ABSTRACT
Individuals with an evening chronotype are at increased risk of experiencing emotional problems, including depressive symptoms. However, the mechanisms underlying these associations remain unclear. The present study aimed to determine whether poor sleep quality, substance use and cognitive emotion regulation difficulties – which have been implicated in the etiology of depression – mediate the relationship between chronotype and depressive symptoms in a student sample, which was assessed cross-sectionally and after 1 year. A total of 742 Dutch students (75% women, mean age 21.4 ± 2.9 years) completed the Quick Inventory of Depressive Symptomatology, the Morningness-Eveningness Questionnaire, the Pittsburgh Sleep Quality Index, a questionnaire assessing alcohol, caffeine, tobacco and cannabis use, the Cognitive Emotion Regulation Questionnaire and the Behavioral Inhibition/Activation Scale. A subsample (n = 115) was assessed 1 year later with the same questionnaires. Cross-sectional analyses showed that evening chronotype was associated with more depressive symptoms, adjusted for age and gender (β = −0.082, p = 0.028). The relationship between eveningness and depressive symptoms was mediated by sleep quality, alcohol consumption and the cognitive emotion regulation strategies of self-blame and positive reappraisal. In longitudinal analyses, eveningness at baseline predicted more depressive symptoms at follow-up, adjusted for age and gender (β = −0.29, p = 0.002); after additional adjustment for baseline depressive symptoms, chronotype remained a significant predictor of depressive symptoms at T2 (β = −0.16, t = −2.01, p = 0.047). Only poor sleep quality at follow-up was a significant mediator of this relationship. Even though the effect is small in terms of explained variance, eveningness is related to depressive symptoms and this relationship is mediated by poor sleep quality, also in a prospective design. Self-blame and reduced positive reappraisal are correlated with eveningness. Further research is needed to assess the efficacy of chronotherapeutic interventions for the prevention of depression, in addition to sleep education and cognitive approaches.
Introduction
Individuals differ in their preferred timing of daily activity patterns, wake-up and bed times. This morning/evening preference is a continuum but is usually divided into three chronotypes: the morning, the evening and the intermediate type (Horne and Östberg Citation1976; Kerkhof Citation1985). Chronotype distribution estimates depend on the population and classification method. In a large student sample, 16% were classified as morning, 60% as intermediate and 24% as evening types (Adan and Natale Citation2002).
Circadian rhythms are regulated by the suprachiasmatic nuclei (SCN) in the hypothalamus. The SCN controls sleep–wake cycles as well as diurnal variations in physiological processes such as body temperature and hormone secretion (Weaver Citation1998; Roenneberg et al. Citation2007) and is entrained by “Zeitgebers”, environmental cues that signal the time of day, most importantly, daylight. Variations in intrinsic circadian rhythms cause variations in chronotype (Duffy et al. Citation2001; Lack et al. Citation2009). Chronotype is related to age, sex (Adan and Natale Citation2002; Roenneberg et al. Citation2007) and other factors such as lifestyle and profession (Adan et al. Citation2012).
The study of chronotypes in relation to psychopathology has received substantial attention and the link between evening preference and depression is well established (Au and Reece Citation2017): having an evening chronotype is associated with a higher prevalence of depressive symptoms (Chelminski et al. Citation1999; Hidalgo et al. Citation2009; Levandovski et al. Citation2011; Merikanto et al. Citation2013), or depressive states (Kitamura et al. Citation2010), also in adolescents (Randler Citation2011). A later chronotype is also associated with having a current diagnosis of major depressive disorder (MDD), having had treatment for depression, or using antidepressants (Merikanto et al. Citation2013; Merikanto et al. Citation2015; Antypa et al. Citation2016), and non-remission of depression (Chan et al. Citation2014). In patients with MDD, evening types reported more suicidal ideation than morning types (Gaspar-Barba et al. Citation2009; Bahk et al. Citation2014), and eveningness and poor sleep quality were both independent predictors of depression severity (Muller et al. Citation2016). In addition, in a recent prospective study in children and adolescents, chronotype (eveningness) longitudinally predicted increases in depressive symptoms and the onset of a depressive episode during a 1-year period, even when prior depression was controlled for (Haraden et al. Citation2017).
Sleep disturbances have been proposed to play a mediating role in the association between eveningness and depressive symptoms (Monteleone and Maj Citation2008). Having an evening chronotype is associated with sleep disturbances, including shorter sleep duration, poorer sleep quality and insufficient sleep (Koskenvuo et al. Citation2007). Police officers who were poor sleepers experienced significant improvements in their sleep quality when they were allowed to work “in phase” with their chronotype (i.e. morning or evening shifts) (Yadav et al. Citation2016). This indicates that a discrepancy between an individual’s chronotype and social obligations, which is more likely to occur in evening types, is related to poor sleep quality (Yadav et al. Citation2016). Subsequently, insufficient sleep leads to worsened mood and decreased ability to regulate negative emotions (Baum et al. Citation2014). Non-depressed people with insomnia have a twofold risk to develop depression, compared to people with no sleep difficulties, according to a meta-analysis of 34 studies (Li et al. Citation2016). However, a study in 1170 adult Japanese subjects showed that the association between extreme evening preference and more depressive symptoms could not be fully explained by mediation by various sleep parameters, including subjective sleep quality (Kitamura et al. Citation2010). Also a study in 756 Hungarian adults showed that both eveningness and sleep complaints were independent risk factors for negative emotionality, and the relation between eveningness and negative emotionality was not explained by sleep complaints (Simor et al. Citation2015).
Because the relationship between chronotype and depression cannot be explained by sleep disturbances alone, additional mechanisms are expected to play a role. Neurobiological explanations have focused on structural and functional brain abnormalities in the circadian machinery (Nestler et al. Citation2002), and various circadian gene polymorphisms have been linked to depression (including CLOCK, PER1 and PER2) (Etain et al. Citation2011). Another potential explanatory mechanism is circadian misalignment or “social jet lag”, which refers to a discrepancy between an individual’s chronotype and conventional social schedules (Adan et al. Citation2012). It has been argued that this discrepancy causes evening people to experience emotional distress (Wittmann et al. Citation2006), supported by a finding that greater misalignment was associated with more severe depression among patients with MDD (Hasler et al. Citation2010b). However, in the abovementioned study in Hungarian adults, circadian misalignment was not an explanatory factor in the relation between chronotype and negative emotionality (Simor et al. Citation2015).
In college students, evening types have been shown to smoke more (Schneider et al. Citation2011) and drink more alcoholic beverages than intermediate or morning types (Taylor et al. Citation2011). Evening types tend to consume more alcohol per occasion and have a higher risk of alcohol-related problems (Prat and Adan Citation2011), which may in turn increase the risk of depressive symptoms. While some have shown decreased psychological well-being in late chronotypes to be mediated by smoking and alcohol consumption (Wittmann et al. Citation2010), others have found the association of late chronotype with depressive symptoms to be independent of smoking status (Levandovski et al. Citation2011).
Evening types consume more caffeinated beverages than intermediate and morning types (Taylor et al. Citation2011) and are more likely to consume caffeine in the evening than intermediate types (Digdon and Rhodes Citation2009), which may contribute to poor sleep in evening persons. With regard to the association between caffeine use and depression, previous studies have yielded mixed findings; however, a recent meta-analysis with dose–response analysis found that coffee and caffeine consumption were significantly associated with decreased risk of depression (Wang, Shen et al., Citation2016).
Psychological explanations for the association between chronotype and depression may include cognitive emotion regulation: the conscious thoughts and cognitive efforts of managing negative emotions following threatening or stressful events (Garnefski et al. Citation2001). A general division between adaptive and maladaptive emotion regulation strategies can be made, and the chronic use of maladaptive emotion regulation strategies such as rumination, catastrophizing and self-blame has been associated with vulnerability to emotional problems after experiencing negative events (Garnefski et al. Citation2001). Internalizing disorders are widely believed to result from difficulties in regulating emotions (Aldao et al. Citation2010; Hofmann et al. Citation2012), and in a study of 116 German adults, deficient emotion regulation was found to predict depressive symptoms at a 5-year follow-up (Berking et al. Citation2014). Emotion regulation difficulties are also a maintaining factor for depression (D’Avanzato et al. Citation2013). A recent study in 150 university students and 90 adult volunteers in the United Kingdom showed impaired emotion regulation in late chronotypes (Watts and Norbury Citation2017). Specifically, they found that morningness was associated with cognitive reappraisal, and eveningness with expressive suppression. Further, eveningness has also been associated with a negative bias in the processing of emotional information among healthy individuals with no history of depression (Berdynaj et al. Citation2016; Horne et al. Citation2016), indicating that eveningness can be associated with affective processing difficulties (such as biased emotion processing) independent of depression. Such disturbances may in turn increase an individual’s risk of subsequent depression and affect regulation difficulties which have been proposed to mediate the relationship between chronotype and depression (Berdynaj et al. Citation2016; Horne et al. Citation2016; Watts and Norbury Citation2017).
Finally, a cross-sectional study showed that behavioral activation mediates the relationship between chronotype and depressive symptoms (Hasler et al. Citation2010a), with eveningness being associated with lower behavioral activation and consequently more depressive symptoms. Further, Hasler and colleagues (2010) also performed reversed mediation analyses and obtained non-significant results supporting their initial hypothesis that it is chronotype that influences depression through reduced behavioral activation, rather than vice versa.
The present study aimed to examine the relationship between chronotype and depressive symptoms, with sleep quality, substance use, behavioral activation and cognitive emotion regulation as potential mediators, in a large Dutch student sample. These associations were examined in cross-sectional and longitudinal data.
Methods
Participants and procedure
Participants were recruited by advertising online and at university buildings in Leiden, The Hague, The Hague, Delft, Rotterdam and Amsterdam in two subsequent years: March 2016 and March 2017. In both years, we ended the data collection in the weekend of the shift to daylight saving time. Being a student of 18 years or older and proficiency in Dutch were inclusion criteria for the study. Participants were provided with a link to the online questionnaires. On the first page, general information about the purpose of the study was given. Participants gave informed consent and were allowed to withdraw from the study at any stage. Participation required approximately 30 min. All data were stored anonymously. Participants who agreed to take part in a follow-up study were asked to provide their email address, which was stored separately from their response data. The study was approved by the Leiden University Institute of Psychology Ethics committee.
A total of 859 individuals participated in the study. After excluding participants who violated the inclusion criteria (not a student [n = 38] or <18 years old [n = 10]) and participants with missing values on the Morningness-Eveningness Questionnaire (MEQ) or QIDS-SR scales (n = 69), the final sample consisted of 742 participants, 74.5% of whom were women. The mean age in the sample was 21.4 ± 2.9, with a range from 18 to 56 years. Most participants (74.8%) were university students; the others were students at other Dutch higher education or vocational training institutions. Data on sleep quality were available for all participants, 9 persons had missing values on substance use, 40 persons had missing values on the Cognitive Emotion Regulation Questionnaire (CERQ) and 59 persons on the Behavioral Inhibition and Behavioral Activation (BIS/BAS) scales. describes the characteristics of the study sample.
Table 1. Characteristics of the sample (N = 742).
The participants who gave consent in 2016 to be approached again 1 year later (n = 272) were invited in March 2017 through e-mail to complete the same questionnaires again. Of those, 126 responded, and 115 fulfilled inclusion criteria. The students (n = 115) who were included in the follow-up study were more often university students (χ² = 9.34, p = 0.009), drank less alcohol (t = 2.98, p = 0.003), had a lower score on positive reappraisal (CERQ) (t = 1.99, p = 0.047), less drive (t = 2.28, p = 0.023) and fun seeking (BAS) (t = 2.07, p = 0.039) and more behavioral inhibition (t = −2.05, p = 0.04) than those who did not participate (n = 627). No differences were found in sex, age, depressive symptoms, chronotype, sleep quality, caffeine use, smoking, cannabis use and the other subscales of CERQ and BAS.
Materials
Data on gender, age and educational level were collected. Depressive symptoms were measured with the Quick Inventory of Depressive Symptomatology – Self-Rated (QIDS-SR, Rush et al. Citation2003), a 16-item self-report measure that assesses frequency and severity of symptoms in the nine DSM-IV domains of depression (sad mood, concentration, self-criticism, suicidal ideation, interest, energy/fatigue, sleep disturbances, changes in appetite/weight and psychomotor agitation/retardation) in the past 7 days. The total score ranges from 0 to 27 (none to severe depressive symptoms). The internal consistency of the QIDS-SR is high, as indicated by Cronbach’s alpha of 0.79 based on our sample.
Chronotype was assessed with the MEQ (Horne and Östberg Citation1976). The MEQ consists of 19 items and is the most widely used measure of chronotype (Adan et al. Citation2012). It contains questions about preferred wake-up and bedtimes and daily activity schedules. A higher total score indicates morningness. MEQ cut-offs are as follows: <42 are evening types and >58 are morning types. The MEQ has a high internal consistency, with Cronbach’s alpha of 0.88 based on our sample.
Subjective sleep quality was assessed with the Pittsburgh Sleep Quality Index (PSQI, Buysse et al. Citation1989). The PSQI measures sleep quality and disturbance retrospectively over a 1-month period, resulting in a global score between 0 and 21, with higher scores indicating poorer sleep quality. It consists of seven components: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, sleep medication use and daytime dysfunction. A global PSQI score of >5 is considered to indicate clinically significant sleep disturbance (Buysse et al. Citation1989). Cronbach’s alpha was 0.66 based on our sample, calculated over the seven component scores of the PSQI.
Substance use during the past month was assessed with a questionnaire (seven questions) about the average number of consumed alcoholic and caffeinated beverages per week; caffeine consumption per week was calculated assuming each cup contained 85 mg of caffeine (Netherlands Nutrition Centre). The amount of tobacco and cannabis use (number of joints) per week was also asked; these last two questions were recoded into dichotomous variables: smoking tobacco yes/no and smoking cannabis yes/no.
The use of cognitive emotion regulation strategies was measured with the CERQ (Garnefski and Kraaij Citation2007). The CERQ consists of 36 questions on nine subscales (four items on each subscale) corresponding to nine different emotion regulation strategies (self-blame, other-blame, rumination, catastrophizing, putting into perspective, positive refocusing, positive reappraisal, acceptance and refocus on planning). Respondents were asked to indicate how they generally tend to think after experiencing negative or unpleasant events, and to answer on a 5-point scale from 1 (almost never) to 5 (almost always) how likely they are to use a certain cognitive strategy in that situation. Items included statements such as “I feel that I am the one to blame for it” (self-blame). A score for each subscale is calculated (ranging from 4 to 20). High scores indicate frequent use of the specific strategy. All subscales had high internal consistency in our sample, with Cronbach’s alphas ranging from 0.75 to 0.83.
Behavioral inhibition and behavioral activation were measured with the BIS/BAS scales (Carver and White Citation1994). The scales contain 24 items to assess endogenous motivational systems, with answers rated on 4-point Likert scales and include two main scales: the BIS scale and the BAS scale. The BIS includes seven items reflecting the response to the anticipation of punishment. The BAS scale comprised three subscales: drive (four items focusing on the constant pursuit of desired goals), fun seeking (four items focusing on the desire for new rewards as well as eagerness to approach a situation) and reward responsiveness (five items reflecting positive reactions to rewards). Four items are used as fillers and are excluded. Cronbach’s alpha of the BIS was 0.86 in our sample; of the three BAS scales, Cronbach’s alpha was 0.72 for drive, 0.63 for fun seeking and 0.57 for reward responsiveness.
Statistical analyses
Descriptive statistics were calculated for all variables. Chronotype was used as a continuous score (MEQ total) for all analyses, apart from descriptives (). Mediation analyses were carried out with PROCESS for SPSS v2.16.3 (Hayes Citation2013) to test whether chronotype was associated with depressive symptoms and whether this relationship was mediated by sleep quality, substance use, cognitive emotion regulation and behavioral activation and inhibition. Four parallel mediation models were run, one for each of the following: (1) sleep quality (total PSQI score), (2) substances: alcohol, caffeine, nicotine, cannabis (3), cognitive emotion regulation: nine CERQ subscales and (4) BIS and BAS subscales: drive, fun seeking, reward responsiveness. All variables that were significant mediators were entered in a final parallel mediation model. The significance of the indirect effects was tested using confidence intervals obtained by the bootstrapping procedure (Hayes Citation2013) with 5000 resamples. An alpha level of 0.05 (two-sided) was used for other statistical tests.
For the subgroup that gave consent for the follow-up study, the significant mediators from the cross-sectional study were again entered in a parallel mediation model, with chronotype at baseline (T1) as the predictor, depressive symptoms after 1 year (T2) as the outcome variable and mediators as measured after 1 year (T2). For the longitudinal analyses, in order to control for the influence of baseline depressive symptoms, depressive symptoms at the first measurement (T1) were entered in the model as covariate. The four QIDS items relating to sleep complaints (items 1–4) were removed from the total score of this covariate due to overlap in variance with one of the mediators (sleep quality; total PSQI score). For depressive symptoms as an outcome measure, the total QIDS score including sleep items was used both in cross-sectional and longitudinal analyses. All analyses were performed with IBM SPSS Statistics 23.0 for Windows (Armonk, NY: IBM Corp.).
Results
Cross-sectional sample
A linear regression analysis, adjusted for age and gender, showed that eveningness was significantly associated with more depressive symptoms (β = −0.082, t = −2.20, p = 0.028), with a small explained variance in this model, R2 = 0.02. Four parallel mediation models were run, one for each of the following: (1) sleep quality (total PSQI score), (2) substances: alcohol, caffeine, nicotine, cannabis, (3) cognitive emotion regulation: each of the CERQ subscales and (4) Behavioral Inhibition and Behavioral Activation (BAS subscales: drive, fun seeking, reward responsiveness). shows the results of the four parallel mediation analyses.
Table 2. Summary of statistical mediation analyses between chronotype (IV) and depressive symptoms (DV) in the cross-sectional sample.
In the initial parallel mediation models, eveningness was associated with poorer sleep quality, more alcohol consumption, more smoking, more self-blame, less refocusing on planning, less positive reappraisal, more blaming others and more fun seeking ().
Poorer sleep quality, less alcohol consumption, more smoking, more self-blame, more rumination, less refocusing on planning, less positive reappraisal, more catastrophizing, more behavioral inhibition and less reward responsiveness were associated with depressive symptoms. Only sleep quality, alcohol consumption, smoking, self-blame, refocusing on planning and positive reappraisal were independent mediators of the association between chronotype and depressive symptoms, in their respective parallel models and adjusted for age and gender. These variables were therefore entered in the final mediation model ().
Table 3. Results of final mediation model (n = 702).
In the final mediation model, the total effect of chronotype on depressive symptoms was borderline significant (effect = −0.033, SE = 0.017, p = 0.05), probably because of the smaller sample size due to missing values on the CERQ (n = 40), even though the regression coefficient was virtually the same as in the total sample. Chronotype was associated with all of the potential mediators in this model: eveningness was associated with poorer sleep quality, more alcohol consumption, more smoking, more self-blame, less refocusing on planning and less positive reappraisal. Poorer sleep quality was associated with more depressive symptoms. Interestingly, less alcohol consumption was associated with more depressive symptoms, and therefore, alcohol consumption acted as a suppressor variable in the relationship between chronotype and depressive symptoms. More self-blame and less positive reappraisal were associated with more depressive symptoms. The completely standardized indirect effects are as follows: sleep quality (effect = −0.083, CI: −0.123, −0.046), alcohol consumption (effect = 0.016, CI: 0.004, 0.033), self-blame (effect = −0.026, CI: −0.052, −0.001) and positive reappraisal (effect = −0.013, CI: −0.028, −0.004). When assessing whether each indirect effect by each mediator was independent from the parallel mediators, we observed independence for all mediating effects, apart from the ones of self-blame and positive reappraisal where effects overlapped and no independence was found (effect = −0.006, CI: −0.018, 0.006). In this multivariate model, smoking and refocusing on planning were not associated with depressive symptoms and were not mediators of the association between chronotype and depressive symptoms.
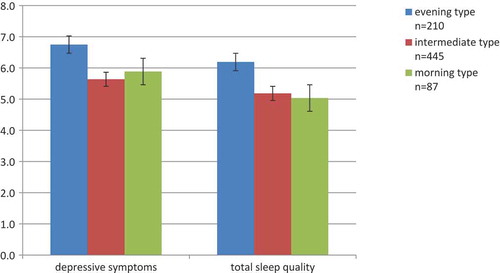
Since the total PSQI score was a significant mediator between chronotype and depressive symptoms, we explored in post-hoc analyses which components of the scale mediated the relationship. A mediation model with all seven components of the PSQI as parallel mediators showed that self-rated subjective sleep quality (effect = −0.02, CI: −0.046, −0.011), sleep onset latency (effect = −0.01, CI: −0.029, −0.005), sleep duration (effect = −0.01, CI: −0.029, −0.005) and daytime dysfunction (effect = −0.05, CI: −0.088, −0.020) were significant mediators (completely standardized indirect effects presented). Finally, in order to understand further how the three chronotype groups differed in terms of level of sleep quality and depressive symptoms, we plotted means and standard errors on these measures (). The mean level of subjective sleep quality observed among evening types was above the clinical threshold (>5) (Buysse et al. Citation1989), whereas morning types scored, on average, below that.
Longitudinal sample
For the 115 participants that completed the follow-up, we performed a multiple linear regression with chronotype, age and gender at T1 as independent variables and depressive symptoms at T2 as the dependent variable. In this model, chronotype at T1 was a significant predictor of depressive symptoms at T2 (β = −0.29, t = −3.10, p = 0.002). When we added baseline depressive symptoms (total QIDS score minus the sleep items) as a covariate, chronotype remained a significant predictor of depressive symptoms at T2 (β = −0.16, t = −2.01, p = 0.047). The correlation between a difference in chronotype between T1 and T2 and a difference in depressive symptoms between T1 and T2 was not significant (r = −0.13, p = 0.15).
Three mediation analyses were also run, with (1) the PSQI total score at T2, (2) alcohol use at T2 and (3) the CERQ subscales at T2 as mediators. Results showed that only total sleep quality at T2 was a significant mediator (standardized indirect effect = −0.11, SE: 0.05, CI: −0.218, −0.040). When depressive symptoms (total QIDS score minus the sleep items) at baseline (T1) were added as a covariate in the mediation model, sleep quality at T2 remained a significant mediator (effect = −0.07, SE: 0.04, CI: −0.164, −0.009).
Discussion
In the present study, the relationship between chronotype and depressive symptoms and its potential mediators was examined in a large sample of Dutch students. The results of the current study supported the previously reported association between the evening chronotype and depressive symptoms (Merikanto et al. Citation2013; Merikanto et al. Citation2015; Antypa et al. Citation2016), even though the proportion of explained variance was small. We found that sleep quality mediated the relationship between chronotype and depressive symptoms, both in the cross-sectional analysis and in the longitudinal analysis. The difference in chronotype between T1 and T2 was not correlated with the difference in depressive symptoms between T1 and T2. Furthermore, alcohol consumption was a significant suppressor variable, and the cognitive emotion regulation strategies of self-blame and positive reappraisal were significant mediators in the cross-sectional study, but not in the longitudinal study. It is possible that the smaller sample size of the prospectively followed subsample and the resulting reduced statistical power, as well as possible selection bias (more depressed students may not have participated in T2), contributed to the fact that we did not find a longitudinal association between a difference in chronotype between T1 and T2 and a difference in depressive symptoms between T1 and T2 and were also unable to replicate mediation by alcohol consumption and cognitive emotion regulation in the longitudinal analyses. Finally, we did not replicate the mediation of the association between eveningness and depressive symptoms by reward responsiveness (from the behavioral activation scale), previously reported in a cross-sectional study (Hasler et al. Citation2010a).
Evening types reported poorer subjective sleep quality, which is in line with prior literature (Kitamura et al. Citation2010; Martin et al. Citation2012; Roeser et al. Citation2012; Rique et al. Citation2014; Yun et al. Citation2015). Post-hoc analyses showed that poorer self-rated subjective sleep quality, longer sleep onset latency, shorter sleep duration and higher daytime dysfunction were significant mediators of the relationship between chronotype and depressive symptoms. Prior research has also shown that eveningness is associated with longer self-reported sleep onset latencies (Taillard et al. Citation2001; Roeser et al. Citation2012) and shorter sleep duration irrespective of whether it was measured on weekdays or weekend days (Park et al. Citation1998; Soehner et al. Citation2011).
Poor sleep quality has been suggested as a prospective predictor (or precursor) of depression in many longitudinal studies (Li et al. Citation2016). EEG research has shown that evening types have different slow wave activity during rapid eye movement (REM) sleep (Chaput et al. Citation2012) and non-REM sleep (Mongrain et al. Citation2006) compared to morning types, and evening types have shown a poorer homeostatic response to sleep disruption (Mongrain and Dumont Citation2007). In our study, it seems that the longer sleep onset latency and shorter sleep duration translated into higher daytime dysfunction and poorer sleep quality in evening types. The literature examining potential mediators of the chronotype–depression relationship has shown mixed results with regard to sleep quality as a mediator. Social jet lag was related to eveningness and depression in a large rural cohort study performed in Brazil (Levandovski et al. Citation2011). However, the association between social jet lag and depression was not replicated in the Netherlands study of Depression and Anxiety (Knapen et al. Citation2018). Also, insomnia scores were higher in morning types in that sample (Antypa et al. Citation2017). Interestingly, in another study, subjective sleep quality (statistically) fully mediated the relationship between chronotype and response to stress (Roeser et al. Citation2012). In a study in patients with MDD, poor sleep quality was not a significant mediator in the relationship between eveningness and depression severity (Muller et al. Citation2016).
Results from our cross-sectional analyses showed associations between the evening chronotype and higher use of maladaptive emotion regulation strategies such as self-blame and lower use of adaptive strategies such as positive reappraisal. This last finding confirmed the results of a recent study in the United Kingdom (Watts and Norbury Citation2017). Our mediation model showed that eveningness was associated with higher use of self-blame and lower use of positive reappraisal, which in turn were associated with reporting more depressive symptoms.
Different emotion regulation strategies are hence differently effective in enhancing – or undermining – an individual’s well-being (Balzarotti et al. Citation2016). Positive reappraisal is defined as “thoughts of creating a positive meaning to the event in terms of personal growth” (Garnefski and Kraaij Citation2007). It helps an individual to reconstruct their initially negative appraisal of a situation and to find positive meaning, which in turn increases motivation and enables coping with an ongoing stressor (Folkman Citation1997). After thinking about stressful life events, experimentally induced positive reappraisal leads to both significantly larger increases in positive affect and decreases in negative affect as compared to strategies such as rumination or acceptance (Rood et al. Citation2012). Individuals who dispositionally used positive reappraisal also had higher levels of both subjective and psychological well-being, including higher levels of positive affect, more positive relations with others and more self-acceptance (Balzarotti et al. Citation2016). Further, depressed patients are less likely to use positive reappraisal as compared to healthy controls (D’Avanzato et al. Citation2013).
Higher self-blame has not been associated with chronotypes before but has been related to increased depression vulnerability in various samples, including adolescents, adults and psychiatric patients (Garnefski and Kraaij Citation2006). Furthermore, it appears that “characterological self-blame” (i.e. blaming one-self and the core of one’s character, in contrast to “behavioral self-blame” which relates to things one did or omitted to do) is specifically related to depressive symptoms in adolescents, as shown in a prospective study (Tilghman-Osborne et al. Citation2008). Self-blame, as assessed with the CERQ, is indeed more related to characterological rather than behavioral self-blame.
In an attempt to better understand the observed relationship between eveningness and the use of these cognitive emotion regulation strategies, some studies in relation to self-control might be relevant. Previous studies have found eveningness to be associated with general difficulties with self-regulation and low self-control (Digdon and Howell Citation2008). Emotion regulation, then, can be seen as part of more general self-control (Tice and Bratslavsky Citation2000). These deficits in emotional control – rather than evening preference per se – then work to increase the individual’s vulnerability to experiencing depressive symptoms, and potentially to developing a depressive disorder.
Students with a later chronotype reported more alcohol consumption, in line with previous research (Taylor et al. Citation2011). Alcohol consumption was a significant suppressor variable: lower amounts of alcohol were associated with more depressive symptoms. Problematic alcohol use has been associated with depressive symptoms in students (Marmorstein Citation2009). However, the mean consumption of about five alcoholic beverages per week in our sample is very low, and therefore, it is unlikely that there were many problematic alcohol users in our sample. A study in American college students, with low endorsement for both depressive symptoms and problematic alcohol use, did not find any association between alcohol consumption and depressive symptoms (Schnetzer et al. Citation2013). The seemingly protective effect of alcohol in our study may also be attributable to a more active social life (Rinker et al. Citation2016), which could in turn be protective against depressive symptoms.
The findings of the present study have some clinical relevance. First, we showed that evening types who experience poor sleep quality are more likely to suffer from depressive symptoms. Identifying individuals at risk for depression based on their chronotype and sleep problems would enable early interventions, addressing their sleep habits as well as their cognitive emotion regulation skills. As discussed elsewhere, current cognitive therapies do not target the specific cognitive emotion regulation strategies individuals use to cope with negative events (Garnefski and Kraaij Citation2007). However, the findings of the current study indicate that these individuals might benefit from more tailored and combined interventions, such as a combination of chronotherapeutics with specific cognitive treatments.
The present study has some strengths and limitations. Strengths include a large sample size and prospective design in a sub-sample, constituting this study as the first prospective study investigating the predictive value of chronotype on depressive symptoms in adults. However, findings can only be generalized to other student samples or young age populations. Future research should test whether the findings of this study can be replicated in a larger sample incorporating a wider age range. Limitations include the absence of objective measures (such as actigraphy) and no distinction of week/weekend sleep patterns in students, which are known to differ (Vitale et al. Citation2015). Furthermore, a recent study on the association between chronotype and depression in students showed that when the concept of morning affect (MA) was differentiated from morning/evening preference, MA was more strongly associated with depressive symptoms than morning/evening preference in itself (Jankowski Citation2016). However, we did not perform such a division and considered chronotype as a unitary construct. Another recent study showed that, in their sample, the association between chronotype and depressive symptom severity was better explained by academic stress (Romo-Nava et al. Citation2016). Although we included a number of potential mediators (BIS/BAS, sleep quality, cognitive emotion regulation, substance use), it is of course possible that the observed relationship between chronotype and depressive symptoms is still better explained by another factor that we did not account for. Furthermore, to construct our mediation model, we made assumptions about the directions of the associations we investigated. However, the cross-sectional design of our main study precluded the inference of temporal or causal relationships. Finally, we have observed only moderate-to-small effects in our analyses, which should be further replicated.
In conclusion, in the present study, we observed that eveningness is related to depressive symptoms in students, both cross-sectionally and longitudinally. A pertinent mediator of this relationship is subjective sleep quality. Further, eveningness is correlated with more use of maladaptive emotion regulation strategies such as self-blame, and lower use of adaptive strategies such as positive reappraisal. Preventive strategies in this age group could involve a combination of chronotherapeutics, sleep education and tailored cognitive approaches.
Declaration of interest
None.
Supplemental Material
Download PDF (204.6 KB)Acknowledgment
The authors would like to thank the Leiden University students who assisted with the data collection.
supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- Adan A, Archer SN, Hidalgo MP, Di Milia L, Natale V, Randler C. 2012. Circadian typology: a comprehensive review. Chronobiol Int. 29:1153–75.
- Adan A, Natale V. 2002. Gender differences in morningness-eveningness preference. Chronobiol Int. 19:709–20.
- Aldao A, Nolen-Hoeksema S, Schweizer S. 2010. Emotion-regulation strategies across psychopathology: a meta-analytic review. Clin Psychol Rev. 30:217–37.
- Antypa N, Verkuil B, Molendijk M, Schoevers R, Penninx B, Van Der Does W. 2017. Associations between chronotypes and psychological vulnerability factors of depression. Chronobiol Int. 34(8): 1125-1135.
- Antypa N, Vogelzangs N, Meesters Y, Schoevers R, Penninx BW. 2016. Chronotype associations with depression and anxiety disorders in a large cohort study. Depress Anxiety. 33:75–83.
- Au J, Reece J. 2017. The relationship between chronotype and depressive symptoms: a meta-analysis. J Affect Disord. 218:93–104.
- Bahk YC, Han E, Lee SH. 2014. Biological rhythm differences and suicidal ideation in patients with major depressive disorder. J Affect Disord. 168:294–97.
- Balzarotti S, Biassoni F, Villani D, Prunas A, Velotti P. 2016. Individual differences in cognitive emotion regulation: implications for subjective and psychological well-being. J Happiness Stud. 17:125–43.
- Baum KT, Desai A, Field J, Miller LE, Rausch J, Beebe DW. 2014. Sleep restriction worsens mood and emotion regulation in adolescents. J Child Psychol Psychiatry. 55:180–90.
- Berdynaj D, Boudissa SN, Grieg MS, Hope C, Mahamed SH, Norbury R. 2016. Effect of chronotype on emotional processing and risk taking. Chronobiol Int. 33:406–18.
- Berking M, Wirtz CM, Svaldi J, Hofmann SG. 2014. Emotion regulation predicts symptoms of depression over five years. Behav Res Ther. 57:13–20.
- Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. 1989. The pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 28:193–213.
- Carver CS, White TL. 1994. Behavioral-inhibition, behavioral activation, and affective responses to impending reward and punishment - the bis bas scales. J Pers Soc Psychol. 67:319–33.
- Chan JW, Lam SP, Li SX, Yu MW, Chan NY, Zhang J, Wing YK. 2014. Eveningness and insomnia: independent risk factors of nonremission in major depressive disorder. Sleep. 37:911–17.
- Chaput JP, McNeil J, Despres JP, Bouchard C, Tremblay A. 2012. Short sleep duration is associated with greater alcohol consumption in adults. Appetite. 59:650–55.
- Chelminski I, Ferraro FR, Petros TV, Plaud JJ. 1999. An analysis of the “eveningness-morningness” dimension in “depressive” college students. J Affect Disord. 52:19–29.
- D’Avanzato C, Joormann J, Siemer M, Gotlib IH. 2013. Emotion regulation in depression and anxiety: examining diagnostic specificity and stability of strategy use. Cognit Ther Res. 37:968–80.
- Digdon NL, Howell AJ. 2008. College students who have an eveningness preference report lower self-control and greater procrastination. Chronobiol Int. 25:1029–46.
- Digdon NL, Rhodes S. 2009. Methods used to cope with sleepiness may perpetuate sleepiness in college students with an evening type circadian preference. Biol Rhythm Res. 40:129–44.
- Duffy JF, Rimmer DW, Czeisler CA. 2001. Association of intrinsic circadian period with morningness-eveningness, usual wake time, and circadian phase. Behav Neurosci. 115:895–99.
- Etain B, Milhiet V, Bellivier F, Leboyer M. 2011. Genetics of circadian rhythms and mood spectrum disorders. Eur Neuropsychopharmacol. 21(Suppl 4):S676–682.
- Folkman S. 1997. Positive psychological states and coping with severe stress. Soc Sci Med. 45:1207–21.
- Garnefski N, Kraaij V. 2006. Relationships between cognitive emotion regulation strategies and depressive symptoms: a comparative study of five specific samples. Pers Individ Dif. 40:1659–69.
- Garnefski N, Kraaij V. 2007. The cognitive emotion regulation questionnaire. Eur J Psychol Assess. 23:141–49.
- Garnefski N, Kraaij V, Spinhoven P. 2001. Negative life events, cognitive emotion regulation and emotional problems. Pers Individ Dif. 30:1311–27.
- Gaspar-Barba E, Calati R, Cruz-Fuentes CS, Ontiveros-Uribe MP, Natale V, De Ronchi D, Serretti A. 2009. Depressive symptomatology is influenced by chronotypes. J Affect Disord. 119:100–06.
- Haraden DA, Mullin BC, Hankin BL. 2017. The relationship between depression and chronotype: a longitudinal assessment during childhood and adolescence. Depress Anxiety. 34:967–76.
- Hasler BP, Allen JJ, Sbarra DA, Bootzin RR, Bernert RA. 2010a. Morningness-eveningness and depression: preliminary evidence for the role of the behavioral activation system and positive affect. Psychiatry Res. 176:166–73.
- Hasler BP, Buysse DJ, Kupfer DJ, Germain A. 2010b. Phase relationships between core body temperature, melatonin, and sleep are associated with depression severity: further evidence for circadian misalignment in non-seasonal depression. Psychiatry Res. 178:205–07.
- Hayes AF. 2013. Introduction to mediation, moderation, and conditional process analysis - a regression-based approach. New York: Guilford Press.
- Hidalgo MP, Caumo W, Posser M, Coccaro SB, Camozzato AL, Chaves ML. 2009. Relationship between depressive mood and chronotype in healthy subjects. Psychiatry Clin Neurosci. 63:283–90.
- Hofmann SG, Sawyer AT, Fang A, Asnaani A. 2012. Emotion dysregulation model of mood and anxiety disorders. Depress Anxiety. 29:409–16.
- Horne CM, Marr-Phillips SDM, Jawaid R, Gibson EL, Norbury R. 2016. Negative emotional biases in late chronotypes. Biol Rhythm Res. 48:151–55.
- Horne JA, Östberg O. 1976. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 4:97–110.
- Jankowski KS. 2016. Morningness-eveningness and depressive symptoms: test on the components level with CES-D in Polish students. J Affect Disord. 196:47–53.
- Kerkhof GA. 1985. Inter-individual differences in the human circadian system: a review. Biol Psychol. 20:83–112.
- Kitamura S, Hida A, Watanabe M, Enomoto M, Aritake-Okada S, Moriguchi Y, Kamei Y, Mishima K. 2010. Evening preference is related to the incidence of depressive states independent of sleep-wake conditions. Chronobiol Int. 27:1797–812.
- Knapen SE, Antypa N, Meesters YSchoevers RA. 2018. Social jetlag and depression status: results obtained from the netherlands study of depression and anxiety. Chronobiol Int. 35(1): 1–7.
- Koskenvuo M, Hublin C, Partinen M, Heikkilä K, Kaprio J. 2007. Heritability of diurnal type: a nationwide study of 8753 adult twin pairs. J Sleep Res. 16:156–62.
- Lack L, Bailey M, Lovato N, Wright H. 2009. Chronotype differences in circadian rhythms of temperature, melatonin, and sleepiness as measured in a modified constant routine protocol. Nat Sci Sleep. 1:1–8.
- Levandovski R, Dantas G, Fernandes LC, Caumo W, Torres I, Roenneberg T, Hidalgo MP, Allebrandt KV. 2011. Depression scores associate with chronotype and social jetlag in a rural population. Chronobiol Int. 28:771–78.
- Li L, Wu C, Gan Y, Qu X, Lu Z. 2016. Insomnia and the risk of depression: a meta-analysis of prospective cohort studies. BMC Psychiatry. 16:375.
- Marmorstein NR. 2009. Longitudinal associations between alcohol problems and depressive symptoms: early adolescence through early adulthood. Alcohol Clin Exp Res. 33:49–59.
- Martin JS, Hebert M, Ledoux E, Gaudreault M, Laberge L. 2012. Relationship of chronotype to sleep, light exposure, and work-related fatigue in student workers. Chronobiol Int. 29:295–304.
- Merikanto I, Kronholm E, Peltonen M, Laatikainen T, Vartiainen E, Partonen T. 2015. Circadian preference links to depression in general adult population. J Affect Disord. 188:143–48.
- Merikanto I, Lahti T, Kronholm E, Peltonen M, Laatikainen T, Vartiainen E, Salomaa V, Partonen T. 2013. Evening types are prone to depression. Chronobiol Int. 30:719–25.
- Mongrain V, Carrier J, Dumont M. 2006. Difference in sleep regulation between morning and evening circadian types as indexed by antero-posterior analyses of the sleep EEG. Eur J Neurosci. 23:497–504.
- Mongrain V, Dumont M. 2007. Increased homeostatic response to behavioral sleep fragmentation in morning types compared to evening types. Sleep. 30:773–80.
- Monteleone P, Maj M. 2008. The circadian basis of mood disorders: recent developments and treatment implications. Eur Neuropsychopharmacol. 18:701–11.
- Muller MJ, Kundermann B, Cabanel N. 2016. Eveningness and poor sleep quality independently contribute to self-reported depression severity in psychiatric inpatients with affective disorder. Nord J Psychiatry. 70:329–34.
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. 2002. Neurobiology of Depression. Neuron. 34:13–25.
- Park YM, Matsumoto K, Seo YJ, Shinkoda H, Park KP. 1998. Sleep in relation to age, sex, and chronotype in Japanese workers. Percept Mot Skills. 87:199–215.
- Prat G, Adan A. 2011. Influence of circadian typology on drug consumption, hazardous alcohol use, and hangover symptoms. Chronobiol Int. 28:248–57.
- Randler C. 2011. Association between morningness-eveningness and mental and physical health in adolescents. Psychol Health Med. 16:29–38.
- Rinker DV, Krieger H, Neighbors C. 2016. Social network factors and addictive behaviors among college students. Curr Addict Rep. 3:356–67.
- Rique GL, Fernandes Filho GM, Ferreira AD, De Sousa-Munoz RL. 2014. Relationship between chronotype and quality of sleep in medical students at the Federal University of Paraiba, Brazil. Sleep Sci. 7:96–102.
- Roenneberg T, Kuehnle T, Juda M, Kantermann T, Allebrandt K, Gordijn M, Merrow M. 2007. Epidemiology of the human circadian clock. Sleep Med Rev. 11:429–38.
- Roeser K, Meule A, Schwerdtle B, Kubler A, Schlarb AA. 2012. Subjective sleep quality exclusively mediates the relationship between morningness-eveningness preference and self-perceived stress response. Chronobiol Int. 29:955–60.
- Romo-Nava F, Tafoya SA, Gutierrez-Soriano J, Osorio Y, Carriedo P, Ocampo B, Bobadilla RI, Heinze G. 2016. The association between chronotype and perceived academic stress to depression in medical students. Chronobiol Int. 33:1359–68.
- Rood L, Roelofs J, Bogels SM, Arntz A. 2012. The effects of experimentally induced rumination, positive reappraisal, acceptance, and distancing when thinking about a stressful event on affect states in adolescents. J Abnorm Child Psychol. 40:73–84.
- Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, Markowitz JC, Ninan PT, Kornstein S, Manber R, et al. 2003. The 16-Item quick inventory of depressive symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 54:573–83.
- Schneider ML, Vasconcellos DC, Dantas G, Levandovski R, Caumo W, Allebrandt KV, Doring M, Hidalgo MP. 2011. Morningness-eveningness, use of stimulants, and minor psychiatric disorders among undergraduate students. Int J Psychol. 46:18–23.
- Schnetzer LW, Schulenberg SE, Buchanan EM. 2013. Differential associations among alcohol use, depression and perceived life meaning in male and female college students. J Subst Use. 18:311–19.
- Simor P, Zavecz Z, Pálosi V, Török CKöteles F. 2015. The influence of sleep complaints on the association between chronotype and negative emotionality in young adults. Chronobiology International. 32(1): 1–10. doi:10.3109/07420528.2014.935786
- Soehner AM, Kennedy KS, Monk TH. 2011. Circadian preference and sleep-wake regularity: associations with self-report sleep parameters in daytime-working adults. Chronobiol Int. 28:802–09.
- Taillard J, Philip P, Chastang JF, Diefenbach K, Bioulac B. 2001. Is self-reported morbidity related to the circadian clock? J Biol Rhythms. 16:183–90.
- Taylor DJ, Clay KC, Bramoweth AD, Sethi K, Roane BM. 2011. Circadian phase preference in college students: relationships with psychological functioning and academics. Chronobiol Int. 28:541–47.
- Tice DM, Bratslavsky E. 2000. Giving in to feel good: the place of emotion regulation in the context of general self-control. Psychol Inq. 11:149–59.
- Tilghman-Osborne C, Cole DA, Felton JW, Ciesla JA. 2008. Relation of guilt, shame, behavioral and characterological self-blame to depressive symptoms in adolescents over time. J Soc Clin Psychol. 27:809–42.
- Vitale JA, Roveda E, Montaruli A, Galasso L, Weydahl A, Caumo A, Carandente F. 2015. Chronotype influences activity circadian rhythm and sleep: differences in sleep quality between weekdays and weekend. Chronobiol Int. 32:405–15.
- Wang L, Shen X, Wu Y, Zhang D. 2016. Coffee and caffeine consumption and depression: a meta-analysis of observational studies. Aust N Z J Psychiatry. 50:228–42.
- Watts AL, Norbury R. 2017. reduced effective emotion regulation in night owls. J Biol Rhythms. 32:369–75.
- Weaver DR. 1998. The suprachiasmatic nucleus: a 25-year retrospective. J Biol Rhythms. 13:100–12.
- Wittmann M, Dinich J, Merrow M, Roenneberg T. 2006. Social jetlag: misalignment of biological and social time. Chronobiol Int. 23:497–509.
- Wittmann M, Paulus M, Roenneberg T. 2010. Decreased psychological well-being in late ‘chronotypes’ is mediated by smoking and alcohol consumption. Subst Use Misuse. 45:15–30.
- Yadav A, Rani S, Singh S. 2016. Working “out-of-phase” with reference to chronotype compromises sleep quality in police officers. Chronobiol Int. 33:151–60.
- Yun JA, Ahn YS, Jeong KS, Joo EJ, Choi KS. 2015. The relationship between chronotype and sleep quality in Korean Firefighters. Clin Psychopharmacol Neurosci. 13:201–08.