Abstract
In alpine ecosystems, imbibed seeds are often exposed to temperatures as low as −35 °C, challenging their survival in the soil. Here, we show that seeds have mechanisms to survive cold climate prevalent in alpine ecosystems and have identified three such mechanisms from existing literature, including two forms of freezing avoidance (the presence of water impermeable seed coats, and the supercooling of seed tissues) and one form of freezing tolerance (by extracellular-freezing). Experimentally-derived published data on the lowest temperature recorded at which 50% of a seed sample survived (i.e., lethal temperature; LT50) was used to generate a dataset of 24 species across low altitude, boreal and alpine environments. We assumed that the ability of seeds to maintain viability at very low temperatures would increase in species associated with higher altitudes conferring a competitive advantage that would be lost under projected climate change. However, our results reveal to underpin that seeds from boreal species survive relatively better at lower temperatures than those of alpine species. Paradoxically, a warming climate could lead to alpine seed death due to extremes of cold at the soil surface resulting from snow cover loss, whilst the declining snow cover may facilitate boreal forest colonization above the current treeline.
I. Introduction
Alpine vegetation (>2200 m a.s.l.) is diverse and globally distributed, but typified by various adaptations to extreme cold due to an increase in altitude and concurrent decrease in temperature by 5 to 10°C km−1 (Körner, Citation2003). Plant species composition in alpine regions has been well-catalogued, starting almost two centuries ago, e.g., Blytt (Citation1876 and references therein). Subsequent studies have revealed that the alpine flora consists of more than 10,000 plant species including long-lived shrubs, herbs and grasses but no trees, as conditions limit tree growth to below the lower boundary of alpine vegetation (Körner, Citation1999; Citation2003). At higher altitudes plants may be exposed to temperatures as low as −40 °C during winter and the prospect of experiencing sub-zero temperatures at any time of the year (Lütz, Citation2011). In effect, such extreme temperatures place an absolute limit on plant survival, distribution pattern and, thus, community structure. Therefore, species richness and diversity declines with altitude up to the permanent snow line, which forms the upper boundary (Körner, Citation1999).
Considerations of how plants adapt to survive at such low, extreme temperatures have been proffered for more than a century, via a plethora of studies (see Burke et al., Citation1976 and references therein; Körner, Citation2003; Lütz, Citation2011). Recently, it has been proposed that there are three keys to the radiation of angiosperms into freezing environments: deciduousness during winter and the presence of narrow water-conducting cells in woody species; and perennation of herbs underground through winter (Zanne et al., Citation2014). Exposing plants to a low enough temperature tends to inexorably result in intracellular ice formation (IIF), which decimates plant tissues at the cellular level and formation of ice in critical structures leads to cell death. Nonetheless, plants adapted to alpine locations have mechanisms to alleviate IIF, markedly increasing their survival. The strategies that plants (and most other over-wintering organisms) use to assuage IIF can be grouped as freezing avoidance by supercooling and freezing tolerance by extracellular-freezing. The parts of the plant that survive freezing environments by supercooling include the xylem ray parenchyma cells (Burke et al., Citation1976; Ristic and Ashworth, Citation1994). In contrast, many hardy wood tissues (Lütz, Citation2011) tolerate freezing by extracellular freeze-desiccation, where ice formation is restricted to external structures and the water from internal structures are desiccated to a low level, ultimately leading to amorphous solidification (glass formation); e.g., in twigs of winter-hardened Populus (Sakai, Citation1960). In rhododendron flower buds, modest supercooling is combined with spatially-limited extracellular and intracellular freezing, which causes the internal tissues of the bud to freeze-dehydrate and reduce the likelihood of IIF (Lütz, Citation2011; Larcher, Citation2012).
For angiosperms to inhabit cold environments the vegetative or woody tissue gained new structural and functional traits (Zanne et al., Citation2014), and the ability of plants to survive alpine climates has been very well documented in the literature (Amen, Citation1966; Bliss, Citation1971; Körner, Citation2003; Björk and Molau, Citation2007; Lütz, Citation2011). However, very little attention has been given to the reproductive biology of these high altitude floras, in particular, how dispersed seeds tolerate cold winters (Neuner, Citation2014). This is mainly due to the notion that most of the alpine plants undergo vegetative, i.e., asexual reproduction by producing clonal populations, rather than sexual reproduction and the dispersal of seeds. The latter was thought to be a rare occurrence (Bliss, Citation1963; Billings and Mooney, Citation1968), despite several earlier studies reporting the production of viable seeds that germinated in alpine environments (see Marchand and Roach, Citation1980). Nevertheless, it is becoming apparent that many species, nearly 2500 (Jaganathan and Dalrymple, Citation2019), of alpine plants are known to reproduce by seeds. This trait is important as seeds tend to offer some consistency in surviving harsh, freezing climates (Körner, Citation2003). Almost all alpine plants produce viable seeds during late summer/autumn (Baskin and Baskin, Citation2014) and seed shedding is normally immediately followed by a harsh winter, hindering seed germination until spring, with germination and seedling emergence occurring rapidly at warmer temperatures and in light (Jaganathan et al., Citation2015). Seed persistence in the soil from primary seed dispersal to germination can vary between one year and centuries (McGraw et al., Citation1991) in the in situ ‘soil seed bank’. Therefore, it is reasonable to assume that over-wintering seeds must tolerate the alpine winter, which is characterized by various factors: intensely cold nights at sub-zero temperatures (air and exposed ground temperatures) and desiccating winds. Dense snow cover is also a factor, although seeds underneath are held in stasis and show significantly lower levels of damage and mortality than seeds kept under artificially snow free conditions (Williams, Citation2006).
Recent studies have shown an increase in species richness over the past several decades across the global alpine regions, e.g., European alps (Steinbauer et al., Citation2018), Himalayas (Hamid et al., Citation2020), with the encroachment of low-altitude species into alpine vegetation. These studies have inter alia considered the effects of climate change on various plant parts, including seedlings (Sierra-Almeida and Cavieres, Citation2012; Rosbakh et al., Citation2020), but not seeds (Briceño et al., Citation2015). For seeds in alpine environments specifically, a changing climate is likely to have indirect and potentially complex impacts upon seed survival. Firstly, there will be a wide variation in conditions experienced by the seed depending on microhabitats. For example, seeds dispersed onto wind-blown ridges or thin snow cover areas may be exposed to soil temperatures often lower than −25°C and air temperatures as low as −30°C (Bai et al., Citation1999; Gusta et al., Citation2006; Larcher et al., Citation2010). Secondly, during the onset of the growing season the risk of seeds being exposed to regular freeze-thawing excursions is increased, as the insulation effect of the snow cover is lost. Such scenarios are expected to prevail in most of the alpine landscapes and early snow-melt resulting from the future climatic conditions means that the seeds will be at extreme risk (Lütz, Citation2011; Mondoni et al., Citation2012). Thirdly, climatic warming is expected to reduce the thickness of snow cover and directly expose the seeds to lower night air temperature (Björk and Molau, Citation2007; Wipf and Rixen, Citation2010; López-Moreno et al., Citation2011) at various times during winter, depending on altitude, aspect, etc. Given these varying impacts, over very small spatial scales, it is important to better understand the potential mechanisms of resilience to such stresses, and how they might vary amongst species. A second consideration is whether species with traits that contribute to invasiveness − such as hard and persistent seeds (e.g., many Fabaceae) or seeds with physiological dormancy (e.g., some Asteraceae) − might be able to rapidly adapt to climate change, thereby accelerating the need of proactive conservation measures.
The main purpose of this review is to provide an insight into the mechanisms by which imbibed, nongerminating seeds might cope in alpine regions when exposed to sub-zero temperatures. For this review, we have used ‘seeds’ in a broad sense, which includes all the embryo, endosperm (if present), perisperm, seed coat, and any seed dispersal/external structures, e.g., hairs. Whilst freezing injury in hydrated seeds has been a subject of considerable investigation, including as a means of judging the success of cryopreservation (Brown and Escombe, Citation1897; Busse, Citation1930; Rossman, Citation1949), freezing tolerance or avoidance in seeds has received relatively little consideration (Jaganathan and Dalrymple, Citation2019). Despite studies in the late 70s and early 80s in a handful of species (Junttila and Stushnoff, Citation1977; Ishikawa and Sakai, Citation1978; Keefe and Moore, Citation1981; Roos and Stanwood, Citation1981) there have been very few attempts (Finch-Savage and McQuistan, 1988; Bai et al., Citation1998; Vernon et al., Citation1999; Hawkins et al., Citation2003; Gusta et al., Citation2006; Marcante et al., Citation2012; Jaganathan et al. Citation2016) in the following decades addressing freezing tolerance in seeds. Undoubtedly, this neglected area should see a resurgence in research attention, and for the purpose of the present review, we have included those earlier studies and referenced all relevant recent studies. Given the association of alpine species with extreme cold, we also wanted to explore if climate change might exacerbate or alleviate the selection pressures caused by differential seed tolerances of freezing, as this might have implications for shifting plant distributions in high altitude environments. We expected that a positive relationship exists between cold tolerance in laboratory-based experimental work and ambient minimum temperatures in the coldest regions of the planet.
II. Seed development, hydration levels and freezing
Seed development involves the following three distinguishable phases regardless of the climate in which the parent plant grows (Bewley and Black, Citation1994; Bewley et al., Citation2013): (1) histogenesis, during which the main structures of seeds including the seed coat, endosperm (if present), other nutrient reserves and embryo differentiate; (2) seed maturation, where the fertilized ovule undergoes rapid multiplication and the individual seed structures become apparent as dry weight accumulates; and (3) maturation drying, in which water content of the seeds declines as the storage reserve deposition slows down, the vascular connections to the parent plant are broken, and the seeds equilibrate to the environmental relative humidity (RH), before being dispersed. In the majority of extant angiosperms, the water content of the seed drops significantly to below 15% (on a fresh weigh basis, hereafter fwb.) before detaching from the parent plant. These desiccation-tolerant (or orthodox) seeds contrast with approximately 8–10% of the global species that produce seeds that are shed at higher water content (c.25–40%) and are desiccation-sensitive (recalcitrant) (Roberts, Citation1973). Although recalcitrant species are absent in alpine ecosystems, seed development in high-altitude plants is complicated by the prevailing low temperature throughout the year (Wagner and Mitterhofer, Citation1998; Forbis, Citation2003; Stinson, Citation2004). Seed development occurs during the warm period of year, generally beginning in mid-summer. At that point, the ovary would be more susceptible to freezing because of the rapid cell division and associated cell expansion (Neuner et al., Citation2013) and a high hydration level. From this point of vulnerability, seeds then gradually acquire freezing tolerance during maturation drying, especially at the final stages which coincide with seed dispersal (Wagner et al., Citation2012).
Dry seeds with a water content c. 3–10% can survive temperatures as low as those achieved by direct immersion in liquid nitrogen (−196°C). For the so-called hard seeds that are impermeable to water, i.e., have physical dormancy (PY), freezing stress will be avoided until this PY is removed and water is absorbed. Thereafter, for all seeds the ability to tolerate low temperature decreases with an increase in water content. During storage in situ in the soil, this will be a function of many parameters, including environmental conditions, seed morphology and the distribution of water in the seed tissues. In addition, imbibed nonhard seeds that enter the first stages of germination have a much higher level of unbound (sensu Wolfe et al., Citation2002), or freezable, water (Junttila and Stushnoff, Citation1977; Bewley and Black, Citation1994; Bewley et al., Citation2013).
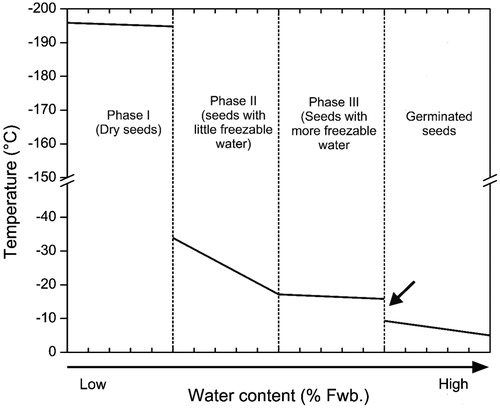
Based on the level of hydration, three distinct phases of imbibition can be recognized and related to freezing tolerance, as described by Junttila and Stushnoff (Citation1977) for lettuce (). In phase I, seeds contain no freezable water (i.e., dry) and therefore withstand any low temperature down to −196°C. Seeds at the top of phase II may have small quantities of freezable water (that is difficult to detect using differential thermal analysis) and seed viability is affected negatively when cooling to between −40 and −20°C. Finally, phase III represents fully imbibed seeds with a much increased amount of freezable water, as the seed nears visible germination, and ice formation temperature becomes prominent at higher sub-zero temperatures, c. between −5 and −18°C, as shown in lettuce (Junttila and Stushnoff, Citation1977).
Figure 1. Typical relationship between water content of seeds and temperature at which seeds incur mortality. Water content in each phase is species-specific. The amount of water increases from phase I through phase III, decreasing the freezing tolerance ability. In phase I, seeds do not have any freezable water and therefore survive low temperatures, even direct exposure to liquid nitrogen. Increase in water content leads to decrease in survival, thus seeds with little freezable water (phase II) do not survive ultra-low temperature but maintain viability when exposed to sub-zero temperatures. Seeds in phase III, i.e., fully imbibed but ungerminated, have mechanisms to survive exposure to sub-zero temperature. In radicle protruded seeds (phase IV), there is a sudden loss in freezing tolerance (indicated with an arrow).
The range of water contents that constitute these three phases varies between species. In Lactuca sativa cv. Great Lakes, for example, seeds with 5-13% WC survived liquid nitrogen exposure, but a slight increase to 16% WC decreases the freezing tolerance to −40°C; and as 20% MC is approached the freezing tolerance declines such that >80% of seeds die around −18°C (Junttila and Stushnoff, Citation1977; Keefe and Moore, Citation1981; Roos and Stanwood, Citation1981). Similarly, seeds of Arabidopsis thaliana cv. Columbia at 18% WC survived exposure to −75°C, but only 36% of seeds germinated after the same cooling when at 27% WC (Vernon et al., Citation1999). When the water content of these seeds was >33%, mortality becomes apparent at −26°C due to ice formation (Vernon et al., Citation1999).
The ability to tolerate low temperatures not only decreases with an increase in WC but this trend also continues throughout germination (Junttila and Stushnoff, Citation1977; Finch-Savage and Mcquistan, 1988; Vernon et al., Citation1999; Hawkins et al., Citation2003; Marcante et al., Citation2012). Thus, germinating seeds have a very limited capacity to survive even just a few degrees below 0°C (Ladinig et al., Citation2013; Marcante et al., Citation2012; Phartyal et al., Citation2018). The pattern of decline in freezing tolerance in germinating seeds cannot be explained by an increase in water content alone, because there is no significant change in water content between completely imbibed and the germinating stage (Bewley and Black, Citation1994; Bewley et al., Citation2013). Hence, the decline in freezing tolerance during germination must involve additional factors, including increased metabolism and/or the onset of cell division and vacuolation in the radicle during the early phase of emergence (Marcante et al., Citation2012). In addition, the accumulation of ice nucleating agents due to bacterial colonization of growing seedlings might be responsible for the loss of supercooling capacity in germinated seeds, where the radicle has just protruded the coat (Vernon et al., Citation1999).
III. Seed survival mechanisms in alpine ecosystems
A. Physical dormancy
In some species, an impermeable seed coat develops during the maturation drying phase of seed development (Baskin et al., Citation2000). This imposes physical dormancy (PY) on the seeds and water uptake is prevented potentially over a long time interval even in field conditions. In some cases, the embryos of seeds with an impermeable coat, also have physiological dormancy (PD) resulting from hormonal imbalances, i.e., combinational dormancy (PY + PD) (Baskin and Baskin, Citation2004). While there may be some water present at the seed surface, i.e., in the testa, the internal tissues, including the endosperm and embryo, remain in a dry state (3–10% WC). Once PY is broken, the seed coat becomes permeable to water and internal tissues can hydrate during episodes of rainfall. This then increases the risk of freezing injury if low temperatures are experienced by the nondormant seed, particularly for seeds >15% WC, although the actual WC threshold depends on seed oil content. Therefore, one ecological strategy to avoid freezing is for species to produce PY seeds.
Baskin and Baskin (Citation2014) reported PY is present in 18 families of the world flora. Amongst those families, only Cistaceae, Convolvulaceae, Geraniaceae, Fabaceae and Malvaceae occur in alpine environments (Jaganathan and Dalrymple, Citation2019). Seed coat impermeability in the Fabaceae is conferred by an epidermal palisade layer of thick-walled Malpighian cells (Baskin et al., Citation2000; Rolston, Citation1978). In the Geraniaceae, PY tends to be caused by sclerotized cells of the inner epidermis of the outer integument, and sclerotized cells of the outer epidermis of the inner integument (Baskin et al., Citation2000). Excluding the Fabaceae, the number of species exhibiting PY in alpine environments in the remaining four families is very low but further study is needed to ascertain whether this is a true representation of PY dormancy in alpine environments. Indeed, Schwienbacher et al. (Citation2011) found that only two Fabaceae species in their survey of 28 species from the central alps of Austria had PY. Similarly, the only Geraniaceae that has been confirmed as developing PY seeds in alpine environments is Geranium albiflorum (). However, all studied Geraniaceae seeds produce PY or PY + PD seeds (see Baskin and Baskin, Citation2014). Some of the other families that occur in alpine regions, including Juncaceae and Onagraceae, may also produce PY seeds, but this requires clarification. In addition, it is not clear if any of the species within alpine ecosystem produce seeds that have PY + PD.
Table 1. Species producing physical dormant (PY) seeds in alpine environments.
Information available on seed developmental studies could help explain the relative infrequency of PY species in high-altitudes. Seeds with PY occur mostly in tropical environments and the abundance of PY species declines toward the pole (Jaganathan, Song, et al., Citation2017). Because PY is induced only after the water content drops to species-specific thresholds, the higher humidity prevalent in temperate regions compared to tropical drylands might reduce the selection pressure to develop impermeability (Jaganathan, Citation2016). Given that seed maturation occurs in a narrow time-span in alpine regions in a high humidity environment, the same might hold true for alpine regions, explaining the lower number of species with PY in high-altitudes. In spite of this theoretical overview, there appears to be no study that has documented the water content of potentially PY seeds in species of alpine regions.
Seeds with PY can persist in soil for many years before germination (Jaganathan et al., Citation2019). In order to germinate, dormancy must be broken and imbibition take place. Scarification of the seed coat is an important process by which PY seeds become water-permeable (Baskin et al., Citation2000; Van Assche et al., Citation2003) and this can be accomplished by continuous freeze/thaw cycle at the beginning of the growing season (Amen, Citation1965; Citation1966; Bell and Amen, Citation1970; Bliss, Citation1971; Baskin et al., Citation2000). For example in Luzula spicata, scarification by continuous freeze-thaw cycles early in the spring increases the proportion of germinated seed (Bell and Amen, Citation1970). Further examples illustrating the effects of freeze-thaw cycle alleviating PY in seeds can be found in: Midgley (Citation1926), Busse (Citation1930), Brant et al. (Citation1971), Rolston (Citation1978), Pritchard et al. (Citation1988), and Shibata et al. (Citation1995). A major limitation of those studies is that the freeze-thaw cycle used extremely low temperature, e.g., direct plunging of seeds in to liquid nitrogen and thawing. This does not reflect natural, ecological conditions for breaking dormancy. Moreover, little is known about the number of natural freeze-thaw cycles required to break PY in the climatic conditions prevailing in alpine environments. It is likely though that the number of freeze-thaw cycles required for dormancy alleviation depends on species in relation to the depth of PY present (Baskin and Baskin, Citation2014). Pritchard et al. (Citation1988) showed that seeds of Trifolium arvense with PY became permeable to water after four cycles of liquid nitrogen exposure. But approximately 40 freeze-thaw cycles were required to break dormancy in T. arvense if the low temperature was limited to −40°C, instead of −196°C. In the alpine landscapes, however, the range of temperature fluctuation is narrow compared to the empirical ranges employed. It is therefore likely that the low temperature excursion to remove PY in seeds requires multiple cycles.
B. Freezing avoidance-supercooling
Beyond PY dormant seeds, most seeds on the soil surface will be in equilibrium with the hydration state of the soil. In the late winter the seeds will be imbibed due to snow melting (Fenner and Thompson, Citation2005; Marcante et al., Citation2012). In imbibed seeds, internal ice formation becomes the single most critical factor determining viability. Water in seeds can, however, supercool to many degrees below the melting point without freezing (Junttila and Stushnoff, Citation1977; Bourque and Wallner, Citation1982) and this constitutes a method of freezing avoidance. During supercooling, potentially freezable water present in the external seed tissues, e.g., endosperm or perisperm is cooled below its melting point (which is 0 °C), but ice formation becomes inevitable when temperatures are reduced to c. −18 °C, because of the presence of ice nucleation factors, e.g., bacteria. When such nucleation is absent, the freezing of supercooled water occurs as low as −41.15 °C, where water itself forms ice due to homogenous ice nucleation (Moore and Molinero, Citation2011). In seeds, ice formation in the internal structures might be avoided (or delayed) if heterogenous ice nucleation occurs is limited in extent and location. Such heterogenous ice nucleation occurs in seeds and other living entities during cooling as water does not occur in its pure form and often exists in macromolecular structures (Angell, Citation1995).
Freezing avoidance in seeds differs from other plant parts in numerous ways. Exceeding the supercooling point (SCP) is known to be lethal to the above-ground tissue of freeze-avoiding plants (Beck et al., Citation1984; Wisniewski et al., Citation2003). However, the location of ice formation within seeds can determine whether viability is lost (). Thus, ice in the endosperm and other external structures can be survived, and delay ice formation within the embryo. But IIF in the embryo marks the death of seeds (Junttila and Stushnoff, Citation1977; Keefe and Moore, Citation1981; Bourque and Wallner, Citation1982). Using differential thermal analysis (DTA) analysis, Lactuca sativa seeds cooled at 5°Ch−1 (Keefe and Moore, Citation1983), 6°Ch−1 (Keefe and Moore, Citation1983) 20°Ch−1 (Junttila and Stushnoff, Citation1977; Keefe and Moore, Citation1981; Citation1983), and 120°Ch−1 (Jaganathan, Citation2009) generate a high temperature exotherm (HTE) in the perisperm a few degrees below zero (between −3 and −10°C). A second, and smaller, low temperature exotherm (LTE) is detected around −16 to −18 °C, presumably as the freeze-dehydrated embryo supercools further than the other seed tissues and freezes independently. Seeds cooled below the HTE temperature were able to germinate, but none survived cooling below the LTE (Keefe and Moore, Citation1981).
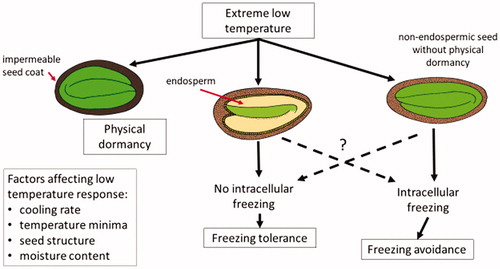
Figure 2. Hypothesized pathways of survival against low temperatures in alpine seeds. Three mechanisms of survival identified includes impermeable seed coat, freezing avoidance and freezing tolerance. Four factors that might affect the response of seeds are identified, signifying particular mechanisms relating to cooling rate, temperature minima, seed structure, and the moisture content of the seeds. For freezing tolerance, freezing is limited to extracellular regions or non-essential tissues. For freezing avoidance, supercooling delays the onset of intracellular freezing.
Seeds in phase II of imbibition can be at various water contents. On the lower end of this continuum, the seeds may have so little freezable water, that it is difficult to detect as an exotherm by DTA. However, small quantities of ice might nucleate and grow, perhaps explaining the sudden loss of seed viability when exposed to −196°C, but not at −30°C. Thus, partially hydrated seeds could supercool to temperatures below −18°C (Ishikawa and Sakai, Citation1978; Stushnoff and Junttila, Citation1978; Angell, Citation1995). At higher water contents, the amount of freezable water is sufficient for heterogeneous ice nucleation to occur at a high sub-zero temperature (Ishikawa and Sakai, Citation1978; ). For example, in the case of Oryza sativa, seeds with a water content up to 18% (the phase II/I transition of water imbibition) do not readily form ice until −35°C; whereas 23% WC seeds (in phase II of imbibition) have an exotherm around −18°C (Ishikawa and Sakai, Citation1978). Similar results were observed for Triticum aestivum (Ishikawa and Sakai, Citation1978).
C. Freezing tolerance-freeze desiccation
Although the extent of freezing avoidance by determining SCP has been studied in seeds of some species, relatively few observations have been made of seed freezing tolerance by the process of freeze desiccation (Keefe and Moore, Citation1983; Bai et al., Citation1998; Gusta et al., Citation2006). In freezing tolerance, the formation of extracellular ice is restricted to noncritical tissue (). Ice forms in some of the seed tissues/cells as the temperature is lowered. More generally, as freezing starts in the extracellular space, through supercooling and then nucleation, the developing vapor pressure differential between the extracellular and intracellular water drives freeze-desiccation (Mazur, Citation1984). Being exothermic, such freezing may result in localized internal heating of the tissue thereby reducing the risk of IIF. When this happens in hydrated seeds, the water content of the embryo is reduced and ice formation is prevented until lower temperatures; thus creating an extra temperature range for seed survival.
There is strong evidence that such freeze desiccation in seeds is highly dependent on ecologically meaningful cooling rates, i.e., <4° Ch−1 (Keefe and Moore, Citation1981, Citation1983; Bai et al., Citation1998; Gusta et al., Citation2006). For example, when fully imbibed Lactuca sativa seeds were cooled at <4°Ch−1, seeds remained viable below −18°C by the mechanism of freeze desiccation (Keefe and Moore, Citation1981, Citation1983). The tolerance mechanism was shown to depend on the migration of embryo water to ice in nonembryo tissue (the perisperm), with freeze desiccating L. sativa seeds surviving down to −50°C (Ishikawa and Sakai, Citation1982). Previous DTA studies showed no LTE in fully hydrated L. sativa with WC c. 50% fwb cooled to −45°C at 2°Ch−1, although some seeds died around −40°C, presumably due to small amounts of homogenous ice formation (Jaganathan et al., Citation2016; Jaganathan, Han, et al., Citation2017). It seems survival depends on how equilibrium is achieved between permissible extracellular and extraneous tissue freezing and IIF in critical tissues (Mazur, Citation1963; Citation1966; Citation1984). During fast cooling, i.e., >4° Ch−1, there is insufficient time for embryo water to migrate to the perisperm and equilibrium is only established by forming ice in the embryo (Keefe and Moore, Citation1981; Citation1982; Jaganathan et al., Citation2016; Jaganathan, Han, et al., Citation2017). Consequently, faster nonecological cooling rates may limit wet seed survival of cold temperatures to an avoidance mechanism governed by the tissues’ supercooling capability and delimited by heterogeneous nucleation events.
A broadly similar freeze desiccation pattern as in L. sativa has been demonstrated in winterfat (Eurotia lanata (Pursh) Moq.). Seeds cooled at 2.5°Ch−1 displayed two exotherms (Bai et al., Citation1998), yet seeds survived at temperatures below the second exotherm. The authors explained ‘that the hairy surface of the winterfat pericarp provides a safe place for ice crystals to form’, suggesting that the first, HTE could relate to the surface of the pericarp, or to the space between the pericarp and the bract wall or between the testa and pericarp wall (Booth, Citation1988). Whilst the authors contended that the LTE related to ice formation in the embryo and that this was survivable, it seems possible that the LTE in E. lanata occurred in noncritical tissues such as the perisperm. In order to better understand the mechanism of survival, we need additional spatial and temporal studies on ice formation using low temperature scanning electron microscopy (LT-SEM). Recent studies on desiccation-sensitive, i.e., recalcitrant, seeds using LT-SEM, have shown that small quantities of very small sized ice can be tolerated in embryo tissues (Wesley-Smith et al., Citation2014; Wesley-Smith et al., Citation2015). However, such tolerance is dependent on extremely fast cooling (e.g., 97 or 3.3 °C s−1) that does not relate to natural ecology and overwintering mechanisms of alpine species’ seed.
IV. Alpine seeds in a changing climate
Climate change studies have tended to focus on the positive or negative impacts of a warming climate on the growth of plants, whilst the ability of seeds to survive low temperatures is assumed to be a weak filter of plant distribution. Consequently, little attention has been paid to the potential for climate change to impact seed survival in cold environments. Because temperature decreases with altitude, we expected that hydrated seeds of high altitude plants could tolerate exposure to lower temperatures than those of boreal and lowland species. Moreover, we postulated that a gradual warming of alpine environments would release these stress-imposed limitations on lower altitude species facilitating their colonization of the upland environment. Thus, we anticipated a positive relationship between cold tolerance of the seeds and the ambient minimum temperatures. The focus on minimum rather than average winter temperature is important as seeds at high altitudes may be covered under a snow blanket during winter and thermally buffered. However, partial and localized melting of snow at the beginning of growing season would expose the seeds to low freezing temperature. Under such circumstances, if the seeds lack a capacity to survive freezing, then presumably seed mortality increases, resulting in the loss of offspring. In contrast, boreal or low altitude species are not likely to be exposed to temperatures below −15 °C during the onset of growing season (Bonan and Shugart, Citation1989; Jungqvist et al., Citation2014); thus avoidance or tolerance (or both) of moderate freezing temperatures would be sufficient to ensure seed survival.
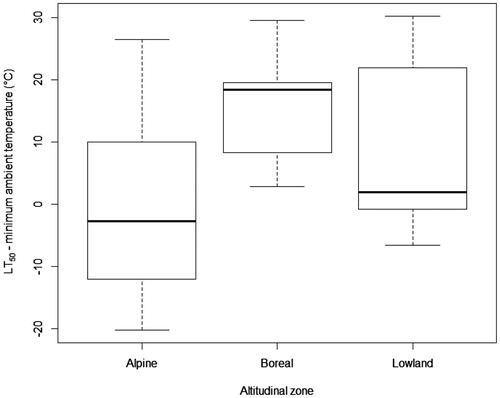
A thorough literature search was conducted in Web of Science using string terms ‘freezing tolerance in seeds’, ‘supercooling in seeds’ and ‘freeze desiccation in seeds’, to identify relevant studies. Experimentally-derived data on the lowest temperature recorded at which 50% of the seeds survived (LT50) were compiled, resulting in a dataset of 24 taxa representing eight families (). Values of LT50 were derived as means but most of these were reported without sample size and/or measures of variance so that our analysis used LT50 as a single value. We assumed that the location at which each taxa achieved its maximum altitude would accurately represent the harshest climate conditions in which they can successfully complete a life cycle. We used the Global Biodiversity Index Facility (GBIF), to identify the location at which the highest elevation was attained by each taxa and then derived the minimum temperature for the coldest month (°C) for each of these locations using Worldclim data (in DIVA-GIS version 7.5.0), hereafter referred to as the minimum ambient temperature. The difference between LT50 and the minimum ambient temperature was calculated for each species by subtracting LT50 from the minimum ambient temperature. When the difference between LT50 and the minimum ambient temperature is positive, this indicates that seed cold tolerance is adequate to survive the temperature minima experienced by seeds. When the difference is a negative value, the temperature at which half the seeds die is higher than that to which the seeds might be exposed and therefore high mortality is likely. The mean difference between LT50 and the minimum ambient temperature was determined by altitudinal zone (lowland, boreal and alpine) and compared using analysis of variance following a Fligner-Killeen test (R version 3.4.0; R Core Team, Citation2018) to ensure that the data did not break assumptions of homogeneity of variance.
Table 2. List of species for which LT50 temperature is available and their distribution assessed on GBIF.
When initiating this analysis, we expected that there would be a significant correlation between minimum ambient temperature and LT50 values because plants that survived in colder environments would have evolved seeds with tolerances for lower temperatures than those from warmer climates. However, this was not evident from our analysis and instead the correlation coefficient was negative and relatively small (R = −0.5194879). Alpine species’ seeds underwent 50% mortality at higher temperatures than boreal or lowland species (mean of alpine LT50= −11.8 °C ± 7.9 compared to boreal mean= −22.4 °C ± 4.2 and lowland mean= −18.7 °C ± 5.4); and the mean difference between alpine species’ LT50 and the minimum ambient temperature was only −0.85 °C (). These findings indicate that freezing tolerances of alpine species might not be adequate to guarantee more than 50% survival in ambient minimum temperatures in a future climate with snow cover loss. Boreal and lowland species respectively () had a positive difference between LT50 and minimum ambient temperature indicating that their physiological ability, and potential environmental plasticity, for survival of extremely low temperatures surpassed that of alpine species. When the difference between LT50 and minimum ambient temperature was statistically compared between altitudinal zones, there was a marginally statistically significant distinction as determined by one-way ANOVA (F (2,21)=3.402, p = 0.052). Our conclusion that alpine seeds have relatively modest low temperature tolerance, implies an extreme risk of extirpation in the face of climate change due to snow cover loss exposing the seeds to critically low temperatures. This finding mirrors the recent observations on seedlings (Rosbakh et al., Citation2020).
V. Implications of climate change for alpine plants
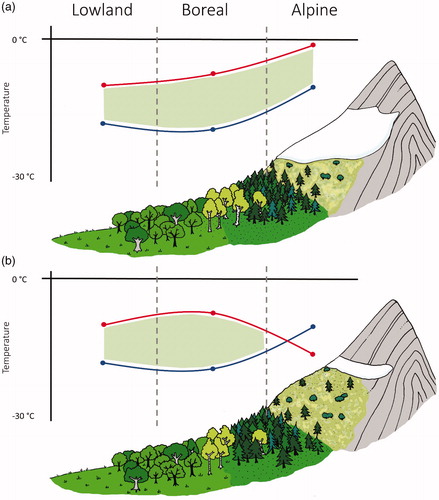
The very short snow-free growing season in alpine environments necessitates the completion of photosynthesis and reproductive cycles in a constrained time interval (Shimono and Kudo, Citation2003; Shimono and Kudo, Citation2005; Holtmeier and Broll, Citation2007). This means that seeds from alpine regions need to survive potential exposure to low temperatures over a long period. Consequently, our hypothesis was that seeds of alpine species would have lower LT50 than boreal or lowland region plants. This hypothesis was not supported. Instead, boreal species, particularly conifer trees, seem to have seeds better adapted to very low temperatures. One likely explanation for this result is that the insulating effects of snow cover have reduced the potential for selection of alpine species for freeze tolerance during the seed phase of the life cycle (,). So climate warming induced reduction in snow cover will increasingly remove this benign environment during winter and increase the risk of failure to regenerate from the soil seed bank in high altitude locations. Future climatic warming is expected to cause the loss of snow volume and an upward snowline movement by as much as 300–600 m from its current positions (Gobiet et al., Citation2014). Even if the snowline remains at the same elevation, earlier snowmelt is widely predicted (Wipf and Rixen, Citation2010) leading to an extended growing season (Björk and Molau, Citation2007). Nonetheless, these changes in temperature and snow cover pose a greater risk to seed survival ().
Figure 4. Hypothesized relationship between LT50 of seeds from lowland, boreal and alpine plant species (blue line) relative to the ambient minimum temperature associated with the same species (red line; see text for details). Figure (a) conveys the current conditions where snow cover maintains an ambient minimum temperature close to freezing in the alpine zone, (b) shows the decrease in ambient temperatures when snow cover has been lost. Green hatched area indicates the conditions where LT50 exceeds the ambient minimum temperature and seeds are therefore within the tolerable temperature range to survive; alpine species in figure b are exposed to temperatures beyond the tolerable freezing range.
Warming conditions are likely to have two significant effects on the vegetation of alpine environments. First, alpine species may have reduced seed banks due to the risk presented by direct exposure of seeds to freezing temperature, which has implications for population survival. Warmer ambient conditions could paradoxically lower soil temperature due to snow cover loss and expose the seeds to much lower temperatures. Secondly, the reduced pool of alpine species able to survive the extreme conditions imposed by loss of snow cover will have to compete with the migrating seeds of species from adjacent snowfree areas, but also the possible invasion of lower altitude species. The loss of snowbeds may be an opportunity for establishment of lower altitude species if they can tolerate the freezing temperatures that may occur sporadically in the spring after snow melt.
Recent changes in snow bed communities indicate a shift toward alpine grasslands facilitated by the proximity of such communities to snow beds (Holtmeier and Broll, Citation2007). The invasion of seeds over greater distances into alpine environments and the survival of seeds during the winter are not considered to be limiting the colonization of alpine vegetation by boreal trees (Grace et al., Citation2002). However, the tolerance of seeds to freezing temperatures has not been mentioned in reviews of treeline advance (Holtmeier and Broll, Citation2007) and this mechanism represents a novel contribution to understanding community change in alpine environments. The limited capacity of alpine seeds to survive freezing temperature in their imbibed state, might be due to the evolutionary response of snow cover protecting seeds, which might not be the case for some low altitude species, e.g., pines. This could possibly explain the tree-line advancements recorded in some of the studies (Moen et al., Citation2004; Piotti et al., Citation2009; Cudlín et al., Citation2017; Shen et al., Citation2018; Sigdel et al., Citation2018). Given the smaller data set reported in this work might present some limitation, we stress that more effort is needed to test the freezing tolerance in seeds, particularly from alpine and boreal ecosystem across the globe. Such effort will likely inform us of the role of evolution in freezing tolerance in seeds and also help identify the ‘species at risk’. This is particularly important in alpine ecosystem, because seeds can persist in the soil even when all the standing vegetation has died off, allowing an opportunity for those species to colonize (Arroyo et al., Citation1999). However, this will largely depend on the effects of climate change altering the seeds from dispersal to germination.
Acknowledgment
We are indebted to Professor Brian Grout and an anonymous reviewer for their comments on an earlier version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Amen, R. D. 1965. Seed dormancy in the alpine rush, Luzula Spicata L. Ecology. 46: 361–364.
- Amen, R. D. 1966. The extent and role of seed dormancy in alpine plants. Q. Rev. Biol. 41: 271–281.
- Angell, C. A. 1995. Formation of glasses from liquids and biopolymers. Science. 267: 1924–1935.
- Arroyo, M. T. K., Cavieres, L. A., Castor, C., and Humaña, A. M. 1999. Persistent soil seed bank and standing vegetation at a high alpine site in the central Chilean Andes. Oecologia. 119: 126–132.
- Bai, Y., Booth, D., and Romo, J. 1998. Winterfat (Eurotia lanata (Pursh) Moq.) seedbed ecology: low temperature exotherms and cold hardiness in hydrated seeds as influenced by imbibition temperature. Ann. Bot. 81: 595–602.
- Bai, Y., Booth, D. T., and Romo, J. 1999. Imbibition temperature affects winterfat (Eurotia lanata (Pursh) Moq.) seed hydration and cold-hardiness response. J. Range Manage. 52: 271–274.
- Baskin, C. C. and Baskin, J. M. 2014. Seeds: ecology, biogeography, and evolution of dormancy and germination. Academic Press: San Diego, USA.
- Baskin, J. M. and Baskin, C. C. 2004. A classification system for seed dormancy. Seed Sci. Res. 14: 1–16.
- Baskin, J. M., Baskin, C. C., and Li, X. 2000. Taxonomy, anatomy and evolution of physical dormancy in seeds. Plant Species Biol. 15: 139–152.
- Beck, E., Schulze, E.-D., Senser, M., and Scheibe, R. 1984. Equilibrium freezing of leaf water and extracellular ice formation in Afroalpine 'giant rosette' plants. Planta. 162: 276–282.
- Bell, K. L. and Amen, R. D. 1970. Seed dormancy in Luzula spicata and L. parviflora. Ecology. 51: 492–496.
- Bewley, J. D. and Black, M. 1994. Seeds: physiology of development and germination. Springer: New York, USA.
- Bewley, J. D., Bradford, K. J., Hilhorst, H. W., and Nonogaki, H. 2013. Environmental regulation of dormancy and germination seeds. Springer: New York, USA.
- Billings, W. D. and Mooney, H.A. 1968. The ecology of arctic and alpine plants. Biol. Rev. 43: 481–529.
- Björk, R.G. and Molau, U. 2007. Ecology of alpine snowbeds and the impact of global change. Arct. Antarct. Alp. Res. 39: 34–43.
- Bliss, L. C. 1963. Alpine plant communities of the presidential range, New Hampshire. Ecology. 44: 678–697.
- Bliss, L. C. 1971. Arctic and alpine plant life cycles. Annu. Rev. Ecol. Syst. 2: 405–438.
- Blytt, A. G. 1876. Essay on the immigration of the Norwegian flora during alternating rainy and dry periods. A. Cammermeyer: Christiania.
- Bock, J. 1976. The effects of increased snowpack on the phenology and seed germinability of selected alpine species. Ecological impacts of snowpack augmentation in the San Juan Mountains of Colorado. US Department of the Interior, Division of Atmospheric Water Resources Management, Bureau of Reclamation: Denver, Colorado, USA, pp 265–271.
- Bonan, G. D. and Shugart, H. H. 1989. Environmental factors and ecological processes in Boreal forests. Annu. Rev. Ecol. Syst. 20: 1–28.
- Booth, D. T. 1988. Winterfat diaspore morphology. J. Range Manage. 41: 351–353.
- Bourque, J. E. and Wallner, S. J. 1982. Endosperm and pericarp involvement in the supercooling of imbibed lettuce seeds. Plant Physiol. 70:1571–1573.
- Brant, R., McKee, G., and Cleveland, R. 1971. Effect of chemical and physical treatment on hard seed of Penngift crownvetch. Crop Sci. 11: 1–6.
- Briceño, V. F., Hoyle, G. L., and Nicotra, A. B. 2015. Seeds at risk: how will a changing alpine climate affect regeneration from seeds in alpine areas? Alp Bot. 125: 59–68.
- Brown, H. T. and Escombe, F. 1897. Note on the influence of very low temperatures on the germinative power of seeds. Proc Royal Soc London. 62: 160–165.
- Burke, M., Gusta, L., Quamme, H., Weiser, C., and Li, P. 1976. Freezing and injury in plants. Annu. Rev. Plant. Physiol. 27: 507–528.
- Busse, W. 1930. Effect of low temperatures on germination of impermeable seeds. Bot Gazette. 89: 169–179.
- Cudlín, P., Cudlín, P., Cudlín, P., Tognetti, R., Malis, F., Alados, C. L., Bebi, P., Grunewald, K., Zhiyanski, M., Andonowski, V., La Porta, N., Bratanova-Doncheva, S., Kachaunova, E., Edwards-Jonášová, M., Ninot, J. M., Rigling, A., Hofgaard, A., Hlásny, T., Skalák, P., and Wielgolaski, F. E. 2017. Drivers of treeline shift in different European mountains. Clim. Res. 73: 135–150.
- Fenner, M. and Thompson, K. 2005. The ecology of seeds. Cambridge University Press: Cambridge, UK.
- Finch-Savage, W., and McQuistan, C. 1988. The potential for newly-germinated cabbage seed survival and storage at sub-zero temperatures. Ann. Bot. 62: 509–512.
- Forbis, T. A. 2003. Seedling demography in an alpine ecosystem. Am. J. Bot. 90: 1197–1206.
- Gobiet, A., Kotlarski, S., Beniston, M., Heinrich, G., Rajczak, J., and Stoffel, M. 2014. 21st century climate change in the European Alps-a review. Sci. Total Environ. 493: 1138–1151.
- Grace, J., Berninger, F., and Nagy, L. 2002. Impacts of climate change on the tree line. Ann. Bot. 90: 537–544.
- Gusta, L. V., Gao, Y. P., and Benning, N. T. 2006. Freezing and desiccation tolerance of imbibed canola seed. Physiol Plant. 127: 237–246.
- Hamid, M., Khuroo, A. A., Malik, A. H., Ahmad, R., Singh, C. P., Dolezal, J., and Haq, S. M. 2020. Early evidence of shifts in Alpine summit vegetation: a case study from Kashmir Himalaya. Front Plant Sci. 11: 421.
- Hawkins, B., Guest, H., and Kolotelo, D. 2003. Freezing tolerance of conifer seeds and germinants. Tree Physiol. 23: 1237–1246.
- Holtmeier, F.-K. and Broll, G. 2007. Treeline advance–driving processes and adverse factors. LO. 1: 1–32.
- Hu, X., Li, T., Wang, J., Wang, Y., Baskin, C. C., and Baskin, J. M. 2013. Seed dormancy in four Tibetan Plateau Vicia species and characterization of physiological changes in response of seeds to environmental factors. Seed Sci. Res. 23: 133–140.
- Ishikawa, M. and Sakai, A. 1978. Freezing avoidance in rice and wheat seeds in relation to water content. Low Temp. Sci. Ser. B. 36: 39–49.
- Ishikawa, M. and Sakai, A. 1982. Characteristics of freezing avoidance in comparison with freezing tolerance: a demonstration of extraorgan freezing. Plant cold hardiness and freezing stress. Academic Press: New York, USA.
- Jaganathan, G., Dalrymple, S., and Liu, B. 2015. Towards an understanding of factors controlling seed bank composition and longevity in the alpine environment. Bot. Rev. 81: 70–103.
- Jaganathan, G. K. 2009. Towards an understanding of seed survival mechanism in alpine seed bank. MSc. Thesis, University of Bedfordshire.
- Jaganathan, G. K. 2016. Influence of maternal environment in developing different levels of physical dormancy and its ecological significance. Plant Ecol. 217: 71–79.
- Jaganathan, G. K., Boenisch, G., Kattge, J., and Dalrymple, S. E. 2019. Physically, physiologically and conceptually hidden: improving the description and communication of seed persistence. Flora. 257: 151413.
- Jaganathan, G. K. and Dalrymple, S. E. 2019. Internal seed structure of alpine plants and extreme cold exposure. Data. 4: 107.
- Jaganathan, G. K., Han, Y., Li, W., Song, D., Song, X., Shen, M., Zhou, Q., Zhang, C., and Liu, B. 2017. Physiological mechanisms only tell half story: multiple biological processes are involved in regulating freezing tolerance of imbibed Lactuca sativa seeds. Sci. Rep. 7: 44166.
- Jaganathan, G. K., Han, Y., Wu, G., and Liu, B. 2016. Freezing tolerance in hydrated lettuce (Lactuca sativa) seeds is dependent on cooling rate but not imbibition temperature. Acta Physiol. Plant. 38: 35.
- Jaganathan, G. K., Song, D., and Liu, B. 2017. Diversity and distribution of physical dormant species in relation to ecosystem and life-forms. Plant Sci. Today. 4: 55–63.
- Jungqvist, G., Oni, S. K., Teutschbein, C., and Futter, M. N. 2014 .Effect of climate change on soil temperature in Swedish boreal forests. PLOS One. 9: e93957.
- Junttila, O., and Stushnoff, C. 1977. Freezing avoidance by deep supercooling in hydrated lettuce seeds. Nature. 269: 325–327.
- Kaye, T. N. 1997. Seed dormancy in high elevation plants: implications for ecology and restoration. Conservation and management of native plants and fungi. Native Plant Society of Oregon: Corvallis, OR.
- Keefe, P. and Moore, K. 1981. Freeze desiccation: a second mechanism for the survival of hydrated lettuce (Lactuca sativa L.) seed at sub-zero temperatures. Ann. Bot. 47: 635–645.
- Keefe, P. and Moore, K. 1982. Frost damage during stratification: mechanism and protection in Pinus sylvestris seed. Seed Sci. Tech. 10:485–495.
- Keefe, P. and Moore, K. 1983. Freezing tolerance in hydrated Lactuca sativa (L) seed: a model to explain observed variation between seed lots. Ann. Bot. 51: 373–383.
- Körner, C. 1999. Alpine plant life: functional plant ecology of high mountain ecosystems. Springer-Verlag: New York, USA.
- Körner, C. 2003. Alpine plant life: functional plant ecology of high mountain ecosystems. Springer Verlag: New York, USA.
- Ladinig, U., Hacker, J., Neuner, G., and Wagner, J. 2013. How endangered is sexual reproduction of high-mountain plants by summer frosts? Frost resistance, frequency of frost events and risk assessment. Oecol. 171: 1–18.
- Larcher, W. 2012. Bioclimatic temperatures in the high alps. Plants in alpine regions: cell physiology of adaption and survival stratergies. Springer: Vienna.
- Larcher, W., Kainmüller, C., and Wagner, J. 2010. Survival types of high mountain plants under extreme temperatures. Flora Morphol. Distrib. Funct. Ecol. Plants. 205: 3–18.
- López-Moreno, J. I., Goyette, S., Vicente-Serrano, S., and Beniston, M. 2011. Effects of climate change on the intensity and frequency of heavy snowfall events in the Pyrenees. Clim. Change. 105:489–508
- Lütz, C. 2011. Plants in alpine regions: cell physiology of adaption and survival strategies. Springer: New York, USA.
- Marcante, S., Sierra‐Almeida, A., Spindelböck, J. P., Erschbamer, B., and Neuner, G. 2012. Frost as a limiting factor for recruitment and establishment of early development stages in an alpine glacier foreland? J. Veg. Sci. 23: 858–868.
- Marchand, P. J. and Roach, D. A. 1980. Reproductive strategies of pioneering alpine species: seed production, dispersal, and germination. Arct. Alp. Res. 12: 137–146.
- Mazur, P. 1963. Kinetics of water loss from cells at subzero temperatures and the likelihood of intracellular freezing. J. Gen. Physiol. 47: 347–369.
- Mazur, P. 1966. Physical and chemical basis of injury in single-celled microorganisms subjected to freezing and thawing. Cryobiol. 213: 213–315.
- Mazur, P. 1984. Freezing of living cells: mechanisms and implications. Am. J. Physiol. 247: C125–C142.
- McGraw, J., Vavrek, M., and Bennington, C. 1991. Ecological genetic variation in seed banks I. Establishment of a time transect. J Ecol. 79: 617–625.
- Midgley, A. 1926. Effect of alternate freezing and thawing on the impermeability of alfalfa and dodder seeds. Agron. J. 18: 1087–1098.
- Moen, J., Aune, K., Edenius, L., and Angerbjörn, A. 2004. Potential effects of climate change on treeline position in the Swedish mountains. Ecol. Soc. 9: 16.
- Mondoni, A., Rossi, G., Orsenigo, S., and Probert, R.J. 2012. Climate warming could shift the timing of seed germination in alpine plants. Ann. Bot. 110: 155–164.
- Moore, E. B. and Molinero, V. 2011. Structural transformation in supercooled water controls the crystallization rate of ice. Nature. 479: 506–508.
- Neuner, G. 2014. Frost resistance in alpine woody plants. Front. Plant Sci. 5: 654.
- Neuner, G., Erler, A., Ladinig, U., Hacker, J., and Wagner, J. 2013. Frost resistance of reproductive tissues during various stages of development in high mountain plants. Physiol. Plant. 147: 88–100.
- Phartyal, S. S., Rosbakh, S., and Poschlod, P. 2018. Seed germination ecology in Trapa natans L., a widely distributed freshwater macrophyte. Aquat. Bot. 147: 18–23.
- Piotti, A., Leonardi, S., Piovani, P., Scalfi, M., and Menozzi, P. 2009. Spruce colonization at treeline: where do those seeds come from? Heredity. 103: 136–145.
- Pritchard, H., Manger, K., and Prendergast, F. 1988. Changes in Trifolium arvense seed quality following alternating temperature treatment using liquid nitrogen. Ann. Bot. 62: 1–11.
- R Core Team. 2018. R: A language and environment for statistical computing; R Foundation for Statistical Computing: Vienna, Austria.
- Ristic, Z. and Ashworth, E. N. 1994. Response of xylem ray parenchyma cells of Red Osier Dogwood (Cornus sericea L.) to freezing stress (microscopic evidence of protoplasm contraction). Plant Physiol. 104: 737–746.
- Roberts, E. 1973. Predicting the storage life of seeds. Seed Sci. Tech. 1: 499–514.
- Rolston, M. P. 1978. Water impermeable seed dormancy. Bot. Rev. 44: 365–396.
- Roos, E. and Stanwood, P. 1981. Effects of low temperature, cooling rate and moisture content on seed germination of lettuce. J. Am. Soc. Hort. Sci. 106: 30–34.
- Rosbakh, S., Margreiter, V., and Jelcic, B. 2020. Seedlings of alpine species do not have better frost-tolerance than their lowland counterparts. Alpine Bot. 130: 1–7.
- Rossman, E. 1949. Freezing injury of inbred and hybrid maize seed. Agron.J. 41: 574–583.
- Sakai, A. 1960. Survival of the twig of woody plants. Nature. 185: 393–394.
- Schwienbacher, E., Navarro-Cano, J.A., Neuner, G., and Erschbamer, B. 2011. Seed dormancy in alpine species. Flora-Morphology, Distribution, Functional Ecology of Plants. 206: 845–856. doi:10.1016/j.flora.2011.05.001
- Shen, W., Zhang, L., Guo, Y., and Luo, T. 2018. Causes for treeline stability under climate warming: Evidence from seed and seedling transplant experiments in southeast Tibet. For. Ecol. Manage. 408: 45–53.
- Shibata, T., Sakai, E., and Shimomura, K. 1995. Effect of rapid freezing and thawing on hard-seed breaking in Astragalus mongholicus Bunge (Leguminosae). J. Plant Physiol. 147: 127–131.
- Shimono, Y. and Kudo, G. 2003. Intraspecific variations in seedling emergence and survival of Potentilla matsumurae (Rosaceae) between alpine fellfield and snowbed habitats. Ann. Bot. 91: 21–29.
- Shimono, Y. and Kudo, G. 2005. Comparisons of germination traits of alpine plants between fellfield and snowbed habitats. Ecol Res. 20: 189–197.
- Sierra-Almeida, A., and Cavieres, L. A. 2012. Summer freezing resistance of high-elevation plant species changes with ontogeny. Environ. Exp. Bot. 80: 10–15.
- Sigdel, S. R., Wang, Y., Camarero, J. J., Zhu, H., Liang, E., and Peñuelas, J. 2018. Moisture-mediated responsiveness of treeline shifts to global warming in the Himalayas. Glob. Chang. Biol. 24: 5549–5559.
- Steinbauer, M. J., Grytnes, J.-A., Jurasinski, G., Kulonen, A., Lenoir, J., Pauli, H., Rixen, C., Winkler, M., Bardy-Durchhalter, M., Barni, E., Bjorkman, A. D., Breiner, F. T., Burg, S., Czortek, P., Dawes, M. A., Delimat, A., Dullinger, S., Erschbamer, B., Felde, V. A., Fernández-Arberas, O., Fossheim, K. F., Gómez-García, D., Georges, D., Grindrud, E. T., Haider, S., Haugum, S. V., Henriksen, H., Herreros, M. J., Jaroszewicz, B., Jaroszynska, F., Kanka, R., Kapfer, J., Klanderud, K., Kühn, I., Lamprecht, A., Matteodo, M., di Cella, U. M., Normand, S., Odland, A., Olsen, S. L., Palacio, S., Petey, M., Piscová, V., Sedlakova, B., Steinbauer, K., Stöckli, V., Svenning, J.-C., Teppa, G., Theurillat, J.-P., Vittoz, P., Woodin, S. J., Zimmermann, N. E., and Wipf, S.2018. Accelerated increase in plant species richness on mountain summits is linked to warming. Nature. 556: 231–234.
- Stinson, K. A. 2004. Natural selection favors rapid reproductive phenology in Potentilla pulcherrima (Rosaceae) at opposite ends of a subalpine snowmelt gradient. Am. J. Bot. 91: 531–539.
- Stushnoff, C. and Junttila, O. 1978. Resistance to low temperature injury in hydrated lettuce seed by supercooling. Plant cold hardiness and freezing stress. Mechanisms and crop implications. Academic Press, Elsevier Inc: London.
- Van Assche, J. A., Debucquoy, K. L., and Rommens, W. A. 2003. Seasonal cycles in the germination capacity of buried seeds of some Leguminosae (Fabaceae). New Phytol. 158: 315–323.
- Vernon, P., Vannier, G., and Arondel, V. 1999. Supercooling capacity of seeds and seedlings in Arabidopsis thaliana. Cryobiology. 39: 138–143.
- Wagner, J., Ladinig, U., Steinacher, G., and Larl, I. 2012. From the flower bud to the mature seed: timing and dynamics of flower and seed development in high-mountain plants. Plants in alpine regions. Springer: Vienna.
- Wagner, J., and Mitterhofer, E. 1998. Phenology, seed development, and reproductive success of an alpine population of Gentianella germanica in climatically varying years. Botanica Acta. 111: 159–166.
- Wesley-Smith, J., Berjak, P., Pammenter, N., and Walters, C. 2014. Intracellular ice and cell survival in cryo-exposed embryonic axes of recalcitrant seeds of Acer saccharinum: an ultrastructural study of factors affecting cell and ice structures. Ann. Bot. 113: 695–709.
- Wesley-Smith, J., Walters, C., Pammenter, N., and Berjak, P. 2015. Why is intracellular ice lethal? A microscopical study showing evidence of programmed cell death in cryo-exposed embryonic axes of recalcitrant seeds of Acer saccharinum. Ann. Bot. 115: 991–1000.
- Williams, R. 2006. Patterns of air temperature and accumulation of snow in subalpine heathlands and grasslands on the Bogong High Plains, Victoria. Aust. J. Ecol. 12: 153–163.
- Wipf, S., and Rixen, C. 2010. A review of snow manipulation experiments in Arctic and alpine tundra ecosystems. Polar Res. 29:95–109.
- Wisniewski, M., Bassett, C., and Gusta, L. V. 2003. An overview of cold hardiness in woody plants: seeing the forest through the trees. Hort Sci. 38: 952–959.
- Wolfe, J., Bryant, G., and Koster, K. L. 2002. Whatis 'unfreezable water', how unfreezable is it, and how much is there? Cryo Lett. 23:157–166.
- Zanne, A. E., Tank, D. C., Cornwell, W. K., Eastman, J. M., Smith, S. A., FitzJohn, R. G., McGlinn, D. J., O'Meara, B. C., Moles, A. T., Reich, P. B., Royer, D. L., Soltis, D. E., Stevens, P. F., Westoby, M., Wright, I. J., Aarssen, L., Bertin, R. I., Calaminus, A., Govaerts, R., Hemmings, F., Leishman, M. R., Oleksyn, J., Soltis, P. S., Swenson, N. G., Warman, L., and Beaulieu, J. M. 2014. Three keys to the radiation of angiosperms into freezing environments. Nature. 506: 89–92.