and three residual statistics, Mahalanobis distance, Cook's distance and Standarized DFFit, were used to detect the presence of outliers and to explain their influences on model predictability.
TABLE 1 New records of apomict-containing families added since Carman (Citation1997)
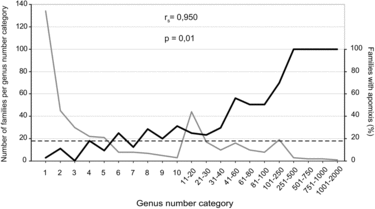
Families were grouped into 21 categories based on numbers of genera per family (family richness). The percentage of families in each category that contain apomicts was then calculated (apomixis presence), and family richness and apomixis presence were compared by Spearman's rank correlation. Three families, Asteraceae (eudicot with gametophytic apomixis), Poaceae (monocot with gametophytic apomixis), and Orchidaceae (monocot with sporophytic apomixis), were then analyzed for intra-familial patterns of apomixis by regressing numbers of apomict-containing genera per subfamily against total numbers of genera per subfamily. Genera in Poaceae were according to A World-wide Phylogenetic Classification of Poaceae (http://archive.is/4yN5E), and genera in Asteraceae and Orchidaceae were according to the Angiosperm Phylogeny Website (http://www.mobot.org/MOBOT/research/APweb/). Phylogenetic trees were modified after Panero and Funk (Citation2008) for Asteraceae, the Grass Phylogeny Working Group (GPWG II, 2012) for Poaceae, and Freudenstein et al. (Citation2004) for Orchidaceae.
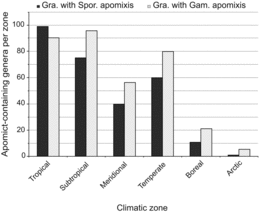
Biogeography information for apomict-containing genera was taken from Mabberley (Citation2008) and Tropicos.org (Missouri Botanical Garden, http://www.tropicos.org) with minor taxonomic adjustments as described above. Distributions of these genera were then classified according to floral zone (Meusel, 1978) with the goal of determining whether numbers of apomict-containing genera increase or decrease when moving from warmer to colder climates. This analysis was based on apomict-containing genera per climatic zone only, not frequencies of apomict-containing genera relative to total numbers of genera in the respective zones. The six climatic zones included were: arctic (including the antarctic region), boreal, temperate (including submeridional, warm temperate, and austral zones), meridional (including the Mediterranean region), subtropical (including N- or boreosubtropical and S- or austrosubtropical zone), and tropical.
Statistical analyses were performed using IBM® SPSS® Statistics, Version 20 for Windows. For all evaluated cases, significance was tested at the p ≤ 0.05 (significant) and p ≤ 0.01 (highly significant) levels of probability.
III. PHYLOGENETIC DISTRIBUTIONS
A. Novel Records
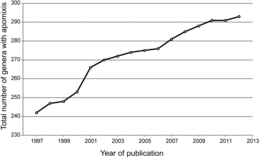
Thirty-two orders (52%), 78 families (19%) and 293 genera (≈2.2%) were found to contain apomictic species (http://www.apomixis.uni-goettingen.de). Total numbers of genera varied from 12,758 to 13,150 depending on circumscription. Fifty-one genera were added to the list of Carman (Citation1997) as newly identified apomicts (; 67 papers), and another 35 were identified that had been reported between 1918 and 1997 but were not included in Carman (Citation1997). Thus, 86 genera have been added. These represent 12 new apomict-containing families from among 10 orders (). According to our updated list, adventitious embryony, apospory, diplospory, and apospory and diplospory together occur in 148, 110, 68, and 17 genera, respectively. Adventitious embryony and gametophytic apomixis also occur together in some genera, i.e., they are not exclusive traits. The type of apomixis is not known for a few genera where experimental evidence confirms apomixis, and an additional five genera, results of which were published from 1998-2012, express developmental features suggestive of apomixis but verification studies have not yet been conducted. We recorded these in the database as “uncertain.”
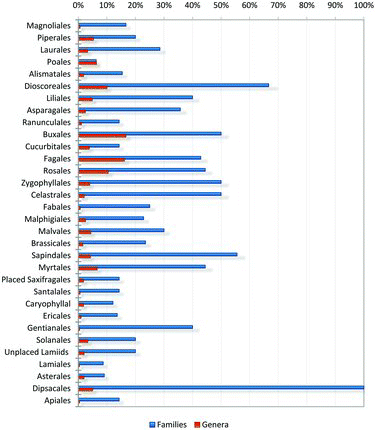
Apomixis occurs scattered over the whole phylogeny and appears both in basal stem groups and in the derived clades of monocots and dicots (). Adventitious embryony is the predominant mode of apomixis in fabids, malvids, and lamiids. Other than this, clear phylogenetic tendencies are not apparent, i.e., apospory, diplospory and adventitious embryony occur in all seven major clades of angiosperms and in most large orders (). These three forms of apomixis occur in 24, 17, and 28 orders, respectively, which parallels distributions at the family and genus levels. In some clades, apomixis is reconstructed as ancestral. But here, the trait is often lost on certain terminal branches, e.g., Canellales, Arecales, Oxalidales, Cornales and Garryales ().
B. Apomixis in Genera, Families and Orders
Among the 32 apomict-containing orders, apomict-containing families ranged from 6.25–100% with a mean of 28.3% (). Interestingly, the three orders containing the three most apomixis-populated families, Poaceae, Asteraceae, and Rosaceae, also contain multiple families in which apomixis has not been reported. To date, Poaceae and Asteraceae are the only apomict-containing families in the Poales (16 families) and Asterales (11 families). Four of nine families in the Rosales contain apomicts. Orders with the highest frequencies of apomict-containing families were small, e.g., Dipsacales (both families), Dioscoreales (2 of 3 families), and Sapindales (5 of 9 families).
Numbers of apomict-containing genera in apomict-containing orders were positively correlated with total numbers of genera in the respective orders. This occurred regardless as to whether the total number of genera was considered to be 13,150 (, rs = 0.66, p < 0.01) or 12,758 (rs = 0.68, p < 0.01). However, frequency values (numbers of apomict-containing genera divided by total numbers of genera in the respective orders) were negatively correlated with total numbers of genera (rs = −0.513, p < 0.11). Two orders were largely responsible for this negative correlation: Asterales, with 1829 genera but with only one of 11 families containing apomicts (1.5% of genera), and Poales, with 930 genera but with only one of 16 families containing apomicts (6.25% of genera). In contrast, high frequencies of apomict-containing genera per order were observed in small orders, e.g., Buxales (16.7%) where six genera and 100 species are grouped into two families. Frequencies of apomict-containing genera in most orders were low, which fits a pattern of a broad taxonomic distribution for apomixis among many orders rather than a concentration of apomixis in few orders. Spearman's rank correlations between numbers of apomict-containing genera and total numbers of genera among apomict-containing orders revealed similar highly significant correlation coefficients for gametophytic (rs = 0.477, p < 0.05) and sporophytic (rs = 0.475, p < 0.05) apomixis.
As shown by Q-Q plot analysis of the complete dataset and Shapiro-Wilk tests of sister orders, normality at the 1–α = 0.99 confidence level (SW = 0.757; P < 0.01) could not be assumed. Wilcoxon tests revealed that median values for families and genera of sister clades (W = 3, Z = −2.8241, p = 0.0024, N = 12) are significantly higher in apomictic clades, and they depict an exceptionally low probability of randomness for these values. Hence, apomixis is strongly associated with biodiversity.
C. Family Diversity and Apomixis
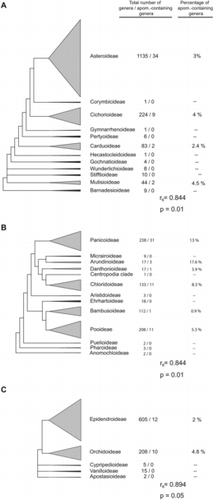
Most families of angiosperms lack diversity, e.g., 252 families (61%) contain only 1–5 genera. In this respect, presence of apomixis in families was positively correlated with numbers of genera per family (). Fifty percent of families with > 40 genera contained at least one apomict. This increased to 63% for families with > 80 genera, and all families with > 250 genera contained apomicts. Likewise, case studies of three highly diverse families revealed strong correlations between presence of apomixis in intra-familial taxa and total numbers of genera in these taxa (). However, these correlations do not tell the whole story. Average levels of biodiversity among families are also important. For example, a genus selected at random from a large group of genera composed of a combination of low diversity families, e.g., 1–5 genera each, was less likely to be apomictic than if it were selected from a similar-sized group of genera taken from among families or sub-families that contain many genera.
D. Biogeography and Apomixis
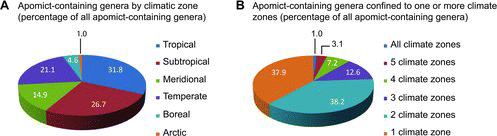
Apomict-containing genera were distributed worldwide with 38, 13, and 11% occurring in 2, 3, and > 3 climatic zones, respectively. They also tended to occur mostly in tropical to temperate zones. Few apomict-containing genera were found in boreal to arctic zones compared to lower latitude zones (). Sporophytic and gametophytic apomixis exhibited similar patterns of distribution across climatic zones (parametric Spearman correlation r = 0.95, p < 0.05) with sporophytic apomixis being slightly more abundant in the tropics and gametophytic apomixis being slightly more abundant elsewhere ().
IV. APOMIXIS AND EVOLUTION
A. Patterns at Higher Taxonomic Levels
Apomixis is widespread and scattered taxonomically among orders, and we found strong evidence that it has arisen multiple times with no clear ties to specific plant families. However, the phylogenetic tree () does suggest that apomixis is potentially ancient in some major clades especially those leading to the commelinids, fabids and lamiids. While sexuality is ancestral in angiosperms, how and when apomixis arose in this group is less certain. What we can say is that stable forms of apomixis either repeatedly evolved de novo during angiospermous evolution (hypothesis of multiple de novo origins) or its expression in typically sexual lineages was repeatedly triggered during evolution (conserved pathways hypothesis). The latter suggests unreduced gamete formation coupled with parthenogenesis (basic apomixis) is an ancient epigenetic competency inherited from angiospermous ancestors with innovations in types of apomixis arising thereafter (Carman et al., Citation2011). In this respect, it is remarkable that adventitious embryony is the most frequent type of apomixis. The direct development of embryos has been reported from other plant tissues, e.g., from anthers (Soriano et al., Citation2013), leaves, and other organs (Tisserat et al., Citation1979). In general, embryony can be activated in many plant cells independent of fertilization (Carman, Citation1990).
The second aspect of gametophytic apomixis, i.e., parthenogenetic development of an egg cell, also occurs spontaneously in otherwise sexual plants (Asker and Jerling, Citation1992). In this respect, methylation patterns and other epigenetic marks are known to participate in regulating embryo and endosperm development (Law and Jacobsen, Citation2010). In contrast with mammals, where a parental contribution is essential for embryony, genomic imprinting in plants appears to be required for endosperm formation only, and not for embryo formation (Engelstädter, Citation2008). Independence from a paternal epigenomic contribution may allow for more flexibility in the evolution of alternative asexual reproductive pathways.
Strikingly, our data indicate that apospory is more frequent than diplospory. Apospory involves activation of gametophyte development in a nucellar cell rather than in the megaspore, and it also involves the differential expression of gene families that regulate sexual development (Grimanelli, Citation2012). Thus, the totipotency and epigenetic flexibility of plant cells makes various routes to apomixis possible. A direct intervention during meiosis, as occurs in diplospory, is perhaps more difficult to achieve in natural plant populations. Thus, diplospory is the least frequent pathway. The artificial MiMe system of producing clonal embryos in seeds of Arabidopsis requires a combination of mutations in three meiosis genes (d’Erfurth et al., Citation2009). As pointed out earlier, such combinations of mutations are unlikely to occur in natural populations because selection would act against each intermediate step (Van Dijk and Vijverberg, Citation2005). Meiosis itself is highly conserved in eukaryotes (Malik et al., Citation2007), and in facultatively diplosporous plants, steps of meiosis tend to be skipped rather than altered (Carman, Citation1997).
Like apomixis in general, apospory, diplospory and adventitious embryony are each taxonomically widespread (), and their genetic or epigenetic regulatory mechanisms differ (Ozias-Akins and van Dijk, Citation2007). The hypothesis of a taxonomic predisposition for the evolution of apomixis within certain clades with apomixis-inducing genetic factors moving rapidly within these clades (Van Dijk and Vijverberg, Citation2005) is not supported by our compilation. In contrast, our data suggest that angiosperms in general have a capacity to switch from sex to apomixis. But this capacity, particularly with regard to gametophytic apomixis, is more pronounced in large, diverse and genomically-complex clades ( and ).
B. Apomixis-sex Systems
The low frequency of apomixis we observed at the genus level (about 2%) is likely due to (i) under-sampling, as suggested by the linear increase in newly discovered apomicts per year between 1997 and 2012 () coupled with methodological problems of recognizing apomixis (Leblanc, 2000; Matzk et al., Citation2000; Hörandl et al., Citation2008), (ii) natural constraints that inhibit shifts from sexuality to apomixis (van Dijk and Vijverberg, Citation2005; Engelstädter, Citation2008; Hörandl et al., Citation2008; Hörandl, Citation2009), and (iii) spread of apomictic lineages followed by reversions to sexuality and further diversification. According to the latter possibility, high taxa diversity coupled with the presence of apomixis may be a normal and expected diversification signature that results from cycles of neopolyploidy-originated apomixis followed by reversions to sexuality (Carman, Citation1997; Hörandl and Hojsgaard, Citation2012). Since points (i) and (ii) have been discussed elsewhere, we discuss here the novel hypothesis that apomixis enhances diversification.
Diversification results from speciation minus extinction. If apomixis leads to high rates of extinction, all else being equal, then taxa with apomixis should be less diverse than taxa without it. However, the apomixis occurrence data reviewed herein suggest the opposite. Families with apomixis must have either extraordinarily high sexual speciation rates, to compensate for extinction rates, or apomixis somehow plays a positive and previously-undetected role in evolution. The prevalent distribution of apomixis among large angiospermous families suggests a positive role. Correlations between numbers of apomict-containing genera and total numbers of genera across apomict-containing families (or sub-families) were high ( and ). Interestingly, a similar correlation was observed among polystichoid ferns, i.e., between species richness and the occurrence of apogamy (apospory followed by adventitious embryony from a non-gametic cell of the unreduced gametophyte). However, apomixis in this group is not associated with higher diversification rates but rather with the reticulate evolution and establishment of polyploid hybrids (Liu et al., Citation2012).
The high frequencies of apomict-containing genera found in genus-rich subfamilies () may occur simply because of increased opportunities to shift to apomixis. Thus, we cannot infer that apomixis alone causes diversification. However, a combination of both traits could be superior to obligate sexuality (Cosendai et al., Citation2013). In this respect, it has been hypothesized that recombination among facultative apomicts coupled with wide hybridization increases genetic diversity and adaptive potential (Hörandl and Paun, Citation2007). Furthermore, apomictic taxa may diversify rapidly by accumulating mutations that could lead to advantageous (e.g., Mark Welch and Meselson, 2001; Pellino et al., 2013) as well as deleterious (i.e., Muller's ratchet) allelic divergences. Partial apomixis adds a further level of complexity, as combinations of apomeiosis with syngamy or meiosis with parthenogenesis may rapidly increase ploidy diversity (Bicknell and Koltunow, Citation2004; Cosendai and Hörandl, Citation2010; Paule et al., Citation2011). Such flexibility of developmental pathways begs the discussion of novel hypotheses to explain putative relationships between apomixis and biodiversity.
A model for apomixis as a facilitator of diversification through cycles of polyploidy and reversions to sex was proposed by Carman (Citation1997). Hörandl and Hojsgaard (Citation2012) expanded on this idea by suggesting that apomictic polyploids serve as pioneer explorers of new niches whereby they rapidly expand the distribution areas of their progenitor sexual populations by occupying new ecological and geographical niches (geographical parthenogenesis). Thereafter, reversals to complete sexuality, accompanied by and possibly caused by substantial cytogenetic and genomic perturbations (Carman, Citation1997), allow for the establishment of new sexual populations in new habitats without the long-term disadvantages of asexuality. The new sexual recombinants, potentially new species or even new genera, are genetically isolated from the original sexual populations and predisposed to a divergent evolutionary destiny. Empirical examples of reversals from apomixis to sex have been reported for Pennisetum (Taliaferro and Bashaw, Citation1966), Pilosella (Chapman et al., Citation2003) and Paspalum (Ortiz et al., Citation2013), which supports the notion that sexuality is the default mode in facultative apomicts (Koltunow et al., Citation2011). Hence, reversals from facultative apomixis to obligate sex, with accompanying expansions of species diversity, seem likely. This possibility has been largely overlooked by evolutionary biologists (Carman, Citation1997). To what extent it may explain ancient divergences within large clades, which today continue to maintain high frequencies of apomixis, e.g., asterids, rosids and monocots (), is a difficult but important question for future research to address.
Many species of Oenothera mimic asexuality through systems of permanent translocation heterozygosity, which restrict recombination to telomeres. Balanced lethals in these systems enforce inbreeding through the formation of unique, genetically compatible male and female gametes. Recent studies suggest that functional asexuality in Oenothera coupled with reversions to sexual reproduction accelerate species diversification (Johnson et al., Citation2011). Williams (Citation1975) proposed a general model explaining the effects of shifts between modes of reproduction. If shifts from asexuality to sex are more frequent than shifts from sex to asexuality, then the frequency of sex at the tips of the phylogeny will be higher than the frequency of apomixis. However, the latter may also be explained by sexual speciation rates that exceed rates of apomict formation. These arguments may explain why in cases of highly diverse families, apomixis frequencies remain low, even among the most diversified subfamilies. If apomixis occasionally serves as a transitional stage that promotes further sexuality and speciation (Carman, Citation1997), then the observed low frequencies of apomixis would be expected because aging apomictic lineages will have reverted to sexuality or will have been purged by Muller's ratchet, i.e., accumulation of deleterious mutations (Muller, Citation1964). The lack of efficient DNA repair mechanisms during meiosis is probably another selective disadvantage to apomixis (Hörandl and Hadacek, Citation2013). Collectively, these considerations suggest that high numbers of genera per family or sub-family may in some cases represent a signature for past apomixis-to-sex reversals followed by rapid speciation especially in families or sub-families where apomixis is prevalent.
C. Apomixis Biodiversity Parallels General Biodiversity
Because of their greater ecological breadth and taxonomic stability, genera were used instead of species to characterize diversity. Our results indicate apomixis biodiversity in general parallels total biodiversity, i.e., the highest numbers of apomict-containing genera occur in the tropics, and this was true for both sporophytic and gametophytic apomixis ( and ). Thus, the postulate that gametophytic apomixis is more abundant in colder regions while sporophytic apomixis is more abundant in the tropics (Bierzychudek, Citation1985; Richards, Citation1997) is not supported. In terms of total numbers of apomict-containing genera, both forms are more abundant in the tropics. However, general speculations that apomict-containing genera represent larger percentages of total genera in higher-latitude compared to lower-latitude climatic zones (Bierzychudek, Citation1985; Asker and Jerling, Citation1992) remain likely, and these speculations will become quantifiable once reasonable estimates of total numbers of genera residing in each climatic zone are obtained. In the tropics, apomixis may confer a selective advantage by providing reproductive assurance in situations of pollen limitation (Whitton et al., Citation2008), which occur in genera-rich rainforests because of low species densities and irregular flowering times. Adventitious embryony, which is especially frequent in the tropics, is often coupled with polyembryony (Naumova, Citation1992), which could increase seedling production and reproductive fitness.
Carman (Citation1997) observed that sporophytic apomicts tend to be diploidized paleopolyploids (diploids with high chromosome base numbers), while gametophytic apomicts tend to be neopolyploids (polyploids with low chromosome base numbers). Hence, the higher frequencies of neopolyploids in temperate to arctic regions (Brochmann et al., Citation2004) may explain the higher frequencies of gametophytic compared to sporophytic apomixis in these regions. Increased rates of polyploidization occur in higher latitudes for at least two reasons. First, cold temperatures enhance unreduced pollen formation, which is a major contributor to polyploidization (Ramsey and Schemske, Citation1998). Second, climatic oscillations during multiple episodes of Pleistocene glaciation caused large-scale plant migrations accompanied by extensive secondary contact hybridization. Many of the resulting semi-sterile to sterile wide hybrids achieved high levels of fertility following polyploidization (Stebbins, Citation1985; Carman, Citation1997; Hewitt, Citation2004).
Apomixis occurs in genetically diverse genera but also in highly cosmopolitan genera, i.e., those whose species span multiple ecological niches and cover large geographic areas. In this regard, 62% of apomict-containing genera were found to occupy multiple climatic zones (). The tendency for apomicts to colonize larger areas than their sexual relatives has been noted by many scientists (Bierzychudek, Citation1985; Bayer et al., Citation1991; Urbani et al., Citation2002; Hörandl, Citation2006), and this is further mirrored by their strong invasiveness (Peters, Citation2001; Chapman et al. Citation2003; Brock Citation2004; Rambuda and Johnson, Citation2004; Hao et al., Citation2011). Plant populations exhibiting facultative apomixis benefit from both modes of reproduction: while apomixis enables rapid range expansion, sexuality maintains sufficient genetic variation to respond to environmental variability (Cosendai et al., Citation2013). Taken together, apomixis allows for rapid spatial expansions and, upon reversals to sexuality, it appears to lead to the formation of new geographically isolated sexual lineages that diversify further by ecological and allopatric speciation (Hörandl and Hojsgaard, Citation2012).
D. The Expanded Transition Theory
The evolutionary and biogeographical information contained in the database supports an expanded version of Carman's (1997) transition-phase hypothesis. As outlined by Hörandl and Hojsgaard (Citation2012), this expansion involves a series of evolutionary steps:
Polyploidization and/or hybridization-associated shifts to facultative apomixis
Diversification of apomictic lineages by mutation, chromosome re-arrangements and aneuploidy, and hybridization and backcrossing, which accompany facultative sexuality
Range expansions of agamic complexes
Occasional re-stabilization of meiotic pathways in some lineages
Complete reversal to monosporic sexuality or polysporic sexuality, which may evolve as a consequence of asynchronous reproductive development in some lineages
Allopatric speciation of newly formed and geographically isolated sexual populations
Further diversification of biological species and the eventual evolution of new genera
At the opposite extreme of this evolutionary process is extinction. But the presence of apomixis in plant families with the highest levels of diversity supports the hypothesis that apomixis often enhances diversification. The Asteraceae, the largest family of angiosperms with c. 23,000 species, serves as an example. All developmental phases (initial apomixis, functional apomixis, polyspory) are present in the major clades of Asteraceae (Hörandl and Hojsgaard, Citation2012), which supports the transition-phase hypothesis. Furthermore, all four major clades of the Asteraceae reflect a strong correlation between apomixis and diversity (). Asteraceae is distributed in all continents except Antarctica (Panero and Funk, Citation2008), and geographical parthenogenesis is frequently observed (Bierzychudek, Citation1985; Hörandl et al., Citation2008). In fact, 34 of 39 documented cases of geographical parthenogenesis in angiosperms are from the Asteroideae and Cichorioideae subfamilies of Asteraceae (Hörandl et al., Citation2008). The predominance of autonomous apomixis in Asteraceae, where the need for pollen is completely eliminated, clearly enhances the likelihood of colony establishment in remote areas.
In Poaceae, three cases of geographical parthenogenesis are known (Hörandl et al., Citation2008). Here, the respective genera also belong to the largest clades, i.e., Pooideae and Panicoideae (). Moreover, the panicoid genus Paspalum contains the largest number of apomictic species of any angiospermous genus (47 species; Ortiz et al., Citation2013). The most frequent genetic system in Paspalum consists of diploid sexual cytotypes, which in general are geographically restricted, and polyploid apomictic cytotypes (mainly tetraploids), which usually occupy larger geographic areas. However, in four of 23 species that form agamic complexes via sexual-apomictic cytotype associations, sexual cytotypes are represented by tetraploids. So far, diploids of these species have not been found. Interestingly, the apomicts exhibit higher ploidy levels than their tetraploid sexual counterparts (Ortiz et al., Citation2013). Cytologically, these sexual tetraploids show partial apomixis (e.g., Quarin, 1994). In the framework of the expanded transition hypothesis, these data are interpreted as representing cases where tetraploid Paspalum cytotypes have undergone a reversal to sexuality followed by (i) morphological divergence from the ancient parental diploid species and (ii) formation of new agamic complexes at higher levels of polyploidization.
With regard to sporophytic apomixis, the geographical parthenogenesis database is insufficiently complete to draw conclusions. Like reversals to sexuality, geographical parthenogenesis may be an opportunistic strategy that is not necessarily realized by every complex.
Stebbins (Citation1950) stated that apomixis has never resulted in the evolution of new genera. This is probably incorrect. The evidence reviewed herein suggests that apomixis perpetuates long-lasting, reproductively-stable plants that generally produce clones but may also infrequently, yet perpetually, spin out new and unique genetic variants, i.e., gene and genome recombinants, that get tested by natural selection in new and diverse habitats. Many of these may revert to sexuality whereupon the evolutionary process continues, perhaps in some cases at accelerated rates. In this respect, apomixis may function as a reproductively-stable springboard in the evolution of new species and genera with wide hybridization, polyploidy, genetic and genomic rearrangements, and reversals to sexuality, all of which may promote divergence at the genetic, genomic and habitat-adaptation levels.
V. PERSPECTIVES FOR FUTURE RESEARCH
The database of apomict-containing genera, which we have assembled, is readily accessible and should stimulate further research on model and non-model systems. Verification of uncertain records in the database is an important priority. In forthcoming versions, we aim to include species-level records, which will allow further fine-tuning of biodiversity patterns. The dataset also serves as a backbone for accommodating additional information such as chromosome numbers, ploidy levels, incompatibility systems and life forms.
Apomicts occur in some of the most species-rich genera and genera-rich families. Examples include Paspalum, Panicum and Axonopus in Poaceae, Senecio, Taraxacum, Hieracium, Eupatorium and Antennaria in Asteraceae, and Ranunculus in Ranunculaceae. An ambitious task for future analyses will be to determine if intra-generic species richness correlates with numbers of apomictic species as expected according to the apomixis-sex reversal hypothesis. This will require large-scale embryological screenings and progeny testing coupled with phylogenetic studies. Such studies are expected to yield important insights concerning the role of apomixis in the reticulate evolution and diversification of angiospermous species and genera.
Finally we hope to stimulate more in-depth biogeographical analyses of apomictic taxa. Population-level assessments of apomixis are crucial for understanding distribution patterns within and among species, and our database provides a starting point for such detailed and comparative studies.
ACKNOWLEDGMENTS
We thank the editor, Dennis J. Gray, for the invitation to present this review article. We thank Timothy Sharbel for critically reading the manuscript.
FUNDING
This work was supported by the Austrian Science Fund (FWF, Project I 310 B-16 to E.H.), the European Union (COST action FA0903, Harnessing Plant reproduction for crop improvement HAPRECI to E.H. and J.C., Short Term Scientific Missions from COST FA0903 to D.H.), and the Utah Agricultural Experiment Station to J.C.
REFERENCES
- Aliyu, O.M., Schranz, M.E., and Sharbel, T.F. 2010. Quantitative variation for apomictic reproduction in the genus Boechera (Brassicaceae). Amer. J. Bot. 97: 1719–1731.
- Asker, S.E. and Jerling, L. 1992. Apomixis in Plants. CRC Press, Boca Raton, FL. Pp. 298.
- Bashaw, E.C., Hussey, M.A., and Hignight, K.W. 1992. Hybridization (n + n and 2n + n) of facultative apomictic species in the Pennisetum agamic complex. Int. J. Pl. Sci. 153: 466–470.
- Bayer, R.J., Purdy, B.G., and Lebedyk, D.G. 1991. Niche differentiation among eight sexual species of Antennaria Gaertner (Asteraceae, Inuleae) and A. rosea, their allopolyploid derivative. Evol. Trends Plants 5: 109–123.
- Bicknell, R.A. and Koltunow, A.M. 2004. Understanding apomixis: Recent advances and remaining conundrums. Pl. Cell 16: S228–S245.
- Bierzychudek, P. 1985. Patterns in plant parthenogenesis. Experientia 41: 1255–1264.
- Bremer, B., Bremer, K., Chase, M.W., Fay, M.F., Reveal, J.L., Soltis, D.E., Soltis, P.S., Stevens, P.F., Anderberg, A.A., Moore, M.J., Olmstead, R.G., Rudall, P.J., Sytsma, K.J., Tank, D.C., Wurdack, K., Xiang, J.Q. Y., Zmarzty, S., and Angiosperm Phylogeny Group. 2009. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Bot. J. Linn. Soc. 161: 105–121.
- Brochmann, C., Brysting, A.K., Alsos, I.G., Borgen, L., Grundt, H.H., Scheen, A.C., and Elven, R. 2004. Polyploidy in arctic plants. Biol. J. Linn. Soc. 82: 521–536.
- Brock, M.T. 2004. The potential for genetic assimilation of a native dandelion species, Taraxacum ceratophorum (Asteraceae), by the exotic congener T. officinale. Amer. J. Bot. 91: 656–663.
- Carman, J.G. 1990. Embryogenic cells in plant tissue cultures: Occurrence and behavior. In Vitro Cell. Devel. Biol. 26: 746–753.
- Carman, J.G. 1997. Asynchronous expression of duplicate genes in angiosperms may cause apomixis, bispory, tetraspory, and polyembryony. Biol. J. Linn. Soc. 61: 51–94.
- Carman, J.G., Jamison, M., Elliott, E., Dwivedi, K.K., and Naumova, T.N. 2011. Apospory appears to accelerate onset of meiosis and sexual embryo sac formation in sorghum ovules. BMC Plant Biology 11: 9.
- Chapman, H., Houliston, G.J., Robson, B., and Iline, I. 2003. A case of reversal: The evolution and maintenance of sexuals from parthenogenetic clones in Hieracium pilosella. Int. J. Pl. Sci. 164: 719–728.
- Cosendai, A.C. and Hörandl, E. 2010. Cytotype stability, facultative apomixis and geographical parthenogenesis in Ranunculus kuepferi (Ranunculaceae). Ann. Bot. 105: 457–470.
- Cosendai, A.C., Wagner, J., Ladinig, U., Rosche, C., and Hörandl, E. 2013. Geographical parthenogenesis and population genetic structure in the alpine species Ranunculus kuepferi (Ranunculaceae). Heredity 110: 560–569.
- Darlington, C.D. 1939. The Evolution of Genetic Systems. Cambridge University Press, Cambridge. Pp. 254.
- D’Erfurth, I., Jolivet, S., Froger, N., Catrice, O., Novatchkova, M., and Mercier, R. 2009. Turning meiosis into mitosis. PLoS Biology 7: e1000124. DOI: 10.1371/journal.pbio.1000124
- Engelstädter, J. 2008. Constraints on the evolution of asexual reproduction. Bioessays 30: 1138–1150.
- Freudenstein, J.V., van den Berg, C., Goldman, D.H., Kores, P.J., Molvray, M., and Chase, M.W. 2004. An expanded plastid DNA phylogeny of Orchidaceae and analysis of jackknife branch support strategy. Amer. J. Bot. 91: 149–157.
- Grass Phylogeny Working G, II. 2012. New grass phylogeny resolves deep evolutionary relationships and discovers C4 origins. New Phytol. 193: 304–312.
- Grant, V. 1981. Plant Speciation. Columbia University Press, New York. Pp. 435.
- Grimanelli, D. 2012. Epigenetic regulation of reproductive development and the emergence of apomixis in angiosperms. Curr. Opin. Pl. Biol. 15: 57–62.
- Gustafsson, Å. 1946. Apomixis in higher plants. I. The mechanism of apomixis. Lunds Univ. Årsskrift 42: 1–67.
- Gustafsson, Å. 1947a. Apomixis in higher plants. II. The causal aspect of apomixis. Lunds Univ. Årsskrift 43: 69–179.
- Gustafsson, Å. 1947b. Apomixis in higher plants. III. Biotype and species formation. Lunds Univ. Årsskrift 43: 181–370.
- Hao, J.H., Qiang, S., Chrobock, T., van Kleunen, M., and Liu, Q.Q. 2011. A test of Baker's law: breeding systems of invasive species of Asteraceae in China. Biol. Invasions 13: 571–580.
- Hewitt, G.M. 2004. Genetic consequences of climatic oscillations in the Quaternary. Phil. Trans. Roy. Soc. London B Biol. Sci. 359: 183–195.
- Heywood, V.B., Brummitt, R.K., Culham, A., and Seberg, O. 2007. Flowering Plant Families of the World. Royal Botanical Gardens, Kew. Pp. 424.
- Hojsgaard, D.H., Martinez, E.J., and Quarin, C.L. 2013. Competition between meiotic and apomictic pathways during ovule and seed development results in clonality. New Phytologist 197: 336–347.
- Hörandl, E. 1998. Species concepts in agamic complexes: Applications in the Ranunculus auricomus complex and general perspectives. Folia Geobot. 33: 335–348.
- Hörandl, E. 2006. The complex causality of geographical parthenogenesis. New. Phytol. 171: 525–538.
- Hörandl, E. 2009. A combinational theory for maintenance of sex. Heredity 103: 445–457.
- Hörandl, E., Cosendai, A.-C., and Temsch, E.M. 2008. Understanding the geographic distributions of apomictic plants: a case for a pluralistic approach. Plant Ecol. Div. 1: 309–320.
- Hörandl, E. and Hadacek, F. 2013. The oxidative damage initiation hypothesis for meiosis. Pl. Repr. 26: 351–367.
- Hörandl, E. and Hojsgaard, D. 2012. The evolution of apomixis in angiosperms: A reappraisal. Pl. Biosystems 146: 681–693.
- Hörandl, E. and Paun, O. 2007. Patterns and sources of genetic diversity in apomictic plants: implications for evolutionary potentials In: Apomixis: Evolution, Mechanisms and Perspectives, pp. 169–194, eds. Hörandl, E., Grossniklaus, U., Van Dijk, P.J., Sharbel, T. International Association for Plant Taxonomy, Ruggel.
- Johnson, M.T. J., FitzJohn, R.G., Smith, S.D., Rausher, M.D., and Otto, S.P. 2011. Loss of sexual recombination and segregation is associated with increased diversification in evening primroses. Evolution 65: 3230–3240.
- Kearney, M. 2005. Hybridization, glaciation and geographical parthenogenesis. Trends Ecol. Evol. 20: 495–502.
- Koltunow, A.M. G., Johnson, S.D., Rodrigues, J.C. M., Okada, T., Hu, Y.K., Tsuchiya, T., Wilson, S., Fletcher, P., Ito, K., Suzuki, G., Mukai, Y., Fehrer, J., and Bicknell, R.A. 2011. Sexual reproduction is the default mode in apomictic Hieracium subgenus Pilosella, in which two dominant loci function to enable apomixis. Plant J. 66: 890–902.
- Law, J.A. and Jacobsen, S.E. 2010. Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nature Rev. Genetics 11: 204–220.
- Leblanc, O. and Mazzucato, A. 2000. Screening procedures to identify and quantify apomixis In: The Flowering of Apomixis: From Mechanisms and Genetic Engineering, pp. 121–136, Savidan, Y., Carman, J.G., Dresselhaus, T., Eds. CIMMYT, Mexico D.F.
- Liu, H.M., Dyer, R.J., Guo, Z.Y., Meng, Z., Li, J.H., and Schneider, H. 2012. The evolutionary dynamics of apomixis in ferns: a case study from Polystichoid ferns. J. Bot. 2012: 1–11.
- Mabberley, D. 2008. Mabberley's Plant-Book. A Portable Dictionary of Plants, Their Classification and Uses. Cambridge University Press, Cambridge. Pp. 1040.
- Malik, S.B., Ramesh, M.A., Hulstrand, A.M., and Lodgson, J.M. 2007. Protist homologs of the meiotic Spo11 gene and topoisomerase VI reveal an evolutionary history of gene duplication and lineage-specific loss. Molecular Biol. and Evol. 24: 2827–2841.
- Mark Welch, D.B. and Meselson, M. 2000. Evidence for the evolution of Bdelloid rotifers without sexual reproduction or genetic exchange. Science 288: 1211–1215.
- Matzk, F., Meister, A., and Schubert, I. 2000. An efficient screen for reproductive pathways using mature seeds of monocots and dicots. Plant J. 21: 97–108.
- Meusel, H.J. E., Rauschert, S., and Weinert, E. 1978. Vergleichende Chorologie der Zentraleuropäischen Flora. Gustav Fischer Verlag, Jena. Pp. 418.
- Mogie, M. 1992. The Evolution of Asexual Reproduction in Plants. Chapman and Hall, London. Pp. 292.
- Muller, H.J. 1964. The relation of recombination to mutational advance. Mutation Res. 106: 2–9.
- Naumova, T.N. 1992. Apomixis in Angiosperms. Nucellar and Integumentary Embryony. CRC Press, Boca Raton. Pp. 144.
- Nogler, G.A. 2006. The lesser-known Mendel: His experiments on hieracium. Genetics 172: 1–6.
- Noyes, R.D. 2007. Apomixis in the Asteraceae: diamonds in the Rough. Funct. Pl. Science Biotechnol. 1: 207–222.
- Ogawa, D., Johnson, S.D., Henderson, S.T., and Koltunow, A.M. G. 2013. Genetic separation of autonomous endosperm formation (AutE) from the two other components of apomixis in Hieracium. Plant Repr. 26: 113–123.
- Ortiz, J.P. A., Quarin, C.L., Pessino, S.C., Acuna, C., Martinez, E.J., Espinoza, F., Hojsgaard, D.H., Sartor, M.E., Caceres, M.E., and Pupilli, F. 2013. Harnessing apomictic reproduction in grasses: what we have learned from Paspalum. Ann. Bot. 112: 767–787.
- Ozias-Akins, P. 2006. Apomixis: Developmental characteristics and genetics. Crit. Rev. Pl. Sci. 25: 199–214.
- Ozias-Akins, P. and van Dijk, P.J. 2007. Mendelian genetics of apomixis in plants. Ann. Rev. Genetics 41: 509–537.
- Panero, J.L. and Funk, V.A. 2008. The value of sampling anomalous taxa in phylogenetic studies: Major clades of the Asteraceae revealed. Molec. Phylogen. Evol. 47: 757–782.
- Paule, J., Sharbel, T.F., and Dobes, C. 2011. Apomictic and sexual lineages of the Potentilla argentea L. group (Rosaceae): Cytotype and molecular genetic differentiation. Taxon 60: 721–732.
- Paun, O., Greilhuber, J., Temsch, E.M., and Hörandl, E. 2006. Patterns, sources and ecological implications of clonal diversity in apomictic Ranunculus carpaticola (Ranunculus auricomus complex, Ranunculaceae). Molec. Ecol. 15: 897–910.
- Peters, H.A. 2001. Clidemia hirta invasion at the Pasoh Forest Reserve: an unexpected plant invasion in an undisturbed tropical forest. Biotropica 33: 60–68.
- Quarin, C.L. 1994. A tetraploid cytotype of Paspalum durifolium: cytology, reproductive behavior and its relationship to diploid P. intermedium. Hereditas 121: 115–118.
- Rambuda, T.D. and Johnson, S.D. 2004. Breeding systems of invasive alien plants in South Africa: does Baker's rule apply? Divers. Distrib. 10: 409–416.
- Ramsey, J. and Schemske, D.W. 1998. Pathways, mechanisms, and rates of polyploid formation in flowering plants. Ann. Rev. Ecol. Syst. 29: 467–501.
- Richards, J.A. 1997. Plant Breeding Systems. Chapman and Hall, London. Pp. 529.
- Richards, J.A. 2003. Apomixis in flowering plants: an overview. Trans. R. Soc. London B 358: 1085–1093.
- Rodriguez-Leal, D. and Vielle-Calzada, J.-P. 2012. Regulation of apomixis:learning from sexual experience. Curr. Opin. Pl.Biol. 15:549–555.
- Soriano, M., Li, H., and Boutilier, K. 2013. Microspore embryogenesis:establishment of embryo identity and pattern in culture. Plant Repr. 26: 181–196.
- Stebbins, G.L. 1941. Apomixis in the angiosperms. Bot. Rev. 7: 507–542.
- Stebbins, G.L. 1950. Variation and Evolution in Plants. Columbia University Press, New York. Pp. 643.
- Stebbins, G.L. 1985. Polyploidy, hybridization, and the invasion of new habitats. Ann. Missouri Bot. Gard. 72: 824–832.
- Taliaferro, C.M. and Bashaw, E.C. 1966. Inheritance and control of obligate apomixis in breeding buffelgrass Pennisetum ciliare. Crop Science 6: 473–480.
- Tisserat, B., Esan, E.B., and Murashige, TG. 1979. Somatic embryogenesis in angiosperms. Hort. Rev. 1: 1–77.
- Tucker, M.R. and Koltunow, A.M. G. 2009. Sexual and asexual (apomictic) seed development in flowering plants: molecular, morphological and evolutionary relationships. Funct. Pl. Biol. 36: 490–504.
- Urbani, M.H., Quarin, C.L., Espinoza, F., Penteado, M.I. O., and Rodrigues, I.F. 2002. Cytogeography and reproduction of the Paspalum simplex polyploid complex. Pl. Syst. Evol. 236: 99–105.
- Van der Hulst, R.G. M., Mes, T.H. M., den Nijs, J.C. M., and Bachmann, C. 2000. Amplified fragment length polymorphism (AFLP) markers reveal that population structure of triploid dandelions (Taraxacum officinale) exhibits both clonality and recombination. Molec. Ecol 9: 1–8.
- Van Dijk, P.J. and Vijverberg, K. 2005. The significance of apomixis in the evolution of the angiosperms: A reappraisal. In: Plant Species-level Systematics. New Perspectives on Pattern & Process, pp. 101–116, T., B. F., Chatrou, L.W. , Gravendeel, B., Pelser, P.B., Eds. A.R.G. Gantner Verlag, Ruggell, Liechtenstein.
- Whitton, J., Sears, C.J., Baack, E.J., and Otto, S.P. 2008. The dynamic nature of apomixis in the angiosperms. Int. J. Pl. Sciences 169: 169–182.
- Williams, G.C. 1975. Sex and Evolution. Princeton University Press, Princeton, N.J. Pp. 200.