Abstract
Since 1998, genetically engineered Bt maize varieties expressing the insecticidal Cry1Ab protein (i.e. event MON 810) have been grown in the European Union (EU), mainly in Spain. These varieties confer resistance against the European and Mediterranean corn borer (ECB and MCB), which are the major lepidopteran maize pests in the EU, particularly in Mediterranean areas. However, widespread, repeated and exclusive use of Bt maize is anticipated to increase the risk of Cry1Ab resistance to evolve in corn borer populations. To delay resistance evolution, typically, refuges of non-Bt maize are planted near or adjacent to, or within Bt maize fields. Moreover, changes in Cry1Ab susceptibility in field populations of corn borers and unexpected damage to maize MON 810, due to corn borers, are monitored on an annual basis. After two decades of Bt maize cultivation in Spain, neither resistant corn borer populations nor farmer complaints on unexpected field damage have been reported. However, whether the resistance monitoring strategy followed in Spain, currently based on discriminating concentration bioassays, is sufficiently sensitive to timely detect early warning signs of resistance in the field remains a point of contention. Moreover, the Cry1Ab resistance allele frequency to Bt maize, which has recently been estimated in MCB populations from north-eastern Spain, might exceed that recommended for successful resistance management. To ensure Bt maize durability in Spain, it is key that adequate resistance management approaches, including monitoring of resistance and farmer compliance with refuge requirements, continue to be implemented and are incorporated in integrated pest management schemes.
Introduction
Genetically engineered (GE) crops expressing insecticidal proteins from the biocontrol agent Bacillus thuringiensis (Bt) have been cultivated globally for 25 years over a continuously growing area. In 2019, the worldwide surface of insect-resistant Bt crops, alone or in combination with other traits such as herbicide tolerance, has exceeded 107 million ha [Citation1]. Commercialized Bt crops mostly include maize, soybean, cotton, and rice, and, to a lesser extent, the eggplant (brinjal). Cultivation of other Bt crops such as cowpea in Nigeria might soon follow [Citation2].
Bt proteins expressed in GE crops confer protection against either certain lepidopteran or coleopteran pests or both, offering a complementary means of control against crop insect pests. The presumed mode of action of Bt proteins is to bind selectively to specific receptors on the epithelial surface of the midgut of susceptible insect species, leading to death through pore formation, cell burst and subsequent septicemia [Citation3–7]. Owing to the targeted specificity of insecticidal Bt proteins expressed in Bt crops to insects from the order of Lepidoptera and Coleoptera, such crops are considered less harmful to other (valued or beneficial) insects (i.e. non-target organisms) and the ecosystem services they contribute to (such as biological control, pollination or decomposition) than broad-spectrum insecticides [Citation8–16]. Deployment of Bt crops has led to some agronomic, environmental and economic benefits compared to conventional crops and their associated farm management practices [Citation17–28]. For example, their adoption has been associated with a reduction in the use of insecticides that are more harmful to the environment, because less or no treatments with soil or broad-spectrum foliar insecticides may be needed [Citation29]. However, there is concern that Bt crops could adversely affect some susceptible non-target insect species when they are exposed to harmful amounts of Bt protein through ingestion/feeding [e.g. Citation30,Citation31]. Moreover, the widespread and repeated use of Bt crops expressing the same Bt protein by individual farmers as the sole pest management option against target insect pests will create significant selection pressure, increasing the potential for pests to evolve resistance to the Bt protein (i.e. heritable reduction in the susceptibility of the target insect pest population to the Bt protein) [Citation32,Citation33]. Resistance evolution in target insect pests is not a direct environmental harm, but resistant populations may require altered pest management practices due to their reduced susceptibility to the Bt protein expressed in a GE crop. Thus, farmers may need to revert to the previously used pest management tools that are more harmful to the environment, and ultimately alter cultivation/farming systems, which may decrease farm income [Citation34] and affect the sustainability of the cropping system. Therefore, in some jurisdictions, the susceptibility of target insect pests to Bt proteins, including those expressed in GE crops, is viewed as a common good to preserve [Citation35,Citation36].
To delay the potential resistance evolution of target insect pests to Bt crops, globally, risk managers typically require that their cultivation is accompanied by the implementation of insect resistance management (IRM) plans, including monitoring strategies. In general, these strategies consist of planting “structured” refuges (i.e. areas with the crop that does not express Bt proteins that are active against the target insect pest) near or adjacent to, or within the Bt crop fields (e.g. in large blocks or as row strips) or mixing non-Bt with Bt seeds in each sowing seed bag. In addition, changes in Bt protein susceptibility in field populations of the targeted insect pests and unexpected damage to GE crops due to the pest are monitored regularly (see below for further details). Despite the adoption of IRM strategies, there are at least 20 instances of field-evolved resistance documented worldwide at present (April 2021). These cases involve eight major agricultural insect pests, nine Bt proteins and in six countries, with reduced pesticide efficacy and practical consequences for pest control [Citation37,Citation38]. The main reasons for the cases of field-evolved reported resistance are the deployment of Bt crop varieties that do not express the Bt protein(s) at a concentration high enough to kill most of the individuals of the targeted pest population and the insufficient planting of non-Bt crop refuges. While the instances of field-evolved resistance have steadily increased over the last decade [Citation39], there are examples of successful implementation of IRM programs accompanying the long-lasting cultivation of Bt crops. In the European Union (EU), this is the case for Spain, where after more than two decades of continuous cultivation of Bt maize (mainly maize event MON 810 that expresses the Cry1Ab protein against the European corn borer (ECB), Ostrinia nubilalis (Lepidoptera: Crambidae), and the Mediterranean corn borer (MCB), Sesamia nonagrioides (Lepidoptera: Noctuidae)), there have been no reports of resistant corn borer populations [Citation40–42]. However, the resistance allele frequency recently estimated in MCB populations from north-eastern Spain might exceed that recommended for a successful implementation of the IRM strategy [Citation43]. Moreover, an unresolved point of contention is whether the current monitoring activities performed in Spain are sufficiently sensitive to timely detect possible field-evolved resistance before becoming irreversible and irremediable [Citation44]. The belated detection of early signs of resistance will significantly hamper the timely implementation of remedial measures to appropriately respond to confirmed resistance, and hence prevent the spread of resistance to other areas or eradicate resistance. Therefore, after briefly reporting on the main lepidopteran pests of maize and the adoption of Bt maize for cultivation in the EU, we: (i) review all relevant ECB and MCB resistance monitoring data gathered for the cultivation of Bt maize in the EU since 1998, focusing on north-eastern Spain where adoption rates of Bt maize are the highest, (ii) discuss whether IRM measures implemented for Bt maize in Spain are adequate to delay resistance evolution, (iii) present the main limitations encountered in the monitoring and management of resistance evolution to Bt maize in Spain so far, and (iv) present recommendations on how to overcome shortcomings to ensure the durability of Bt maize.
Lepidopteran pests of maize in the European Union
ECB and MCB are the most damaging lepidopteran pests to maize in the EU. ECB is widespread throughout Europe, while MCB has a narrower geographical distribution being solely present in the Mediterranean region [Citation45,Citation46]. However, climate changes may favor the further expansion of MCB to other EU territories where it could establish as a new agricultural pest [Citation47]. Both species complete a variable number of generations per year depending on the latitude, ranging from one generation in northern countries (for ECB) up to three generations in Spain and Portugal (for MCB) [Citation48,Citation49].
ECB and MCB larvae cause direct damage to maize plants (i.e. reduced physical strength of the stalk that ultimately may lead to plant lodging, significant metabolic and physiological alterations due to disrupted transport and use of nutrients and water) by boring into the plant stem (tunneling) and feeding on it for most of their larval stage. Occasionally, second-generation larvae feed on maize ears causing wounds through which fungal pathogens can enter the maize plant. Some of these pathogens produce mycotoxins that are toxic to vertebrates [Citation50–52]. Thus, damage to maize plants caused by corn borers may reduce yield, and lead to broken stalks, dropped ears and low grain quality. Insecticidal sprays are frequently ineffective against corn borers because once a larva has entered the maize stem, it is no longer exposed to the chemical treatment and thus protected from the insecticide sprays. In contrast, lepidopteran-active Bt maize plants directly target ECB/MCB larvae feeding on them through the Bt proteins’ expression in plant tissues, so they can be a highly efficient pest control measure against corn borers [Citation53].
ECB and MCB have been defined by Bt crop developers as the main target insect pests of Bt maize in the EU (i.e. target organisms). Oher noctuids such as Sesamia cretica, Agrotis spp., Helicoverpa armigera and Mythimna unipuncta that occasionally cause damage to maize in certain EU regions [Citation45,Citation46] are not considered the target pests of Bt maize: they are not prevalent over the entire European maize growing area, do not regularly occur at high population densities, or cause damage to maize only in certain seasons [Citation54]. The potential for Bt maize resistance evolution in non-target lepidopteran pest species is not addressed in this review.
Cultivation of Bt maize in the European Union
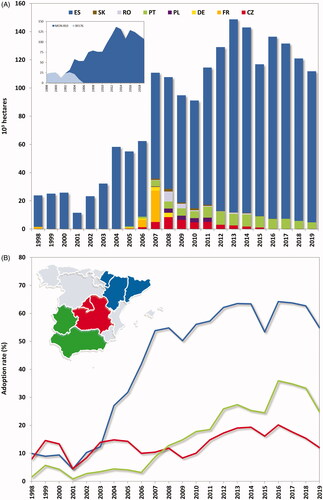
Since 1998, the European Commission has approved the cultivation of two single Bt maize events, i.e. Bt176 and MON 810. Both events express the Cry1Ab protein conferring protection against ECB and MCB larvae. Maize hybrids derived from the event Bt176 were grown in France in 1998, Portugal in 1999, and Spain between 1998 and 2005. Cultivation of maize Bt176 in the EU was discontinued because it contained an antibiotic-resistant marker gene [Citation55], and failed to provide season-long protection against corn borers in some cases [Citation42]. Maize MON 810 cultivation in the EU started in 2003 in Spain when the de facto moratorium on new approvals of genetically modified organisms introduced by the Member States was lifted following the adoption of new legislation. Since then, maize MON 810 varieties have been grown at different commercial scales in nine Member States (). However, the cultivation of Bt maize remains a controversial issue in the EU [Citation56]. Following the adoption of new legislation in 2015 allowing individual Member States to ban or restrict the cultivation of GE crops in their territory, 17 EU Member States and one region have prohibited Bt maize cultivation on their territory (https://ec.europa.eu/food/plant/gmo/authorisation/cultivation/geographical_scope_en). In 2019, only two Member States have cultivated MON 810, with significant acreage in Spain (107,127 ha) and less in Portugal (4,718 ha). Bt maize represents approximately 35% of Spain’s total maize area and less than 10% in Portugal [Citation27]. However, in regions with a high incidence of corn borer infestation such as the Ebro basin in north-eastern Spain, adoption rates of Bt maize can exceed 60% (https://www.mapa.gob.es/).
Figure 1. (A) Total area (103 ha) devoted to Bt maize varieties in the European Union since 1998. The small graph on the upper left represents the area (103 ha) planted with varieties Bt maize events Bt176 and MON 810 in Spain. (B) Adoption rate of Bt maize in the main maize growing areas in Spain since 1998. CZ: Czech Republic; DE: Germany; ES: Spain; FR: France; PL: Poland; PT: Portugal; RO: Romania; SK: Slovakia.
Insect resistance management for Bt maize
In most jurisdictions, the cultivation of GE crops is subject to risk assessment and regulatory approval. In the risk analysis process, the role of risk assessors such as the European Food Safety Authority (EFSA) is to assess any plausible risk that the cultivation of a GE crop may pose to human and animal health and the environment. Decisions to approve cultivation, given potential risk management, are taken by risk managers (i.e. European Commission, EU Member States). As part of the regulatory approval process of Bt crops in the EU and other jurisdictions, applicants (also termed registrants) submitting an application/dossier for cultivation proactively provide an IRM plan. IRM plans are designed to prevent or at least delay resistance evolution in the target insect pests and extend Bt crop durability [Citation34,Citation57–59].
In 2003, the European Association for Bioindustries (EuropaBio) designed a long-term, EU-wide, IRM plan for various lepidopteran-active Bt maize events, including MON 810 (and derived varieties). The IRM plan proposed a standard methodology to manage and monitor potential resistance evolution to Cry1Ab and Cry1F proteins in ECB and MCB populations in the EU [Citation58]. Four main pillars underpin the IRM plan: (i) compliance with refuge obligations, (ii) establishment of baseline susceptibility of corn borers to the Bt protein and monitoring of resistance evolution, (iii) communication and education of farmers, and (iv) remedial actions in case of field-evolved resistance.
High-dose refuge strategy to delay resistance evolution
Currently implemented IRM measures for Bt crops typically are based on the ‘high-dose refuge’ (HDR) strategy, which also forms the cornerstone of the harmonized IRM plan for Bt maize in the EU. This strategy requires that: (i) Bt plants produce sufficiently high concentrations of the Bt protein (i.e. 25 times the amount needed to kill 99% of susceptible individuals and 95% of heterozygous individuals for resistance alleles [Citation59]) so that heterozygotes do not survive exposure in the Bt crop, thus making resistance functionally recessive, and (ii) refuge areas of non-Bt host plants are planted near or adjacent to, or within, the Bt crop fields [Citation60–63]. Refuge areas provide a reservoir of susceptible target insects that will mate with those rare resistant individuals emerging from Bt crop fields. If resistance is a recessive trait, then the heterozygous offspring produced by such crosses will not survive on Bt plants. The optimal size and configuration of refuge areas and their distance to the Bt crop fields depends on: population size, feeding habits and the dispersal abilities of the target insect pest [Citation64,Citation65].
The underlying assumptions of the HDR strategy are that: (i) the Bt protein is expressed at appropriate levels in relevant plant parts, (ii) alleles conferring resistance to Bt proteins are rare or absent in pest populations (ideally their frequency should be below 10−3), and (iii) random mating occurs between resistant insects emerging in Bt crops and susceptible insects preserved on refuges at sufficient levels [Citation34]. Whether the underlying assumptions of the HDR strategy are met for ECB and MCB and Bt maize MON 810 in Spain is described below.
Bt protein is expressed at appropriate levels in relevant parts to the plant:
Cry1Ab protein expression levels in Bt maize may fluctuate during the growing season, resulting in variable selection pressure on different annual ECB/MCB generations. In maize Bt176 varieties, levels of expression of the Cry1Ab protein decreased after anthesis [Citation66]. This decline allowed heterozygous ECB and MCB larvae from the second and third generations to complete their development on those Bt plants, thus accelerating selection for resistant populations [Citation67]. In maize MON 810 plants, however, high concentrations of the Cry1Ab protein are maintained throughout vegetative and reproductive development stages of maize [Citation68,Citation69], conferring season-long protection against all generations of ECB and MCB [Citation67,Citation70,Citation71]. While the genetic background of a maize MON 810 variety, environmental conditions and/or agricultural practices can cause variation in Cry1Ab protein concentration levels, such levels remain sufficiently high to maintain any resistance alleles in corn borers functionally recessive [Citation72].
Initial resistance alleles are rare in the targeted insect pest populations
Allele frequencies for Cry1Ab resistance as expressed in Bt maize were first estimated in MCB populations from north-eastern Spain in the 2004 and 2005 growing seasons [Citation73], following seven and eight successive years of cultivation (). No resistance alleles were detected in any of the 85 isofemale lines tested by F2 screening [Citation74] and the frequency was estimated to be 2.9 × 10−3, with 95% credibility intervals (CI) between 0 and 8.6 × 10−3 (Supplementary material, Table S1). The number of lines tested by Andreadis et al. [Citation73] was insufficient to confirm whether the initial frequency of resistance was below the recommended value of 10−3. However, considering the relatively low adoption rate of Bt maize in that area at that time (∼35%) and the initial allele frequencies estimated in other southern MCB populations [Citation73], the actual frequency was presumed to be lower than the estimated 2.9 × 10−3. More than a decade later, Camargo et al. [Citation43] re-calculated the allele frequency in MCB populations collected in 2016 from the same area. One of the 137 lines screened was shown to carry a resistance allele. The updated frequency was estimated to be 3.6 × 10−3 (95% CI between 4 × 10−4 and 10−2) (Supplementary material, Table S1). These most recent estimates indicate that the frequency of resistance alleles in MCB populations from north-eastern Spain might exceed the value recommended for effective implementation of the HDR strategy. The authors attributed the increased frequency of Cry1Ab resistance alleles to maize MON 810 to the strong selective pressure caused by the widespread, repeated and exclusive use of Bt maize in the Ebro basin.
Table 1. Historical data on Cry1Ab susceptibility from concentration-response assays with Mediterranean and European corn borer populations collected in maize fields from north-eastern Spain between 1998 and 2015.
Unlike MCB, there are no recent estimates of the frequency of Cry1Ab resistance alleles to maize MON 810 in Spanish populations of ECB. This parameter has previously been estimated in populations of ECB collected in: France, Germany, Italy and Slovakia between 1999 and 2004 before Bt maize was grown in these countries. There was no evidence of resistance to Bt maize in any of the 2156 lines tested from those populations, and the expected frequency of resistance alleles was estimated to be 0.0001, with a 95% CI between 0 and 0.0003 [Citation75,Citation76]. Considering the low genetic differentiation of ECB populations in Europe [Citation77], a similarly low initial rate of Cry1Ab resistance alleles was assumed for Spanish populations.
Random mating occurs between resistant insects emerging in Bt crops and susceptible insects preserved on refuges at sufficient levels
For refuges to be effective, their placement, configuration and size should ensure that resistant and susceptible insects mate randomly and that refuges harbor a sufficiently large population of susceptible insects to outnumber resistant ones. How much mingling and mating would occur between individuals emerging from refuges and Bt crop fields is determined by the scale of adult movement.
A series of release and recapture studies, carried out in the United States using light and pheromone traps, showed that adult dispersal for ECB was influenced by agronomic practices and environmental conditions [Citation78]. Adults could move over 40 km in short periods [Citation79], though most males were often recaptured at shorter distances, in the range of 200–800 m from the release site [Citation78,Citation79]. Allozyme analyses of several ECB populations collected from maize fields across different European countries revealed an extensive level of gene flow and thus dispersal between regions [Citation77,Citation80]. In the case of MCB, data from studies carried out in north-eastern Spain using pheromone traps showed that most MCB adults disperse at least 400 m away from emergence points [Citation81,Citation82].
A concern may be the non-synchronous emergence of insects from refuges and Bt crop fields, as this could result in nonrandom (assortative) mating and accelerate resistance development. For MCB, random mating between individuals emerging from refuges and Bt maize fields is assumed to be driven by the dispersal of adult males, because adult females rarely disperse before mating [Citation81–85]. By contrast, adult females of ECB might move out of their fields of emergence before mating [Citation86,Citation87], though virgin females disperse less often than males and mated females [Citation88]. Since field-evolved resistance to Bt maize has not yet occurred in corn borers in the EU, the level of potential local assortative mating remains uncertain. Consequently, this is an important assumption to test for reducing uncertainty about the potential of the HDR strategy to delay resistance evolution should resistance occur.
Refuge requirements
In the EU, farmers growing more than 5 ha of Bt maize must plant structured refuges equivalent to at least 20% of the total Bt maize area. Refuge areas should be planted near (within a 750 m distance) or adjacent to, or within the Bt maize fields either as blocks or strips of non-Bt maize. For clusters of Bt maize fields with an aggregated area greater than 5 ha, EFSA advocated planting refuges, irrespective of individual field and farm size [Citation89]. Whenever possible, refuge maize should be selected based on equivalent maturity to Bt maize and be planted with the same planting window as Bt maize. Moreover, farmers should manage refuges using comparable agronomic practices (fertilization, weed and pest management and irrigation) to the Bt maize areas, and avoid the spraying of microbial Bt formulations [Citation90].
Considering the host range of ECB and MCB, the use of wild or cultivated plants other than maize is not considered a suitable alternative to a structured refuge for both corn borers [Citation91,Citation92]. While ECB may lend itself to the seed blend concept (also termed seed mixtures or “refuge-in-the-bag”) [Citation93], the use of seed blends is not recommended in the EU [Citation40].
Resistance and compliance monitoring
IRM programs for Bt crops require routine monitoring for resistance evolution, so that early warning signs, indicating a decrease in susceptibility to the Bt protein expressed in Bt crops in field populations of the target insect pests, are detected [Citation94]. Timely detection of such signs enables taking action to limit the survival of resistant insects, and slow or prevent their spread should resistance have already evolved [Citation95]. Data generated through resistance monitoring also allows researchers, risk assessors, risk managers and regulators to assess whether the HDR strategy delays resistance evolution in the target insect pest adequately and efficiently. Moreover, since ensuring high levels of compliance with refuge requirements is a critical factor contributing to the success of IRM plans, monitoring activities are also tailored to collect information on the implementation of refuges, Bt crop adoption levels and farmer use patterns (such as applied pest management practices). This information gives relevant indications on whether farmers follow and adhere to the refuge requirements, and on compliance levels. An overview of the two programs that have been implemented in the EU to monitor the resistance evolution of target insect pest populations to Bt maize, as well as the available data on farmer’s compliance with refuge requirements, are presented below.
Resistance monitoring
Resistance monitoring programs for Bt crops usually follows a two-pronged approach, consisting of (i) regular monitoring for changes in susceptibility to the Bt protein in field populations of the target insect pest, and (ii) monitoring of unexpected field damage caused by the insect pest [Citation57,Citation58,Citation96,Citation97].
Baseline and monitoring target insect pest susceptibility to Bt maize
Two programs for monitoring the evolution of Cry1Ab resistance in corn borer populations have been put in place in the EU: (i) one runby the Spanish authorities between 1998 and 2011, and (ii) an ongoing one conducted by the applicants marketing maize MON 810 since 2004.
Spanish resistance monitoring program (1998–2011)
In 1998, following the commercial cultivation of maize Bt176, the Spanish authorities initiated resistance monitoring activities for corn borers. Before the widespread cultivation of Bt maize varieties, baseline susceptibility to Cry1Ab and natural variability in ECB and MCB field populations was established for many maize-growing regions based on the highest anticipated levels of adoption [Citation98]. For both pest species, geographical differences in the susceptibility to Bt protein among populations were low. The observed differences were attributed to natural variations due to a lack of usage of previous Bt spray formulations in the studied areas.
Changes in baseline susceptibility were measured periodically by collecting between 300 and 500 corn borer larvae of the last generation from maize fields (either from Bt176, non-Bt maize fields or refuge areas in the three regions where Bt maize was grown, i.e. north-eastern, central and south-eastern Spain). The concentration causing 50% mortality (LC50) was then estimated in diet-overlay concentration/response bioassays with microbially-produced Cry1Ab protein using the progeny of the field-collected individuals. Reference susceptible populations, initiated in 1998 and 2000 from MCB and ECB larvae collected from non-Bt maize fields, and maintained in the laboratory ever since without any exposure to the Cry1Ab protein, were used in the bioassays as an additional comparator. To preserve its vigor and avoid inbreeding depression, populations were refreshed periodically with new individuals.
The results of the Spanish monitoring programs indicated that susceptibilities of field populations of ECB and MCB to Bt maize remained within a narrow range for thirteen years (1999–2011) and were comparable to those of the relevant laboratory reference populations ().
Seed-companies program (2004–present)
As required by the EU decision approving the cultivation of maize MON 810, the applicant marketing maize MON 810 commenced a resistance monitoring program in 2004. Baselines of susceptibility to the Cry1Ab protein were established for ECB and MCB populations from several EU maize growing areas in accordance with the geographic distribution of corn borers. Small variations in susceptibility of populations (no higher than 6.6-fold for ECB and 9-fold for MCB) were observed across the continent. Given that the cultivation of maize MON 810 varieties has only continued in Portugal and Spain, yet on a small scale (i.e. less than 10% of maize cultivation) in Portugal, routine monitoring efforts for insect resistance have focused primarily on Spain.
Between 2004 and 2015, ECB and MCB populations were sampled from the same three geographical areas included in the Spanish monitoring program and their susceptibility was assessed using the same concentration/response bioassay. For each area and target insect pests, between 300 and 500 corn borer larvae were collected at least every second year from a minimum of three refuge areas or non-Bt maize fields. Then, the Cry1Ab concentration causing 50% and 90% of molt inhibition (MIC50,90), denoted as larvae that had either died or failed to molt to the second instar, was estimated after seven days of exposure using the progeny of the field-collected individuals. MIC50,90 values were also estimated from laboratory reference populations and served as additional comparators to account for any potential issues derived from the unavailability of a stable purified protein over time [Citation40].
In 2016, EuropaBio revised the sampling strategy and monitoring protocol of the IRM plan accounting for the experience gained with its practical implementation, several recommendations made by EFSA [Citation99] and new available scientific evidence. From then onwards, monitoring has focused on the Ebro basin (north-eastern Spain), as this is the area with the highest adoption rate of maize MON 810 in the EU (>60% between 2015 and 2019) and where both ECB and MCB complete two generations per year. Adhering to the revised plan, corn borer populations were collected every year from at least three 10 km × 10 km areas within that region. To increase the sensitivity of the monitoring strategy, the sampling size has been increased with an annual goal of at least 1000 larvae of each target insect pest, and bioassays using a single concentration, which is expected to discriminate between susceptible and (homozygous) resistant individuals, have superseded concentration/response tests.
In addition to the bioassays with microbially–produced Cry1Ab protein, supplementary tests using maize MON 810 leaves have been performed since 2011 with ECB and MCB larvae that survive the diagnostic concentration as well as with spare larvae not used in the bioassays. These tests aim to verify whether resistant individuals are already present in the field-collected populations.
Results of insect bioassays
Table S2 of the Supplementary material provides an overview of the different types of bioassays that have been performed within the seed companies monitoring program, covering: (i) concentration/response, (ii) diagnostic concentration, and (iii) plant-tissue assays.
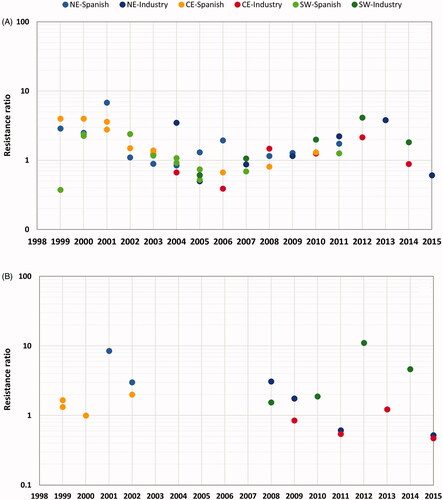
Concentration/response assays: The outcome of the concentration/response assays does not show any trends in Cry1Ab susceptibility of corn borers that were sampled and monitored in different geographical areas in Spain for more than 10 years (). Estimated MIC50 values did not fluctuate considerably as the difference between the most and least susceptible samples in a given area ranged between 2.0- and 3.9-fold in ECB, and 2.8- and 7.0-fold in MCB. Oscillations in Cry1Ab susceptibility were attributed to natural variability, as they are comparable to the changes observed in the respective susceptible reference strains (reared in the laboratory without any exposure to Bt proteins) and to historical values of field populations collected in Europe [Citation40,Citation41]. Similar conclusions can be drawn from the results of the concentration/response studies performed in both programs, regardless of the endpoint measured and the source of the microbially-produced Cry1Ab protein used in each program. While fluctuations occurred over time (), LC/MIC values followed no trend. Moreover, resistance ratios, which indicate the number of times that the susceptibility of the field population is higher than the laboratory susceptible population, never exceeded 10 (). They were much lower compared to those reported for instances of field-evolved and laboratory-selected resistance of lepidopteran pests to Bt proteins [e.g. Citation37,Citation39].
Figure 2. Changes in susceptibility to the Cry1Ab protein for Mediterranean corn borer (A) and European corn borer (B) in populations from north-eastern (NE), central (CE) and south-western (SW) Spain. Resistance ratios were calculated based on Cry1Ab concentration causing 50% mortality (LC50) (Spanish monitoring plan) and inhibition (MIC50) (seed-companies monitoring plan).
Diagnostic concentration assays: Diagnostic concentration assays revealed no symptoms of Cry1Ab resistance in corn borer populations collected in north-eastern Spain between 2016 and 2019. Molt inhibition values of ECB larvae exposed to the discriminating concentration for seven days were always higher than the expected >99% (Table 4). For MCB populations, molt inhibition rates averaged for the three sampling zones were lower than the expected >99% in all four years (). However, statistically significant differences between the field-collected population and the susceptible reference strain were only observed in 2017. Follow-up studies using plant material indicated that none of the second instars that survived the diagnostic concentration in that each year reached the third instar after feeding on maize MON 810 leaves [Citation100].
Plant tissue assays: Larvae that survived the concentration/response or the diagnostic concentration bioassays with Cry1Ab and spare individuals from those assays were fed maize MON 810 leaves in plant tissue assays. None of the tested larvae were able to molt to the following instar and all died within a few days ().
Table 2. Molting inhibition (%) of European and Mediterranean corn borer populations from north-eastern Spain tested with a diagnostic concentration of the Cry1Ab protein.
Table 3. Number of European and Mediterranean corn borer neonates fed maize MON 810 leaf tissue from the offspring of field-collected populations collected between 2011 and 2019.
Unexpected field damage caused by corn borers
Applicants marketing maize MON 810 seeds have established a reporting system allowing farmers to report complaints about product performance and related issues to seed suppliers via the local sales representatives or customer service routes. Complaints can include unexpected crop damage to Bt maize possibly caused by corn borers due to product failure. So far, none of the complaints received by seed industries (5417 over the 2016–2019 growing seasons) have been attributed to the loss of efficacy of Bt maize varieties in Portugal and Spain [Citation44,Citation100,Citation101].
Evaluating the results of the resistance monitoring program
As required by EU legislation on GE plants, IRM activities accompanying the cultivation of Bt maize in the EU and their outcomes are reported to the European Commission and the EU Member States on a yearly basis in annual post-market environmental monitoring (PMEM) reports. Since 2010, EFSA has assessed the methodology and results of the IRM activities associated with the cultivation of maize MON 810, issued scientific outputs on each of the annual PMEM reports, and made concrete recommendations on how to further improve the implementation and reporting of the resistance monitoring strategy [Citation44,Citation100–109].
Based on the analysis of the resistance monitoring data provided in the 2009–2018 PMEM reports, EFSA concluded that there are no indications of decreased Cry1Ab susceptibility in Spanish ECB and MCB populations. However, due to the number of field-collected larvae represented in the diagnostic concentration bioassays and limitations of those bioassays to detect recessive resistance alleles at low frequencies [Citation95], EFSA is of the opinion that the current monitoring strategy for maize MON 810 is not sufficiently sensitive to enable timely detection of a surge of field-evolved resistance. Therefore, the applicant marketing maize MON 810 has been recommended to (i) increase the number of larvae sampled in the field across relevant geographical areas and reduce their mortality during rearing under laboratory conditions (which reached approximately 50% for ECB and between 40 and 60% for MCB in 2017–2019) prior to diagnostic concentration assays, or (ii) replace the diagnostic bioassay by a more sensitive testing method. Yet, finding sampling sites with sufficient corn borer larvae and reducing larval mortality before laboratory testing is a challenge. So, the alternative is the replacement of current resistance monitoring activities with the use of the F2 screen that would provide an estimation of resistance alleles in corn borer populations [Citation74,Citation94,Citation95]. Such data could be gathered on a periodic basis, as resistance allele frequencies are not expected to increase rapidly from one year to another, provided that enough isolines are screened.
Monitoring of compliance with refuge requirements
The evolution of resistance to Bt crops can rapidly accelerate if farmers fail to plant refuges. Thus, ensuring and monitoring compliance with refuge requirements, especially in hotspot areas where target insect pest populations are subject to significant selection pressure and where resistance is most likely to arise, is key to the success of IRM [Citation39,Citation110–114]. Seed companies, national competent authorities and farmers’ associations play a pivotal role in reinforcing grower’s awareness of the importance to follow refuge obligations. Besides education (i.e. training and stewardship activities), compliance can be maximized via farmer contracts, certification tests, audits, rewards for compliance, crop insurance for refuges, databases of non-compliant farmers, sales restrictions, and fines for noncompliance. Some stakeholders have also proposed refuge planting to be a prerequisite for direct payments under the EU’s common agricultural policy (https://ec.europa.eu/info/food-farming-fisheries/key-policies/common-agricultural-policy/) or other national rules.
If a structured refuge is to be planted, then monitoring the farmer’s compliance with refuge obligations is an important element of the IRM plan. The reporting of noncompliance with refuge requirements, especially in areas where the uptake of Bt crops is high, may serve as a trigger to strengthen education (training) programs that aid farmers understanding the importance of adhering to refuge requirements and to impose penalties for noncompliance (i.e. sales restrictions and fines).
Data on compliance with refuge requirements by growers planting Bt maize in Spain are reported by applicants in annual PMEM reports and typically gathered through farmer questionnaires. During the first eight years of cultivation of Bt maize, only around 60% of Spanish farmers required to plant refuge areas followed this requirement. However, since 2009, annual surveys have reported consistently greater levels (∼90%) of compliance by Spanish growers (Supplementary material, Table S3), perhaps associated with the implementation of training programs directed at personnel, distributors, cooperatives and individual farmers to raise awareness on the importance of refuge compliance. Overall, Head and Greenplate [Citation61] indicated that monitoring farmer compliance remains challenging due to the resources required to visit an appropriate number of farmers, and the potential bias present in telephone- and computer-based surveys.
Currently, farmers that grow less than 5 ha of Bt maize are not required to plant refuges. However, exempting “small” farmers from planting refuges may be an important bottleneck for IRM. Such farmers represent a significant portion of Bt maize growers [Citation44], so the effective percentage of refuges planted would be less than the expected 20% of Bt maize growing area. Therefore, EFSA considers that refuge requirements should also apply to clusters of small Bt maize fields in which the aggregated area is greater than 5 ha, irrespective of individual field and farm size [Citation89], but acknowledges that the implementation of this recommendation may be challenging. It would require adequate information systems on GE crop cultivation [Citation44] and incentives for the farmers who plant refuges, e.g. rebate on seed costs [Citation115].
Simulations with resistance evolution models
Mathematical models have been widely used to predict the possible adaptation of target insects to Bt crops [e.g. Citation62,Citation114,Citation116,Citation117], and design IRM strategies to delay resistance evolution [e.g. Citation118–120].
Simulations to predict resistance occurrence
A resistance evolution model was developed by Castañera et al. [Citation71] for MCB and maize cropping systems in north-eastern Spain, the EU area with the highest adoption rates of Bt maize. This model has considered a wide range of parameters related to the biology, ecology and genetics of the pest and agricultural practices, among others. By modifying some of the parameters of the model (refuge compliance, female dispersal, assortative mating, local colonization, adoption rate of Bt maize events Bt176 and MON 810), the authors established which factors have contributed to the success of IRM, and those expected to accelerate the resistance evolution. Model simulations revealed that local assortative mating (nonrandom mating between susceptible and resistant individuals) has more influence in accelerating the development of resistance than any other factor. The low initial adoption rates of Bt maize varieties and the full replacement of maize Bt176 by MON 810, in which season-long protection is ensured through the continued Cry1Ab expression throughout the entire plant life cycle, have been pivotal to delay resistance evolution in MCB populations.
Camargo et al. [Citation43] ran simulations with the same model as Castañera et al. [Citation71], but using the latest estimates of resistance allele frequency in MCB populations collected in northeast Spain in 2016. The new simulations indicated that resistance is not evolving faster than the initial projections and that field-evolved resistance to maize MON 810 varieties in MCB in the Ebro basin still might take 25 years or more to appear.
Simulations to design appropriate insect resistance management strategies
Predictive models for resistance evolution have been used to determine and design proper IRM strategies. In 2015, using a resistance evolution model [Citation99], EFSA tested several scenarios of density-dependent mortality for ECB under different adoption rates of Bt maize. The outcomes of the scenario analysis enabled the estimation of the time needed for resistance to evolve and evaluate whether the IRM approach implemented in the EU allows for the early detection of resistance. For instance, for a maize MON 810 adoption rate of 60%, the estimated number of generations of ECB populations required the detection of a resistance allele frequency of 3% and 5% to reach field-evolved resistance (corresponding to the frequency of 50%) is 7–10 and 5–7, respectively (Figure 1S). Based on these figures and acknowledging that there should be a correct balance between sampling efforts and timely detection of a surge of field resistance, EFSA advocated setting a maximum detection limit for resistance allele frequency at 3% in areas with 60% maize MON 810 adoption rates. EFSA also recommended annual sampling of populations of both target insect pests in areas where the maize MON 810 adoption rate is at least 60% of the total area cropped to maize. Since 2016, this latter recommendation has been followed by the applicant marketing maize MON 810 [Citation101].
Concluding remarks
The number of instances of field-evolved resistance to Bt crops documented in agricultural pests globally has risen in the last decade [Citation39]. In most cases, resistance evolution has been attributed to the deployment of non-high dose Bt crop events and the lack of or only partial compliance with refuge requirements. In Spain, however, there have been no reports of resistant corn borer populations after more than 20 years of cultivation of Bt maize varieties expressing the Cry1Ab protein. Resistance monitoring data and the lack of farmer complaints on unexpected damage to Bt maize caused by corn borers confirm maintenance of ECB and MCB susceptibility to Cry1Ab [Citation40–42,Citation44]. While resistance is not evolving faster than predicted by initial model simulations, the most recent estimation of Cry1Ab resistance allele frequency in MCB populations from north-eastern Spain might exceed the value recommended for the successful implementation of the HDR approach [Citation43]. Relevant factors contributing to the current durability of Bt maize in Spain include gradual adoption rates of Bt maize, replacement of Bt maize event Bt176 by the high-dose event MON 810, the low initial frequency of resistance alleles in corn borer populations, and increased levels of growers’ compliance with refuge requirements [Citation71].
The IRM strategy for Bt maize has been implemented successfully in Spain for more than 20 years, with consistently elevated levels (∼90%) of compliance with refuge requirements since 2009. Moreover, the IRM strategy has been regularly updated in the light of the resistance monitoring data gathered annually, the periodic assessment of IRM appropriateness and cost-effectiveness, EFSA’s recommendations for methodological improvements and new relevant evidence published in the scientific literature. Despite the diligent implementation of the IRM strategy, and some efforts to further fine-tune insect resistance monitoring, an unresolved point of contention between EFSA and the applicant is whether the resistance monitoring strategy followed in Spain, based on discriminating concentration bioassays, is sufficiently sensitive to timely detect early warning signs of resistance in the field [Citation44]. EFSA considers this a concern, as the belated detection of early signs of resistance will significantly hamper the timely implementation of remedial measures to appropriately respond to confirmed resistance, and hence prevent the spread of resistance to other areas or eradicate resistance [Citation99]. However, it is also acknowledged that tailoring monitoring activities to the early detection of resistance would demand additional sampling and monitoring efforts, which may raise practical challenges.
To achieve the recommended 3% resistance allele frequency, EFSA previously recommended using a more sensitive testing strategy that combines periodic estimations of allele frequencies for Cry1Ab resistance to Bt maize through F2 screening, with a robust farmer alert system for reports of unexpected damage [Citation44]. Alternatively, resistance allele frequencies could be estimated by F1 screening of a colony set up with a proper number of field-collected larvae in case that resistant corn borer populations are available (e.g. through laboratory selection) [Citation121]. Matched F2 and F1 screens are regularly conducted to manage resistance in Helicoverpa sp. to Bt cotton in Australia [Citation122]. Such data enable adjusting predictive models for resistance evolution to account for the new resistance allele frequencies and other changes in model parameters. The outcome of model simulations would help to evaluate whether the frequency of the Cry1Ab resistance alleles is evolving as predicted and decide when to conduct the next F2 screening. Finally, obtaining more precise estimates on some of the factors influencing the evolution of resistance, such as the level of local assortative mating, would be crucial to reduce uncertainties associated with model projections [Citation71].
The recently estimated increase of Cry1Ab resistance allele frequency to Bt maize in MCB populations from north-eastern Spain confirms the continued need to implement adequate IRM approaches (including resistance and compliance monitoring), and incorporate those in IPM schemes to ensure Bt maize durability in Spain. This is particularly pertinent in the EU context as current chemical and biological control and cultural methods against ECB and MCB are subject to limitations in Spain [Citation46], while no new GE lepidopteran-active maize events (except for the Bt maize event 1507 that expresses the Cry1F protein, see below) for cultivation are in the regulatory approval pipelines to offer additional means for managing corn borers and resistance to Bt maize (Supplementary material, Table S4). Such new products are at various development stages or approved for use in non-EU jurisdictions (https://www.isaaa.org/gmapprovaldatabase/default.asp). These GE maize events either rely on new Bt proteins, combine existing ones, or are based on alternative strategies involving a different mode of action than Bt proteins (e.g. chimeric proteins). For instance, Bt maize pyramids (such as Bt maize 1507 × MON 810 expressing the Cry1F and Cry1Ab proteins and Bt maize MON 89034 expressing the Cry1A.105 and Cry2Ab2 proteins), which expresses at least two existing Bt proteins with different modes of action targeting the same insect pests [Citation123], can effectively delay the evolution of resistance to each Bt protein if most individuals that are resistant to one Bt protein are killed by the other, and when selection for resistance to one of the Bt proteins does not cause cross-resistance to the other. Under such conditions, IRM could be greatly simplified with the possibilities of reduced refuge amounts. An exception to this is the Bt maize 1507 for which regulatory approval is pending in the EU. If approved for cultivation, this Bt maize could be grown in rotation with maize MON 810 enabling the alternation of Bt proteins with a different mode of action, thus avoiding repeated selection pressure encountered in continuous maize MON 810 cultivation, as is often practiced in Spain [Citation71]. Additional approaches, such as combining the HDR strategy with mass releases of sterile insects [Citation124] or male GE insects with a female-specific self-limiting gene [Citation125,Citation126] have been proposed or applied for simultaneously managing agricultural insect pest populations and resistance to Bt crops. In the southwestern United States and northern Mexico, for instance, the combined use of Bt cotton and mass release of sterile pink bollworm (Pectinophora gossypiella) moths has enabled the successful suppression of this invasive pest [Citation124] and the replacement of refuges with mass releases of sterile insects.
Education (training) programs aiding farmers to understand the importance of adhering to IRM requirements are essential to the success of the HDR strategy and thus should continue to form an integral part of IRM plans for Bt maize, in order to maximize compliance with refuge requirements, especially in hotspot areas (i.e. north-eastern Spain) where corn borer populations are subject to significant selection pressure and resistance is most likely to arise.
Author contributions
Fernando Álvarez-Alfageme: Conceptualization, Formal analysis, Writing – Original Draft, Writing – Review and Editing; Yann Devos: Conceptualization, Writing – Sections of Original Draft, Writing – Review and Revising; Ana M. Camargo: Review and Editing; Salvatore Arpaia: Review and Editing; Antoine Messéan: Review and Editing.
Supplemental Material
Download MS Word (114 KB)Disclosure statement
No potential conflict of interest was reported by the author(s). The views expressed in this publication are those from the authors and do not necessarily represent the official position of the European Food Safety Authority (EFSA). EFSA assumes no responsibility or liability for any errors or inaccuracies that may appear. Part of this review paper builds on EFSA (2019, 2020), to which the authors contributed actively.
References
- ISAAA (International Service for the Acquisition of Agri-biotech Applications). Global Status of Commercialized Biotech/GM Crops. 2019. Ithaca (NY): ISAAA; 2020. Brief No. 55
- Addae PC, Ishiyaku MF, Tignegre JB, et al. Efficacy of a cry1Ab gene for control of Maruca vitrata (Lepidoptera: Crambidae) in cowpea (Fabales: Fabaceae). J Econ Entomol. 2020;113(2):974–979.
- OECD (Organisation for Economic Co-operation and Development). Consensus document on the safety information on transgenic plants expressing Bacillus thuringiensis-derived insect control proteins. OECD Papers. 2007. Vol. 7/11. Available at:.
- Sanahuja G, Banakar R, Twyman RM, et al. Bacillus thuringiensis: a century of research, development and commercial applications. Plant Biotechnol J. 2011;9(3):283–300.
- Bravo A, Gómez I, Porta H, et al. Evolution of Bacillus thuringiensis Cry toxins insecticidal activity. Microb Biotechnol. 2013;6(1):17–26.
- Vachon V, Laprade R, Schwartz JL. Current models of the mode of action of Bacillus thuringiensis insecticidal crystal proteins: a critical review. J Invertebr Pathol. 2012;111(1):1–12.
- Soberón M, Monnerat R, Bravo A. Mode of action of cry toxins from Bacillus thuringiensis and resistance mechanisms. In: Gopalakrishnakone P, Stiles B, Alape-Girón A, et al., editors. Microbial toxins. Toxicology. Dordrecht: Springer; 2018. p. 1–28.
- Marvier M, McCreedy C, Regetz J, et al. A meta-analysis of effects of Bt cotton and maize on nontarget invertebrates. Science. 2007;316(5830):1475–1477.
- Duan JJ, Marvier M, Huesing J, et al. A meta-analysis of effects of Bt crops on honey bees (Hymenoptera: Apidae). PLOS One. 2008;3(1):e1415.
- Naranjo SE. Impacts of Bt crops on non-target organisms and insecticide use patterns. CAB Reviews. 2009;4(011):23.
- Comas C, Lumbierres B, Pons X, et al. No effects of Bacillus thuringiensis maize on nontarget organisms in the field in southern Europe: a meta-analysis of 26 arthropod taxa. Transgenic Res. 2014;23(1):135–143.
- NAS (National Academies of Sciences, Engineering, and Medicine). Genetically engineered crops: Experiences and prospects. Washington, DC: The National Academies Press; 2016.
- Dang C, Lu Z, Wang L, et al. Does Bt rice pose risks to non-target arthropods? Results of a meta-analysis in China. Plant Biotechnol J. 2017;15(8):1047–1053.
- Pellegrino E, Bedin S, Nuti M, et al. Impact of genetically engineered maize on agronomic, environmental and toxicological traits: a meta-analysis of 21 years of field data. Sci Rep. 2018;8(1):3113.
- Romeis J, Naranjo SE, Meissle M, et al. Genetically engineered crops help support conservation biological control. Biol Control. 2019;130:136–154.
- Krogh PH, Kostov K, Damgaard CF. The effect of Bt crops on soil invertebrates: a systematic review and quantitative meta-analysis. Transgenic Res. 2020;29(5–6):487–498.
- Gómez-Barbero M, Berbel J, Rodríguez-Cerezo E. Bt corn in Spain-the performance of the EU’s first GM crop. Nat Biotechnol. 2008;26(4):384–386.
- Qaim M. The economics of genetically modified crops. Annu Rev Resour Econ. 2009;1(1):665–694.
- Carpenter JE. Peer-reviewed surveys indicate positive impact of commercialized GM crops. Nat Biotechnol. 2010;28(4):319–321.
- Hutchison W, Burkness E, Mitchell P, et al. Areawide suppression of European corn borer with Bt maize reaps savings to non-Bt maize growers . Science. 2010;330(6001):222–225.
- Lu Y, Wu L, Jiang Y, et al. Widespread adoption of Bt cotton and insecticide decrease promotes biocontrol services. Nature. 2012;487(7407):362–365.
- Wan P, Huang Y, Tabashnik BE, et al. The halo effect: suppression of pink bollworm on non-Bt cotton by Bt cotton in China. PLOS One. 2012;7(7):e42004.
- Areal FJ, Riesgo L, Rodríguez-Cerezo E. Economic and agronomic impact of commercialized GM crops: a metaanalysis. J Agric Sci. 2013;151(1):7–33.
- Brookes G, Barfoot P. Key global environmental impacts of genetically modified (GM) crop use 1996-2012. GM Crops Food. 2014;5(2):149–160.
- Klumper W, Qaim M. A meta-analysis of the impacts of genetically modified crops. PLOS One. 2014;9(11):e111629.
- Brookes G, Barfoot P. Environmental impacts of genetically modified (GM) crop use 1996-2016: Impacts on pesticide use and carbon emissions. GM Crops Food. 2018;9(3):109–139.
- Brookes G. Twenty-one years of using insect resistant (GM) maize in Spain and Portugal: farm-level economic and environmental contributions. GM Crops Food. 2019;10(2):90–101.
- Yu J, Hennessy DA, Wu F. The impact of Bt corn on aflatoxin-related insurance claims in the United States. Sci Rep. 2020;10(1):10046.
- Porter P, Cullen E, Sappington T, et al. Comment submitted by Patrick Porter, North Central Coordinating Committee NCCC46 and other corn entomologists. 2012. EPA Docket: EPAHQ-OPP-2011–0922. Available at: http://www.regulations.gov/#!documentDetail;D=EPA-HQ-OPP-2011-0922-0013
- Lang A, Otto M. A synthesis of laboratory and field studies on the effects of transgenic Bt-maize on nontarget Lepidoptera. Entomol Exp Appl. 2010;135(2):121–134.
- Baudrot V, Walker E, Lang A, et al. When the average hides the risk of Bt-corn pollen on non-target Lepidoptera: application to Aglais io in Catalonia. Ecotoxicol Environ Saf. 2021;207:111215.
- Siegfried BD, Meinke LJ, Scharf ME. Resistance management concerns for areawide management programs. J Agric Urban Entomol. 1998;15:359–369.
- Tabashnik BE, Mota-Sanchez D, Whalon ME, et al. Defining terms for proactive management of resistance to Bt crops and pesticides. J Econ Entomol. 2014;107(2):496–507.
- Andow DA. The risk of resistance evolution in insects to transgenic insecticidal crops. Collect Biosafety Rev. 2008;4:142–199.
- Gassmann AJ, Hutchison WD. Bt crops and insect pests: past successes, future challenges and opportunities. GM Crops Food. 2012;3(3):139.
- Hurley TM, Babcock BA, Hellmich RL. Bt corn and insect resistance: an economic assessment of refuges. J Agric Resour Econ. 2001;26:176–194.
- Smith JL, Farhan Y, Schaafsma AW. Practical resistance of Ostrinia nubilalis (Lepidoptera: Crambidae) to Cry1F Bacillus thuringiensis maize discovered in Nova Scotia, Canada. Sci Rep. 2019;9(1):18247.
- Tabashnik BE, Carrière Y. Global patterns of resistance to Bt crops highlighting pink bollworm in the United States, China, and India. J Econ Entomol. 2019;112(6):2513–2523.
- Tabashnik BE, Carrière Y. Surge in insect resistance to transgenic crops and prospects for sustainability. Nat Biotechnol. 2017;35(10):926–935.
- Farinós GP, Hernandez-Crespo P, Ortego F, et al. Monitoring of Sesamia nonagrioides resistance to MON 810 maize in the European Union: lessons from a long-term harmonized plan . Pest Manag Sci. 2018;74(3):557–568.
- Thieme TGM, Buuk C, Gloyna K, et al. Ten years of MON 810 resistance monitoring of field populations of Ostrinia nubilalis in Europe. J Appl Entomol. 2018;142(1–2):192–200.
- Farinós GP, Ortego F. [Pest resistance to Bt maize: current status and monitoring plans in Spain]. Boletín SEEA. 2019;4:52–57. Spanish.
- Camargo AM, Andow DA, Castañera P, et al. First detection of a Sesamia nonagrioides resistance allele to Bt maize in. Sci Rep. 2018;8(1):3977.
- Álvarez F, Georgiadis M, Messéan A, et al. Assessment of the 2018 post market environmental monitoring report on the cultivation of genetically modified maize MON810 in the EU. EFSA J. 2020;18:6245.
- Meissle M, Romeis J, Bigler F. Bt maize and integrated pest-management – a European perspective. Pest Manag Sci. 2011;67(9):1049–1058.
- Meissle M, Álvarez-Alfageme F, Malone LA, et al. Establishing a database of bio-ecological information on non-target arthropod species to support the environmental risk assessment of genetically modified crops in the EU. EFSA Support Publ. 2012;9EN 334:1–170.
- Maiorano A, Cerrani I, Fumagalli D, et al. New biological model to manage the impact of climate warming on maize corn borers. Agron Sustain Dev. 2014;34(3):609–621.
- Velasco P, Revilla P, Monetti L, et al. Corn borers (Lepidoptera:Noctuidae; Crambidae) in northwestern Spain: population dynamics and distribution. Maydica. 2007;52:195–203.
- Eizaguirre M, Fantinou AA. Abundance of Sesamia nonagrioides (Lef.) (Lepidoptera: Noctuidae) on the edges of the Mediterranean Basin. Psyche. 2012;2012:1–7.
- Munkvold GP, Hellmich RL, Rice LG. Comparison of fumonisin concentrations in kernels of transgenic Bt maize hybrids and nontransgenic hybrids. Plant Dis. 1999;83(2):130–138.
- Avantaggiato G, Quaranta F, Desiderio E, et al. Fumonisin contamination of maize hybrids visibly damaged by Sesamia. J Sci Food Agric. 2003;83(1):13–18.
- Papst C, Utz HF, Melchinger AE, et al. Mycotoxins produced by Fusarium spp. in isogenic Bt vs. non-Bt maize hybrids under European corn borer pressure. Agron J. 2005;97:219–224.
- Hellmich RL, Albajes R, Bergvinson D, et al. The present and future role of insect-resistant GM crops in maize IPM. In: Romeis J, Shelton AM, Kennedy GG, editors. Integration of insect-resistant genetically modified crops within IPM programs. Dordrecht (Netherlands): Springer; 2008. p. 119–158.
- EFSA GMO Panel (EFSA Panel on Genetically Modified Organisms). Scientific opinion supplementing the conclusions of the environmental risk assessment and risk management recommendations for the cultivation of the genetically modified insect resistant maize Bt11 and MON810. EFSA J. 2012;10:3016.
- EFSA (European Food Safety Authority). Opinion of the scientific panel on genetically modified organisms on the use of antibiotic resistance genes as marker genes in genetically modified plants. EFSA J. 2004;48:1–18.
- Lucht JM. Public acceptance of plant biotechnology and GM crops. Viruses. 2015;7(8):4254–4281.
- Bates SL, Zhao J-Z, Roush RT, et al. Insect resistance management in GM crops: past, present and future. Nat Biotechnol. 2005;23(1):57–62.
- Alcalde E. Post-market monitoring plans of Bt-176 in Spain: 1998–2005. J Verbr Lebensm. 2006;1(S1):102–105.
- US EPA (United States Environmental Protection Agency). White paper on resistance in lepidopteran pests of Bacillus thuringiensis (Bt) plant-incorporated protectants in the United States. 2018. Available from: https://www.epa.gov/sites/production/files/2018-07/documents/position_paper_07132018.pdf
- MacIntosh SC. Managing the risk of insect resistance to transgenic insect control traits: practical approaches in local environments. Pest Manag Sci. 2010;66(1):100–106.
- Head GP, Greenplate J. The design and implementation of insect resistance management programs for Bt crops. GM Crops Food. 2012;3(3):144–153.
- Gould F. Sustainability of transgenic insecticidal cultivars: integrating pest genetics and ecology. Annu Rev Entomol. 1998;43:701–726.
- Matten SR, Frederick RJ, Reynolds AH. United States Environmental protection agency insect resistance management programs for plant incorporated protectants and use of simulation modeling. In: Wozniak C, McHughen A, editors. Regulation of agricultural biotechnology: the United States and Canada. Dordrecht (Netherlands): Springer; 2012. p. 175–267.
- Han L, Jiang X, Peng Y. Potential resistance management for the sustainable use of insect-resistant genetically modified corn and rice in China. Curr Opin Insect Sci. 2016;15:139–143.
- Li Y, Gao Y, Wu K. Function and effectiveness of natural refuge in IRM strategies for Bt crops. Curr Opin Insect Sci. 2017;21:1–6.
- Fearing PL, Brown D, Vlachos D, et al. Quantitative analysis of CryIA(b) expression in Bt maize plants, tissues, and silage and stability of expression over successive generations. Mol Breed. 1997;3(3):169–176.
- Walker KA, Hellmich RL, Lewis LC. Late-instar European corn borer (Lepidoptera: Crambidae) tunneling and survival in transgenic corn hybrids. J Econ Entomol. 2000;93(4):1276–1285.
- Nguyen HT, Rehle JA, Rheinpfalz DLR. Quantitative analysis of the seasonal and tissue specific expression Cry1Ab in transgenic maize MON810. J Plant Dis Prot. 2007;114(2):82–87.
- Székács A, Lauber É, Juracsek J, et al. Cry1Ab toxin production of MON 810 transgenic maize. Environ Toxicol Chem. 2010;29(1):182–190.
- Siegfried BD, Hellmich RL. Understanding successful resistance management: the European corn borer and Bt corn in the United States. GM Crops Food. 2012;3(3):184–193.
- Castañera P, Farinós GP, Ortego F, et al. Sixteen years of Bt maize in the EU hotspot: why has resistance not evolved? PLOS One. 2016;11(5):e0154200.
- EFSA (European Food Safety Authority). Relevance of a new scientific publication (Trtikova et al. 2015) on previous EFSA GMO Panel conclusions on the risk assessment of maize MON 810 and other Cry1Ab-expressing Bt-maize events. EFSA Support Publ. 2015;11:EN–878.
- Andreadis SS, Álvarez-Alfageme F, Sánchez-Ramos I, et al. Frequency of resistance to Bacillus thuringiensis toxin Cry1Ab in Greek and Spanish population of Sesamia nonagrioides (Lepidoptera: Noctuidae). J Econ Entomol. 2007;100(1):195–201.
- Andow DA, Alstad DN. The F2 screen for rare resistance alleles. J Econ Entomol. 1998;91(3):572–578.
- Bourguet D, Chaufaux J, Séguin M, et al. Frequency of alleles conferring resistance to Bt maize in French and US corn belt populations of the European corn borer, Ostrinia nubilalis. Theor Appl Genet. 2003;106(7):1225–1233.
- Engels H, Bourguet D, Cagáň L, et al. Evaluating resistance to Bt toxin Cry1Ab by F2 screen in European populations of Ostrinia nubilalis (Lepidoptera: Crambidae). Jnl Econ Entom. 2010;103(5):1803–1809.
- Gaspers C. The European corn borer (Ostrinia nubilalis, Hbn.), its susceptibility to the Bt-toxin Cry1F, its pheromone races and its gene flow in Europe in view of an insect resistance management [dissertation]. Aachen (Germany): Rheinisch-Westfälischen Technischen Hochschule; 2009.
- Hun TE, Higley LG, Witkowski JF, et al. Dispersal of adult european corn borer (Lepidoptera: Crambidae) within and proximal to irrigated and non-irrigated corn. J Econ Entomol. 2001;94(6):1369–1377.
- Showers WB, Hellmich RL, Derrick-Robinson ME, et al. Aggregation and dispersal behaviour of marked and released European corn borer (Lepidoptera: Crambidae) adults. Environ Entomol. 2001;30(4):700–710.
- Bourguet D, Bethenod MT, Trouvë C, et al. Host-plant diversity of the European corn borer Ostrinia nubilalis: what value for sustainable transgenic insecticidal Bt maize? Proc Biol Sci. 2000;267(1449):1177–1184.
- Albajes R, Eras J, López C, et al. Testing rubidium marking for measuring adult dispersal of the corn borer Sesamia nonagrioides: first results. IOBC/WPRS Bull. 2004;27:15–22.
- López C, Hernández-Escareño G, Eizaguirre M, et al. Antixenosis and larval and adult dispersal in the Mediterranean corn borer, Sesamia nonagrioides, in relation to Bt maize. Entomol Exp Appl. 2013;149(3):256–264.
- López C, Sans A, Eizaguirre M. [Influence of maize plant on mating of Sesamia nonagrioides Lefèbvre (Lepidoptera: Noctuidae)]. Inv Agr Prod Prot Veg. 1999;14:415–422. Spanish.
- Eizaguirre M, López C, Albajes R. Dispersal capacity in the Mediterranean corn borer, Sesamia nonagrioides. Entomol Exp Appl. 2004;113(1):25–34. h
- López C, Eizaguirre M, Albajes R. Courtship and mating behaviour of the Mediterranean corn borer, Sesamia nonagrioides (Lepidoptera: Noctuidae). Span J Agric Res. 2003;1(1):43–51.
- Dorhout DL, Sappington TW, Rice ME. Evidence for obligate migratory flight behavior in young European corn borer (Lepidoptera: Crambidae) females. Environ Entomol. 2008;37(5):1280–1290.
- Hu Y, Andow DA. Field observations of Ostrinia nubilalis eclosion and post‐eclosion activity of females around their natal plants. Insect Sci. 2011;18(6):712–7718.
- Qureshi JA, Buschman LL, Throne JE, et al. Adult dispersal of Ostrinia nubilalis Hübner (Lepidoptera: Crambidae) and its implications for resistance management in Bt‐maize. J Appl Entomology. 2005;129(6):281–292.
- EFSA (European Food Safety Authority). Scientific opinion of the panel on genetically modified organisms on applications (EFSA-GMO-RX-MON810) for the renewal of authorisation for the continued marketing of (1) existing food and food ingredients produced from genetically modified insect resistant maize MON810; (2) feed consisting of and/or containing maize MON810, and maize MON810 for feed use (including cultivation); and of (3) food additives and feed materials produced from maize MON810, all under Regulation (EC) No 1829/2003 from Monsanto. EFSA J. 2009;1149:1–85.
- EuropaBio. Harmonised insect resistance management (IRM) plan for cultivation of Bt maize (single insecticidal trait) in the EU. 2019. Available from: https://ec.europa.eu/food/plant/gmo/post_authorisation/plans_reports_opinions/report_2019_mon_810_en
- Camargo AM, Arias-Martín M, Castañera P, et al. Performance of Sesamia nonagrioides on cultivated and wild host plants: implications for Bt maize resistance management. Pest Manag Sci. 2020;76(11):3657–3666.
- Losey JE, Calvin DD, Carter ME, et al. Evaluation of noncorn host plants as a refuge in a resistance management program for European corn borer (Lepidoptera: Crambidae) on Bt-corn. Environ Entomol. 2001;30(4):728–735.
- Onstad DW, Crespo AL, Pan Z, et al. Blended refuge and insect resistance management for insecticidal corn. Environ Entomol. 2018;47(1):210–219.
- Siegfried BD, Spencer T. Bt resistance monitoring in European corn borers and western corn rootworms. In: Oliver M, Li Y, editors. Gene containment. New York (NY): Wiley; 2012. Chapter 3.
- Siegfried BD, Spencer T, Crespo AL, et al. Ten years of Bt resistance monitoring in the European corn borer: what we know, what we don’t know, and what we can do better? Am Entomol. 2007;53(4):208–214.
- Wilhelm R, Sanvido O, Castañera P, et al. Monitoring the commercial cultivation of Bt maize in Europe – conclusions and recommendations for future monitoring practice. Environ Biosafety Res. 2009;8(4):219–225.
- Glaser JA, Matten SR. Sustainability of insect resistance management strategies for transgenic Bt corn. Biotechnol Adv. 2003;22(1–2):45–69.
- González-Núñez M, Ortego F, Castañera P. Susceptibility of Spanish populations of the corn borers Sesamia nonagrioides (Lepidoptera: Noctuidae) and Ostrinia nubilalis (Lepidoptera: Crambidae) to a Bacillus thuringiensis endotoxin. J Econ Entomol. 2000;93(2):459–463.
- EFSA (European Food Safety Authority). Clarifications on EFSA GMO Panel recommendations on the Insect Resistance Management plan for genetically modified maize MON810. EFSA Support Publ. 2015;12:EN–842.
- EFSA (European Food Safety Authority). Assessment of the 2017 post-market environmental monitoring report on the cultivation of genetically modified maize MON810. EFSA J. 2019;17:5742.
- EFSA (European Food Safety Authority). Statement on annual post-market environmental monitoring report on the cultivation of genetically modified maize MON810 in 2016. EFSA J. 2018;16:5287.
- EFSA GMO Panel (EFSA Panel on Genetically Modified Organisms). Scientific opinion on the annual post-market environmental monitoring (PMEM) report from Monsanto Europe S.A. on the cultivation of genetically modified maize MON810 in 2009. EFSA J. 2011;9:2376.
- EFSA GMO Panel (EFSA Panel on Genetically Modified Organisms). Scientific Opinion on the annual Post-Market Environmental Monitoring (PMEM) report from Monsanto Europe S.A. on the cultivation of genetically modified maize MON810 in 2010. EFSA J. 2012;10:2610.
- EFSA GMO Panel (EFSA Panel on Genetically Modified Organisms). Scientific Opinion on the annual Post-Market Environmental Monitoring (PMEM) report from Monsanto Europe S.A. on the cultivation of genetically modified maize MON810 in 2011. EFSA J. 2013;11:3500.
- EFSA GMO Panel (EFSA Panel on Genetically Modified Organisms). Scientific Opinion on the annual post-market environmental monitoring (PMEM) report from Monsanto Europe S.A. on the cultivation of genetically modified maize MON810 in 2012. EFSA J. 2014;12:3704.
- EFSA GMO Panel (EFSA Panel on Genetically Modified Organisms). Scientific Opinion on the annual post-market environmental monitoring (PMEM) report from Monsanto Europe S.A. on the cultivation of genetically modified maize MON810 in 2013. EFSA J. 2015;13:4039.
- EFSA GMO Panel (EFSA Panel on Genetically Modified Organisms). Scientific opinion on the revised annual post-market environmental monitoring (PMEM) report on the cultivation of genetically modified maize MON810 in 2013 from Monsanto. Europe S.A. EFSA J. 2015;13:4295.
- EFSA GMO Panel (EFSA Panel on Genetically Modified Organisms). Scientific Opinion on the annual post-market environmental monitoring (PMEM) report from Monsanto Europe S.A. on the cultivation of genetically modified maize MON810 in 2014. EFSA J. 2016;14:4446.
- EFSA GMO Panel (EFSA Panel on Genetically Modified Organisms). Scientific Opinion on the annual post-market environmental monitoring (PMEM) report on the cultivation of genetically modified maize MON810 in 2015 from Monsanto. Europe S.A. EFSA J. 2017;15:4805.
- Huang F, Andow AA, Buschman LL. Success of the high dose/refuge resistance management strategy after 15 years of Bt crop use in North America. Entomol Exp Appl. 2011;140(1):1–16.
- Storer NP, Babcock JM, Schlenz M, et al. Discovery and characterization of field resistance to Bt maize: Spodoptera frugiperda (Lepidoptera: Noctuidae) in Puerto Rico. J Econ Entomol. 2010;103(4):1031–1038.
- Farias JR, Andow DA, Horikoshi RJ, et al. Field-evolved resistance to Cry1F maize by Spodoptera frugiperda (Lepidoptera: Noctuidae) in Brazil. Crop Prot. 2014;64:150–158.
- Omoto C, Bernardi O, Salmeron E, et al. Field-evolved resistance to Cry1Ab maize by Spodoptera frugiperda in Brazil. Pest Manag Sci. 2016;72(9):1727–1736.
- Alstad DN, Andow DA. Managing the evolution of insect resistance to transgenic plants. Science. 1995;268(5219):1894–1896.
- Carrière Y, Brown ZS, Downes SJ, et al. Governing evolution: a socioecological comparison of resistance management for insecticidal transgenic Bt crops among four countries. Ambio. 2020;49(1):1–16.
- Gould F. Simulation models for predicting durability of insect-resistant germ plasm: a deterministic diploid, two-locus model. Environ Entomol. 1986;15(1):1–10.
- Arpaia S, Chiriatti K, Giorio G. Predicting the adaptation of Colorado potato beetle (Coleoptera: Chrysomelidae) to transgenic eggplants expressing CryIII toxin: the role of gene dominance, migration, and fitness costs. J Econ Entomol. 1998;91(1):21–29.
- Tabashnik BE. Delaying insect resistance to transgenic crops. Proc Natl Acad Sci USA. 2008;105(49):19029–19030.
- Caprio M. Evaluating resistance management strategies for multiple toxins in the presence of external refuges. J Econ Entomol. 1998;91(5):1021–1031.
- Andow DA, Pueppke SG, Schaafsma AW, et al. Early detection and mitigation of resistance to Bt maize by western corn rootworm (Coleoptera: Chrysomelidae). J Econ Entomol. 2016;109(1):1–12.
- Gould F, Anderson A, Jones A, et al. Initial frequency of alleles for resistance to Bacillus thuringiensis toxins in field populations of Heliothis virescens. Proc Natl Acad Sci U S A. 1997;94(8):3519–3523.
- Downes S, Walsh T, Tay WT. Bt resistance in Australian insect pest species. Curr Opin Insect Sci. 2016;15:78–83.
- Carriere Y, Fabrick JA, Tabashnik BR. Can pyramids and seed mixtures delay resistance to Bt crops? Trends Biotechnol. 2016;34(4):291–302.
- Tabashnik BE, Liesner LR, Ellsworth PC, et al. Transgenic cotton and sterile insect releases synergize eradication of pink bollworm a century after it invaded the United States. Proc Natl Acad Sci USA. 2021;118(1):e2019115118.
- Zhou L, Alphey N, Walker AS, et al. Combining the high-dose/refuge strategy and self-limiting transgenic insects in resistance management-A test in experimental mesocosms. Evol Appl. 2018;11(5):727–738.
- Brewer TR, Bonsall M. Combining refuges with transgenic insect releases for the management of an insect pest with non-recessive resistance to Bt crops in agricultural landscapes . J Theor Biol. 2021;509:110514.
- Farinós GP, de la Poza M, Ortego F, et al. Monitoring corn borers resistance to Bt-maize in Spain. In: Miklau M, Gaugitsch H, Heissenberger A, editors. Proceedings EU Workshop: Monitoring of environmental impacts of genetically modified plants; 2000 Nov 9–10; Berlin (Germany): German Federal Environmental Agency; 2001. p. 114–118.
- Farinós GP, de la Poza M, González Núñez M, et al. Research programme to monitor corn borer resistance to Bt-maize in Spain. IOBC/WPRS Bull. 2004;27:7377.
- Farinós GP, De la Poza M, Hernndez-Crespo P, et al. Resistance monitoring of field populations of the corn borers Sesamia nonagrioides and Ostrinia nubilalis after five years of Bt maize cultivation in Spain. Entomol Exp Appl. 2004;110(1):23–30.
- Farinós GP, Andreadis SS, de la Poza M, et al. Comparative assessment of the field-susceptibility of Sesamia nonagrioides to the Cry1Ab toxin in areas with different adoption rates of Bt maize and in Bt-free areas. Entomol Exp Appl. 2011;30(7):902–906.
