Abstract
The coming century in agriculture will be marked by increasing exposure of crops to abiotic stress and disease due to climate change. The plant traits with the strongest potential to mitigate these stresses are complex, and are increasingly recognized to involve interaction with the microbiome. Through symbiosis with soil fungi, plants form arbuscular mycorrhizae (AM) that can alleviate nutrient, water, and temperature stress, and can confer pathogen resistance and increased yield. The portfolio of advantages offered by AM overlaps with the benefits of agriculturally useful plant traits that have been the subject of decades of intensive biotechnological efforts, such as C4 photosynthesis and rhizobial nitrogen fixation. In this article we illustrate the prospective benefits of genetic engineering to produce AM in nonmycorrhizal plants and modify AM in already-mycorrhizal crops. We highlight recent advances which have clarified the key genetic and metabolic components of AM symbiosis, and show that many of these components are involved in other plant biological processes and have already been subject to extensive genetic engineering in nonsymbiotic contexts. We provide a theoretical research roadmap to accomplish engineering of AM into the nonmycorrhizal model Arabidopsis including specific molecular genetic approaches. We conclude that AM is potentially more tractable than other complex plant traits, and that a concerted research initiative for biotechnological manipulation of AM could fill unique needs for agricultural resilience. Finally, we note that engineering of AM provides a potential back door into manipulation of other essential plant traits, including carbon storage, and beneficial microbiome assembly.
I. Background
Arbuscular mycorrhizae (AM) are a symbiotic relationship in which Glomeromycotan fungi form nutrient exchange structures within the cells of plant roots (Schüβler et al., Citation2001). The plant provides fixed carbon in the form of lipids to the fungus, and receives nutrients and water in return (Oldroyd, Citation2013; Rich et al., Citation2017b). AM symbiosis can also confer resistance to pathogens, temperature stress, and soil toxins to the host plant. The AM host trait dates back to the original colonization of land by plants, and has accompanied diverse plant species throughout their evolutionary adaptations to a wide range of stresses and environments (Delaux et al., Citation2013; Genre et al., Citation2020; Radhakrishnan et al., Citation2020). However, this trait has been lost in many species, adding up to over 25% of modern plants (Cosme et al., Citation2018).
Non-AM crops worldwide occupied more than a hundred million acres in 2019, an area larger than the nation of Sweden or the state of California, producing a harvest of almost half a billion tonnes (FAO, Citation2021). While non-AM plants are agriculturally and phylogenetically in the minority, the raw scale of their cultivation indicates significant potential for gains from agricultural improvement and raises the question of whether absence of AM reduces productivity or resilience in these crops.
In this paper we explore the prospect of engineering of a synthetic AM symbiosis system. Such a system could provide agricultural benefits to nonhost species, such as the many crops in the families Brassicaceae and Amaranthaceae (). Engineered mycorrhizae could also be useful in crops which are natural AM hosts, but where the native symbiotic trait is not optimized to contribute to improved yield in a modern agricultural context (Berruti et al., Citation2016; Calvo-Polanco et al., Citation2016). The latter extends the potential application of synthetic AM to almost all modern varieties of nominally AM-host crop species, for which the prospect of AM as biofertilizers has spurred significant recent interest.
Table 1. Global abundance of non-AM crops in 2021 (FAO, Citation2021).
II. The benefits of engineered AM
Mycorrhizal inoculation of host crops is generally beneficial, though the extent and reliability of the effect is the subject of significant debate (Rouphael et al., Citation2015; Calvo-Polanco et al., Citation2016; Hijri, Citation2016; Chen et al., Citation2018; Ryan and Graham, Citation2018). In typical measures of AM benefits, a fungus inoculation treatment produces an increase in mycorrhization above the background level that is then compared to the benefits seen (Berruti et al., Citation2016). These can include up to an 80% increase in above-ground biomass, and yield increases greater than 100% (Pellegrino et al., Citation2015; Berruti et al., Citation2016; Hijri, Citation2016; Rillig et al., Citation2019). AM colonization has also been reported to increase the size and nutritional content of fruits and grains (Eo and Eom, Citation2009; Lehmann and Rillig, Citation2015; Bona et al., Citation2017; Fiorilli et al., Citation2018). Mycorrhizae contribute to nutrient requirements by improving access to what is already present in the soil, including all three macronutrients, with the strongest effect in phosphorus nutrition (Bucher, Citation2007; Nouri et al., Citation2014; Giovannetti et al., Citation2019) (). Unlike Green Revolution breeding advances, the yield benefit of AM is therefore tied to reduced, rather than increased, inputs of expensive and environmentally damaging synthetic fertilizer (Pingali and Rosegrant, Citation1994; Borlaug, Citation2002; De Schutter and Vanloqueren, Citation2011). The presumptive slate of benefits offered by engineered AM is the same as those observed in studies of natural mycorrhization, but could exceed them in scale. In the case of non-AM plants, conferring a trait where there was none before provides more prospective improvement than quantitative increase of an existing trait. In AM host crops, the engineered system can provide a quantitative increase in mycorrhization, but also a means of stabilizing the variability in AM response and incompatibility with fertilization and culture practices that has plagued past attempts to harness the benefits of AM at scale ().
Figure 1. AM in host plants can improve a remarkable range of growth traits including yield, resilience against climate stressors and diseases. However, in agriculture these benefits are unavailable to non-AM crops grown at scale (), and even in host species are contingent on establishment of colonization over the growing season and limited by breeding and cultivation practices that do not factor in mycorrhizae.
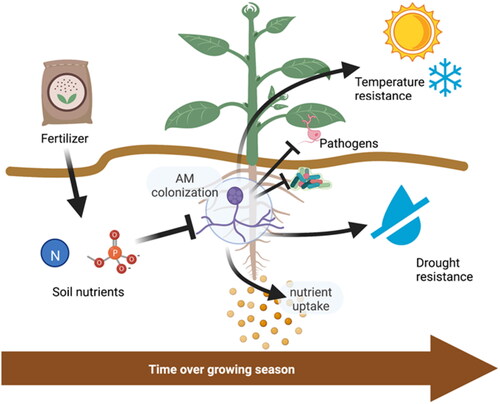
The increased yields of AM-host crops are also due to a reduction of crop loss through pests, pathogens, and abiotic stress (Begum et al., Citation2019) (). Via the phenomenon of mycorrhiza-induced resistance (MIR), AM colonization is documented to defend plants against a vast array of microbial and insect threats (Pozo and Azcón-Aguilar, Citation2007; Jung et al., Citation2012; Walters et al., Citation2013). The protective effect of MIR extends to major agricultural pathogens like powdery mildew and black root rot (fungal diseases), tomato spotted wilt virus, insect pests such as cabbage looper, and root parasitic nematodes (Vos et al., Citation2012; Citation2013; Hayek et al., Citation2014; Mustafa et al., Citation2017; Schoenherr et al., Citation2019). Mycorrhizal plants maintain higher biomass production under heat and cold stress than controls, and show reduced ROS and other markers of metabolic distress (Zhu et al., Citation2010; Abdel Latef and He, Citation2011; Yu et al., Citation2014; Jajoo and Mathur, Citation2021). Plants hosting AM have shown up to 46% higher water uptake under drought stress than controls, including 20% directly attributable to water flow through hyphae (Ruth et al., Citation2011; Zhang et al., Citation2018). Mycorrhizal crop plants also maintain higher stomatal conductance, photosynthesis rate, and yield under drought (Al-Karaki et al., Citation2004; Subramanian et al., Citation2006; Yooyongwech et al., Citation2016). Mycorrhizal plants also maintain higher hydraulic conductance under flood stress, and produce more biomass than controls across a wide variety of crops (Wu et al., Citation2013; Calvo-Polanco et al., Citation2014).
We focus here on the example of engineering an AM host trait into nonhost plants, the most extreme potential application. A genetically engineered system enabling AM in nonhost plants must be fully self-contained or rely only on interaction with conserved native genes to establish and maintain the symbiosis. Because it is not species context-dependent, such a system could also be applied in whole or in part to genetic engineering of natural AM hosts to optimize AM specific functions, expanding the relevance to essentially all crops. Such a system would be defined by the symbiotic outcome rather than a precise recapitulation of known mechanisms from AM models, which diverges from natural AM genetics at many points but accomplishes the same endpoint biological tasks. While the stress resistance benefits of AM may be of even greater interest to agriculture than the effect on nutrient uptake, we treat nutrient exchange as the defining aspect of the system because it is strictly necessary for survival of the symbiotic fungus within roots.
III. Engineering approach
While the evolutionary context giving rise to non-AM plants varies across the many independent non-AM groups, the proximate genetic causes for mycorrhizal loss are clear and remarkably consistent, with strong implications for engineering. A shared set of core genes covering three parts of the AM genetic program have been lost in all independent evolutions of nonhosting (Wang and Qiu, Citation2006; Delaux et al., Citation2014; Favre et al., Citation2014; Walder and van der Heijden, Citation2015; Bravo et al., Citation2016; Radhakrishnan et al., Citation2020).
Genes were lost from the Common Symbiosis Pathway ('CSP') that mediates signal transduction for AM and other intracellular endosymbioses including nitrogen-fixing rhizobia. Some CSP gene losses are perfectly correlated with loss of AM: no plant missing any one of these genes is known to form AM (). These include the cell-surface receptor SymRK, the ion channels CASTOR/POLLUX, nuclear kinase CCaMK, and the transcription factor CYCLOPS (Delaux et al., Citation2014; Bravo et al., Citation2016; Radhakrishnan et al., Citation2020). SymRK helps activate AM signaling in response to multiple types of chitin molecules and lipo-chitooligosaccharides, likely in complex with other receptors that provide greater specificity (Demchenko et al., Citation2004; Pan et al., Citation2018; Feng et al., Citation2019). Binding of microbial signals to the plant surface receptors initiates an early cytoplasmic signal transduction pathway, which ultimately results in calcium spiking in the nucleus mediated by CASTOR. CCaMK is activated by binding to the released calcium, upon which it phosphorylates CYCLOPS, enabling DNA binding to downstream targets (Oldroyd, Citation2013; Pimprikar and Gutjahr, Citation2018).
The term CSP (also SYM Pathway or Common Symbiosis Signaling Pathway) in the literature may be used with different levels of specificity. In the strictest sense it refers to the signal transduction genes leading from SymRK to CYCLOPS activation which are (a) shared gene-for-gene by AM and rhizobia and (b) canonically connected to each other through known mechanisms (Gutjahr et al., Citation2008; Parniske, Citation2008; Held et al., Citation2010; Genre and Russo, Citation2016; Radhakrishnan et al., Citation2020) (). The CSP may also be seen to include genes shared across multiple intracellular symbioses but not acting primarily in signal transduction (e.g. VAPYRIN in (C) below), or refer sensu lato to the outcome of CSP activation in a specific type of symbiosis (Harrison, Citation2012; Roy et al., Citation2021). Here we focus on the specific genes identified through function-agnostic phylogenetic analysis of non-AM plants, but early signaling and canonical understanding of the CSP is the subject of very active research; see, e.g. Venturi and Keel (Citation2016), Wu et al. (Citation2021), Waters and Nelson (Citation2023), Charpentier et al. (Citation2016), Duszyn et al. (Citation2019), and many others.
Genes involved in the provision of lipids to AM fungi as a carbon source are lost. These include the GRAS transcription factors RAM1 and RAD1 and their targets: the lipid transporters STR and STR2, and lipid synthesis genes RAM2, FatM, and DIS (Delaux et al., Citation2014; Bravo et al., Citation2016; Radhakrishnan et al., Citation2020). Although the order of events is not clear, these genes were lost with AM even if another endosymbiosis is present, pointing to the unique role of lipids in AM nutrient exchange (Rich et al., Citation2017a; Citation2021).
Genes involved in vesicle trafficking to the peri-arbuscular membrane are lost. These include CERBERUS, VAPYRIN, and SYP132a (Bravo et al., Citation2016; Huisman et al., Citation2016; Radhakrishnan et al., Citation2020). CERBERUS and VAPYRIN act in complex to form VAPYRIN-bodies that are actively transported to the peri-arbuscular membrane (PAM) (Bapaume et al., Citation2019). These structures are likely involved in the delivery and localization of PAM-specific proteins including nutrient transporters (Luginbuehl and Oldroyd, Citation2017). SYP132a is a t-SNARE protein that is expressed at high levels in arbusculated cells and accumulates around arbuscules, in contrast to VAPYRIN through a passive mechanism (Huisman et al., Citation2020). These genes as part of the CSP are implicated in other intracellular symbioses and are conserved in plants having such relationships even in the absence of AM.
Figure 2. Interactions of the Common Symbiosis Pathway (CSP) genes with non-CSP components. From top left: AM requires both the receptor kinases SymRK, and CERK1, a central immune receptor acting in many biotic interactions and conserved in plants regardless of AM status. Activation of the CSP culminates in upregulation of RAM1 by the CYCLOPS transcription factor complex including DELLA protein. Treating cyclops mutant plants with the GA inhibitor paclobutrazol restores mycorrhization (Pimprikar et al., Citation2016). In addition to regulation by the CSP, RAM1 and the related protein RAD1 are direct targets of the phosphate-sensitive transcription factor PHR. PHR regulates other AM targets including VAPYRIN and PT4. Overexpression of PHR can triple mycorrhizal colonization (Shi et al., Citation2021). RAM1, RAD1, and PHR also form a positive feedback loop with transcription of the lipid synthesis master regulator WRINKLED family (Jiang et al., Citation2018). AMF interact with multiple plant hormones including strigolactone signaling components (Carotenoid Cleavage Dioxygenase 7 (CCD7) and CCD8, Strigolactone receptor D14, and F-box protein MORE AXILLARY GROWTH2 (MAX2) and Jasmonic acid (JA) pathway elements (LOX;Lipoxygenases, OPR3; OPDA reductases, COI1; JA co-receptor complex F-box protein CORONATINE INSENSITIVE1), all of which have important roles in plant development, defense, and cell wall modifications (EXPAs; expansins, CESAs; Cellulose synthases; XTHs, Xyloglucan endotranglucosylase/hydrolases) (Mayzlish-Gati et al., Citation2012; Hou and Tsuda, Citation2022).
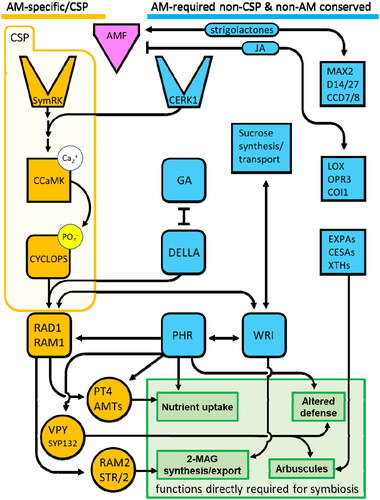
The functional genetic literature on AM has historically centered on genes like those of the CSP with isolable functions in AM (Oldroyd, Citation2013). However, as evidenced by the small number of genes identified as truly AM-specific by phylogenetics, most AM functions are executed by genes shared with other plant processes, which are conserved in plants regardless of AM status and regulated by factors that go well beyond the symbiotic interaction (). Major metabolic networks such as WRINKLED-mediated lipid synthesis and PHR/SPX phosphate sensing act directly in AM symbiosis and manipulating them has larger quantitative effects on mycorrhization than some canonical AM genes (Jiang et al., Citation2018; Shi et al., Citation2021). Initiation of AM symbiotic signaling depends on the major defense receptor CERK1 (Zhang et al., Citation2015; Leppyanen et al., Citation2017; Desaki et al., Citation2018). Strigolactones, with an essential role in AMF recruitment to the root, also serve as plant hormones, and auxin, cytokinin, jasmonic and salicylic acid hormones are heavily implicated in successful AM formation as well (Ludwig-Müller et al., Citation2002; Besserer et al., Citation2006; Hanlon and Coenen, Citation2011; Decker et al., Citation2017; Rudaya et al., Citation2021; Hou and Tsuda, Citation2022). Expansins and SNAREs common to many growth processes act in arbuscule formation (Balestrini et al., Citation2005; Choi et al., Citation2006; Cosgrove, Citation2015; Huisman et al., Citation2020). This is made even more striking by the fact that of the three groups of truly AM-specific genes lost from non-AM species, none provide a biochemically unique function necessary to the fundamental task of symbiosis; they act either as an induction system (the CSP) or as specially-regulated gene duplicates that provide familiar cellular functions at the right place and time.
The reliance on non-AM-specific genes for the majority of AM functions is a point of convergence for three key findings in the AM literature that directly inform the engineering perspective:
As a trait inherited from the first common ancestor of all land plants, the presence of AM is a conserved trait in plants and their loss or inhibition is the derived state (Delaux et al., Citation2013; Citation2015). It should therefore be possible to enable AM using only "parts" familiar to the biology of all plants.
The greatest genetic novelty in AM biology relates to the mechanisms that induce and regulate the process. The downstream metabolic and cellular functions are executed by recruiting non-AM-specific genetic components that have often been well-characterized, and even used in applied genetic engineering, in their nonsymbiotic contexts.
Canonically non-AM plants, including Arabidopsis, are able to be colonized by AM fungi under the right conditions and even form AM-like structures, albeit to a much lesser degree and without symbiotic exchange (Brundrett, Citation2009; Veiga et al., Citation2013; Cosme et al., Citation2018; Fernández et al., Citation2019).
A number of aspects of AM formation are not discussed in detail here, including membrane remodeling and vesicle transport, the signaling and fungus-nutritional role of hexoses, hormonal patterns, and of course the common symbiosis pathway to name just a few. Mycorrhization is both a complex plant trait and an ecological process, which is subject to potentially helpful manipulation on many fronts, from selection of elite fungal inocula to changes in mechanical cultivation practices. Here, we advocate for the understanding that the benefits of mycorrhizae are also subject to genetic engineering, often through the use of familiar, well-studied targets of plant biotechnology that now need only be brought together in a new way.
For the purpose of this review, we divide the theoretical engineering task into five parts:
Make non-hosts compatible with AM symbionts,
feed the fungus;
nutrient uptake from the fungus;
structural formation of the arbuscule, and
induction/regulation.
Each of these goals produces outcomes that can be measured in isolation as well as in interaction with each other (). In the subsequent sections we describe candidate genes and synthetic biology tools to achieve each functional sub-part of AM symbiosis. We highlight informative background literature, points of connection between native AM genetics and other plant functions, and contrast our theoretical engineered system with mechanisms and outcomes of natural AM.
Figure 3. Candidate transgenes (1st column) for 4 modular engineering targets, their desired effects (2nd column), and their Mechanisms (3rd column). Transgenes in 1st column and their protein products in the 3rd column are color-matched. (A) the AM phenotype of unmodified Arabidopsis; plants can be colonized by AM fungi nursed from a host plant, but without symbiosis and resulting in a defense response. (B) Arabidopsis modified for increased compatibility with AM fungi by secretion of strigolactones (‘Strig.’) from root hairs, liberation of scopoletin (yellow) from its glycoside, and defense reduction in response to lipochitooligosaccharides (purple). (C) Arabidopsis modified to synthesize and export symbiotic 2-MAGs into the root apoplast where they meet carbon needs of AM fungi; by upregulation of general lipid synthesis (WRI1, FatB), 16:0 and 14:0 FA synthesis (AtFatB, CnFATB3), a G3P acyltransferase (GPAT6), cutin ABC transporter for MAG export from the cell membrane (ABCG13), and lipid transfer protein (LTPG1) to export MAGs across the cell wall. (D) Driving transport of nutrients secreted into the intercellular apoplast by expressing a single plasma membrane proton pump (AtAHA2); protons are cotransported with nutrients and also upregulate transporter expression. (E) Alteration of cell wall stiffness to allow AM fungi to enter the cell. Right, top: a stiff cell wall with tightly packed xyloglucan/hemicellulose (blue chain) and cellulose microfibrils (green) that prevent access by hyphopodia (purple). Bottom: xyloglucanase (MtXTH1) modifies hemicellulose chain lengths and hemicellulose-cellulose interfaces to provide binding sites of expansin (AtEXPA1) that increases wall flexibility to allow hyphae in.
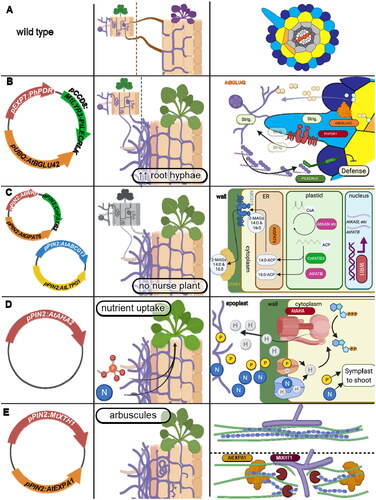
A. Making nonhost roots compatible with AM fungi
In field studies and 'nurse plant’ systems, mature hyphae supported by carbon from symbiosis with an existing host show the ability to colonize the cortex of nonhost plants with hyphae in the intercellular space and a range of other 'rudimentary AM' phenotypes (Veiga et al., Citation2013; Cosme et al., Citation2018; Fernández et al., Citation2019). Arabidopsis in these systems can be colonized by R. irregularis at 5–20% of root length (Fernández et al., Citation2019; Cosme et al., Citation2021; Wang et al., Citation2023). While this is well below the amount of mycorrhization that can be seen in host plants, it resulted in a dramatic phenotypic response and activation of defense mechanisms.. Above-ground growth is reduced by 50% in colonized Arabidopsis, late-acting defense genes are upregulated, and the plants show induced resistance to pathogens, suggesting significant diversion of resources to systemic defenses (Fernández et al., Citation2019; Wang et al., Citation2023). Thus the first subgoal of engineering AM is to increase the root colonization by nurse-supported AM fungi of Arabidopsis, while mitigating the defense response to the symbiont.
The first route to achieving increased colonization is constitutively altering the composition of root exudates for increased scopoletin (a coumarin) and strigolactones (). Coumarins are already noted as multifunctional beneficial exudates that mobilize iron and promote beneficial soil microbes (Clemens and Weber, Citation2016; Stringlis et al., Citation2019). Growth of AM fungi is encouraged by coumarins and enriched in the Arabidopsis rhizosphere under iron starvation, while growth of pathogenic fungi is suppressed (Fries et al., Citation1997; Rehman et al., Citation2005; Stringlis et al., Citation2018; Stassen et al., Citation2021).
Overexpression of the native Arabidopsis gene b-glucosidase 42 (BGLU42) which hydrolyzes the glycoside of scopoletin to enable its secretion, increased intraradical hyphae of AM fungi in Arabidopsis from less than 5% to 20% root length in a nurse system (Cosme et al., Citation2021). BGLU42 and related genes also contribute to induced systemic resistance, and its overexpression has been shown to enhance resistance of Arabidopsis to multiple pathogens (Zamioudis et al., Citation2014; Trapet et al., Citation2021). Root or whole-plant overexpression of BGLU42 is therefore appealing as a transgene that is already shown to enhance Arabidopsis AM compatibility without obvious downsides such as disease susceptibility ().
Strigolactones are well-known mediators of plant communication with AMF, which follow a strigolactone concentration gradient in soil to reach host plants (Akiyama et al., Citation2005; Besserer et al., Citation2006). Strigolactones are upregulated by phosphate deficiency in both mycorrhizal and nonmycorrhizal plants and are strongly involved in coordinating their systemic response (Kohlen et al., Citation2011; Mayzlish-Gati et al., Citation2012; Decker et al., Citation2017; Bürger and Chory, Citation2020). In rice, the smax1 mutant (suppressor of max 1) shows derepression of strigolactone synthesis associated with a 50% increase in mycorrhization, showing that quantitative increase of strigolactones in host plants can indeed effect a quantitative increase in mycorrhization (Choi et al., Citation2020).
As with coumarins, strigolactones mediate some amount of interaction with AM fungi in Arabidopsis despite its nonhost status. Strigolactone synthesis genes Carotenoid Cleavage Dioxygenase 7 (CCD7) and CCD8 are upregulated in roots upon nursed colonization with AMF (Fernández et al., Citation2019). However, though strigolactone synthesis is induced in Arabidopsis by both AM-conducive conditions and the presence of AMF, Arabidopsis shows a much lower export of strigolactones into the rhizosphere than do host plants (Yoneyama et al., Citation2008; Kohlen et al., Citation2011). Overexpression of the ATP-Binding Cassette transporter PDR1 from petunia showed reduced retention of the preloaded synthetic strigolactone GR24 and increased tolerance to externally applied GR24. These two observations are best explained with an increased export of native strigolactone in the OE-PhPDR1 plants (Kretzschmar et al., Citation2012). Constitutive expression of the petunia strigolactone transporter PhPDR1 in Arabidopsis could therefore enable higher exudation of strigolactones from the root. This gene is therefore the second component we propose for engineering of Arabidopsis AM compatibility. In addition to taking advantage of the existing AM-fungus-induced strigolactone accumulation in Arabidopsis, PDR1 as a transporter may be less disruptive to other plant functions than overexpression of biosynthesis enzymes. Strigolactones have a conserved role as plant hormones that affect architecture and germination, which could result in pleiotropies if disturbed (Brewer et al., Citation2013). Native strigolactone synthesis is also self-limiting via direct sensing of strigolactone accumulation (Mashiguchi et al., Citation2009; Kretzschmar et al., Citation2012; Soundappan et al., Citation2015; Choi et al., Citation2020). In an ideal situation, therefore, PDR1 expression can enable a continuous outflow of strigolactones while autoregulation of biosynthesis maintains a cellular pool of the metabolite at a level dictated by native processes ().
Figure 4. Multiple uses for the AM-inducible expression of native strigolactone synthesis gene CCD8. (A) Transgenic expression in trichoblasts (light blue) of the strigolactone exporter PhPDR1 allows their secretion into the soil rather than accumulation upon sensing of AM fungus. This also mitigates negative autoregulation of strigolactone synthesis due to intracellular accumulation, and permits a positive feedback loop to form between secretion of strigolactones and recruitment of AM fungus. Limiting expression to trichoblasts via cell-type-specific promoter such as pEXP7 avoids secretion from atrichoblasts (dark blue) or cortical cells (yellow), reducing undesired effects from the plant hormone activity of strigolactones. (B) Expression of the proposed AM-fungus-specific chimeric sensor kinase MtLYR3-PtLecRLK under the native pCCD8 promoter places regulation of MtLYR3-PtLecRLK under the negative feedback loop that governs strigolactone accumulation in all cells except modified trichoblasts as described in (A). This provides a means to spatially and temporally limit the potentially dangerous defense-reducing effects of MtLYR3-PtLecRLK to sites of active AM fungus colonization.
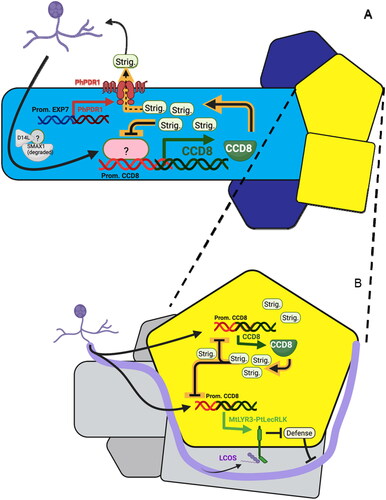
To further minimize perturbation to the hormonal role of strigolactones, PDR1 can be controlled by a cell-type-specific promoter for trichoblasts, which contribute about 90% of root surface area. As the primary site for other rhizosphere interactions, trichoblasts represent a natural target for engineering of the exudate and have been used for this purpose before (Mudge et al., Citation2003; Zimmermann et al., Citation2003). Multiple trichoblast-specific promoters are available, such as EXP7 and PHT1-2 (Zimmermann et al., Citation2003; Marquès-Bueno et al., Citation2016).
Alterations to the root exudate can attract AMF to the root and increase its hospitability for them. However, as shown experimentally in previous work these metabolites do not change the defense response of Arabidopsis to AM colonization which results in extreme growth reduction (Fernández et al., Citation2019; Cosme et al., Citation2021; Wang et al., Citation2023). Salicylic and jasmonic acid and the Pep/PEPR immunity triggering system are all strongly implicated in the nonhost defense response to AMF (Fernández et al., Citation2019; Hornstein et al., Citation2023; Wang et al., Citation2023). The precise mechanisms of this defense response are not fully known, as is also the case for AM host plant mechanisms of AMF/pathogen discrimination and mycorrhizae-induced resistance (Jung et al., Citation2012; Robe et al., Citation2021). However, the implication of major, conserved defense pathways in the nonhost plant’s response suggests that AMF fail to be distinguished from pathogens by important parts of the plant defense system.
Recent work in poplar has identified the Lectin Receptor-like Kinase 1 (LECRLK1) as essential for defense control during ectomycorrhizal symbiosis with Laccaria bicolor (Labbé et al., Citation2019). Remarkably, transgenic expression of this single gene in Arabidopsis led to suppression of defense genes during interaction with L. bicolor that enabled intraradical colonization and formation of ectomycorrhiza-like structures in Arabidopsis roots (Labbé et al., Citation2019). We suggest that PtLECRLK1 could also be used to turn down the defense response to AM fungi, in parallel with the metabolomic changes that promote their colonization.
PtLECRLK1 differs structurally from receptor kinases characterized in AM and likely binds a different ligand (Bellande et al., Citation2017; Choi et al., Citation2018; Pan et al., Citation2018; Labbé et al., Citation2019). Chitin signals that activate the CSP, however, are less unique than once thought, involving a range of molecules produced by most fungi (He et al., Citation2019; Sun et al., Citation2020; Zhang et al., Citation2021a). While it is unlikely that PtLECRLK1 signaling operates in exactly the same way as natural AM signaling, the L. bicolor ligand may already be produced by AM fungi. It will be essential to further characterize the mechanisms of PtLECRLK1, but a range of engineering options already exist to make use of this gene as an AM engineering component. A constitutively activated or kinase-only version of PtLECRLK1 could provide defense reduction regardless of the protein’s ability to bind AMF signals. However, this would relinquish the ability to selectively discriminate against signals from nonsymbiotic fungi. An alternative is to create a fusion protein that replaces the extracellular domain of PtLECRLK1 with one specific to a known AMF molecule.
Prior work demonstrates that the extracellular and kinase domains of receptors acting in defense and symbiosis are modular and amenable to domain swap. This feature has been used in the past to alter defensive signal response in Arabidopsis, demonstrate conservation of symbiotic receptors in AM species, alter rhizobial symbiont specificity, and even switch receptor specificity from pathogens to rhizobial symbiotic signals (Brutus et al., Citation2010; Li et al., Citation2018; Bozsoki et al., Citation2020). LysM Receptor-Like Kinase 3 of Medicago truncatula (MtLYR3) is a promising donor for an extracellular domain that acts in AM signaling to bind with high specificity to lipo-chitooligosaccharides (LCOs) that are elevated in the exudates of AMF relative to other fungi (Fliegmann et al., Citation2013; Citation2016; Malkov et al., Citation2016). In this case LYR3 is useful more for its biochemical LCO specificity than the strength of its role in natural AM signaling, which falls behind other receptors (Shinya et al., Citation2015). A theoretical MtLYR3-PtLECRLK1 fusion kinase would respond to LCOs in a lock-and-key mechanism more similar to that involved in perception of nitrogen-fixing bacteria for nodule formation, and would open similar prospects for tuning of symbiont-host pairings by altering ligand specificity (Bozsoki et al., Citation2017; Citation2020). To avoid increased susceptibility to pathogens, we suggest that the PtLECRLK1 (or its fusion kinase) can be placed under the control of a promoter from one of the strigolactone synthesis genes (e.g. pAtCCD8), already known to be responsive to AM fungi in Arabidopsis (Fernández et al., Citation2019). This will allow the existing feedback mechanisms for strigolactone induction and autorepression under AMF exposure to provide dynamic regulation of defense alteration in close connection to exudate changes ().
Numerous other candidates exist for engineering of an AM-specific defense reduction, which are best viewed in the context of non-AM plants’ interactions with unrelated endophytic fungi. Arabidopsis, for example, is colonized intraradically by multiple Trichoderma species and Serendipita (Piriformospora) indica, and in some cases even intracellularly (Peškan-Berghöfer et al., Citation2004; Contreras-Cornejo et al., Citation2009; Salas-Marina et al., Citation2011; Fesel and Zuccaro, Citation2016; Tseng et al., Citation2020). These relationships have effects reminiscent of mycorrhizal symbiosis, but which are context dependent and may tip into parasitism in some settings (Fesel and Zuccaro, Citation2016; Conchillo, Haro, and Benito, Citation2021; Opitz et al., Citation2021). Serendipita colonization of Arabidopsis is increased by plant stress and can provide soil nutrients to the host in return for sugars, much as AMF colonization is increased by nutrient stress (Nouri et al., Citation2014; Hiruma et al., Citation2016; Vahabi et al., Citation2016). In another case, Arabidopsis appears to have a co-opted a pathogenic fungus, Colletotrichum tofieldiae, for phosphate uptake in a nutrient-dependent, AM-like manner, but with apparent species-level dependence on glucosinolate metabolism of the plant alongside cutin perception by the fungus (Bonfante and Genre, Citation2015; Wang and Wang, Citation2016; Delaux, Citation2017). Also like AM, Serendipita and Trichoderma can provide growth benefits under stress, which include pathogen resistance through an apparent ability to activate systemic resistance while still successfully colonizing the root (Contreras-Cornejo et al., Citation2009; Salas-Marina et al., Citation2011; Mathys et al., Citation2012; Gill et al., Citation2016; Tseng et al., Citation2020). These effects involve alterations to salicylic and jasmonic acid signaling, as is observed both in AM and in the Arabidopsis response to AMF (Salas-Marina et al., Citation2011; Mathys et al., Citation2012; Morán-Diez et al., Citation2012; Vahabi et al., Citation2016; Hornstein et al., Citation2023).
These fungi are not specific to non-AM host plants, rather, they are extremely nonselective in hosts (to the extent that no nonhost of Serendipita has ever been found) and apparently possess a colonization mechanism so robust it extends equally to AM and non-AM plants (Jacobs et al., Citation2011). Though many details of this mechanism have not been characterized, it is of high interest for the potential to inform engineering of routes to restore AMF access (Xu et al., Citation2018). Colonization of Arabidopsis by both Serendipita and Trichoderma relies on suppression of salicylic acid host immunity, which occurs via alterations to host plant gibberellin, DELLA, and jasmonic acid signaling (Camehl et al., Citation2011; Jacobs et al., Citation2011; Tseng et al., Citation2020; Opitz et al., Citation2021). The immunity-suppressing effects might be narrowly targeted to colonization sites, as SA can be found to be upregulated by Trichoderma colonization in other tissues of the plant in at least some cases (Salas-Marina et al., Citation2011). Calcium signaling is also required for colonization and defense reduction in Serendipita and Trichoderma colonization (Navazio et al., Citation2007; Nizam et al., Citation2019; Jogawat et al., Citation2020). Importantly, while the signal molecules and full mechanism of calcium signaling in these endophytic interactions have not been characterized, they are known to be selective against at least some pathogens, for example, being induced in Arabidopsis by Trichoderma exudates but not mixed exudates of Trichoderma and the necrotrophic pathogen Botrytis cinerea (Navazio et al., Citation2007). The involvement of induced calcium signaling and DELLAs is of course reminiscent of the CCaMK-CYCLOPS-RAM1 module in the CSP, and an analogous mechanism might be reconstructed in Arabidopsis if, for example, a kinase acting in Serendipita or Trichoderma signal perception can be used as a component of an AMF-sensitive chimeric protein in the same way as described above for PtLECRLK1.
Arabidopsis also possesses one of the strictly required signal receptors for AM, CERK1, though it lacks many others. CERK1 acts in both symbiosis and immunity signaling in both AM and non-AM plants, and presumably has not been lost from Arabidopsis due to its role in defense (Miyata et al., Citation2014; Zhang et al., Citation2015; Carotenuto et al., Citation2017; Huang et al., Citation2020). CERK1 suppression in rice nearly eliminates AM colonization, and at the molecular level is required to activate the AMF-responsive calcium signaling that acts in the CAP (Zhang et al., Citation2015; Carotenuto and Genre, Citation2017). It is therefore possible that CERK1’s ability to activate AM-responsive signaling in Arabidopsis might be restored by expression of some of the lost receptors which work in concert with CERK1 in AM host plants; this would be analogous to the approach in engineering of rhizobia in which addition of NFR receptors enables mycorrhizal (but not rhizobial) species to perceive rhizobial chitin-based Nod factors in addition to mycorrhizal Myc factors. Selecting candidates for this approach is challenging due to the combinatorial and multifunctional roles of defense/symbiosis receptors in AM plants, and would rely on significant basic research to further understand symbiotic signal perception. An immediate candidate, however, is SymRK, which relative to other receptors acting in AM is distinct in structure, has not undergone extensive duplication, and is evolutionarily perfectly correlated with AM symbiosis (Demchenko et al., Citation2004; Gherbi et al., Citation2008; Li et al., Citation2018; Radkahrishan et al., Citation2020). Constitutive expression of SymRK’s truncated kinase domain leads to autoactivation of CSP signaling and formation of nodules in Medicago even in the absence of symbiotic bacteria (Saha et al., Citation2014; Ried, Antolín-Llovera, and Parniske, Citation2014). Although some of the activity of SymRK relates to induction of the CSP and subsequent transcriptional response, it also directly reduces the immune response by physically interacting with defensive receptors such as BAK1 (Feng et al., Citation2021). The kinase domain of SymRK, like PtLecRLK and unknown receptors in the Trichoderma/Serendipita symbioses, therefore becomes another excellent candidate for defense suppression to enable AMF. However, inducibility remains the key issue, to avoid universally lowering the engineered plants’ defenses against pathogens. In this regard SymRK, despite its distinctive nature among receptors, would also require significant advances in basic work (or trial and error) to use in an engineering application, as its ligand and mechanism of action in symbiotic signaling remain enigmatic (Holsters, Citation2008; Miyata et al., Citation2023).
B. Engineering biosynthesis and export of MAGs to feed AMF
Discoveries in recent years make clear that the strict host dependency of the fungal partner in AM is due to its genetic inability to synthesize its own fatty acids, which must be supplied to it by the plant (Trépanier et al., Citation2005; Wewer et al., Citation2014; Keymer et al., Citation2017; Luginbuehl et al., Citation2017; Rich et al., Citation2017b). The lipid species exported by the plant for this purpose are distinctive medium-chain 2-monoacylglycerols (2-MAGs) which are also precursors of cutin (Schreiber, Citation2010; Yeats et al., Citation2014; Keymer et al., Citation2017). Cutin is an extracellular wax found in the casparian strip and above-ground epidermis. Production, synthesis and transport of cutin is conserved in all land plants, and most genes involved in AM-specific lipid synthesis have confirmed functional orthologs involved in cutin metabolism of the nonhost plant Arabidopsis, but are not expressed in the root cortex where AM symbiosis occurs in host plants (Todd et al., Citation1999; Wang et al., Citation2012; He et al., Citation2020; Rich et al., Citation2021). Specialization of lipid synthesis genes for AM (or not) is therefore primarily in transcription pattern, not coding sequence.
1. Lipid synthesis
The chain length of the lipid used for AM symbiotic exchange is highly specific, a 16:0 2-MAG (palmitate), exported by the plant in arbusculated cells and taken up by the fungus (Keymer et al., Citation2017; Rich et al., Citation2021). Asymbiotic growth of AM fungi is possible on yeast medium supplemented only with 14:0 fatty acid, but not 16:0, as a sole carbon source (Kameoka et al., Citation2019; Sugiura et al., Citation2020; Tanaka et al., Citation2022). The 2-carbon difference in chain length identified by these two lines of evidence could indicate different requirements for the fungus in and outside of arbuscules. Synthesis of 2-MAGs in arbuscocytes of model plants is accomplished by specialized copies of lipid metabolism genes whose nonsymbiotic orthologs are functionally conserved in Arabidopsis. In Lotus, these AM-specific lipid genes include orthologs of Ketoacyl Synthase (LjKASI; LjDIS) and Fatty Acid Thioesterase B (FatB; LjFATM), located in the plastid, and an sn-2 Glycerol-3-Phosphate Acyl Transferase Required for Arbuscular Mycorrhizae 2 (GPAT; LjRAM2), located in the endoplasmic reticulum (Yang et al., Citation2010; Bravo et al., Citation2017; Brands et al., Citation2018).
KASI in general lipid synthesis extends acyl-ACP carbon chains from 4:0 to 16:0 using malonyl-ACP (He et al., Citation2020). FatBs terminate fatty acid elongation by cleaving the acyl chain of ACP to produce a free fatty acid for export from the plastid. The FatBs show high substrate specificity for fatty acid chain length (He et al., Citation2020). Complementation analysis shows that when placed under appropriate promoters, generic KASI and FatB genes, including those from Arabidopsis, are interchangeable for the AM-active orthologs DIS and FatM and able to support fully effective symbiosis (Dörmann et al., Citation2000; Bravo et al., Citation2017; Brands et al., Citation2018).
RAM2, like other sn-2 GPATs, preferentially transfers a fatty acid from Acyl-CoA to the middle carbon atom of glycerol-3-phosphate; RAM2 also cleaves phosphate from the third carbon of glycerol, to produce a 2-MAG in a single step (Bravo et al., Citation2017; Keymer et al., Citation2017). Arabidopsis GPATs 4, 6, and 8 are active in cutin synthesis and possess the same 2-acylating, 3-dephosphorylating activity as RAM2 (Philippe et al., Citation2020). AtGPAT6, normally expressed in reproductive organs and essential for seed and pollen development, functionally complements ram2 knockouts of Medicago truncatula when driven by the MtRAM2 promoter: again, use of a native Arabidopsis coding sequence is possible for the final step of symbiotic 2-MAG synthesis (Li et al., Citation2012; Wang et al., Citation2012; Petit et al., Citation2016).
Total fatty acid biosynthesis in the Arabidopsis root will need to be increased to provide sufficient precursor metabolites and transit proteins for synthesis of the AM-specialized lipids. In model host plants this is driven by AM-induced GRAS and WRINKLED (WRI) family transcription factors (TFs) (Zhang et al., Citation2020). The GRAS TFs RAM1 and RAD1 provide points of intersection for complex regulation by the Common Symbiosis Pathway and plant hormones, and their effect on lipid synthesis is not clearly conserved in non-AM species. In contrast, the activity of WRI proteins as master regulators of lipid synthetic genes is highly conserved across all plant families, including those that have lost mycorrhizae (Radhakrishnan et al., Citation2020; Rich et al., Citation2021). Transgenic expression of AtWRI1 robustly drives lipid accumulation in new tissues and species, for example in tobacco leaf, potato root, and seeds of multiple Brassicaceae, and directly regulates KASI and FatB (Reynolds et al., Citation2015). Native Arabidopsis WRI1 is likely to be similarly able to drive FA synthesis in the root cortex if expressed there, providing a means of broadly upregulating fatty acid metabolism genes for engineered AM without the need to transgenically express multiple biosynthetic enzymes. For nonhost species with multiple cortical cell layers like radish (Raphanus sativus L.), it will be important to confirm the expression of WRI1 in the correct outer cortical cell layer (Mitsui et al. Citation2015).
For 16:0 FA synthesis in the root driven by expression of AtWRI1, a root-specific promoter which provides cortex expression is needed, and ideally will also avoid expression in above-ground somatic tissues that could unnecessarily divert carbon used for lipid synthesis. The promoters of PIN2 (pPIN2) or certain root pectate lyase genes (pPLy), which show widespread expression in root tissues but only limited above-ground expression, are good candidates (Abas et al., Citation2006; Sun and van Nocker, Citation2010). Should 14:0 2-MAG be required to support AMF, especially in the context of an intermediate engineering stage proposed here that involves uptake by extracellular hyphae, chain length can be altered by expression of a FatB from a species with an appropriate FA profile, an extension of lipid quality engineering that is already a common target of plant biotechnology (Haslam et al., Citation2016). An appropriate candidate in this case could be FatB3 of Cocos nucifera (coconut), which is already documented to mediate 14-carbon FA synthesis both in situ and a transgene (Yuan et al., Citation2017b). Such a transgene would simply be placed under a promoter regulated by WRI1, enabling its expression in concert with native lipid machinery.
GPAT6 is not controlled by WRI1 and must be separately reassigned to root expression. This could be accomplished via a whole-plant constitutive promoter, resulting in synthesis of 2-MAGs in superfluous above-ground tissues as well as the root, which may be an advantageous trait. Whole-plant overexpression of foreign GPATs in Arabidopsis has shown beneficial effects, such as increased salt and drought tolerance (Chen et al., Citation2011; Sui et al., Citation2017). In a detailed study, stable overexpression of NbGPAT6a under the 35S promoter in tobacco led to a large accumulation in leaves of unpolymerized cutin monomers, as well as changes to cell wall and cuticle morphology. Notably, these changes had no obvious negative effects to the plant and led to increased pathogen resistance (Fawke et al., Citation2019). In Arabidopsis, 35S:RAM2 and 35S:GPAT6 overexpression had the same results as in tobacco, of increased leaf accumulation of cutin precursors (Wang et al., Citation2012). However, there is at least once report of 2-MAG synthesis increasing susceptibility to a pathogen. The ram2 mutant of Medicago shows improved resistance to the oomycete pathogen Phytophthora palmivora, which was reversed by addition of exogenous 16:0, indicating a need to carefully evaluate unintended consequences of GPAT6 expression under whatever promoter is initially chosen (Wang et al., Citation2012).
2. Transport of 2-MAGs for symbiosis
Lipid transfer in an arbusculated cell involves export from the plant cell membrane and uptake by the fungal hyphae that make up the arbuscule. Transit of metabolites in arbusculated cells does not involve the plant cell wall, as the fungus is already on its inner side. However, arbuscule-independent nourishment of the fungus as a self-contained engineering module () requires that 2-MAGs pass through the cell wall; this requires an extra transport step (Pollard et al., Citation2008).
In natural AM plants, export of 16:0 2-MAG through the plasma membrane is accomplished by ABCG proteins that preferentially transport medium-chain MAGs and are localized specifically to the arbuscular interface on the cell membrane. These AM-specific ABC transporters belong to the STR subfamily, which is distinctive in its high conservation and lack of duplication among AM plants, and is not present in Arabidopsis (Zhang et al., Citation2010). As Arabidopsis cutin synthesis in most parts of the plant is dominated by 18-carbon precursors, it is possible that native Arabidopsis transporters are not suitable for export of 16:0 species (Beisson et al., Citation2012; Philippe et al., Citation2020). Expression of Medicago orthologs MtSTR and MtSTR2, which act as a heterodimer, in Arabidopsis leaves led to extracellular accumulation of cutin monomers including 16:0-α,ω-dicarboxylic acid (DCA), the same effect as in Medicago itself (Jiang et al., Citation2017). The Medicago STR transporters could be used as transgenes for 2-MAG transport in Arabidopsis roots given the evidence of their function in Arabidopsis. Another option is to use native Arabidopsis STR transporters associated with flowers, where 16:0 MAG production by GPAT6 corresponds locally to a cuticle composed primarily of 16-carbon monomers. ABCG13 is well documented to mediate cutin transport for Arabidopsis floral cuticles and is presumably competent for transport of 16:0 given the local prevalence of this chain length (Panikashvili et al., Citation2011; Takeda et al., Citation2015; Do et al., Citation2018). We include AtABCG13 expression under a root-specific promoter as the first gene on the transport sub-cassette (). AtABCG13 has also been documented in other tissues including roots, supporting the prospects for its ability to function in root cells, although its substrate outside the flower has not been confirmed (Bird et al., Citation2007; Panikashvili et al., Citation2007). Another benefit of using this Arabidopsis gene as opposed to MtSTR1/2 is that ABCG13 is known to function as a homodimer, removing the need to express both halves of a heterodimer complex (Do et al., Citation2018).
We must also provide a mechanism for lipids to transit the cell wall, as they do not readily diffuse into the intercellular apoplast (Shepherd and Wynne Griffiths, Citation2006). This mechanism will be rendered unnecessary in the future if lipid synthesis is coupled with a means of producing arbuscules. Cross-wall transport of 2-MAG is accomplished in the process of cutin synthesis when monomers are transported to the outermost plant surface prior to polymerization; this is believed to be mediated by nonspecific lipid transfer proteins (nsLTPs) which we propose to use for the same purpose within the cortex (Liu et al., Citation2015).
NsLTPs bind to wax monomers in vitro, are closely associated with sites of cutin synthesis, and are known to be secreted into the intercellular matrix; nsLTP knock-out mutants show decreased cuticular wax (DeBono et al., Citation2009; Liu et al., Citation2015). However, the exact mechanism of nsLTPs in mediating transit and subsequent release of cutin monomers has not been clarified, and multiple nsLTPs likely work together to bring lipids fully across the cell wall. LTPs also work in signaling, pathogen resistance, and cell structure (Maldonado et al., Citation2002; Jacq et al., Citation2017; Edqvist et al., Citation2018). Further investigation of LTP transport mechanisms will likely be required for an optimal engineering strategy, but current information provides key early guidance. Consistent with the distribution of their substrates in wax synthesis, LTPs have specific binding activity with cutin-monomer-transporting LTPs found in the shoot, and suberin-transporting LTPs in the root (Edqvist et al., Citation2018). Reassigning a cutin-transporting LTP from root to shoot expression is the most essential element due to the substrate specificity involved. AtLTPG1 and AtLTPG2 are both shown experimentally to mediate cutin deposition in Arabidopsis leaves and stems, as have their homologs in Brassica napus and B. rapa (Maldonado et al., Citation2002; DeBono et al., Citation2009; Li-Beisson et al., Citation2009; Kim et al., Citation2012; Liu et al., Citation2014; Jacq et al., Citation2017; Edqvist et al., Citation2018). We therefore suggest AtLTPG1 as the first candidate LTP for export of cutin into the extracellular apoplast, acting synergistically with membrane export by ABCG13.
C. Driving macronutrient uptake with proton export
Transgenic expression of AM-specific nutrient transporters is not appealing because they interact with structural components of the arbuscule and fail to function independently from other aspects of symbiosis (Parniske, Citation2008; Kobae and Hata, Citation2010). Cortical expression of nonsymbiotic transporters acting in general root nutrient uptake is possible, however, generic nutrient transporter overexpression is a common engineering target with a history of mixed results. Targeting transport proteins directly also guarantees the need to include a large number of transgenes to mediate uptake of different nutrient molecules under different conditions. Phosphate, nitrate, and ammonium each require dedicated transporters, and these are further divided into high and low affinity families containing many members, multiple of which might be required to coordinate full assimilation (Bustos et al., Citation2010; Bao et al., Citation2011; Krapp et al., Citation2014; Hao et al., Citation2020). High-affinity phosphate and low-affinity ammonium transporters, prominent mediators of symbiotic update, are highly sensitive to pH, requiring further coexpression of an H + ATPase to function (Krajinski et al., Citation2014; Wang et al., Citation2014a; Liu et al., Citation2020b). Rather than engineering specific transporters with an elaborate multi-gene system, targeted expression of this proton pump alone could broadly enhance nutrient uptake in the tissues subject to synthetic AM colonization.
Active maintenance of the proton gradient across the plasmamembrane is essential and potentially limiting for uptake from fungi in natural AM. Rice, Medicago, Lotus, and tomato all have H + ATPase genes with a dedicated function in AM and which, like AM-specific nutrient transporters, locate specifically on the peri-arbuscular membrane (Wang et al., Citation2014a; Liu et al., Citation2016; Citation2020b). Silencing or knock-out of these genes results in altered arbuscule morphology, decreased nutrient uptake, and essentially abolishes the benefits of mycorrhization, while overexpression of the AM-specific H + ATPase in tomato led to increased N and P uptake and increased colonization (ibid).
Protons in the apoplast enable multiple mechanisms for uptake of N, P, and K that are relatively well known, and conserved between AM-specialized and other homologs of these genes. Phosphate and nitrate are taken up via H + symporters, requiring one proton per phosphate, and two per nitrate (Nussaume, Citation2011; Wang et al., Citation2018). Potassium is also transported by HAK proteins that are driven by membrane potential from extracellular H+ (Grabov, Citation2007). Hyperpolarized membrane potential also opens the AKT1 channel complex, a separate route of K uptake (Wang and Wu, Citation2013). Nitrogen in the form of ammonium is transported by AMT proteins that do not cotransport protons but require low extracellular pH (Koegel et al., Citation2013; Hao et al., Citation2020).
Increasing the concentration of protons in the general root apoplast can also drive signaling and expression of genes for uptake and assimilation (Palmgren, Citation2001; Ding et al., Citation2021). In rice, overexpressing a single gene for plasma membrane H + ATPase led to upregulation of multiple classes of nitrate, ammonium, and phosphate transporters, as well as transporters for potassium and amino acids (Zhang, Wang, et al., Citation2021). Importantly and in contrast to individual nutrient transporters, this transgenic H + ATPase expression also led to upregulation of macronutrient assimilation pathways. Treatments with chemicals and nanomaterials that result in increased H + ATPase expression have shown similar effects (Gévaudant et al., Citation2007; Kim et al., Citation2015; Zhang, Wang, et al., Citation2021). The coordinated multi-nutrient uptake-assimilation response likely involves interaction of extracellular H + with auxin signaling, which in turn implicates transcription factors such as GRF4 that are known to be involved in high-level regulation of nutrient uptake (Siao et al., Citation2020; Ren et al., Citation2021). Taken together, these facts suggest that not only is expression of a proton pump necessary to enable symbiotic nutrient uptake, it may also be sufficient. We therefore identify cortical expression of a native H + ATPase as a single genetic modification that can enable a meaningful level of uptake of multiple nutrients secreted into the apoplast by mycorrhizal fungi ().
Vacuolar and plasma membrane H + ATPases have been investigated as transgenes for other purposes. Constitutive plasma membrane H + ATPase expression is associated with increased salt, acid, and heavy metal resistance, and increased stomatal opening leading to increased growth (Young et al., Citation1998; Wang et al., Citation2014a; Janicka-Russak and Kabała, Citation2015; Zhang et al., Citation2017; Fan et al., Citation2018; Zhang, Wang, et al., Citation2021). However, overexpression in the shoot has also resulted in deformities of leaf architecture, impaired pollen production, and undesirable water loss from stomata. These deleterious effects seem to be avoided in above-ground applications that use cell-specific promoters, and so it is preferable to similarly limit expression of a transgenic H + ATPase to desired tissues of the root (Gévaudant et al., Citation2007; Wang et al., Citation2014a; Zhang et al., Citation2017; Ren et al., Citation2021; Toh et al., Citation2021; Zhang, Wang, et al., Citation2021). This can be achieved using any of the previously described root-specific promoters, or a combination of promoters to adjust levels in individual cell layers.
Plasma membrane H + ATPases are subject to extensive regulation by protein-protein interactions. The N and C termini both extend into the cytosol and contain autoinhibitory domains that can be enhanced or inhibited by numerous factors depending on subspecialization in these domains (Young et al., Citation1998; Baekgaard et al., Citation2005; Ekberg et al., Citation2010; Wang, Noguchi, et al., Citation2014; Wielandt et al., Citation2015; Falhof et al., Citation2016). Symbiosis-specialized H + ATPases, for example, are phosphorylated at specific residues in the C-terminus following symbiotic signal perception (Nguyen et al., Citation2015). In the initial engineering approach, consistent activity of a transgenic H + ATPase can be provided by simply truncating the inhibitory domain of a well-known Arabidopsis homolog such as AHA2, which acts in root growth and guard cells (Haruta et al., Citation2010; Yuan et al., Citation2017a; Hoffmann et al., Citation2019; Ding et al., Citation2021; Ren et al., Citation2021). In that case, we would rely solely on transcriptional control to adjust the level of proton exudation into the apoplast. Post-translational modification could itself provide a way to fine-tune activity or control pleiotropic effects. For example, selecting particular H + ATPases or applying rational modification could tie H + export to specific stimuli via interaction with known receptor kinases or signaling proteins. H + ATPases with differences in the transmembrane domain may also be of interest, for example to regulate fine localization to regions of the cell membrane or make proton export self-limiting to avoid over-acidification (Haruta et al., Citation2010; Nguyen et al., Citation2015).
D. Building the arbuscules
Despite the structural complexity of the arbuscule and loss of associated genes in non-AM plants, arbuscule-like intracellular structures form in the roots of nonhost plants upon nursed colonization (Veiga et al., Citation2013; Cosme et al., Citation2018; Fernández et al., Citation2019; Wang et al., Citation2023) (). This is consistent with the fact that functional AM symbiosis also occurs through a wide diversity of intracellular structures in host plants (Dickson, Citation2004). We therefore focus on improving access of AMF through the cell wall to increase the ease and frequency with which these structures can form; even if not fully sufficient for functioning arbuscules, cell wall passage is a necessary first step that will enable further understanding of the intracellular structure.
Figure 5. Multiple reports of intraradical and intracellular AMF colonization structures formed in non-AM plants. (A) confocal fluorescent images of canonical arbuscules (A, white arrowheads) formed by Funneliformis mosseae in roots of AM model plant Lotus japonicus (Carotenuto and Genre, 2020); (B) confocal fluorescent images of stunted arbuscule-like structures (SAC) formed inside root cells of Arabidopsis by AM fungus Rhizophagus irregularis (Wang et al., Citation2023). (C) light micrograph of canonical arbuscules (A) with subtending hyphae (H) and vesicle (V) formed by R. irregularis in cortical cells of AM model plant Medicago truncatula (Cosme et al., Citation2021); (D) light micrograph of arbuscule-like structures (A) with subtending hypha (H) formed by R. irregularis in cortical cells (CC) of Arabidopsis (Cosme et al., Citation2018). (E, F) light micrographs of intraradical hyphae (H) and vesicles (V) formed by R. irregularis in Brassica napus and B. campestris, respectively (Wang et al., Citation2023). (G) Hyphophodia (Hp) formed on cells of Arabidopsis roots by R. irregularis (Fernández et al., Citation2019). (H) Entry point of fungal hypha (F) via a root hair (arrow) and formation of intraradical vesicles by R. irregularis in Arabidopsis roots (Veiga et al., Citation2013). (I) Cross section reconstructed from confocal imaging and corresponding diagram showing hyphae (H) of R. irregularis inside and outside of an Arabidopsis epidermal cell (Veiga et al., Citation2013). Anatomical position and naming of intraradical structures is reported as determined by original authors; image annotations in B and D-H follow original publications, annotations in A and I have been modified.
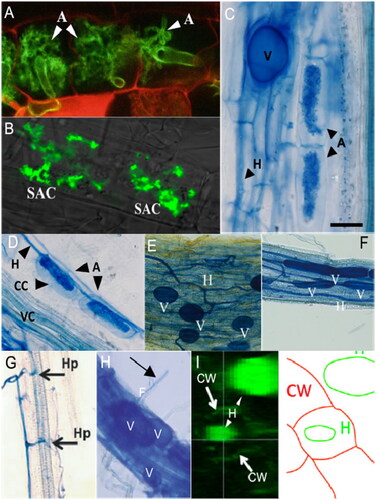
Cell entry in AM hosts is mediated by signaling and subsequent cellular restructuring by the host plant to form a prepenetration apparatus (PPA) which contributes to initial access through epidermal cells to reach layers below (Genre and Bonfante, Citation2005; Genre et al., Citation2008; Luginbuehl and Oldroyd, Citation2017). This process initially involves expanding and increasing plasticity of the cell wall by CSP-mediated expression of expansins, cellulose synthase, and endotransgycosylases, which are expressed tissue specifically or in individual cells upon contact with the fungus (Liu et al., Citation2003; Küster et al., Citation2007; Siciliano et al., Citation2007; Dermatsev et al., Citation2010; Bapaume and Reinhardt, Citation2012; Balestrini and Bonfante, Citation2014). These cell wall-related genes are universally present in all plants, though as in other cases, mycorrhizal species may have copies that are dedicated solely to symbiosis. Among the various cell wall remodeling genes implicated in arbuscule formation, xyloglucan endotransglucosylase/hydrolases (XTHs) and expansins show promise for engineering because they seem to work in conjunction with the localized presence of adhering fungal hyphae, which can transmit physical pressure agnostic of metabolic and genetic functions (Balestrini et al., Citation2005; Siciliano et al., Citation2007; Van Sandt et al., Citation2007; Dermatsev et al., Citation2010; Wiśniewska and Golinowski, Citation2011).
XTH expression and enzymatic activity is observed to increase sharply in roots upon mycorrhization (Maldonado-Mendoza et al., Citation2005; Sampedro et al., Citation2007; Nanjareddy et al., Citation2017; Zhang, Gao, et al., 2021). Although the increase in XTH expression of mycorrhizal roots can be attributed to gene copies with AM-specific transcription, their expression is induced across the entire root system and not tightly confined to arbuscocytes as with many other AM-specific genes (Maldonado-Mendoza et al., Citation2005). It has been proposed that the AM-specific but whole-root expression pattern of MtXTH1 in symbiosis means it does not explicitly loosen the cell wall to enable penetration by AM fungi, but may more subtly alter wall chemistry to allow general proliferation of the fungus and passage through the wall without compromising cellular integrity (Maldonado-Mendoza et al., Citation2005). This idea is consistent with the evolution in understanding XTHs that these proteins are secondary actors in cell wall structure whose exact effect is determined by their role as enablers or modifiers of other proteins’ activity (Kaewthai et al., Citation2013; Cosgrove, Citation2016; Ishida and Yokoyama, Citation2022).
The XTH family has at least 33 members in Arabidopsis that are subspecialized in expression and biochemical function (Vissenberg et al., Citation2005; Ishida and Yokoyama, Citation2022). XTHs are ubiquitously expressed in primary growth, where they have been implicated in relaxation and loosening, as well as thinning and thickening of cell walls (Van Sandt et al., Citation2007; Maris et al., Citation2009; Miedes et al., Citation2013; Cosgrove, Citation2016; Niraula et al., Citation2021). The variable contributions of this gene family may relate to individual members’ balance of endohydrolase activity, which terminates a xyloglucan chain after cleavage, and endotransglycosylase activity, which ligates the cleaved molecule to another xyloglucan mid-chain, as well as what other cell wall modifying enzymes XTHs are coexpressed with (Eklöf and Brumer, Citation2010; Cosgrove, Citation2016). MtXTH1 has greatest sequence similarity to the relatively poorly studied XTH6 of Arabidopsis, which is associated with cell wall loosening in abscission zones during flower development (Rose et al., Citation2002; Becnel et al., Citation2006; Yang et al., Citation2007; Lashbrook and Cai, Citation2008; Ishida and Yokoyama, Citation2022). AtXTH6 is also upregulated as part of an increased growth phenotype induced by transgenic expansin expression (Ilias et al., Citation2019).
If XTHs function as auxiliary proteins that are necessary but not sufficient for cell wall passage, their enablement of AM formation should intersect with expression of another cell wall modifying enzyme(s). These include expansins, particularly ɑ-expansins, which are consistently found to be upregulated in transcriptomes of mycorrhizal roots and arbusculated cells, while silencing of these ɑ-expansins leads to stunting of arbuscules (Weidmann et al., Citation2004; Siciliano et al., Citation2007; Dermatsev et al., Citation2010; Vangelisti et al., Citation2018; Liu et al., Citation2020a). ɑ-expansins are a large subgroup of the even larger expansin gene family (Choi et al., Citation2006). In general, they are structural proteins that interact with cell wall components to relax the wall in a pH-dependent manner, with the effect becoming stronger at lower pH (Cosgrove, Citation2015; Marowa et al., Citation2016). Unlike XTHs, the cell wall loosening effect of expansins is direct, and they have been shown to alter growth via cell elongation in many transgenic studies (Boron et al., Citation2015; Marowa et al., Citation2016; Ilias et al., Citation2019; Muthusamy et al., Citation2020). It is likely that the expression of XTH relates to enabling expansin activity by increasing accessibility of microfibril contact points, leading to increased cell wall flexibility to enable penetration (Park and Cosgrove, Citation2012; Citation2015; Cosgrove, Citation2014; Wang et al., Citation2016).
In the absence of more specific information about the biochemical properties of AM-specific XTH proteins, the most reasonable option for engineering is use of a known homolog from a symbiosis model, such as Medicago truncatula MtXTH1 under a promoter conferring expression in all root tissues (Maldonado-Mendoza et al., Citation2005). The choice of expansin is similar, with small differences between localization of multiple expansins being shown in some cases of AM colonization but difference in functions not biochemically described (Balestrini et al., Citation2005; Wiśniewska and Golinowski, Citation2011). A dependency on the intersection of multiple expansins with biomechanical force is also consistent with results for expansins’ effect on general growth (Caderas et al., Citation2000; Vogler et al., Citation2003; Rebocho et al., Citation2017; Samalova et al., Citation2020). The choice of gene and promoter is clearly essential to producing the desired cell wall softening effect, but as with XTH, we note that in the absence of more information testing of different expansins for this engineering step will be necessary.
E. Control and cross-talk
In the simplest manifestation of synthetic AM, mycorrhization may be set to a static, constitutively high level conferred by the promoters of the various genes used. Even in the course of producing a static overall phenotype, however, it will be necessary to balance the expression of the component genes against each other and any deleterious effects on plant metabolism. AM engineering must also be balanced against any potentially deleterious effects from manipulations of host plant immunity used to enable AM fungus colonization. Where possible in the above sections we proposed specific promoters that are highly tissue- and cell-type specific and/or subject to dynamic regulation from underlying plant metabolism to provide this balance. In this section, we discuss two additional aspects of control and regulation: areas of potential cross-talk between modules when they are coexpressed, and more sophisticated options for control of engineered AM than constitutive expression.
1. Cross-talk
The most significant point of intersection between all four engineering modules is pH. We propose driving nutrient exchange by expressing a proton exporter. This will have a direct effect of enhancing cell wall softening for arbuscule formation because the activity of expansins is pH dependent (Cosgrove, Citation2015; Marowa et al., Citation2016). It is conceivable that reduced pH alone will enable fungi to cross the cell wall, or, on the other hand, that expression of expansion and XTH will be effective only when combined with reduced pH. pH-induced cell wall alterations that change porosity will also affect the diffusion of apoplastic nutrients and signaling metabolites. While the H + ions are not intended for secretion into the soil, it is also likely that the root exudate will be acidified, which may in turn affect the diffusion of strigolactones and scopoletin into the soil that are enabled by the metabolite engineering module (Clemens and Weber, Citation2016; Stringlis et al., Citation2018; Abedini et al., Citation2021). General upregulation of oil synthesis in the root and its export to fungus will also increase its strength as a sink tissue (Durand et al., Citation2018). This is expected to lead to increased sucrose flux from the shoot to the root (Schulz et al., Citation2011). Some but not all mechanisms of phloem loading and unloading are mediated by pH-sensitive sugar transporters and pH-regulated activity of cell wall invertase (Krausgrill et al., Citation1996; Hothorn et al., Citation2010). This intersection of lipid and pH manipulation, affecting the fundamental issue of source-sink flow of nutrients and photosynthate, may require direct manipulation of sugar transporters or other mediators of carbon flux to bring into balance.
The intersection of all of these traits with pH is also of concern for potentially increased pathogen susceptibility, given that similar patterns of cell wall flexibility, and increased sink strength accompany successful infections (Malinowski et al., Citation2019; Ried et al., Citation2019). However, the extensive opportunities described under the nutrient uptake section for selecting a transgene or posttranscriptionally regulating H + ATPase activity to stay within a particular pH range offer at least one direct means of controlling these effects.
2. Inducible control
Recent detailed work has revealed a previously uncharacterized degree of control of AM establishment by the deeply conserved SPX/PHR phosphate sensing system whose activation is determined by phosphate starvation (Shi et al., Citation2021; Das and Gutjahr, Citation2022). This SPX/PHR phosphate sensing system includes regulation of CSP-specific transcription factors such as RAM1, RAD1, and CYCLOPS; WRINKLED and RAM2 controlling lipid synthesis; sugar transporters; and nutrient transporters (Shi et al., Citation2021) (). The same study identified the P1BS promoter element as the binding site for these PHR-regulated AM genes, and showed that knock-out of SPX or overexpression of PHR could greatly increase mycorrhizae under high phosphate fertilization that otherwise lead to strong autoinhibition of AM. Considering that PHR regulation of the P1BS element is conserved and well known in Arabidopsis, this finding is quite significant in showing that one of the main control mechanisms of AM is intact and available in Arabidopsis (Bustos et al., Citation2010; Oropeza-Aburto et al., Citation2012; Sobkowiak et al., Citation2012; Carbonnel and Gutjahr, Citation2014). In engineered synthetic AM that are already outside of CSP control, the addition of P1BS elements to some or all of the constitutive promoters used would have the effect of enhancing the baseline response when the plants encounter unexpectedly low phosphate.
The DNA-binding domain of CYCLOPS constitutively expressed in Arabidopsis can induce extensive gene regulation despite the absence of its canonical targets (Hornstein et al., Citation2023). If this truncated CYCLOPS gene were controlled by a PHR-responsive promoter, it could serve as a master regulator for downstream regulation of genes engineered for synthetic AM. The placement of other transgenes under CYCLOPS-regulated promoters providing a simplified facsimile of the phosphate-responsive regulation underlying natural AM (Singh et al., Citation2014; Pimprikar et al., Citation2016; Rudaya et al., Citation2021).
A conserved promoter element could also be used to connect AM regulation directly to the availability of photosynthate. The calcium-sensing kinase DMI3 has recently been found to interact with an ortholog of the mobile transcription factor HY5 that is transported from the shoot in response to a wide array of stimuli (Wang et al., Citation2021). HY5 is responsible for coordinating root traits with photosynthesis during general growth, when HY5 moves from the shoot to the root in response to light, where it regulates hormones, nutrient, and sucrose transport including control of phloem loading to provide photosynthate to the root (Chen et al., Citation2016; Gangappa and Botto, Citation2016). In synthetic AM, the latter function of HY5 could be used to tie mycorrhiza formation to the availability of carbon in order to avoid the high cost of constitutively supporting AM. This is appealing as a means of dealing with circumstances arising later in the season where mycorrhizae might compete for photosynthate with seeds or other harvested products.
Balance of engineered AM and pathogen susceptibility will never be perfect across the range of plants and pathogens found in the field. To address this, engineering should include a 'fallback’ that ties a shutdown of symbiotic machinery into the response to local pathogen infection. This could be achieves for a large grouping of pathogens via the Damage Associated Molecular Pattern (DAMP) or hypersensitivity response (HR). Because AM do not breach the cell membrane, and do not naturally result in a hypersensitive response, the distinction between signals involved in DAMP sensing and in AM function is quite large, for example with extracellular ATP, inceptin, and GRI/GRIp protein serving as non-AM-induced markers of infection (Medina-Castellanos et al., Citation2014; Hou et al., Citation2019; Jewell et al., Citation2019). The HR response is mediated by NLR sensor proteins, whose binding to pathogen virulence proteins results in local cell death (Mohr et al., Citation2010; Lai and Eulgem, Citation2018; Balint‐Kurti, Citation2019). In case of either DAMP or HR related signaling, the promoter of key genes in the synthetic AM system could be designed to include a target site for a plant-foreign negative regulator such as TetR, while TetR itself is placed under a promoter induced by one of the existing defense responses (Gossen et al., Citation1995). This would allow for suppression of the AM system and its potentially vulnerability-inducing components in cells near sites where the native defense system identifies a bona fide infection as taking place. This approach would not defend against biotrophic pathogens that neither damage cells nor induce a cell death response, however, if TetR regulation is built into the engineered system from the start, the ‘failsafe’ could be easily tailored by altering the expression conditions of TetR, without needing to rework other gene cassettes.
IV. Conclusions
In devising an engineered system explicitly intended to escape millions of years of evolved adaptation, we should be aware of the possibility of creating a 'Potemkin village’ system that looks like mycorrhizae but lacks their remarkable ability to coordinate responses to multiple stresses. While we have illustrated reasons to swap a conservative regulation system for a constitutive or more liberal induction of synthetic AM, it could be that the seemingly byzantine regulation of natural AM is more beneficial than we think. Aside from recognizing that this is the case if experimental results show it to be, we should consider that real-world applications in crops that already have mycorrhizae likely would not involve replacing the native system with synthetic AM, but deploying synthetic AM alongside the native system. In the simplest conception, the two systems are separated and work in parallel to expand the benefits of AM.
The benefits of engineered AM also go beyond what was discussed here, especially in terms of long-term change to farm soils, the agroecosystem, and even land use. A recent study estimated that over 1.2 billion acres of cropland expansion into natural areas could be avoided through 2050 by a 7% net increase in phosphate fertilizer, primarily in sub-Saharan Africa (Mogollón et al., Citation2021). Achieving some or all of this effect by improved fertilizer use efficiency is even more advantageous, as it avoids a tradeoff with the harms of fertilizer extraction and the projected limits to phosphate production and access (Obersteiner et al., Citation2013; Scholz et al., Citation2013; Herrera-Estrella and López-Arredondo, Citation2016; Folberth et al., Citation2020). Over many years, mycorrhizae also improve soil structure, water retention, and organic nitrogen availability (Gosling et al., Citation2006; Berruti et al., Citation2016). Rotation of nonmycorrhizal crops into fields reduces the population of AM fungi, limiting their benefits to the soil as well as the future speed of colonization and benefit provided to host crops planted on the same soil (Karasawa and Takebe, Citation2012; Berruti et al., Citation2018). Engineering of mycorrhizae in non-AM crops could therefore encourage crop rotations and directly increase yield of not only the newly-mycorrhizal species, but also the naturally AM plants they rotate with. For similar reasons, even naturally mycorrhizal crops can benefit from the use of an engineered system that lifts the suppression of mycorrhization imposed by modern farming conditions and enables higher colonization and consistent AM benefits. This is especially true in regard to stress resistance aspects of AM, for which the symbiosis must be established well in advance of the stress occurring ().
The effect of mycorrhization on fertilizer use is particularly synergistic in regard to carbon emissions and climate change. The reduction in nutrients relative to yield means less fertilizer must be mined (P), synthesized (N), and transported, all at a significant carbon cost. Mycorrhizal plants also continuously transfer a significant fraction of fixed carbon into AM fungi, where it is sequestered in the living extraradical hyphae as well as the durable glycoprotein substance glomalin which takes up to 40 years to degrade (Wright and Nichols, Citation2002; Verbruggen et al., Citation2013; Holátko et al., Citation2021). Finally, mycorrhizae mediate the transfer of photosynthate to other microbes, and richness of fungi in the soil microbiome is associated with more durable accumulation of biomass (D’Hondt et al., Citation1998; Kaiser et al., Citation2015; Domeignoz-Horta et al., Citation2021).
Acknowledgements
The authors wish to thank F.G., R.S., M.C., R.S., and B.E. for their thoughtful comments on this manuscript.
Declaration of interest
The authors declare no conflict of interest.
Additional information
Funding
References
- Abas, L., Benjamins, R., Malenica, N., Paciorek, T., Wiśniewska, J., Moulinier-Anzola, J. C., Sieberer, T., Friml, J., and Luschnig, C. 2006. Intracellular trafficking and proteolysis of the Arabidopsis auxin-efflux facilitator PIN2 are involved in root gravitropism. Nat. Cell Biol. 8: 249–256. doi:10.1038/ncb1369
- Abdel Latef, A. A. H., and He, C. 2011. Arbuscular mycorrhizal influence on growth, photosynthetic pigments, osmotic adjustment and oxidative stress in tomato plants subjected to low temperature stress. Acta Physiol. Plant. 33: 1217–1225. doi:10.1007/s11738-010-0650-3
- Abedini, D., Jaupitre, S., Bouwmeester, H., and Dong, L. 2021. Metabolic interactions in beneficial microbe recruitment by plants. Curr. Opin. Biotechnol. 70: 241–247. doi:10.1016/j.copbio.2021.06.015
- Akiyama, K., Matsuzaki, K., and Hayashi, H. 2005. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature. 435: 824–827. doi:10.1038/nature03608
- Al-Karaki, G., McMichael, B., and Zak, J. 2004. Field response of wheat to arbuscular mycorrhizal fungi and drought stress. Mycorrhiza. 14: 263–269. doi:10.1007/s00572-003-0265-2
- Baekgaard, L., Fuglsang, A. T., and Palmgren, M. G. 2005. Regulation of plant plasma membrane H+- and Ca2+-ATPases by terminal domains. J. Bioenerg. Biomembr. 37: 369–374. doi:10.1007/s10863-005-9473-0
- Balestrini, R., and Bonfante, P. 2014. Cell wall remodeling in mycorrhizal symbiosis: a way towards biotrophism. Front. Plant Sci. 5: e00237. doi:10.3389/fpls.2014.00237
- Balestrini, R., Cosgrove, D. J., and Bonfante, P. 2005. Differential location of ?-expansin proteins during the accommodation of root cells to an arbuscular mycorrhizal fungus. Planta. 220: 889–899.
- Balint‐Kurti, P. 2019. The plant hypersensitive response: concepts, control and consequences. Mol. Plant Pathol. 20: 1163–1178. doi:10.1111/mpp.12821
- Bao, S., An, L., Su, S., Zhou, Z., and Gan, Y. 2011. Expression patterns of nitrate, phosphate, and sulfate transporters in Arabidopsis roots exposed to different nutritional regimes. Botany. 89: 647–653. doi:10.1139/b11-053
- Bapaume, L., Laukamm, S., Darbon, G., Monney, C., Meyenhofer, F., Feddermann, N., Chen, M., and Reinhardt, D. 2019. VAPYRIN marks an endosomal trafficking compartment involved in arbuscular mycorrhizal symbiosis. Front. Plant Sci. 10: 666. doi:10.3389/fpls.2019.00666
- Bapaume, L., and Reinhardt, D. 2012. How membranes shape plant symbioses: signaling and transport in nodulation and arbuscular mycorrhiza. Front. Plant Sci. 3: e00223. doi:10.3389/fpls.2012.00223
- Becnel, J., Natarajan, M., Kipp, A., and Braam, J. 2006. Developmental expression patterns of arabidopsis XTH genes reported by transgenes and genevestigator. Plant Mol. Biol. 61: 451–467. doi:10.1007/s11103-006-0021-z
- Begum, N., Qin, C., Ahanger, M. A., Raza, S., Khan, M. I., Ashraf, M., Ahmed, N., and Zhang, L. 2019. Role of arbuscular mycorrhizal Fungi in plant growth regulation: implications in abiotic stress tolerance. Front. Plant Sci. 10: 1068. doi:10.3389/fpls.2019.01068
- Beisson, F., Li-Beisson, Y., and Pollard, M. 2012. Solving the puzzles of cutin and suberin polymer biosynthesis. Curr. Opin. Plant Biol. 15: 329–337. doi:10.1016/j.pbi.2012.03.003
- Bellande, K., Bono, J.-J., Savelli, B., Jamet, E., and Canut, H. 2017. Plant lectins and lectin receptor-like kinases: How do they sense the outside? 18: 1164. doi:10.3390/ijms18061164
- Berruti, A., Bianciotto, V., and Lumini, E. 2018. Seasonal variation in winter wheat field soil arbuscular mycorrhizal fungus communities after non-mycorrhizal crop cultivation. Mycorrhiza 28: 535–548. doi:10.1007/s00572-018-0845-9
- Berruti, A., Lumini, E., Balestrini, R., and Bianciotto, V. 2016. Arbuscular mycorrhizal Fungi as natural biofertilizers: let’s benefit from past successes. Front. Microbiol. 1559. 6: e01559. doi:10.3389/fmicb.2015.01559
- Besserer, A., Puech-Pagès, V., Kiefer, P., Gomez-Roldan, V., Jauneau, A., Roy, S., Portais, J.-C., Roux, C., Bécard, G., and Séjalon-Delmas, N. 2006. Strigolactones stimulate arbuscular mycorrhizal fungi by activating mitochondria. PLoS Biol. 4: e226. doi:10.1371/journal.pbio.0040226
- Bird, D., Beisson, F., Brigham, A., Shin, J., Greer, S., Jetter, R., Kunst, L., Wu, X., Yephremov, A., and Samuels, L. 2007. Characterization of arabidopsis ABCG11/WBC11, an ATP binding cassette (ABC) transporter that is required for cuticular lipid secretion†: WBC11 is required for cuticular lipid transport. Plant J. 52: 485–498. doi:10.1111/j.1365-313X.2007.03252.x
- Bona, E., Cantamessa, S., Massa, N., Manassero, P., Marsano, F., Copetta, A., Lingua, G., D'Agostino, G., Gamalero, E., and Berta, G. 2017. Arbuscular mycorrhizal fungi and plant growth-promoting pseudomonads improve yield, quality and nutritional value of tomato: a field study. Mycorrhiza. 27:1–11. doi:10.1007/s00572-016-0727-y
- Bonfante, P., and Genre, A. 2015. Arbuscular mycorrhizal dialogues: Do you speak ‘plantish’ or ‘fungish’? Trends Plant Sci. 20: 150–154. doi:10.1016/j.tplants.2014.12.002
- Borlaug, N. E. 2002. Feeding a world of 10 billion people: the miracle ahead. In Vitro Cell. Dev. Biol. – Plant 38: 221–228. doi:10.1079/IVP2001279
- Boron, A. K., Van Loock, B., Suslov, D., Markakis, M. N., Verbelen, J.-P., and Vissenberg, K. 2015. Over-expression of AtEXLA2 alters etiolated arabidopsis hypocotyl growth. Ann. Bot. 115: 67–80. doi:10.1093/aob/mcu221
- Bozsoki, Z., Cheng, J., Feng, F., Gysel, K., Vinther, M., Andersen, K. R., Oldroyd, G., Blaise, M., Radutoiu, S., and Stougaard, J. 2017. Receptor-mediated chitin perception in legume roots is functionally separable from Nod factor perception. Proc. Natl. Acad. Sci. USA. 114: 1706795. doi:10.1073/pnas.1706795114
- Bozsoki, Z., Gysel, K., Hansen, S. B., Lironi, D., Krönauer, C., Feng, F., de Jong, N., Vinther, M., Kamble, M., Thygesen, M. B., Engholm, E., Kofoed, C., Fort, S., Sullivan, J. T., Ronson, C. W., Jensen, K. J., Blaise, M., Oldroyd, G., Stougaard, J., Andersen, K. R., and Radutoiu, S. 2020. Ligand-recognizing motifs in plant LysM receptors are major determinants of specificity. Science 369: 663–670. doi:10.1126/science.abb3377
- Brands, M., Wewer, V., Keymer, A., Gutjahr, C., and Dörmann, P. 2018. The Lotus japonicus acyl-acyl carrier protein thioesterase FatM is required for mycorrhiza formation and lipid accumulation of Rhizophagus irregularis. Plant J. 95: 219–232. doi:10.1111/tpj.13943
- Bravo, A., Brands, M., Wewer, V., Dörmann, P., and Harrison, M. J. 2017. Arbuscular mycorrhiza‐specific enzymes FatM and RAM 2 fine‐tune lipid biosynthesis to promote development of arbuscular mycorrhiza. New Phytol. 214: 1631–1645. doi:10.1111/nph.14533
- Bravo, A., York, T., Pumplin, N., Mueller, L. A., and Harrison, M. J. 2016. Genes conserved for arbuscular mycorrhizal symbiosis identified through phylogenomics. Nat. Plants. 2: 15208. doi:10.1038/nplants.2015.208
- Brewer, P. B., Koltai, H., and Beveridge, C. A. 2013. Diverse Roles of Strigolactones in Plant Development. Mol. Plant. 6: 18–28. doi:10.1093/mp/sss130
- Brundrett, M. C. 2009. Mycorrhizal associations and other means of nutrition of vascular plants: understanding the global diversity of host plants by resolving conflicting information and developing reliable means of diagnosis. Plant Soil 320: 37–77. doi:10.1007/s11104-008-9877-9
- Brutus, A., Sicilia, F., Macone, A., Cervone, F., and De Lorenzo, G. 2010. A domain swap approach reveals a role of the plant wall-associated kinase 1 (WAK1) as a receptor of oligogalacturonides. Proc. Natl. Acad. Sci. USA. 107: 9452–9457. doi:10.1073/pnas.1000675107
- Bucher, M. 2007. Functional biology of plant phosphate uptake at root and mycorrhiza interfaces. New Phytol. 173: 11–26. doi:10.1073/pnas.1000675107
- Bürger, M., and Chory, J. 2020. The Many Models of Strigolactone Signaling. Trends Plant Sci. 25: 395–405. doi:10.1016/j.tplants.2019.12.009
- Bustos, R., Castrillo, G., Linhares, F., Puga, M. I., Rubio, V., Pérez-Pérez, J., Solano, R., Leyva, A., and Paz-Ares, J. 2010. A central regulatory system largely controls transcriptional activation and repression responses to phosphate starvation in arabidopsis. PLoS Genet. 6: e1001102. doi:10.1371/journal.pgen.1001102
- Caderas, D., Muster, M., Vogler, H., Mandel, T., Rose, J. K. C., McQueen-Mason, S., and Kuhlemeier, C. 2000. Limited correlation between expansin gene expression and elongation growth rate. Plant Physiol. 123: 1399–1414. doi:10.1104/pp.123.4.1399
- Calvo-Polanco, M., Molina, S., Zamarreño, A. M., García-Mina, J. M., and Aroca, R. 2014. The symbiosis with the arbuscular mycorrhizal fungus rhizophagus irregularis drives root water transport in flooded tomato plants. Plant Cell Physiol. 55: 1017–1029. doi:10.1093/pcp/pcu035
- Calvo-Polanco, M., Sánchez-Romera, B., Aroca, R., Asins, M. J., Declerck, S., Dodd, I. C., Martínez-Andújar, C., Albacete, A., and Ruiz-Lozano, J. M. 2016. Exploring the use of recombinant inbred lines in combination with beneficial microbial inoculants (AM fungus and PGPR) to improve drought stress tolerance in tomato. Environ. Exp. Bot. 131: 47–57. doi:10.1016/j.envexpbot.2016.06.015
- Camehl, I., Drzewiecki, C., Vadassery, J., Shahollari, B., Sherameti, I., Forzani, C., Munnik, T., Hirt, H., and Oelmüller, R. 2011. The OXI1 kinase pathway mediates piriformospora indica-induced growth promotion in arabidopsis. PLoS Pathog. 7: e1002051. doi:10.1371/journal.ppat.1002051
- Carbonnel, S., and Gutjahr, C. 2014. Control of arbuscular mycorrhiza development by nutrient signals. Front. Plant Sci. 5: 462. doi:10.3389/fpls.2014.00462
- Carotenuto, G., Chabaud, M., Miyata, K., Capozzi, M., Takeda, N., Kaku, H., Shibuya, N., Nakagawa, T., Barker, D. G., and Genre, A. 2017. The rice LysM receptor‐like kinase Os CERK 1 is required for the perception of short‐chain chitin oligomers in arbuscular mycorrhizal signaling. New Phytol. 214: 1440–1446. doi:10.1111/nph.14539
- Carotenuto, G., and Genre, A. 2017. Fluorescent staining of arbuscular mycorrhizal structures using Wheat Germ Agglutinin (WGA) and propidium iodide. In Arbuscular Mycorrhizal Fungi, Ferrol, N., and L. Lanfranco, Eds., New York, NY: Springer US, p. 53–59.
- Charpentier, M., Sun, J., Martins, T.V., Radhakrishnan, G.V., Findlay, K., Soumpourou, E., Thouin, J., Véry, A.-A., Sanders, D., Morris, R.J., et al. 2016. Nuclear-localized cyclic nucleotide—gated channels mediate symbiotic calcium oscillations. Science 352: 1102–1105.
- Chen, A., Gu, M., Sun, S., Zhu, L., Hong, S., and Xu, G. 2011. Identification of two conserved cis ‐acting elements, MYCS and P1BS, involved in the regulation of mycorrhiza‐activated phosphate transporters in eudicot species. New Phytol. 189: 1157–1169. doi:10.1111/j.1469-8137.2010.03556.x
- Chen, M., Arato, M., Borghi, L., Nouri, E., and Reinhardt, D. 2018. Beneficial services of arbuscular mycorrhizal fungi – from ecology to application. Front. Plant Sci. 9: 1270. doi:10.3389/fpls.2018.01270
- Chen, X., Yao, Q., Gao, X., Jiang, C., Harberd, N. P., and Fu, X. 2016. Shoot-to-root mobile transcription factor HY5 coordinates plant carbon and nitrogen acquisition. Curr. Biol. 26: 640–646. doi:10.1016/j.cub.2015.12.066
- Choi, D., Cho, H.-T., and Lee, Y. 2006. Expansins: expanding importance in plant growth and development. Physiol. Plant. 2006: 060127022051001. doi:10.1111/j.1399-3054.2006.00612.x
- Choi, J., Lee, T., Cho, J., Servante, E. K., Pucker, B., Summers, W., Bowden, S., Rahimi, M., An, K., An, G., Bouwmeester, H. J., Wallington, E. J., Oldroyd, G., and Paszkowski, U. 2020. The negative regulator SMAX1 controls mycorrhizal symbiosis and strigolactone biosynthesis in rice. Nat. Commun. 11: 2114. doi:10.1038/s41467-020-16021-1
- Choi, J., Summers, W., and Paszkowski, U. 2018. Mechanisms Underlying Establishment of Arbuscular Mycorrhizal Symbioses. Annu. Rev. Phytopathol. 56: 135–160. doi:10.1146/annurev-phyto-080516-035521
- Clemens, S., and Weber, M. 2016. The essential role of coumarin secretion for Fe acquisition from alkaline soil. Plant Signaling Behav. 11: e1114197. doi:10.1080/15592324.2015.1114197
- Conchillo, L. B., Haro, R., and Benito, B. 2021. K+ nutrition exchange in the serendipita-arabidopsis symbiosis: study of the fungal K+ transporters involved. Front. Ecol. Evol 9: 789371.
- Contreras-Cornejo, H. A., Macías-Rodríguez, L., Cortés-Penagos, C., and López-Bucio, J. 2009. Trichoderma virens, a plant beneficial Fungus, enhances biomass production and promotes lateral root growth through an auxin-dependent mechanism in arabidopsis. Plant Physiol. 149: 1579–1592. doi:10.1104/pp.108.130369
- Cosgrove, D. J. 2014. Re-constructing our models of cellulose and primary cell wall assembly. Curr. Opin. Plant Biol. 22: 122–131. doi:10.1016/j.pbi.2014.11.001
- Cosgrove, D. J. 2015. Plant expansins: diversity and interactions with plant cell walls. Curr. Opin. Plant Biol. 25: 162–172. doi:10.1016/j.pbi.2015.05.014
- Cosgrove, D. J. 2016. Catalysts of plant cell wall loosening. F1000Res. 5: 119. doi:10.12688/f1000research.7180.1
- Cosme, M., Fernández, I., Declerck, S., van der Heijden, M. G. A., and Pieterse, C. M. J. 2021. A coumarin exudation pathway mitigates arbuscular mycorrhizal incompatibility in Arabidopsis thaliana. Plant Mol. Biol. 106: 319–334. doi:10.1007/s11103-021-01143-x
- Cosme, M., Fernández, I., Van der Heijden, M. G. A., and Pieterse, C. M. J. 2018. Non-mycorrhizal plants: the exceptions that prove the rule. Trends Plant Sci. 23: 577–587. doi:10.1016/j.tplants.2018.04.004
- Das, D., and Gutjahr, C. 2022. Old dog, new trick: The PHR-SPX system regulates arbuscular mycorrhizal symbiosis. Mol. Plant. 15: 225–227. doi:10.1016/j.molp.2021.12.010
- De Schutter, O., and Vanloqueren, G. 2011. The new green revolution: How twenty-first-century science can feed the world. Solutions 2: 11.
- DeBono, A., Yeats, T. H., Rose, J. K. C., Bird, D., Jetter, R., Kunst, L., and Samuels, L. 2009. Arabidopsis LTPG Is a Glycosylphosphatidylinositol-anchored lipid transfer protein required for export of lipids to the plant surface. Plant Cell. 21: 1230–1238. doi:10.1105/tpc.108.064451
- Decker, E. L., Alder, A., Hunn, S., Ferguson, J., Lehtonen, M. T., Scheler, B., Kerres, K. L., Wiedemann, G., Safavi-Rizi, V., Nordzieke, S., Balakrishna, A., Baz, L., Avalos, J., Valkonen, J. P. T., Reski, R., and Al-Babili, S. 2017. Strigolactone biosynthesis is evolutionarily conserved, regulated by phosphate starvation and contributes to resistance against phytopathogenic fungi in a moss, Physcomitrella patens. New Phytol. 216: 455–468. doi:10.1111/nph.14506
- Delaux, P. 2017. Comparative phylogenomics of symbiotic associations. New Phytol. 213: 89–94. doi:10.1111/nph.14161
- Delaux, P.-M., Radhakrishnan, G. V., Jayaraman, D., Cheema, J., Malbreil, M., Volkening, J. D., Sekimoto, H., Nishiyama, T., Melkonian, M. Pokorny., and L, 2015. Algal ancestor of land plants was preadapted for symbiosis. Proc. Natl. Acad. Sci. USA. 112: 13390–13395.
- Delaux, P.-M., Séjalon-Delmas, N., Bécard, G., and Ané, J.-M. 2013. Evolution of the plant–microbe symbiotic ‘toolkit. Trends Plant Sci. 18: 298–304. doi:10.1016/j.tplants.2013.01.008
- Delaux, P.-M., Varala, K., Edger, P. P., Coruzzi, G. M., Pires, J. C., and Ané, J.-M. 2014. Comparative phylogenomics uncovers the impact of symbiotic associations on host genome evolution. PLoS Genet. 10: e1004487. doi:10.1371/journal.pgen.1004487
- Demchenko, K., Winzer, T., Stougaard, J., Parniske, M., and Pawlowski, K. 2004. Distinct roles of Lotus japonicus SYMRK and SYM15 in root colonization and arbuscule formation. New Phytol. 163: 381–392. doi:10.1111/j.1469-8137.2004.01123.x
- Dermatsev, V., Weingarten-Baror, C., Resnick, N., Gadkar, V., Wininger, S., Kolotilin, I., Mayzlish-Gati, E., Zilberstein, A., Koltai, H., and Kapulnik, Y. 2010. Microarray analysis and functional tests suggest the involvement of expansins in the early stages of symbiosis of the arbuscular mycorrhizal fungus Glomus intraradices on tomato (Solanum lycopersicum). Mol. Plant Pathol. 11: 121–135. doi:10.1111/j.1364-3703.2009.00581.x
- Desaki, Y., Miyata, K., Suzuki, M., Shibuya, N., and Kaku, H. 2018. Plant immunity and symbiosis signaling mediated by LysM receptors. Innate Immun. 24: 92–100. doi:10.1177/1753425917738885
- D’Hondt, S., Donaghay, P., Zachos, J. C., Luttenberg, D., and Lindinger, M. 1998. Organic Carbon fluxes and ecological recovery from the cretaceous-tertiary mass extinction. Science 282: 276–279. doi:10.1126/science.282.5387.276
- Dickson, S. 2004. The Arum–Paris continuum of mycorrhizal symbioses. New Phytol. 163: 187–200. doi:10.1111/j.1469-8137.2004.01095.x
- Ding, M., Zhang, M., Zeng, H., Hayashi, Y., Zhu, Y., and Kinoshita, T. 2021. Molecular basis of plasma membrane H+-ATPase function and potential application in the agricultural production. Plant Physiol. Biochem. 168: 10–16. doi:10.1016/j.plaphy.2021.09.036
- Do, T. H. T., Martinoia, E., and Lee, Y. 2018. Functions of ABC transporters in plant growth and development. Curr. Opin. Plant Biol. 41: 32–38. doi:10.1016/j.pbi.2017.08.003
- Domeignoz-Horta, L. A., Shinfuku, M., Junier, P., Poirier, S., Verrecchia, E., Sebag, D., and DeAngelis, K. M. 2021. Direct evidence for the role of microbial community composition in the formation of soil organic matter composition and persistence. 1: 64. doi:10.1038/s43705-021-00071-7
- Dörmann, P., Voelker, T. A., and Ohlrogge, J. B. 2000. Accumulation of Palmitate in Arabidopsis Mediated by the Acyl-Acyl Carrier Protein Thioesterase FATB1. Plant Physiol. 123: 637–644. doi:10.1104/pp.123.2.637
- Durand, M., Mainson, D., Porcheron, B., Maurousset, L., Lemoine, R., and Pourtau, N. 2018. Carbon source–sink relationship in Arabidopsis thaliana: the role of sucrose transporters. Planta 247: 587–611. doi:10.1007/s00425-017-2807-4
- Duszyn, M., Świeżawska, B., Szmidt-Jaworska, A., and Jaworski, K. 2019. Cyclic nucleotide gated channels (CNGCs) in plant signalling—Current knowledge and perspectives. Journal of Plant Physiology 241: 153035.
- Edqvist, J., Blomqvist, K., Nieuwland, J., and Salminen, T. A. 2018. Plant lipid transfer proteins: are we finally closing in on the roles of these enigmatic proteins? J. Lipid Res. 59: 1374–1382. doi:10.1194/jlr.R083139
- Ekberg, K., Palmgren, M. G., Veierskov, B., and Buch-Pedersen, M. J. 2010. A Novel mechanism of P-type ATPase autoinhibition involving both termini of the protein. J. Biol. Chem. 285: 7344–7350. doi:10.1074/jbc.M109.096123
- Eklöf, J. M., and Brumer, H. 2010. The XTH gene family: an update on enzyme structure, function, and phylogeny in Xyloglucan remodeling. Plant Physiol. 153: 456–466. doi:10.1104/pp.110.156844
- Eo, J.-K., and Eom, A.-H. 2009. Differential growth response of various crop species to arbuscular mycorrhizal inoculation. Mycobiology 37: 72–76. doi:10.4489/MYCO.2009.37.1.072
- Falhof, J., Pedersen, J. T., Fuglsang, A. T., and Palmgren, M. 2016. Plasma membrane H+-ATPase regulation in the center of plant physiology. Mol. Plant. 9: 323–337. doi:10.1016/j.molp.2015.11.002
- Fan, Y., Wan, S., Jiang, Y., Xia, Y., Chen, X., Gao, M., Cao, Y., Luo, Y., Zhou, Y., and Jiang, X. 2018. Over-expression of a plasma membrane H+-ATPase SpAHA1 conferred salt tolerance to transgenic Arabidopsis. Protoplasma 255: 1827–1837. doi:10.1007/s00709-018-1275-4
- FAO. 2021. https://www.fao.org/faostat/en/#data/QCL (accessed Sep 12, 2022).
- Favre, P., Bapaume, L., Bossolini, E., Delorenzi, M., Falquet, L., and Reinhardt, D. 2014. A novel bioinformatics pipeline to discover genes related to arbuscular mycorrhizal symbiosis based on their evolutionary conservation pattern among higher plants. BMC Plant Biol. 14: 333. doi:10.1186/s12870-014-0333-0
- Fawke, S., Torode, T. A., Gogleva, A., Fich, E. A., Sørensen, I., Yunusov, T., Rose, J. K. C., and Schornack, S. 2019. Glycerol‐3‐phosphate acyltransferase 6 controls filamentous pathogen interactions and cell wall properties of the tomato and Nicotiana benthamiana leaf epidermis. New Phytol. 223: 1547–1559. doi:10.1111/nph.15846
- Feng, F., Sun, J., Radhakrishnan, G. V., Lee, T., Bozsóki, Z., Fort, S., Gavrin, A., Gysel, K., Thygesen, M. B., Andersen, K. R., Radutoiu, S., Stougaard, J., and Oldroyd, G. E. D. 2019. A combination of chitooligosaccharide and lipochitooligosaccharide recognition promotes arbuscular mycorrhizal associations in Medicago truncatula. Nat. Commun. 10: 5047. doi:10.1038/s41467-019-12999-5
- Feng, Y., Wu, P., Liu, C., Peng, L., Wang, T., Wang, C., Tan, Q., Li, B., Ou, Y., and Zhu, H. 2021. Suppression of LjBAK1-mediated immunity by SymRK promotes rhizobial infection in Lotus japonicus. Molecular Plant 14: 1935–1950. doi:10.1038/s41467-019-12999-5
- Fernández, I., Cosme, M., Stringlis, I. A., Yu, K., Wees Jonge, R., van Saskia, C. M., Pozo, M. J., Pieterse, C. M. J., and van der Heijden, M. G. A. 2019. Molecular dialogue between arbuscular mycorrhizal fungi and the nonhost plant Arabidopsis thaliana switches from initial detection to antagonism. New Phytol. 223: 867–881. doi:10.1111/nph.15798
- Fesel, P. H., and Zuccaro, A. 2016. Dissecting endophytic lifestyle along the parasitism/mutualism continuum in Arabidopsis. Curr. Opin. Microbiol. 32: 103–112. doi:10.1016/j.mib.2016.05.008
- Fiorilli, V., Vannini, C., Ortolani, F., Garcia-Seco, D., Chiapello, M., Novero, M., Domingo, G., Terzi, V., Morcia, C., and Bagnaresi, P. 2018. Omics approaches revealed how arbuscular mycorrhizal symbiosis enhances yield and resistance to leaf pathogen in wheat. Sci Rep 8: 9625. doi:10.1016/j.mib.2016.05.008
- Fliegmann, J., Canova, S., Lachaud, C., Uhlenbroich, S., Gasciolli, V., Pichereaux, C., Rossignol, M., Rosenberg, C., Cumener, M., Pitorre, D., Lefebvre, B., Gough, C., Samain, E., Fort, S., Driguez, H., Vauzeilles, B., Beau, J.-M., Nurisso, A., Imberty, A., Cullimore, J., and Bono, J.-J. 2013. Lipo-chitooligosaccharidic symbiotic signals are recognized by LysM receptor-like kinase LYR3 in the legume Medicago truncatula. ACS Chem. Biol. 8: 1900–1906. doi:10.1021/cb400369u
- Fliegmann, J., Jauneau, A., Pichereaux, C., Rosenberg, C., Gasciolli, V., Timmers, A. C. J., Burlet-Schiltz, O., Cullimore, J., and Bono, J.-J. 2016. LYR3, a high-affinity LCO-binding protein of Medicago truncatula, interacts with LYK3, a key symbiotic receptor. FEBS Lett. 590: 1477–1487. doi:10.1002/1873-3468.12191
- Folberth, C., Khabarov, N., Balkovič, J., Skalský, R., Visconti, P., Ciais, P., Janssens, I. A., Peñuelas, J., and Obersteiner, M. 2020. The global cropland-sparing potential of high-yield farming. Nat. Sustain. 3: 281–289. doi:10.1038/s41893-020-0505-x
- Fries, L. L. M., Pacovsky, R. S., Safir, G. R., and Siqueira, J. O. 1997. Plant growth and arbuscular mycorrhizal fungal colonization affected by exogenously applied phenolic compounds. J. Chem. Ecol. 23: 1755–1767. doi:10.1023/B:JOEC.0000006449.09141.cd
- Gangappa, S. N., and Botto, J. F. 2016. The multifaceted roles of HY5 in plant growth and development. Mol. Plant. 9: 1353–1365. doi:10.1016/j.molp.2016.07.002
- Genre, A., and Bonfante, P. 2005. Building a mycorrhizal cell: How to reach compatibility between plants and arbuscular mycorrhizal fungi. J. Plant Interact. 1: 3–13. doi:10.1080/17429140500318986
- Genre, A., and Russo, G. 2016. Does a common pathway transduce symbiotic signals in plant–microbe interactions? Front. Plant Sci. 7: 96. doi:10.3389/fpls.2016.00096
- Genre, A., Chabaud, M., Faccio, A., Barker, D. G., and Bonfante, P. 2008. Prepenetration apparatus assembly precedes and predicts the colonization patterns of arbuscular mycorrhizal fungi within the root cortex of both Medicago truncatula and Daucus carota. Plant Cell. 20: 1407–1420. doi:10.1105/tpc.108.059014
- Genre, A., Lanfranco, L., Perotto, S., and Bonfante, P. 2020. Unique and common traits in mycorrhizal symbioses. Nat. Rev. Microbiol. 18: 649–660. doi:10.1038/s41579-020-0402-3
- Gévaudant, F., Duby, G., Stedingk, E., von, Zhao, R., Morsomme, P., and Boutry, M. 2007. Expression of a constitutively activated plasma membrane H+-ATPase alters plant development and increases salt tolerance. Plant Physiol. 144: 1763–1776. doi:10.1104/pp.107.103762
- Gherbi, H., Markmann, K., Svistoonoff, S., Estevan, J., Autran, D., Giczey, G., Auguy, F., Péret, B., Laplaze, L., Franche, C., Parniske, M., and Bogusz, D. 2008. SymRK defines a common genetic basis for plant root endosymbioses with arbuscular mycorrhiza fungi, rhizobia, and Frankia bacteria. Proc. Natl. Acad. Sci. USA. 105: 4928–4932. doi:10.1073/pnas.0710618105
- Gill, S. S., Gill, R., Trivedi, D. K., Anjum, N. A., Sharma, K. K., Ansari, M. W., Ansari, A. A., Johri, A. K., Prasad, R., Pereira, E., Varma, A., and Tuteja, N. 2016. Piriformospora indica: potential and significance in plant stress tolerance. Front. Microbiol. 332. 7: 458. doi:10.3389/fmicb.2016.00332
- Giovannetti, M., Göschl, C., Dietzen, C., Andersen, S. U., Kopriva, S., and Busch, W. 2019. Identification of novel genes involved in phosphate accumulation in Lotus japonicus through Genome Wide Association mapping of root system architecture and anion content. PLoS Genet. 15: e1008126. doi:10.1371/journal.pgen.1008126
- Gosling, P., Hodge, A., Goodlass, G., and Bending, G. D. 2006. Arbuscular mycorrhizal fungi and organic farming. Agric Ecosyst Environ 113: 17–35. doi:10.1016/j.agee.2005.09.009
- Gossen, M., Freundlieb, S., Bender, G., Müller, G., Hillen, W., and Bujard, H. 1995. Transcriptional activation by tetracyclines in mammalian cells. Science 268: 1766–1769. doi:10.1126/science.7792603
- Grabov, A. 2007. Plant KT/KUP/HAK potassium transporters: single family – multiple functions. Ann. Bot. 99: 1035–1041. doi:10.1093/aob/mcm066
- Gutjahr, C., Banba, M., Croset, V., An, K., Miyao, A., An, G., Hirochika, H., Imaizumi-Anraku, H., and Paszkowski, U. 2008. Arbuscular mycorrhiza–specific signaling in rice transcends the common symbiosis signaling pathway. Plant Cell. 20: 2989–3005. doi:10.1105/tpc.108.062414
- Hanlon, M. T., and Coenen, C. 2011. Genetic evidence for auxin involvement in arbuscular mycorrhiza initiation. New Phytol. 189: 701–709. doi:10.1111/j.1469-8137.2010.03567.x
- Hao, D.-L., Zhou, J.-Y., Yang, S.-Y., Qi, W., Yang, K.-J., and Su, Y.-H. 2020. Function and REGULATION OF AMMONIUM TRANSPORTERS IN PLANTS. Int J Mol Sci. 21: 3557. doi:10.3390/ijms21103557
- Harrison, M. J. 2012. Cellular programs for arbuscular mycorrhizal symbiosis. Curr. Opin. Plant Biol. 15: 691–698. doi:10.1016/j.pbi.2012.08.010
- Haruta, M., Burch, H. L., Nelson, R. B., Barrett-Wilt, G., Kline, K. G., Mohsin, S. B., Young, J. C., Otegui, M. S., and Sussman, M. R. 2010. Molecular characterization of mutant arabidopsis plants with reduced plasma membrane proton pump activity. J. Biol. Chem. 285: 17918–17929. doi:10.1074/jbc.M110.101733
- Haslam, R. P., Sayanova, O., Kim, H. J., Cahoon, E. B., and Napier, J. A. 2016. Synthetic redesign of plant lipid metabolism. Plant J. 87: 76–86. doi:10.1111/tpj.13172
- Hayek, S., Gianinazzi-Pearson, V., Gianinazzi, S., and Franken, P. 2014. Elucidating mechanisms of mycorrhiza-induced resistance against Thielaviopsis basicola via targeted transcript analysis of Petunia hybrida genes. Physiol. Mol. Plant Pathol. 88: 67–76. doi:10.1016/j.pmpp.2014.09.003
- He, J., Zhang, C., Dai, H., Liu, H., Zhang, X., Yang, J., Chen, X., Zhu, Y., Wang, D., Qi, X., Li, W., Wang, Z., An, G., Yu, N., He, Z., Wang, Y.-F., Xiao, Y., Zhang, P., and Wang, E. 2019. A LysM receptor heteromer mediates perception of arbuscular mycorrhizal symbiotic signal in rice. Mol. Plant. 12: 1561–1576. doi:10.1016/j.molp.2019.10.015
- He, M., Qin, C.-X., Wang, X., and Ding, N.-Z. 2020. Plant unsaturated fatty acids: biosynthesis and regulation. Front. Plant Sci. 11: 390. doi:10.3389/fpls.2020.00390
- Held, M., Hossain, M. S., Yokota, K., Bonfante, P., Stougaard, J., and Szczyglowski, K. 2010. Common and not so common symbiotic entry. Trends Plant Sci. 15: 540–545. doi:10.1016/j.tplants.2010.08.001
- Herrera-Estrella, L., and López-Arredondo, D. 2016. Phosphorus: the underrated element for feeding the world. Trends Plant Sci. 21: 461–463. doi:10.1016/j.tplants.2016.04.010
- Hijri, M. 2016. Analysis of a large dataset of mycorrhiza inoculation field trials on potato shows highly significant increases in yield. Mycorrhiza 26: 209–214. doi:10.1007/s00572-015-0661-4
- Hiruma, K., Gerlach, N., Sacristán, S., Nakano, R. T., Hacquard, S., Kracher, B., Neumann, U., Ramírez, D., Bucher, M., O’Connell, R. J., and Schulze-Lefert, P. 2016. Root endophyte colletotrichum tofieldiae confers plant fitness benefits that are phosphate status dependent. Cell. 165: 464–474. doi:10.1016/j.cell.2016.02.028
- Hoffmann, R. D., Olsen, L. I., Ezike, C. V., Pedersen, J. T., Manstretta, R., López-Marqués, R. L., and Palmgren, M. 2019. Roles of plasma membrane proton ATPases AHA2 and AHA7 in normal growth of roots and root hairs in Arabidopsis thaliana. Physiol. Plant. 166: 848–861. doi:10.1111/ppl.12842
- Holátko, J., Brtnický, M., Kučerík, J., Kotianová, M., Elbl, J., Kintl, A., Kynický, J., Benada, O., Datta, R., and Jansa, J. 2021. Glomalin – Truths, myths, and the future of this elusive soil glycoprotein. Soil Biol. Biochem. 153: 108116. doi:10.1016/j.soilbio.2020.108116
- Holsters, M. 2008. SYMRK, an enigmatic receptor guarding and guiding microbial endosymbioses with plant roots. Proc. Natl. Acad. Sci. USA. 105: 4537–4538. doi:10.1073/pnas.0801270105
- Hornstein, E. D., Charles, M., Franklin, M., Edwards, B., Vintila, S., Kleiner, M., and Sederoff, H. 2023. Re-engineering a lost trait: IPD3, a master regulator of arbuscular mycorrhizal symbiosis, affects genes for immunity and metabolism of non-host Arabidopsis when restored long after its evolutionary loss. bioRxiv. 2023.03.06.531368. doi:10.1101/2023.03.06.531368
- Hothorn, M., Van Den Ende, W., Lammens, W., Rybin, V., and Scheffzek, K. 2010. Structural insights into the pH-controlled targeting of plant cell-wall invertase by a specific inhibitor protein. Proc. Natl. Acad. Sci. USA. 107: 17427–17432. doi:10.1073/pnas.1004481107
- Huang, R., Li, Z., Mao, C., Zhang, H., Sun, Z., Li, H., Huang, C., Feng, Y., Shen, X., Bucher, M., Zhang, Z., Lin, Y., Cao, Y., and Duanmu, D. 2020. Natural variation at Os CERK 1 regulates arbuscular mycorrhizal symbiosis in rice. New Phytol. 225: 1762–1776. doi:10.1111/nph.16158
- Hou, S., Liu, Z., Shen, H., and Wu, D. 2019. Damage-associated molecular pattern-triggered immunity in plants. Front. Plant Sci. 10: 646. doi:10.3389/fpls.2019.00646
- Hou, S., and Tsuda, K. 2022. Salicylic acid and jasmonic acid crosstalk in plant immunity. Essays Biochem. 66: 647–656. doi:10.1042/EBC20210090
- Huisman, R., Hontelez, J., Bisseling, T., and Limpens, E. 2020. SNARE complexity in arbuscular mycorrhizal symbiosis. Front. Plant Sci. 11: 354. doi:10.3389/fpls.2020.00354
- Huisman, R., Hontelez, J., Mysore, K. S., Wen, J., Bisseling, T., and Limpens, E. 2016. A symbiosis‐dedicated syntaxin of plants 13II isoform controls the formation of a stable host–microbe interface in symbiosis. New Phytol. 211: 1338–1351. doi:10.1111/nph.13973
- Ilias, I. A., Negishi, K., Yasue, K., Jomura, N., Morohashi, K., Baharum, S. N., and Goh, H.-H. 2019. Transcriptome-wide effects of expansin gene manipulation in etiolated Arabidopsis seedling. J. Plant Res. 132: 159–172. doi:10.1007/s10265-018-1067-0
- Ishida, K., and Yokoyama, R. 2022. Reconsidering the function of the xyloglucan endotransglucosylase/hydrolase family. J. Plant Res. 135: 145–156. doi:10.1007/s10265-021-01361-w
- Jacobs, S., Zechmann, B., Molitor, A., Trujillo, M., Petutschnig, E., Lipka, V., Kogel, K.-H., and Schäfer, P. 2011. Broad-spectrum suppression of innate immunity is required for colonization of arabidopsis roots by the Fungus Piriformospora indica. Plant Physiol. 156: 726–740. doi:10.1104/pp.111.176446
- Jacq, A., Pernot, C., Martinez, Y., Domergue, F., Payré, B., Jamet, E., Burlat, V., and Pacquit, V. B. 2017. The arabidopsis lipid transfer protein 2 (AtLTP2) is involved in cuticle-cell wall interface integrity and in etiolated hypocotyl permeability. Front. Plant Sci. 8: e00263. doi:10.3389/fpls.2017.00263
- Jajoo, A., and Mathur, S. 2021. Role of arbuscular mycorrhizal fungi as an underground saviuor for protecting plants from abiotic stresses. Physiol. Mol. Biol. Plants 27: 2589–2603. doi:10.1007/s12298-021-01091-2
- Janicka-Russak, M., and Kabała, K. 2015. The role of plasma membrane H+-ATPase in salinity stress of plants. In Progress in Botany, Lüttge, U., and W. Beyschlag, Eds., Cham: Springer International Publishing, pp. 77–92.
- Jewell, J. B., Sowders, J. M., He, R., Willis, M. A., Gang, D. R., and Tanaka, K. 2019. Extracellular ATP shapes a defense-related transcriptome both independently and along with other defense signaling pathways. Plant Physiol. 179: 1144–1158. doi:10.1104/pp.18.01301
- Jiang, Y., Wang, W., Xie, Q., Liu, N., Liu, L., Wang, D., Zhang, X., Yang, C., Chen, X., Tang, D., and Wang, E. 2017. Plants transfer lipids to sustain colonization by mutualistic mycorrhizal and parasitic fungi. Science 356: 1172–1175. doi:10.1126/science.aam9970
- Jiang, Y., Xie, Q., Wang, W., Yang, J., Zhang, X., Yu, N., Zhou, Y., and Wang, E. 2018. Medicago AP2-domain transcription factor WRI5a is a master regulator of lipid biosynthesis and transfer during mycorrhizal symbiosis. Molecular Plant 11: 1344–1359. doi:10.1016/j.molp.2018.09.006
- Jogawat, A., Meena, M. K., Kundu, A., Varma, M., and Vadassery, J. 2020. Calcium channel CNGC19 mediates basal defense signaling to regulate colonization by Piriformospora indica in Arabidopsis roots. Journal of Experimental Botany 71: 2752–2768. doi:10.1093/jxb/eraa028
- Jung, S. C., Martinez-Medina, A., Lopez-Raez, J. A., and Pozo, M. J. 2012. Mycorrhiza-induced resistance and priming of plant defenses. J. Chem. Ecol. 38: 651–664. doi:10.1007/s10886-012-0134-6
- Kaewthai, N., Gendre, D., Eklöf, J. M., Ibatullin, F. M., Ezcurra, I., Bhalerao, R. P., and Brumer, H. 2013. Group III-A XTH genes of arabidopsis encode predominant xyloglucan endohydrolases that are dispensable for normal growth. Plant Physiol. 161: 440–454. doi:10.1104/pp.112.207308
- Kaiser, C., Kilburn, M. R., Clode, P. L., Fuchslueger, L., Koranda, M., Cliff, J. B., Solaiman, Z. M., and Murphy, D. V. 2015. Exploring the transfer of recent plant photosynthates to soil microbes: mycorrhizal pathway vs direct root exudation. New Phytol. 205: 1537–1551. doi:10.1111/nph.13138
- Kameoka, H., Tsutsui, I., Saito, K., Kikuchi, Y., Handa, Y., Ezawa, T., Hayashi, H., Kawaguchi, M., and Akiyama, K. 2019. Stimulation of asymbiotic sporulation in arbuscular mycorrhizal fungi by fatty acids. Nat. Microbiol. 4: 1654–1660. doi:10.1038/s41564-019-0485-7
- Karasawa, T., and Takebe, M. 2012. Temporal or spatial arrangements of cover crops to promote arbuscular mycorrhizal colonization and P uptake of upland crops grown after nonmycorrhizal crops. Plant Soil. 353: 355–366. doi:10.1007/s11104-011-1036-z
- Keymer, A., Pimprikar, P., Wewer, V., Huber, C., Brands, M., Bucerius, S. L., Delaux, P.-M., Klingl, V., Röpenack-Lahaye, E. v., Wang, T. L., Eisenreich, W., Dörmann, P., Parniske, M., and Gutjahr, C. 2017. Lipid transfer from plants to arbuscular mycorrhiza fungi. Elife. 6: e29107. doi:10.7554/eLife.29107
- Kim, H., Lee, S. B., Kim, H. J., Min, M. K., Hwang, I., and Suh, M. C. 2012. Characterization of Glycosylphosphatidylinositol-Anchored Lipid Transfer Protein 2 (LTPG2) and overlapping function between LTPG/LTPG1 and LTPG2 in cuticular Wax Export or accumulation in Arabidopsis thaliana. Plant Cell Physiol. 53: 1391–1403. doi:10.1093/pcp/pcs083
- Kim, J.-H., Oh, Y., Yoon, H., Hwang, I., and Chang, Y.-S. 2015. Iron nanoparticle-induced activation of plasma membrane H+-ATPase promotes stomatal opening in Arabidopsis thaliana. Environ. Sci. Technol. 49: 1113–1119. doi:10.1021/es504375t
- Kobae, Y., and Hata, S. 2010. Dynamics of periarbuscular membranes visualized with a fluorescent phosphate transporter in arbuscular mycorrhizal roots of rice. Plant Cell Physiol. 51: 341–353. doi:10.1093/pcp/pcq013
- Koegel, S., Ait Lahmidi, N., Arnould, C., Chatagnier, O., Walder, F., Ineichen, K., Boller, T., Wipf, D., Wiemken, A., and Courty, P. 2013. The family of ammonium transporters (AMT) in S orghum bicolor : two AMT members are induced locally, but not systemically in roots colonized by arbuscular mycorrhizal fungi. New Phytol. 198: 853–865. doi:10.1111/nph.12199
- Kohlen, W., Charnikhova, T., Liu, Q., Bours, R., Domagalska, M. A., Beguerie, S., Verstappen, F., Leyser, O., Bouwmeester, H., and Ruyter-Spira, C. 2011. Strigolactones are transported through the Xylem and play a key role in shoot architectural response to phosphate deficiency in nonarbuscular mycorrhizal host arabidopsis. Plant Physiol. 155: 974–987. doi:10.1104/pp.110.164640
- Krajinski, F., Courty, P.-E., Sieh, D., Franken, P., Zhang, H., Bucher, M., Gerlach, N., Kryvoruchko, I., Zoeller, D., Udvardi, M., and Hause, B. 2014. The H+-ATPase HA1 of Medicago truncatula is essential for phosphate transport and plant growth during arbuscular mycorrhizal symbiosis. Plant Cell. 26: 1808–1817. doi:10.1105/tpc.113.120436
- Krapp, A., David, L. C., Chardin, C., Girin, T., Marmagne, A., Leprince, A.-S., Chaillou, S., Ferrario-Méry, S., Meyer, C., and Daniel-Vedele, F. 2014. Nitrate transport and signalling in Arabidopsis. J. Exp. Bot. 65: 789–798. doi:10.1093/jxb/eru001
- Krausgrill, S., Sander, A., Greiner, S., Weil, M., and Rausch, T. 1996. Regulation of cell wall invertase by a proteinaceous inhibitor. J. Exp. Bot. 47: 1193–1198. doi:10.1093/jxb/47.Special_Issue.1193
- Kretzschmar, T., Kohlen, W., Sasse, J., Borghi, L., Schlegel, M., Bachelier, J. B., Reinhardt, D., Bours, R., Bouwmeester, H. J., and Martinoia, E. 2012. A petunia ABC protein controls strigolactone-dependent symbiotic signalling and branching. Nature. 483: 341–344. doi:10.1038/nature10873
- Küster, H., Becker, A., Firnhaber, C., Hohnjec, N., Manthey, K., Perlick, A. M., Bekel, T., Dondrup, M., Henckel, K., Goesmann, A., Meyer, F., Wipf, D., Requena, N., Hildebrandt, U., Hampp, R., Nehls, U., Krajinski, F., Franken, P., and Pühler, A. 2007. Development of bioinformatic tools to support EST-sequencing, in silico and microarray-based transcriptome profiling in mycorrhizal symbioses. Phytochemistry 68: 19–32. doi:10.1016/j.phytochem.2006.09.026
- Labbé, J., Muchero, W., Czarnecki, O., Wang, J., Wang, X., Bryan, A. C., Zheng, K., Yang, Y., Xie, M., Zhang, J., Wang, D., Meidl, P., Wang, H., Morrell-Falvey, J. L., Cope, K. R., Maia, L. G. S., Ané, J.-M., Mewalal, R., Jawdy, S. S., Gunter, L. E., Schackwitz, W., Martin, J., Le Tacon, F., Li, T., Zhang, Z., Ranjan, P., Lindquist, E., Yang, X., Jacobson, D. A., Tschaplinski, T. J., Barry, K., Schmutz, J., Chen, J.-G., and Tuskan, G. A. 2019. Mediation of plant–mycorrhizal interaction by a lectin receptor-like kinase. Nat. Plants. 5: 676–680. doi:10.1038/s41477-019-0469-x
- Lai, Y., and Eulgem, T. 2018. Transcript‐level expression control of plant NLR genes. Mol. Plant Pathol. 19: 1267–1281. doi:10.1111/mpp.12607
- Lashbrook, C. C., and Cai, S. 2008. Cell wall remodeling in Arabidopsis stamen abscission zones: temporal aspects of control inferred from transcriptional profiling. Plant Signal. Behav. 3: 733–736. doi:10.4161/psb.3.9.6489
- Lehmann, A., and Rillig, M. C. 2015. Arbuscular mycorrhizal contribution to copper, manganese and iron nutrient concentrations in crops – A meta-analysis. Soil Biol. Biochem. 81: 147–158. doi:10.1016/j.soilbio.2014.11.013
- Leppyanen, I., Shakhnazarova, V., Shtark, O., Vishnevskaya, N., Tikhonovich, I., and Dolgikh, E. 2017. Receptor-Like Kinase LYK9 in Pisum sativum L. Is the CERK1-like receptor that controls both plant immunity and AM symbiosis development. 19: 8. doi:10.3390/ijms19010008
- Li, H., Chen, M., Duan, L., Zhang, T., Cao, Y., and Zhang, Z. 2018. Domain swap approach reveals the critical roles of different domains of SYMRK in root nodule symbiosis in Lotus japonicus. Front. Plant Sci. 9: 697. doi:10.3389/fpls.2018.00697
- Li, X.-C., Zhu, J., Yang, J., Zhang, G.-R., Xing, W.-F., Zhang, S., and Yang, Z.-N. 2012. Glycerol-3-Phosphate Acyltransferase 6 (GPAT6) is important for tapetum development in arabidopsis and plays multiple roles in plant fertility. Mol. Plant. 5: 131–142. doi:10.1093/mp/ssr057
- Li-Beisson, Y., Pollard, M., Sauveplane, V., Pinot, F., Ohlrogge, J., and Beisson, F. 2009. Nanoridges that characterize the surface morphology of flowers require the synthesis of cutin polyester. Proc. Natl. Acad. Sci. USA. 106: 22008–22013. doi:10.1073/pnas.0909090106
- Liu, C.-Y., Zhang, F., Zhang, D.-J., Zou, Y.-N., Shu, B., and Wu, Q.-S. 2020a. Transcriptome analysis reveals improved root hair growth in trifoliate orange seedlings by arbuscular mycorrhizal fungi. Plant Growth Regul. 92: 195–203. doi:10.1007/s10725-020-00630-3
- Liu, F., Xiong, X., Wu, L., Fu, D., Hayward, A., Zeng, X., Cao, Y., Wu, Y., Li, Y., and Wu, G. 2014. BraLTP1, a lipid transfer protein gene involved in epicuticular wax deposition, cell proliferation and flower development in Brassica napus. PLoS One. 9: e110272. doi:10.1371/journal.pone.0110272
- Liu, F., Zhang, X., Lu, C., Zeng, X., Li, Y., Fu, D., and Wu, G. 2015. Non-specific lipid transfer proteins in plants: presenting new advances and an integrated functional analysis. J. Exp. Bot. 66: 5663–5681. doi:10.1093/jxb/erv313
- Liu, J., Blaylock, L. A., Endre, G., Cho, J., Town, C. D., VandenBosch, K. A., and Harrison, M. J. 2003. Transcript profiling coupled with spatial expression analyses reveals genes involved in distinct developmental stages of an arbuscular mycorrhizal symbiosis. Plant Cell. 15: 2106–2123. doi:10.1105/tpc.014183
- Liu, J., Chen, J., Xie, K., Tian, Y., Yan, A., Liu, J., Huang, Y., Wang, S., Zhu, Y., Chen, A., and Xu, G. 2020b. A mycorrhiza‐specific H+‐ATPase is essential for arbuscule development and symbiotic phosphate and nitrogen uptake. Plant. Cell Environ. 43: 1069–1083. doi:10.1111/pce.13714
- Liu, J., Liu, J., Chen, A., Ji, M., Chen, J., Yang, X., Gu, M., Qu, H., and Xu, G. 2016. Analysis of tomato plasma membrane H+-ATPase gene family suggests a mycorrhiza-mediated regulatory mechanism conserved in diverse plant species. Mycorrhiza 26: 645–656. doi:10.1007/s00572-016-0700-9
- Ludwig-Müller, J., Bennett, R. N., García-Garrido, J. M., Piché, Y., and Vierheilig, H. 2002. Reduced arbuscular mycorrhizal root colonization in Tropaeolum majus and Carica papaya after jasmonic acid application can not be attributed to increased glucosinolate levels. J. Plant Physiol. 159: 517–523. doi:10.1078/0176-1617-00731
- Luginbuehl, L. H., Menard, G. N., Kurup, S., Van Erp, H., Radhakrishnan, G. V., Breakspear, A., Oldroyd, G. E. D., and Eastmond, P. J. 2017. Fatty acids in arbuscular mycorrhizal fungi are synthesized by the host plant. Science 356: 1175–1178. doi:10.1126/science.aan0081
- Luginbuehl, L. H., and Oldroyd, G. E. D. 2017. Understanding the arbuscule at the heart of endomycorrhizal symbioses in plants. Curr. Biol. 27: R952–R963. doi:10.1016/j.cub.2017.06.042
- Maldonado, A. M., Doerner, P., Dixon, R. A., Lamb, C. J., and Cameron, R. K. 2002. A putative lipid transfer protein involved in systemic resistance signalling in Arabidopsis. Nature 419: 399–403. doi:10.1038/nature00962
- Maldonado-Mendoza, I. E., Dewbre, G. R., Blaylock, L., and Harrison, M. J. 2005. Expression of a xyloglucan endotransglucosylase/hydrolase gene, Mt-XTH1, from Medicago truncatula is induced systemically in mycorrhizal roots. Gene 345: 191–197. doi:10.1016/j.gene.2004.10.028
- Malinowski, R., Truman, W., and Blicharz, S. 2019. Genius architect or clever thief – How Plasmodiophora brassicae reprograms host development to establish a pathogen-oriented physiological sink. Mol. Plant. Microbe Interact. 32: 1259–1266. doi:10.1094/MPMI-03-19-0069-CR
- Malkov, N., Fliegmann, J., Rosenberg, C., Gasciolli, V., Timmers, A. C. J., Nurisso, A., Cullimore, J., and Bono, J.-J. 2016. Molecular basis of lipo-chitooligosaccharide recognition by the lysin motif receptor-like kinase LYR3 in legumes. Biochem. J. 473: 1369–1378. doi:10.1042/BCJ20160073
- Maris, A., Suslov, D., Fry, S. C., Verbelen, J.-P., and Vissenberg, K. 2009. Enzymic characterization of two recombinant xyloglucan endotransglucosylase/hydrolase (XTH) proteins of Arabidopsis and their effect on root growth and cell wall extension. J. Exp. Bot. 60: 3959–3972. doi:10.1093/jxb/erp229
- Marowa, P., Ding, A., and Kong, Y. 2016. Expansins: roles in plant growth and potential applications in crop improvement. Plant Cell Rep. 35: 949–965. doi:10.1007/s00299-016-1948-4
- Marquès-Bueno, M. M., Morao, A. K., Cayrel, A., Platre, M. P., Barberon, M., Caillieux, E., Colot, V., Jaillais, Y., Roudier, F., and Vert, G. 2016. A versatile Multisite Gateway-compatible promoter and transgenic line collection for cell type-specific functional genomics in Arabidopsis. Plant J. 85: 320–333. doi:10.1111/tpj.13099
- Mashiguchi, K., Sasaki, E., Shimada, Y., Nagae, M., Ueno, K., Nakano, T., Yoneyama, K., Suzuki, Y., and Asami, T. 2009. Feedback-regulation of strigolactone biosynthetic genes and strigolactone-regulated genes in Arabidopsis. Biosci. Biotechnol. Biochem. 73: 2460–2465. doi:10.1271/bbb.90443
- Mathys, J., De Cremer, K., Timmermans, P., Van Kerckhove, S., Lievens, B., Vanhaecke, M., Cammue, B. P. A., and De Coninck, B. 2012. Genome-wide characterization of ISR induced in Arabidopsis thaliana by Trichoderma hamatum T382 Against Botrytis cinerea infection. Front. Plant Sci. 3: e00108.
- Mayzlish-Gati, E., De-Cuyper, C., Goormachtig, S., Beeckman, T., Vuylsteke, M., Brewer, P. B., Beveridge, C. A., Yermiyahu, U., Kaplan, Y., Enzer, Y., Wininger, S., Resnick, N., Cohen, M., Kapulnik, Y., and Koltai, H. 2012. Strigolactones are involved in root response to low phosphate conditions in arabidopsis. Plant Physiol. 160: 1329–1341. doi:10.1104/pp.112.202358
- Medina-Castellanos, E., Esquivel-Naranjo, E. U., Heil, M., and Herrera-Estrella, A. 2014. Extracellular ATP activates MAPK and ROS signaling during injury response in the fungus Trichoderma atroviride. Front. Plant Sci. 5: e00659. doi:10.3389/fpls.2014.00659
- Miedes, E., Suslov, D., Vandenbussche, F., Kenobi, K., Ivakov, A., Van Der Straeten, D., Lorences, E. P., Mellerowicz, E. J., Verbelen, J.-P., and Vissenberg, K. 2013. Xyloglucan endotransglucosylase/hydrolase (XTH) overexpression affects growth and cell wall mechanics in etiolated Arabidopsis hypocotyls. J. Exp. Bot. 64: 2481–2497. doi:10.1093/jxb/ert107
- Mitsui, Y., Shimomura, M., Komatsu, K., Namiki, N., Shibata-Hatta, M., Imai, M., Katayose, Y., Mukai, Y., Kanamori, H., Kurita, K., Kagami, T., Wakatsuki, A., Ohyanagi, H., Ikawa, H., Minaka, N., Nakagawa, K., Shiwa, Y., and Sasaki, T. 2015. The radish genome and comprehensive gene expression profile of tuberous root formation and development. Sci. Rep. 5: 10835. doi:10.1038/srep10835
- Miyata, K., Hosotani, M., Akamatsu, A., Takeda, N., Jiang, W., Sugiyama, T., Takaoka, R., Matsumoto, K., Abe, S., Shibuya, N., and Kaku, H. 2023. OsSYMRK Plays an Essential Role in AM Symbiosis in Rice (Oryza sativa). Plant Cell Physiol. 64: 378–391. doi:10.1093/pcp/pcad006
- Miyata, K., Kozaki, T., Kouzai, Y., Ozawa, K., Ishii, K., Asamizu, E., Okabe, Y., Umehara, Y., Miyamoto, A., Kobae, Y., Akiyama, K., Kaku, H., Nishizawa, Y., Shibuya, N., and Nakagawa, T. 2014. The bifunctional plant receptor, OsCERK1, regulates both chitin-triggered immunity and arbuscular mycorrhizal symbiosis in rice. Plant Cell Physiol. 55: 1864–1872. doi:10.1093/pcp/pcu129
- Mogollón, J. M., Bouwman, A. F., Beusen, A. H. W., Lassaletta, L., van Grinsven, H. J. M., and Westhoek, H. 2021. More efficient phosphorus use can avoid cropland expansion. Nat Food 2: 509–518. doi:10.1093/pcp/pcu129
- Mohr, T. J., Mammarella, N. D., Hoff, T., Woffenden, B. J., Jelesko, J. G., and McDowell, J. M. 2010. The Arabidopsis downy mildew resistance Gene RPP8 is induced by pathogens and salicylic acid and is regulated by W Box cis elements. Mol. Plant. Microbe Interact. 23: 1303–1315. doi:10.1094/MPMI-01-10-0022
- Morán-Diez, E., Rubio, B., Domínguez, S., Hermosa, R., Monte, E., and Nicolás, C. 2012. Transcriptomic response of Arabidopsis thaliana after 24h incubation with the biocontrol fungus Trichoderma harzianum. J. Plant Physiol. 169: 614–620. doi:10.1016/j.jplph.2011.12.016
- Mudge, S. R., Smith, F. W., and Richardson, A. E. 2003. Root-specific and phosphate-regulated expression of phytase under the control of a phosphate transporter promoter enables Arabidopsis to grow on phytate as a sole P source. Plant Sci. 165: 871–878. doi:10.1016/S0168-9452(03)00286-3
- Mustafa, G., Khong, N. G., Tisserant, B., Randoux, B., Fontaine, J., Magnin-Robert, M., Reignault, P., and Sahraoui, A. L.-H. 2017. Defence mechanisms associated with mycorrhiza-induced resistance in wheat against powdery mildew. Funct. Plant Biol. 44: 443–454. doi:10.1071/FP16206
- Muthusamy, M., Kim, J. A., Jeong, M.-J., and Lee, S. I. 2020. Blue and red light upregulate α-expansin 1 (EXPA1) in transgenic Brassica rapa and its overexpression promotes leaf and root growth in Arabidopsis. Plant Growth Regul. 91: 75–87. doi:10.1007/s10725-020-00588-2
- Nanjareddy, K., Arthikala, M.-K., Gómez, B.-M., Blanco, L., and Lara, M. 2017. Differentially expressed genes in mycorrhized and nodulated roots of common bean are associated with defense, cell wall architecture, N metabolism, and P metabolism. PLoS One. 12: e0182328. doi:10.1371/journal.pone.0182328
- Navazio, L., Baldan, B., Moscatiello, R., Zuppini, A., Woo, S. L., Mariani, P., and Lorito, M. 2007. Calcium-mediated perception and defense responses activated in plant cells by metabolite mixtures secreted by the biocontrol fungus Trichoderma atroviride. BMC Plant Biol. 7: 41. doi:10.1186/1471-2229-7-41
- Nizam, S., Qiang, X., Wawra, S., Nostadt, R., Getzke, F., Schwanke, F., Dreyer, I., Langen, G., and Zuccaro, A. 2019. Serendipita indica E5′NT modulates extracellular nucleotide levels in the plant apoplast and affects fungal colonization. EMBO Rep. 20: e47430. doi:10.15252/embr.201847430
- Nguyen, T. T., Volkening, J. D., Rose, C. M., Venkateshwaran, M., Westphall, M. S., Coon, J. J., Ané, J.-M., and Sussman, M. R. 2015. Potential regulatory phosphorylation sites in a Medicago truncatula plasma membrane proton pump implicated during early symbiotic signaling in roots. FEBS Lett. 589: 2186–2193. doi:10.1016/j.febslet.2015.06.035
- Niraula, P. M., Zhang, X., Jeremic, D., Lawrence, K. S., and Klink, V. P. 2021. Xyloglucan endotransglycosylase/hydrolase increases tightly-bound xyloglucan and chain number but decreases chain length contributing to the defense response that Glycine max has to Heterodera glycines. PLoS One. 16: e0244305. doi:10.1371/journal.pone.0244305
- Nouri, E., Breuillin-Sessoms, F., Feller, U., and Reinhardt, D. 2014. Phosphorus and Nitrogen Regulate Arbuscular Mycorrhizal Symbiosis in Petunia hybrida. PLoS One. 9: e90841. doi:10.1371/journal.pone.0090841
- Nussaume, L. 2011. Phosphate import in plants: focus on the PHT1 transporters. Front. Plant Sci. 2: e00083. doi:10.3389/fpls.2011.00083
- Obersteiner, M., Peñuelas, J., Ciais, P., van der Velde, M., and Janssens, I. A. 2013. The phosphorus trilemma. Nature Geosci. 6: 897–898. doi:10.1038/ngeo1990
- Oldroyd, G. E. D. 2013. Speak, friend, and enter: signalling systems that promote beneficial symbiotic associations in plants. Nat. Rev. Microbiol. 11: 252–263. doi:10.1038/nrmicro2990
- Opitz, M. W., Daneshkhah, R., Lorenz, C., Ludwig, R., Steinkellner, S., and Wieczorek, K. 2021. Serendipita indica changes host sugar and defense status in Arabidopsis thaliana: Cooperation or exploitation? Planta 253: 74. doi:10.1007/s00425-021-03587-3
- Oropeza-Aburto, A., Cruz-Ramírez, A., Acevedo-Hernández, G. J., Pérez-Torres, C.-A., Caballero-Pérez, J., and Herrera-Estrella, L. 2012. Functional analysis of the Arabidopsis PLDZ2 promoter reveals an evolutionarily conserved low-Pi-responsive transcriptional enhancer element. J. Exp. Bot. 63: 2189–2202. doi:10.1093/jxb/err446
- Palmgren, M. G. 2001. Plant plasma membrane H+-ATPases: powerhouses for nutrient uptake. Annu. Rev. Plant Physiol. Plant Mol. Biol. 52: 817–845. doi:10.1146/annurev.arplant.52.1.817
- Pan, H., Stonoha-Arther, C., and Wang, D. 2018. Medicago plants control nodulation by regulating proteolysis of the receptor-like kinase DMI2. Plant Physiol. 177: 792–802. doi:10.1104/pp.17.01542
- Panikashvili, D., Savaldi-Goldstein, S., Mandel, T., Yifhar, T., Franke, R. B., Höfer, R., Schreiber, L., Chory, J., and Aharoni, A. 2007. The Arabidopsis DESPERADO/AtWBC11 transporter is required for cutin and wax secretion. Plant Physiol. 145: 1345–1360. doi:10.1104/pp.107.105676
- Panikashvili, D., Shi, J. X., Schreiber, L., and Aharoni, A. 2011. The Arabidopsis ABCG13 transporter is required for flower cuticle secretion and patterning of the petal epidermis. New Phytol. 190: 113–124. doi:10.1111/j.1469-8137.2010.03608.x
- Park, Y. B., and Cosgrove, D. J. 2012. Changes in cell wall biomechanical properties in the Xyloglucan-Deficient xxt1/xxt2 mutant of arabidopsis. Plant Physiol. 158: 465–475. doi:10.1104/pp.111.189779
- Park, Y. B., and Cosgrove, D. J. 2015. Xyloglucan and its interactions with other components of the growing cell wall. Plant Cell Physiol. 56: 180–194. doi:10.1093/pcp/pcu204
- Parniske, M. 2008. Arbuscular mycorrhiza: the mother of plant root endosymbioses. Nat. Rev. Microbiol. 6: 763–775. doi:10.1038/nrmicro1987
- Pellegrino, E., Öpik, M., Bonari, E., and Ercoli, L. 2015. Responses of wheat to arbuscular mycorrhizal fungi: a meta-analysis of field studies from 1975 to 2013. Soil Biol. Biochem. 84: 210–217. doi:10.1016/j.soilbio.2015.02.020
- Peškan-Berghöfer, T., Shahollari, B., Giong, P. H., Hehl, S., Markert, C., Blanke, V., Kost, G., Varma, A., and Oelmuller, R. 2004. Association of Piriformospora indica with Arabidopsis thaliana roots represents a novel system to study beneficial plant–microbe interactions and involves early plant protein modifications in the endoplasmic reticulum and at the plasma membrane. Physiol. Plant. 122: 465–477. doi:10.1111/j.1399-3054.2004.00424.x
- Petit, J., Bres, C., Mauxion, J.-P., Wong Jun Tai, F., Martin, L. B. B., Fich, E. A., Joubès, J., Rose, J. K. C., Domergue, F., and Rothan, C. 2016. The glycerol-3-phosphate acyltransferase GPAT6 from tomato plays a central role in fruit cutin biosynthesis. Plant Physiol. 171: 894–913. 2016. doi:10.1104/pp.16.00409
- Philippe, G., Sørensen, I., Jiao, C., Sun, X., Fei, Z., Domozych, D. S., and Rose, J. K. 2020. Cutin and suberin: assembly and origins of specialized lipidic cell wall scaffolds. Curr. Opin. Plant Biol. 55: 11–20. doi:10.1016/j.pbi.2020.01.008
- Pimprikar, P., Carbonnel, S., Paries, M., Katzer, K., Klingl, V., Bohmer, M. J., Karl, L., Floss, D. S., Harrison, M. J., Parniske, M., and Gutjahr, C. 2016. A CCaMK-CYCLOPS-DELLA complex activates transcription of RAM1 to regulate arbuscule branching. Curr. Biol. 26: 987–998. doi:10.1016/j.cub.2016.01.069
- Pimprikar, P., and Gutjahr, C. 2018. Transcriptional regulation of arbuscular mycorrhiza development. Plant Cell Physiol. 59: 673–690. doi:10.1093/pcp/pcy024
- Pingali, P. L., and Rosegrant, M. W. 1994. Confronting the environmental consequences of the green revolution in Asia (No. 581-2016-39465).
- Pollard, M., Beisson, F., Li, Y., and Ohlrogge, J. B. 2008. Building lipid barriers: biosynthesis of cutin and suberin. Trends Plant Sci. 13: 236–246. doi:10.1016/j.tplants.2008.03.003
- Pozo, M. J., and Azcón-Aguilar, C. 2007. Unraveling mycorrhiza-induced resistance. Curr. Opin. Plant Biol. 10: 393–398. doi:10.1016/j.pbi.2007.05.004
- Radhakrishnan, G. V., Keller, J., Rich, M. K., Vernié, T., Mbadinga Mbadinga, D. L., Vigneron, N., Cottret, L., Clemente, H. S., Libourel, C., Cheema, J., Linde, A.-M., Eklund, D. M., Cheng, S., Wong, G. K. S., Lagercrantz, U., Li, F.-W., Oldroyd, G. E. D., and Delaux, P.-M. 2020. An ancestral signalling pathway is conserved in intracellular symbioses-forming plant lineages. Nat. Plants. 6: 280–289. doi:10.1038/s41477-020-0613-7
- Rebocho, A. B., Southam, P., Kennaway, J. R., Bangham, J. A., and Coen, E. 2017. Generation of shape complexity through tissue conflict resolution. ELife 6: e20156. doi:10.7554/eLife.20156
- Rehman, S. U., Chohan, Z. H., Gulnaz, F., and Supuran, C. T. 2005. In-vitro antibacterial, antifungal and cytotoxic activities of some coumarins and their metal complexes. J. Enzyme Inhib. Med. Chem. 20: 333–340. doi:10.1080/14756360500141911
- Ren, Z., Suolang, B., Fujiwara, T., Yang, D., Saijo, Y., Kinoshita, T., and Wang, Y. 2021. Promotion and upregulation of a plasma membrane proton-ATPase strategy: principles and applications. Front. Plant Sci. 12: 749337. doi:10.3389/fpls.2021.749337
- Reynolds, K. B., Taylor, M. C., Zhou, X.-R., Vanhercke, T., Wood, C. C., Blanchard, C. L., Singh, S. P., and Petrie, J. R. 2015. Metabolic engineering of medium-chain fatty acid biosynthesis in Nicotiana benthamiana plant leaf lipids. Front. Plant Sci. 6: e00164. doi:10.3389/fpls.2015.00164
- Rich, M. K., Courty, P.-E., Roux, C., and Reinhardt, D. 2017a. Role of the GRAS transcription factor ATA/RAM1 in the transcriptional reprogramming of arbuscular mycorrhiza in Petunia hybrida. BMC Genomics. 18: 589. doi:10.1186/s12864-017-3988-8
- Rich, M. K., Nouri, E., Courty, P.-E., and Reinhardt, D. 2017b. Diet of arbuscular mycorrhizal fungi: bread and butter? Trends Plant Sci. 22: 652–660. doi:10.1016/j.tplants.2017.05.008
- Rich, M. K., Vigneron, N., Libourel, C., Keller, J., Xue, L., Hajheidari, M., Radhakrishnan, G. V., Le Ru, A., Diop, S. I., Potente, G., Conti, E., Duijsings, D., Batut, A., Le Faouder, P., Kodama, K., Kyozuka, J., Sallet, E., Bécard, G., Rodriguez-Franco, M., Ott, T., Bertrand-Michel, J., Oldroyd, G. E. D., Szövényi, P., Bucher, M., and Delaux, P.-M. 2021. Lipid exchanges drove the evolution of mutualism during plant terrestrialization. Science 372: 864–868. doi:10.1126/science.abg0929
- Ried, M. K., Antolín-Llovera, M., and Parniske, M. 2014. Spontaneous symbiotic reprogramming of plant roots triggered by receptor-like kinases. ELife 3: e03891. doi:10.7554/eLife.03891
- Ried, M. K., Banhara, A., Hwu, F.-Y., Binder, A., Gust, A. A., Höfle, C., Hückelhoven, R., Nürnberger, T., and Parniske, M. 2019. A set of Arabidopsis genes involved in the accommodation of the downy mildew pathogen Hyaloperonospora arabidopsidis. PLoS Pathog. 15: e1007747. doi:10.1371/journal.ppat.1007747
- Rillig, M. C., Aguilar-Trigueros, C. A., Camenzind, T., Cavagnaro, T. R., Degrune, F., Hohmann, P., Lammel, D. R., Mansour, I., Roy, J., van der Heijden, M. G. A., and Yang, G. 2019. Why farmers should manage the arbuscular mycorrhizal symbiosis. New Phytol. 222: 1171–1175. doi:10.1111/nph.15602
- Robe, K., Conejero, G., Gao, F., Lefebvre-Legendre, L., Sylvestre-Gonon, E., Rofidal, V., Hem, S., Rouhier, N., Barberon, M., Hecker, A., Gaymard, F., Izquierdo, E., and Dubos, C. 2021. Coumarin accumulation and trafficking in Arabidopsis thaliana : a complex and dynamic process. New Phytol. 229: 2062–2079. doi:10.1111/nph.17090
- Rose, J. K. C., Braam, J., Fry, S. C., and Nishitani, K. 2002. The XTH family of enzymes involved in xyloglucan endotransglucosylation and endohydrolysis: current perspectives and a new unifying nomenclature. Plant Cell Physiol. 43: 1421–1435. doi:10.1093/pcp/pcf171
- Rouphael, Y., Franken, P., Schneider, C., Schwarz, D., Giovannetti, M., Agnolucci, M., Pascale, S. D., Bonini, P., and Colla, G. 2015. Arbuscular mycorrhizal fungi act as biostimulants in horticultural crops. Sci. Hortic. 196: 91–108. doi:10.1016/j.scienta.2015.09.002
- Roy, S., Breakspear, A., Cousins, D., Torres-Jerez, I., Jackson, K., Kumar, A., Su, Y., Liu, C.-W., Krom, N., Udvardi, M., Xu, P., and Murray, J. D. 2021. Three common symbiotic ABC subfamily B transporters in Medicago truncatula are regulated by a NIN-independent branch of the symbiosis signaling pathway. Mol. Plant. Microbe Interact. 34: 939–951. doi:10.1094/MPMI-02-21-0036-R
- Rudaya, E. S., Kozyulina, P., Yu, Pavlova, O. A., Dolgikh, A. V., Ivanova, A. N., and Dolgikh, E. A. 2021. Regulation of the later stages of nodulation stimulated by IPD3/CYCLOPS transcription factor and cytokinin in Pea Pisum sativum L. Plants 11: 56. doi:10.3390/plants11010056
- Ruth, B., Khalvati, M., and Schmidhalter, U. 2011. Quantification of mycorrhizal water uptake via high-resolution on-line water content sensors. Plant Soil 342: 459–468. doi:10.1007/s11104-010-0709-3
- Ryan, M. H., and Graham, J. H. 2018. Little evidence that farmers should consider abundance or diversity of arbuscular mycorrhizal fungi when managing crops. New Phytol. 220: 1092–1107. doi:10.1111/nph.15308
- Saha, S., Dutta, A., Bhattacharya, A., and DasGupta, M. 2014. Intracellular Catalytic Domain of Symbiosis Receptor Kinase Hyperactivates Spontaneous Nodulation in Absence of Rhizobia. Plant Physiology 166: 1699–1708.
- Salas-Marina, M. A., Silva-Flores, M. A., Uresti-Rivera, E. E., Castro-Longoria, E., Herrera-Estrella, A., and Casas-Flores, S. 2011. Colonization of Arabidopsis roots by Trichoderma atroviride promotes growth and enhances systemic disease resistance through jasmonic acid/ethylene and salicylic acid pathways. Eur. J. Plant Pathol. 131: 15–26. doi:10.1007/s10658-011-9782-6
- Samalova, M., Elsayad, K., Melnikava, A., Peaucelle, A., Gahurova, E., Gumulec, J., Spyroglou, I., Zemlyanskaya, E. V., Ubogoeva, E. V., and Hejatko, J. 2020. Expansin-controlled cell wall stiffness regulates root growth in Arabidopsis Plant Biology.
- Sampedro, I., Aranda, E., Diaz, R., Ocampo, J. A., and Garcia-Romera, I. 2007. Xyloglucanases in the interaction between the arbuscular mycorrhizal fungus Glomus mosseae and Rhizobium. Symbiosis 43: 29–36.
- Schoenherr, A. P., Rizzo, E., Jackson, N., Manosalva, P., and Gomez, S. K. 2019. Mycorrhiza-induced resistance in potato involves priming of defense responses against cabbage looper (Noctuidae: Lepidoptera). Environ. Entomol. 48: 370–381. doi:10.1093/ee/nvy195
- Scholz, R. W., Ulrich, A. E., Eilittä, M., and Roy, A. 2013. Sustainable use of phosphorus: a finite resource. Sci. Total Environ. 461–462: 799–803. doi:10.1016/j.scitotenv.2013.05.043
- Schreiber, L. 2010. Transport barriers made of cutin, suberin and associated waxes. Trends Plant Sci. 15: 546–553. doi:10.1016/j.tplants.2010.06.004
- Schulz, A., Beyhl, D., Marten, I., Wormit, A., Neuhaus, E., Poschet, G., Büttner, M., Schneider, S., Sauer, N., and Hedrich, R. 2011. Proton-driven sucrose symport and antiport are provided by the vacuolar transporters SUC4 and TMT1/2: AtSUC4- and AtTMT1/2-mediated vacuolar sucrose transport. Plant J. 68: 129–136. doi:10.1111/j.1365-313X.2011.04672.x
- Schüβler, A., Schwarzott, D., and Walker, C. 2001. A new fungal phylum, the Glomeromycota: phylogeny and evolution. Mycol. Res. 105: 1413–1421. doi:10.1017/S0953756201005196
- Shepherd, T., and Wynne Griffiths, D. 2006. The effects of stress on plant cuticular waxes. New Phytol. 171: 469–499. doi:10.1111/j.1469-8137.2006.01826.x
- Shi, J., Zhao, B., Zheng, S., Zhang, X., Wang, X., Dong, W., Xie, Q., Wang, G., Xiao, Y., Chen, F., Yu, N., and Wang, E. 2021. A phosphate starvation response-centered network regulates mycorrhizal symbiosis. Cell 184: 5527–5540.e18. doi:10.1016/j.cell.2021.09.030
- Shinya, T., Nakagawa, T., Kaku, H., and Shibuya, N. 2015. Chitin-mediated plant–fungal interactions: catching, hiding and handshaking. Curr. Opin. Plant Biol. 26: 64–71. doi:10.1016/j.pbi.2015.05.032
- Siao, W., Coskun, D., Baluška, F., Kronzucker, H. J., and Xu, W. 2020. Root-Apex proton fluxes at the centre of soil-stress acclimation. Trends Plant Sci. 25: 794–804. doi:10.1016/j.tplants.2020.03.002
- Siciliano, V., Genre, A., Balestrini, R., Cappellazzo, G., deWit, P. J. G. M., and Bonfante, P. 2007. Transcriptome analysis of arbuscular mycorrhizal roots during development of the prepenetration apparatus. Plant Physiol. 144: 1455–1466. doi:10.1104/pp.107.097980
- Singh, S., Katzer, K., Lambert, J., Cerri, M., and Parniske, M. 2014. CYCLOPS, A DNA-binding transcriptional activator, orchestrates symbiotic root nodule development. Cell Host Microbe. 15: 139–152. doi:10.1016/j.chom.2014.01.011
- Sobkowiak, L., Bielewicz, D., Malecka, E. M., Jakobsen, I., Albrechtsen, M., Szweykowska-Kulinska, Z., and Pacak, A. 2012. The role of the P1BS element containing promoter-driven genes in pi transport and homeostasis in plants. Front. Plant Sci. 3: e00058. doi:10.3389/fpls.2012.00058
- Soundappan, I., Bennett, T., Morffy, N., Liang, Y., Stanga, J. P., Abbas, A., Leyser, O., and Nelson, D. C. 2015. SMAX1-LIKE/D53 family members enable distinct MAX2-dependent responses to strigolactones and karrikins in Arabidopsis. Plant Cell. 27: 3143–3159. doi:10.1105/tpc.15.00562
- Stassen, M. J. J., Hsu, S.-H., Pieterse, C. M. J., and Stringlis, I. A. 2021. Coumarin communication along the microbiome–root–shoot axis. Trends Plant Sci. 26: 169–183. doi:10.1016/j.tplants.2020.09.008
- Stringlis, I. A., de. Jonge, R., and Pieterse, C. M. J. 2019. The age of coumarins in plant–microbe interactions. Plant Cell Physiol. 60: 1405–1419. doi:10.1093/pcp/pcz076
- Stringlis, I. A., Yu, K., Feussner, K., Jonge, R., de, Van Bentum, S., Van Verk, M. C., Berendsen, R. L., Bakker, P. A. H. M., Feussner, I., and Pieterse, C. M. J. 2018. MYB72-dependent coumarin exudation shapes root microbiome assembly to promote plant health. Proc. Natl. Acad. Sci. USA. 115: 1722335. doi:10.1073/pnas.1722335115
- Subramanian, K. S., Santhanakrishnan, P., and Balasubramanian, P. 2006. Responses of field grown tomato plants to arbuscular mycorrhizal fungal colonization under varying intensities of drought stress. Sci. Hortic. 107: 245–253. doi:10.1016/j.scienta.2005.07.006
- Sugiura, Y., Akiyama, R., Tanaka, S., Yano, K., Kameoka, H., Marui, S., Saito, M., Kawaguchi, M., Akiyama, K., and Saito, K. 2020. Myristate can be used as a carbon and energy source for the asymbiotic growth of arbuscular mycorrhizal fungi. Proc. Natl. Acad. Sci. USA. 117: 25779–25788. doi:10.1073/pnas.2006948117
- Sui, N., Tian, S., Wang, W., Wang, M., and Fan, H. 2017. Overexpression of Glycerol-3-phosphate acyltransferase from suaeda salsa improves salt tolerance in arabidopsis. Front. Plant Sci. 8: 1337. doi:10.3389/fpls.2017.01337
- Sun, L., and van Nocker, S. 2010. Analysis of promoter activity of members of the PECTATE LYASE-LIKE (PLL) gene family in cell separation in Arabidopsis. BMC Plant Biol. 10: 152. doi:10.1186/1471-2229-10-152
- Sun, Y., Qiao, Z., Muchero, W., and Chen, J.-G. 2020. Lectin receptor-like kinases: the sensor and mediator at the plant cell surface. Front. Plant Sci. 11: 596301. doi:10.3389/fpls.2020.596301
- Takeda, N., Handa, Y., Tsuzuki, S., Kojima, M., Sakakibara, H., and Kawaguchi, M. 2015. Gibberellins interfere with symbiosis signaling and gene expression and alter colonization by arbuscular mycorrhizal fungi in Lotus japonicus. Plant Physiol. 167: 545–557. doi:10.1104/pp.114.247700
- Tanaka, S., Hashimoto, K., Kobayashi, Y., Yano, K., Maeda, T., Kameoka, H., Ezawa, T., Saito, K., Akiyama, K., and Kawaguchi, M. 2022. Asymbiotic mass production of the arbuscular mycorrhizal fungus Rhizophagus clarus. Commun. Biol. 5: 43. doi:10.1038/s42003-021-02967-5
- Todd, J., Post-Beittenmiller, D., and Jaworski, J. G. 1999. KCS1encodes a fatty acid elongase 3-ketoacyl-CoA synthase affecting wax biosynthesis inArabidopsis thaliana. Plant J. 17: 119–130. doi:10.1046/j.1365-313x.1999.00352.x
- Toh, S., Takata, N., Ando, E., Toda, Y., Wang, Y., Hayashi, Y., Mitsuda, N., Nagano, S., Taniguchi, T., and Kinoshita, T. 2021. Overexpression of plasma membrane H+-ATPase in guard cells enhances light-induced stomatal opening, photosynthesis, and plant growth in hybrid Aspen. Front. Plant Sci. 12: 766037. doi:10.3389/fpls.2021.766037
- Trapet, P. L., Verbon, E. H., Bosma, R. R., Voordendag, K., Van Pelt, J. A., and Pieterse, C. M. J. 2021. Mechanisms underlying iron deficiency-induced resistance against pathogens with different lifestyles. J. Exp. Bot. 72: 2231–2241. doi:10.1093/jxb/eraa535
- Trépanier, M., Bécard, G., Moutoglis, P., Willemot, C., Gagné, S., Avis, T. J., and Rioux, J.-A. 2005. Dependence of arbuscular-mycorrhizal fungi on their plant host for palmitic acid synthesis. Appl. Environ. Microbiol. 71: 5341–5347. doi:10.1128/AEM.71.9.5341-5347.2005
- Tseng, Y.-H., Rouina, H., Groten, K., Rajani, P., Furch, A. C. U., Reichelt, M., Baldwin, I. T., Nataraja, K. N., Uma Shaanker, R., and Oelmüller, R. 2020. An endophytic trichoderma strain promotes growth of its hosts and defends against pathogen attack. Front. Plant Sci. 11: 573670. doi:10.3389/fpls.2020.573670
- Vahabi, K., Dorcheh, S. K., Monajembashi, S., Westermann, M., Reichelt, M., Falkenberg, D., Hemmerich, P., Sherameti, I., and Oelmüller, R. 2016. Stress promotes Arabidopsis – Piriformospora indica interaction. Plant Signal. Behav. 11: e1136763. doi:10.1080/15592324.2015.1136763
- Van Sandt, V. S. T., Suslov, D., Verbelen, J.-P., and Vissenberg, K. 2007. Xyloglucan endotransglucosylase activity loosens a plant cell wall. Ann. Bot. 100: 1467–1473. doi:10.1093/aob/mcm248
- Vangelisti, A., Natali, L., Bernardi, R., Sbrana, C., Turrini, A., Hassani-Pak, K., Hughes, D., Cavallini, A., Giovannetti, M., and Giordani, T. 2018. Transcriptome changes induced by arbuscular mycorrhizal fungi in sunflower (Helianthus annuus L.) roots. Sci. Rep. 8: 4. doi:10.1038/s41598-017-18445-0
- Veiga, R. S., Faccio, A., Genre, A., Pieterse, C. M., Bonfante, P., and van der Heijden, M. G. 2013. Arbuscular mycorrhizal fungi reduce growth and infect roots of the non‐host plant A rabidopsis thaliana. Plant. Cell Environ. 36: 1926–1937. doi:10.1111/pce.12102
- Venturi, V., and Keel, C. 2016. Signaling in the rhizosphere. Trends Plant Sci. 21: 187–198. doi:10.1016/j.tplants.2016.01.005
- Verbruggen, E., Veresoglou, S. D., Anderson, I. C., Caruso, T., Hammer, E. C., Kohler, J., and Rillig, M. C. 2013. Arbuscular mycorrhizal fungi – short‐term liability but long‐term benefits for soil carbon storage? New Phytol. 197: 366–368. doi:10.1111/nph.12079
- Vissenberg, K., Oyama, M., Osato, Y., Yokoyama, R., Verbelen, J.-P., and Nishitani, K. 2005. Differential expression of AtXTH17, AtXTH18, AtXTH19 and AtXTH20 genes in arabidopsis roots. physiological roles in specification in cell wall construction. Plant Cell Physiol. 46: 192–200. doi:10.1093/pcp/pci013
- Vogler, H., Caderas, D., Mandel, T., and Kuhlemeier, C. 2003. Domains of expansin gene expression define growth regions in the shoot apex of tomato. Plant Mol. Biol. 53: 267–272. doi:10.1023/b:plan.0000006999.48516.be
- Vos, C., Schouteden, N., Tuinen, D., van, Chatagnier, O., Elsen, A., De Waele, D., Panis, B., and Gianinazzi-Pearson, V. 2013. Mycorrhiza-induced resistance against the root–knot nematode Meloidogyne incognita involves priming of defense gene responses in tomato. Soil Biol. Biochem. 60: 45–54. doi:10.1016/j.soilbio.2013.01.013
- Vos, C., Van Den Broucke, D., Lombi, F. M., De Waele, D., and Elsen, A. 2012. Mycorrhiza-induced resistance in banana acts on nematode host location and penetration. Soil Biol. Biochem. 47: 60–66. doi:10.1016/j.soilbio.2011.12.027
- Walder, F., and van der Heijden, M. G. A. 2015. Regulation of resource exchange in the arbuscular mycorrhizal symbiosis. Nat. Plants. 1: 15159. doi:10.1038/nplants.2015.159
- Walters, D. R., Ratsep, J., and Havis, N. D. 2013. Controlling crop diseases using induced resistance: challenges for the future. J. Exp. Bot. 64: 1263–1280. doi:10.1093/jxb/ert026
- Wang, B., and Qiu, Y.-L. 2006. Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza 16: 299–363. doi:10.1007/s00572-005-0033-6
- Wang, E., Schornack, S., Marsh, J. F., Gobbato, E., Schwessinger, B., Eastmond, P., Schultze, M., Kamoun, S., and Oldroyd, G. E. D. 2012. A common signaling process that promotes mycorrhizal and oomycete colonization of plants. Curr. Biol. 22: 2242–2246. doi:10.1016/j.cub.2012.09.043
- Wang, E., Yu, N., Bano, S. A., Liu, C., Miller, A. J., Cousins, D., Zhang, X., Ratet, P., Tadege, M., Mysore, K. S., Downie, J. A., Murray, J. D., Oldroyd, G. E. D., and Schultze, M. 2014a. A H+-ATPase that energizes nutrient uptake during mycorrhizal symbioses in rice and Medicago truncatula. Plant Cell. 26: 1818–1830. doi:10.1105/tpc.113.120527
- Wang, T., Guo, J., Peng, Y., Lyu, X., Liu, B., Sun, S., and Wang, X. 2021. Light-induced mobile factors from shoots regulate rhizobium-triggered soybean root nodulation. Science 374: 65–71. doi:10.1126/science.abh2890
- Wang, T., Phyo, P., and Hong, M. 2016. Multidimensional solid-state NMR spectroscopy of plant cell walls. Solid State Nucl. Magn. Reson. 78: 56–63. doi:10.1016/j.ssnmr.2016.08.001
- Wang, Y., Chen, H., Shao, M., Zhu, T., Li, S., Olsson, P. A., and Hammer, E. C. 2023. Arbuscular mycorrhizal fungi trigger danger‐associated peptide signaling and inhibit carbon–phosphorus exchange with nonhost plants. Plant. Cell Environ. 46: 2206–2221. doi:10.1111/pce.14600
- Wang, Y., Noguchi, K., Ono, N., Inoue, S., Terashima, I., and Kinoshita, T. 2014. Overexpression of plasma membrane H+-ATPase in guard cells promotes light-induced stomatal opening and enhances plant growth. Proc. Natl. Acad. Sci. USA. 111: 533–538. doi:10.1073/pnas.1305438111
- Wang, C., and Wang, E. 2016. Arabidopsis Farms Colletotrichum tofieldiae for Phosphate Uptake. Mol. Plant. 9: 953–955. doi:10.1016/j.molp.2016.05.007
- Wang, Y., and Wu, W.-H. 2013. Potassium transport and signaling in higher plants. Annu. Rev. Plant Biol. 64: 451–476. doi:10.1146/annurev-arplant-050312-120153
- Wang, Y.-Y., Cheng, Y.-H., Chen, K.-E., and Tsay, Y.-F. 2018. Nitrate transport, signaling, and use efficiency. Annu. Rev. Plant Biol. 69: 85–122. doi:10.1146/annurev-arplant-042817-040056
- Waters, M. T., and Nelson, D. C. 2023. Karrikin perception and signalling. New Phytol. 237: 1525–1541. doi:10.1111/nph.18598
- Weidmann, S., Sanchez, L., Descombin, J., Chatagnier, O., Gianinazzi, S., and Gianinazzi-Pearson, V. 2004. Fungal elicitation of signal transduction-related plant genes precedes mycorrhiza establishment and requires the dmi3 Gene in Medicago truncatula. Mol. Plant. Microbe Interact. 17: 1385–1393. doi:10.1094/MPMI.2004.17.12.1385
- Wewer, V., Brands, M., and Dörmann, P. 2014. Fatty acid synthesis and lipid metabolism in the obligate biotrophic fungus Rhizophagus irregularis during mycorrhization of Lotus japonicus. Plant J. 79: 398–412. doi:10.1111/tpj.12566
- Wielandt, A. G., Pedersen, J. T., Falhof, J., Kemmer, G. C., Lund, A., Ekberg, K., Fuglsang, A. T., Pomorski, T. G., Buch-Pedersen, M. J., and Palmgren, M. 2015. Specific activation of the plant p-type plasma membrane H+-ATPase by lysophospholipids depends on the autoinhibitory N- and C-terminal domains. J. Biol. Chem. 290: 16281–16291. doi:10.1074/jbc.M114.617746
- Wiśniewska, M., and Golinowski, W. 2011. Immunolocalization of α-expansin protein (NtEXPA5) in tobacco roots in the presence of the arbuscular mycorrhizal fungus glomus mosseae Nicol. & Gerd. Acta Biologica Cracoviensia Series Botanica 53: e0034z. doi:10.2478/v10182-011-0034-z
- Wright, S. F., and Nichols, K. A. 2002. Glomalin: hiding place for a third of the world’s stored soil carbon. Agric. Res. 50: 4–7.
- Wu, C., Qu, J., Liu, L., Kang, H., Sun, H., Zhang, Y., Ghorbani, A., and Pehlivan, N. 2021. Quo vadis: signaling molecules and small secreted proteins from mycorrhizal fungi at the early stage of mycorrhiza formation. Symbiosis 85: 123–143. doi:10.1007/s13199-021-00793-1
- Wu, Q.-S., Zou, Y.-N., and Huang, Y.-M. 2013. The arbuscular mycorrhizal fungus Diversispora spurca ameliorates effects of waterlogging on growth, root system architecture and antioxidant enzyme activities of citrus seedlings. Fungal Ecol. 6: 37–43. doi:10.1016/j.funeco.2012.09.002
- Xu, L., Wu, C., Oelmüller, R., and Zhang, W. 2018. Role of phytohormones in piriformospora indica-induced growth promotion and stress tolerance in plants: more questions than answers. Front. Microbiol. 9: 1646. doi:10.3389/fmicb.2018.01646
- Yang, C., Guo, R., Jie, F., Nettleton, D., Peng, J., Carr, T., Yeakley, J. M., Fan, J.-B., and Whitham, S. A. 2007. Spatial Analysis of Arabidopsis thaliana Gene Expression in Response to Turnip mosaic virus Infection. Mol. Plant. Microbe Interact. 20: 358–370. doi:10.1094/MPMI-20-4-0358
- Yang, W., Pollard, M., Li-Beisson, Y., Beisson, F., Feig, M., and Ohlrogge, J. 2010. A distinct type of glycerol-3-phosphate acyltransferase with sn -2 preference and phosphatase activity producing 2-monoacylglycerol. Proc. Natl. Acad. Sci. USA. 107: 12040–12045. doi:10.1073/pnas.0914149107
- Yeats, T. H., Huang, W., Chatterjee, S., Viart, H. M.-F., Clausen, M. H., Stark, R. E., and Rose, J. K. C. 2014. Tomato Cutin Deficient 1 (CD1) and putative orthologs comprise an ancient family of cutin synthase-like (CUS) proteins that are conserved among land plants. Plant J. 77: 667–675. doi:10.1111/tpj.12422
- Yoneyama, K., Xie, X., Sekimoto, H., Takeuchi, Y., Ogasawara, S., Akiyama, K., Hayashi, H., and Yoneyama, K. 2008. Strigolactones, host recognition signals for root parasitic plants and arbuscular mycorrhizal fungi, from Fabaceae plants. New Phytol. 179: 484–494. doi:10.1111/j.1469-8137.2008.02462.x
- Yooyongwech, S., Samphumphuang, T., Tisarum, R., Theerawitaya, C., and Cha-Um, S. 2016. Arbuscular mycorrhizal fungi (AMF) improved water deficit tolerance in two different sweet potato genotypes involves osmotic adjustments via soluble sugar and free proline. Sci. Hortic. 198: 107–117. doi:10.1016/j.scienta.2015.11.002
- Young, J. C., DeWitt, N. D., and Sussman, M. R. 1998. A transgene encoding a plasma membrane H+-ATPase that confers acid resistance in arabidopsis thaliana seedlings. Genetics 149: 501–507. doi:10.1093/genetics/149.2.501
- Yu, N., Luo, D., Zhang, X., Liu, J., Wang, W., Jin, Y., Dong, W., Liu, J., Liu, H., Yang, W., Zeng, L., Li, Q., He, Z., Oldroyd, G. E. D., and Wang, E. 2014. A DELLA protein complex controls the arbuscular mycorrhizal symbiosis in plants. Cell Res. 24: 130–133. doi:10.1038/cr.2013.167
- Yuan, W., Zhang, D., Song, T., Xu, F., Lin, S., Xu, W., Li, Q., Zhu, Y., Liang, J., and Zhang, J. 2017a. Arabidopsis plasma membrane H+-ATPase genes AHA2 and AHA7 have distinct and overlapping roles in the modulation of root tip H+ efflux in response to low-phosphorus stress. J. Exp. Bot. 68: 1731–1741. doi:10.1093/jxb/erx040
- Yuan, Y., Gao, L., Sun, R., Yu, T., Liang, Y., Li, D., and Zheng, Y. 2017b. Seed-specific expression of an acyl-acyl carrier protein thioesterase CnFatB3 from coconut (Cocos nucifera L.) increases the accumulation of medium-chain fatty acids in transgenic Arabidopsis seeds. Sci. Hortic. 223: 5–9. doi:10.1016/j.scienta.2017.05.029
- Zamioudis, C., Hanson, J., and Pieterse, C. M. J. 2014. β-Glucosidase BGLU42 is a MYB72-dependent key regulator of rhizobacteria-induced systemic resistance and modulates iron deficiency responses in Arabidopsis roots. New Phytol. 204: 368–379. doi:10.1111/nph.12980
- Zhang, C., He, J., Dai, H., Wang, G., Zhang, X., Wang, C., Shi, J., Chen, X., Wang, D., and Wang, E. 2021a. Discriminating symbiosis and immunity signals by receptor competition in rice. Proc. Natl. Acad. Sci. USA. 118: e2023738118. doi:10.1073/pnas.2023738118
- Zhang, F., Zou, Y.-N., and Wu, Q.-S. 2018. Quantitative estimation of water uptake by mycorrhizal extraradical hyphae in citrus under drought stress. Sci. Hortic. 229: 132–136. doi:10.1016/j.scienta.2017.10.038
- Zhang, G., Ahmad, M. Z., Chen, B., Manan, S., Zhang, Y., Jin, H., Wang, X., and Zhao, J. 2020. Lipidomic and transcriptomic profiling of developing nodules reveals the essential roles of active glycolysis and fatty acid and membrane lipid biosynthesis in soybean nodulation. Plant J. 103: 1351–1371. doi:10.1111/tpj.14805
- Zhang, J., Wei, J., Li, D., Kong, X., Rengel, Z., Chen, L., Yang, Y., Cui, X., and Chen, Q. 2017. The role of the plasma membrane H+-ATPase in plant responses to aluminum toxicity. Front. Plant Sci. 8: 1757. doi:10.3389/fpls.2017.01757
- Zhang, M., Wang, Y., Chen, X., Xu, F., Ding, M., Ye, W., Kawai, Y., Toda, Y., Hayashi, Y., Suzuki, T., Zeng, H., Xiao, L., Xiao, X., Xu, J., Guo, S., Yan, F., Shen, Q., Xu, G., Kinoshita, T., and Zhu, Y. 2021. Plasma membrane H+-ATPase overexpression increases rice yield via simultaneous enhancement of nutrient uptake and photosynthesis. Nat. Commun. 12: 735. doi:10.1038/s41467-021-20964-4
- Zhang, Q., Blaylock, L. A., and Harrison, M. J. 2010. Two Medicago truncatula half-ABC transporters are essential for arbuscule development in arbuscular mycorrhizal symbiosis. Plant Cell. 22: 1483–1497. doi:10.1105/tpc.110.074955
- Zhang, X., Dong, W., Sun, J., Feng, F., Deng, Y., He, Z., Oldroyd, G. E. D., and Wang, E. 2015. The receptor kinase CERK1 has dual functions in symbiosis and immunity signalling. Plant J. 81: 258–267. doi:10.1111/tpj.12723
- Zhang, X., Gao, H., Liang, Y., and Cao, Y. 2021. Full-length transcriptome analysis of asparagus roots reveals the molecular mechanism of salt tolerance induced by arbuscular mycorrhizal fungi. Environ. Exp. Bot. 185: 104402. doi:10.1016/j.envexpbot.2021.104402
- Zhu, X.-C., Song, F.-B., and Xu, H.-W. 2010. Arbuscular mycorrhizae improves low temperature stress in maize via alterations in host water status and photosynthesis. Plant Soil. 331: 129–137. doi:10.1007/s11104-009-0239-z
- Zimmermann, P., Zardi, G., Lehmann, M., Zeder, C., Amrhein, N., Frossard, E., and Bucher, M. 2003. Engineering the root-soil interface via targeted expression of a synthetic phytase gene in trichoblasts: Engineering the root-soil interface. Plant Biotechnol. J. 1: 353–360. doi:10.1046/j.1467-7652.2003.00033.x
