ABSTRACT
Light is necessary for life, and artificial light improves visual performance and safety, but there is an increasing concern of the potential health and environmental impacts of light. Findings from a number of studies suggest that mistimed light exposure disrupts the circadian rhythm in humans, potentially causing further health impacts. However, a variety of methods has been applied in individual experimental studies of light-induced circadian impacts, including definition of light exposure and outcomes. Thus, a systematic review is needed to synthesize the results. In addition, a review of the scientific evidence on the impacts of light on circadian rhythm is needed for developing an evaluation method of light pollution, i.e., the negative impacts of artificial light, in life cycle assessment (LCA). The current LCA practice does not have a method to evaluate the light pollution, neither in terms of human health nor the ecological impacts. The systematic literature survey was conducted by searching for two concepts: light and circadian rhythm. The circadian rhythm was searched with additional terms of melatonin and rapid-eye-movement (REM) sleep. The literature search resulted to 128 articles which were subjected to a data collection and analysis. Melatonin secretion was studied in 122 articles and REM sleep in 13 articles. The reports on melatonin secretion were divided into studies with specific light exposure (101 reports), usually in a controlled laboratory environment, and studies of prevailing light conditions typical at home or work environments (21 studies). Studies were generally conducted on adults in their twenties or thirties, but only very few studies experimented on children and elderly adults. Surprisingly many studies were conducted with a small sample size: 39 out of 128 studies were conducted with 10 or less subjects. The quality criteria of studies for more profound synthesis were a minimum sample size of 20 subjects and providing details of the light exposure (spectrum or wavelength; illuminance, irradiance or photon density). This resulted to 13 qualified studies on melatonin and 2 studies on REM sleep. Further analysis of these 15 reports indicated that a two-hour exposure to blue light (460 nm) in the evening suppresses melatonin, the maximum melatonin-suppressing effect being achieved at the shortest wavelengths (424 nm, violet). The melatonin concentration recovered rather rapidly, within 15 min from cessation of the exposure, suggesting a short-term or simultaneous impact of light exposure on the melatonin secretion. Melatonin secretion and suppression were reduced with age, but the light-induced circadian phase advance was not impaired with age. Light exposure in the evening, at night and in the morning affected the circadian phase of melatonin levels. In addition, even the longest wavelengths (631 nm, red) and intermittent light exposures induced circadian resetting responses, and exposure to low light levels (5–10 lux) at night when sleeping with eyes closed induced a circadian response. The review enables further development of an evaluation method of light pollution in LCA regarding the light-induced impacts on human circadian system.
Introduction
Artificial light is a necessity in the modern societies. It provides illumination when natural light is not available enabling a multitude of functions after dark. It is intended to improve the visual performance and visibility in indoor and outdoor environments, thus improving safety and comfort of humans. In addition, artificial light may serve as a source for relaxation and beauty, and to improve alertness and productivity.
The intention of artificial light is to be beneficial but accumulating evidence points to adverse impacts on humans (Dominoni et al. Citation2016) and the environment (Irwin Citation2018). Artificial light is suspected to have adverse impacts on humans, animals, plants and ecosystem balance (Tähkämö et al.). Potential environmental impacts of lighting products have been evaluated in several life-cycle assessment (LCA) studies (e.g., Scholand and Dillon Citation2012; Tähkämö and Halonen Citation2015; see reviews in Franz and Wenzl Citation2017; Tähkämö et al. Citation2014). The LCA method is a tool for systematic evaluation of potential environmental impacts – including those on human health – of any type of product system or process. However, previous LCAs of lamps and luminaires exclude the impacts caused by light but they focus on the impacts of other energy and material flows causing a number of environmental impacts, such as global warming, acidification, ozone depletion, resource depletion, ecotoxicity and human toxicity.
Light pollution, that is the sum of all adverse impacts of artificial light (Commission International de l’Eclairage Citation2014), is excluded from prior LCA studies, as current characterization models used in LCA do not enable the quantification and evaluation of the impacts of artificial light on humans or any other species or parts of the environment. It has been acknowledged that an evaluation method should be developed for assessing light pollution in LCAs (Cucurachi et al. Citation2014; European Commission-Joint Research Centre - Institute for Environment and Sustainability Citation2011).
In order to develop an evaluation method for light pollution in LCA, it is necessary to evaluate first the scientific evidence on artificial light negatively affecting the environment (Cucurachi et al. Citation2014). Based on a preliminary literature search (Tähkämö Citation2017), a lot of studies were found on impacts of light on human health, especially related to circadian rhythms. The impacts of artificial light on fauna, flora and the ecosystems were found to be complex and contradictory in the scientific literature. Impacts on animals varied by species and even by population, and the studies on plants and ecosystem chains are scarce. The impacts on human health are likewise complex (Tähkämö Citation2017), but there are preliminary indications of a link between severe health concerns and the light pollution (Bedrosian and Nelson Citation2013; Cho et al. Citation2015; Reiter et al. Citation2007), especially via the circadian rhythm disruption and compromised melatonin excretion as its marker. These are based on an increasing number of individual studies on light-induced impacts on human circadian rhythm, using a variety of experimental setups, study methods and definition of light exposure. However, to the best of our knowledge, there exists no systematic review on the impact of light on circadian rhythm. With regard to previous works on the subject, Cho et al. (Citation2015) reviewed 85 papers, and found adverse health effects in humans, related to melatonin suppression, sleep and alertness. Souman et al. (Citation2018) reviewed 68 papers on light affecting alertness in humans and found that increasing intensity of polychromatic light increased subjective alertness but significant effect was not found in many of the reviewed papers. Hence, this literature review collects and synthesizes the studies on the impacts of light on human circadian rhythm.
Non-visual circadian response in humans
In humans, non-visual information of light is detected by the eyes. The non-visual stimulus is detected by the intrinsically photosensitive retinal ganglion cells (ipRGCs), which contain melanopsin as well as pituitary adenylate cyclase activating polypeptide, and is transmitted directly to the suprachiasmatic nucleus (SCN) of the hypothalamus (Hannibal et al. Citation2004). The SCN acts as the master circadian clock that organizes the daily recurring physiological functions, such as hormone secretion (e.g. melatonin, cortisol) and body temperature. The master circadian clock is synchronized by the light-dark transitions perceived by the eyes, but it can be disturbed by changes in the light-darkness pattern caused by the artificial lighting especially at night. Exposure to artificial light at night (ALAN) has been estimated to increase annually by 3–6% in past decades (Hölker et al. Citation2010) and continues to extend further in space, time, and intensity with more than 2% annual growth in radiance and extent (Kyba et al. Citation2017).
The detection of non-visual information requires higher light levels compared to that of visual information (Brainard et al. Citation1988; Zeitzer et al. Citation2000). In addition to the intensity of light, the spectrum and duration of the light exposure affect the response to non-visual information of light. Also the qualities of the individual, such as age, genes and chronotype (i.e. the behavioral trait of morningness-eveningness), appear to affect sensitivity to the light stimulus eliciting the non-visual responses.
A circadian rhythm can be determined by measuring melatonin levels. Melatonin is a hormone that signals night to the human body. Melatonin secretion is cyclic throughout the day: its secretion is high at night (in the dark) and low during daytime. The level of high melatonin secretion at night is not equal for all people, but it varies depending on age and sex (Follenius et al. Citation1995). Melatonin can be measured from saliva, blood and urine samples. The primary urinary metabolite of melatonin (6-sulphatoxymelatonin), if being collected every 2 to 8 hours over a 24-hour period, is used to estimate the amount of melatonin production per night as well as per day, but more frequent sampling every 10–30 minutes as typically conducted with blood (plasma) or saliva samples is required, if phase changes in the circadian rhythm are in the focus (Benloucif et al. Citation2008). Plasma melatonin concentrations are usually about three times greater than those assessed from saliva. Disruption of normal melatonin secretion is linked with several diseases in humans, most notably some types of cancer, metabolic syndrome and mental disorders (Touitou et al. Citation2017), but causality between the exposure to artificial light and these conditions has not been proved. International Agency for Research on Cancer (IARC) has classified shift work containing circadian disruption as probably carcinogenic to humans, indicating that night-time light exposure may contribute to cancer risk (Straif et al. Citation2007).
Light exposure in the evening and at night can suppress and delay (phase-shift) the normal melatonin secretion. The effect of light exposure on the phase of circadian rhythm of melatonin is described by a phase-response curve (PRC), indicating the magnitude and direction of the phase shift. The PRCs typically show that light exposures during the early biological night delay and exposures during late biological night and early morning advance the circadian phase. PRCs have been determined for various exposure levels and durations (e.g. Khalsa et al. Citation2003; Kripke et al. Citation2007; Revell et al. Citation2012; Rüger et al. Citation2013; St Hilaire et al. Citation2012).
In addition to melatonin secretion, light exposure affects sleep-wakefulness cycle. Thus, as rapid-eye-movement (REM) sleep timing is controlled by the master circadian clock (Lee et al. Citation2009), the circadian rhythm disruption can also be detected by measuring REM sleep parameters with electroencephalogram (EEG).
Method
A systematic literature search was conducted on peer-reviewed scientific journal articles on light-induced circadian rhythm disruption, including suppression and phase-shift of melatonin secretion and impacts on REM sleep parameters. As suggested by Brocke et al. (Citation2009), the literature review shall (1) define the scope of the review, (2) conceptualize the topic, (3) conduct a literature search, (4) analyse and synthesize the found literature, and (5) plan a research agenda. The first four steps are applied in the review, but the fifth one is not, as the intention of this literature review was not to find research gaps but to analyse the existing scientific evidence on the light-induced impacts on circadian rhythm in humans.
Scope
The scope of the survey was the research outcomes, that is the results of the previously-reported research. The literature search was conducted using conceptual organization, dividing the search into certain concepts (instead or historical or methodological organization). The audience of the literature was intended to be scholars in general, and the literature search aims at an exhaustive coverage of the literature.
Concepts
The search was designed to focus on two main concepts: light and circadian rhythm. Hence, the search terms related to light were chosen to be (“light exposure” OR “artificial light”), and those related to circadian rhythm (circadian AND melatonin OR “REM sleep”). The terms were searched in titles, abstracts and keywords of the papers. The search terms did not include only “light” due to its ambiguity and frequent use in idioms and phrases.
Literature search
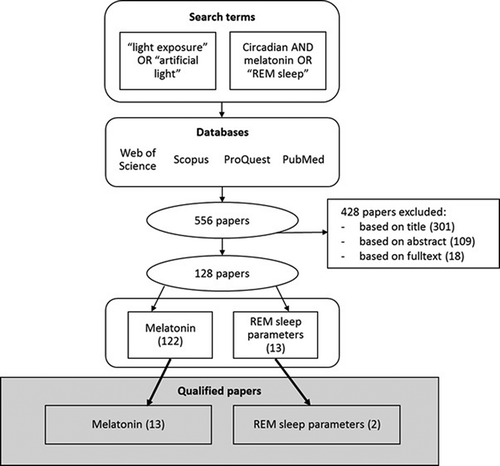
To cover all potentially relevant studies in human health and lighting disciplines, the literature was searched in four databases: PubMed, Scopus, ProQuest and Web of Science. The search was conducted on 1 November 2017. After executing searches, combining the results and removing duplicates, the search resulted to 556 papers (). The papers were deemed relevant on the basis of their title, abstract, and finally full text, resulting to 128 relevant papers.
The inclusion criteria of the papers in the analysis were: (1) scientific papers (excluding conference papers and book chapters), (2) papers presenting original experimental studies (excluding reviews and studies based on data published elsewhere), (3) studies on healthy humans (excluding experiments done on patients and animals), (4) level of light exposure measured (excluding studies where light level was subjectively evaluated or not measured at all), (5) written in English language and (6) measuring either melatonin level or REM sleep or both (excluding other parameters related to circadian rhythm or human health). The light exposures from both artificial and natural sources were included, including displays.
Due to the large number of studies and their variety in quality, research methods and exposure details, additional quality criteria was determined to select the papers of highest quality for more detailed analysis. The quality criteria were (1) a minimum of 20 test subjects, (2) spectral characteristics of light exposure given (spectral power distribution (SPD) or wavelength) and (3) level of exposure indicated in illuminance, irradiance and/or photon density. The quality criteria resulted to 15 qualified papers.
Results
Data collection
The data collected from the 128 full text papers are provided in the supplementary material. Collected data included the number, sex and age of test subjects; intensity, duration, timing, spectral characteristics and lamp type of the light exposure; point of measurement for light level; location of the study (laboratory vs. typical living environment); type of melatonin sample; and/or the provided REM sleep parameters. Many studies provided information on the characteristics of the subjects, such as Body Mass Index, diet and vision, and restricted the experiments on subjects with certain life styles; such as no or little alcohol consumption, no trans-meridian travels, no night shift work, no or little caffeine intake, no smoking, no medication or drugs; and/or recommended the subjects to maintain a certain life style, typically instructed to maintain a regular sleep/wake cycle prior to the experiment. The details of the participant selection or their instructions for behavior prior the experiments were not collected in the literature review.
Supplementary Material 1 (SM 1) presents 101 studies of specific, intended light exposure affecting melatonin secretion. Supplementary Material 2 (SM 2) lists the data collected from 21 studies with prevailing light conditions, in contrast to intended exposures in SM 1. The details of the 13 studies of REM sleep parameters (time, %, latency) are listed in Supplementary Material 3 (SM 3). Of these three lists of papers, only the papers fulfilling the quality criteria are analysed in detail in section of Analysis of qualified papers.
Specific light exposure affecting melatonin secretion
The studies of light-induced impacts on melatonin secretion from intended light exposure (see SM 1) were published between 1980 and 2017. The number of subjects ranged from 4 to 72 being typically between 5 and 20 subjects per study. About 29 out of 101 studies were conducted with a sample size of 10 or less. The age of the subjects was typically between 20 and 30 years, while only few studies were conducted with children younger than 15 years of age or elderly adults above 50 years. Light intensity levels were indicated in illuminance (lx), irradiance (W/m2), and/or photon flux (photons/m2/s), or, very rarely, in luminance (cd/m2). The light levels of the exposures ranged from 0 to several thousands of lux, without any typical range to be identified. Dim light below 10 lx or darkness (0 lx) was frequently used as control or comparison. It was not always stated where the light level was measured but when it was, it was most often measured at the eye of the subject (also cornea, forehead) or near the eye (pendant around neck, wrist). Sometimes it was also indicated that the measurement was conducted at the direction of gaze, when the direction of gaze was usually controlled. The status of the subjects’ eyes was not frequently indicated or controlled, but it was possible to assume it based on the protocol, for example if the subject was remaining awake, asked to gaze at a point, or allowed to read (open). The subjects’ eyes were dilated in eight studies. It is clear that dilation of the pupils increases the resulting responses in melatonin secretion.
In SM 1, temporal characteristics of the exposure varied from pulsed, intermittent exposure to constant exposure over several hours. Duration of the exposure was typically between 1 and 5 hours. Studies included a variety of repetition procedures either during the same day or night or during following days. Detailed time schedules of exposures were not collected in the table. Exposures were scheduled at all times of day, with a clear majority of the studies (71 out of 101 studies) exposing subjects to light in the evening and/or night.
Most studies (76 out of 101 studies) presented in SM 1 were conducted entirely in laboratory or other well-controlled facilities (simulated living facility, hospital, research facility). Only a few studies (4 out of 101 studies) were conducted in work conditions, and some studies allowed the subjects to continue their lives in their typical living environments (including work place and home) either entirely (measurements at home) or partially (measurements in lab). In some cases, the particular place of experiment was not indicated but it was possible to assume based on the protocol, for example a strictly controlled light environment is difficult to obtain in home environment.
Studies listed in SM 1 evaluated melatonin levels from saliva, blood (plasma) or urine samples, with saliva and blood samples being the most frequent. Although the posture of the subject during melatonin sampling may affect the melatonin level (Deacon and Arendt Citation1994), it was not stated in all studies and therefore not collected in SM tables.
The level of spectral characteristics of the light exposure was indicated at very different levels in the studies. Both poly- and monochromatic light were used, and part of the studies experimented with several different monochromatic lights. Only very few studies provided the spectral power distribution of the light exposure or indicated the exact peak wavelengths (with half peak bandwidth) of the radiation. Such accurate measures of spectral characteristics are necessary for the accurate evaluation of the results, as the impacts on human circadian rhythm depend on the spectrum of the light exposure. Correlated colour temperature (CCT), expressed in kelvins (K), is a rough representation of the colour of the light, which can be obtained by very different spectra. CCT is a measure useful for lighting design but not in health-related experiments.
The studies were conducted using a variety of light sources. Most often fluorescent lamps (FL) and light-emitting diodes (LED) were used, while part of the studies used several different light sources from daylight to discharge lamps and incandescent lamps. Filters were also applied in some of the studies to block the radiation at certain wavelengths.
Prevailing light exposure affecting melatonin secretion
About 21 studies are listed in SM 2, as they, instead of experimenting with specific predetermined light exposure, measured or controlled light conditions existing in normal living environments (home, work, school). Thus, the type of light source or its spectral details was not frequently provided in studies listed in SM 2. The studies were published between 1994 and 2017. The range in sample size was large, 5–528 subjects, with 4 out of 21 studies conducted with maximum of 10 subjects. The subjects were usually from 20 to 40 years old, being somewhat older than in those presented in SM 1. One study was conducted with pre-school children (Akacem et al. Citation2016) and one with elderly (Obayashi et al. Citation2014) adults. Light levels were typically measured for 24 h per day providing insight on the long-term light exposure profiles.
Light exposure affecting REM sleep
The studies evaluating the impacts of light on REM sleep parameters are collected in SM 3. They studied the impact of light exposure on duration of REM sleep, REM %, and/or REM sleep latency, and were published between 1995 and 2017. The number of subjects ranged from 8 to 30, and 6 out of 13 studies had sample size 10 or below. The age of subjects was typically in the range of 20–30 years, with only one study conducted on elderly adults (Münch et al. Citation2011). The intensity of light exposure ranged from few dozens to few thousands of lux, with one study comparing the impact of 5 and 10 lux (Cho et al. Citation2016) and another comparing the impact of light from e-book (32 lx) and light reflected from paper book (1 lx) (Chang et al. Citation2015). Light exposures were typically continuous light exposure for 2–8 h at any time of day, except for one study using pulsed light (Zeitzer et al. Citation2014). Subjects were typically awake with eyes open. Only two studies (Cho et al. Citation2016; Zeitzer et al. Citation2014) exposed sleeping subjects to light at night.
Analysis of qualified papers
lists the details of the papers qualifying against the criteria of sample size of at least 20 and providing information on the intensity (illuminance, irradiance or photon density) and spectral characteristics of the exposure (SPD or wavelength). Each qualified paper is briefly introduced here with their main results in terms of melatonin secretion or REM sleep affected by light exposure.
Table 1. Qualifying studies of light exposure affecting melatonin secretion and REM sleep evaluated against quality criteria: at least 20 subjects participating, and intensity and spectral information given of light exposure (illuminance, irradiance or photon density; SPD or wavelength). Light intensity refers to illuminance (lx), irradiance (W/m2), photon density (photons/cm2/s) and/or luminance (cd/m2). Abbreviations: CCT = correlated colour temperature (expressed in kelvins, K), FL = fluorescent lamp, INC = incandescent lamp, LED = light-emitting diode, SPD = spectral power distribution.
Brainard et al. (Citation2001) exposed 72 subjects to full-field, monochromatic light at night (02–03:30 AM) at wavelengths from 420 to 600 nm. The subjects’ pupils were dilated, and blood samples were collected before and after the exposures. Each subject was exposed to at least seven different irradiances of one wavelength at minimum of 1 week between exposures. A fitted action spectrum was constructed from the melatonin suppression data collected from a total of 627 individual experiments indicating the most effective melatonin suppression at wavelength range of 446–477 nm. Thus, the study contributed to the finding of a single photopigment that mediates circadian photoreception in humans and has a peak sensitivity to radiation at different wavelength area than rod and cone cell photopigments responsible for visual response. Significant melatonin suppression was found at irradiances of at least 3.1 µW/cm2.
Thapan et al. (Citation2001) measured melatonin suppression in 22 subjects exposed to a total of 215 cases of monochromatic light, aiming at producing an action spectrum for the spectral sensitivity of light-induced nocturnal melatonin suppression. The subjects received a 30-min light pulse at night (between 23:30 and 02:30 h) with varying peak wavelengths (424, 456, 472, 496, 520 and 548 nm) and irradiances ranging from 0.7 to 65.0 μW/cm2. Subjects completed 1 to 16 sessions of three consecutive nights (19–07 h), including a baseline night without light exposure and two nights with light exposure with dilated pupils. Each wavelength was tested at five to eight irradiance levels, and each irradiance was tested on three to seven subjects. Melatonin levels were assessed from blood collected before (90 and 15 min prior to exposure), during and after (every 15 min for 1.5 h starting from the beginning of light exposure, and at 2 h after) the exposure and from urine for 2 days prior to exposure. They found that plasma melatonin was suppressed with increasing irradiance at all tested wavelengths, indicating an intensity-dependent relationship between melatonin suppression and light exposure. Statistically significant melatonin suppression was obtained at minimum irradiances of 1.9, 2.0, 1.8, 3.0, 7.0 and 7.2 μW/cm2 for 424, 456, 472, 496, 520 and 548 nm, respectively. An action spectrum was generated by fitting irradiance-response curves. The peak of the action spectrum was located more in the short-wavelength range compared to the peaks of visual sensitivity functions (photopic or scotopic), suggesting a new photopigment at short wavelength range. Melatonin was maximally suppressed 30–45 min after the lights on, but it must be noted that the exposure lasted for 30 min. The 30-min exposure was not found to cause long-term suppressing impact on nocturnal melatonin, but the melatonin concentration recovered quite rapidly (within 1 h from the beginning of the exposure). Interestingly and contradictory to the frequently cited fitted action spectrum curve, the maximum melatonin-suppressing effect was found at the shortest tested wavelength (424 nm) followed by, in order, 456 nm, 472 nm, 500 nm, 520 nm and 548 nm. The curved shape of the action spectrum was a result of fitting the irradiance-responses to rhodopsin templates with peak at 459 nm and correcting for lens density.
In a study by Cajochen et al. (Citation2006), 24 subjects were studied in two groups of 12. In light study, 12 male subjects were controlled for 30 h in dim ambient light (<15 lx) and were allowed to sleep for 8 h at habitual bedtime. In the circadian study, 12 female and male subjects were kept in constant recumbent posture in bed in a controlled environment for 16 h starting in the evening and exposing them first to <2 lx for 1.5 h, then to 0 lx for 2 h, followed by light exposure (blue, green or no light) for 2 h, and finally to <2 lx of polychromatic light for 1.5 h. After these dim, dark, light and again dim light conditions, the subjects were allowed to sleep. Melatonin was measured from saliva. About 2-h exposure to blue light (460 nm) in the evening significantly suppressed melatonin (from 12.0 pg⁄mL to 8.8 pg⁄mL) compared to low-melatonin level earlier in the evening, but green light (550 nm) did not.
One of the objectives of the study by Sletten et al. (Citation2009) was to evaluate the effectiveness of blue light in circadian phase advance and how the response may differ with age, as melatonin suppression has been found to reduce with age. Blood was collected to assess dim light melatonin onset (DLMO) from plasma a week before the experiment and before and after the light exposure. About 11 young and 10 elderly adults maintained a consistent sleep-wake schedule from 1 week before and during the study. A 3-day experimental protocol consisted of a baseline night, night of exposure and post-stimulus night. All young subjects and 7 of the 10 elderly subjects participated in 2 laboratory sessions, 1 for each types of light exposures, monochromatic blue (peak 456 nm) and green (peak 548 nm), both at similar level of photon density (6*10^13 photons/cm2/s). The rest of the elderly subjects (3) were exposed only the blue light. The exposures of 2 h were individually timed to begin 8.5 h after their DLMO, that is occurring early in the morning. The pupils of the subjects were dilated during the exposures. The magnitude of phase advance was assessed from the difference in plasma melatonin rhythm phase markers before and after light exposure. The circadian phases were advanced by both blue and green light in both age groups, with slightly larger advance elicited by blue than green light but not significantly. The phase advance in the young was larger (DLMO 2104 ± 0043 h, n = 11), but not in a statistical manner, compared to the elderly (DLMO 2136 ± 0074 h, n = 15). They found that light-induced circadian phase advance was not significantly impaired with age.
Crowley and Carskadon (Citation2010) conducted two experiments of which experiment 2 is included in this further analysis. The experiment 2 examined the impact of short-wavelength light on weekend mornings on circadian rhythm in 33 young (15–17 a) subjects. The experiment lasted for 2 weeks and was conducted as a between-subjects design with 2 groups: typical light exposure (“TYPICAL”) and specifically designed light exposure (“LIGHT”). Both groups went to sleep at home 1.5 h later and woke up 3 h later on weekends compared to weeknights, providing 1.5 h longer sleeping time at weekends than on weeknights. Subjects visited the laboratory before (Friday) and after (Sunday) each weekend to assess DLMO phase from saliva. 14 subjects in the TYPICAL group and 14 in the LIGHT group had useable salivary DLMO phase data on both nights for analysis. The LIGHT group received light exposure either at 30% intensity level (4 subjects) or at 100% level (10 subjects). DLMO phase delayed over TYPICAL weekend in experiment 2 by 46 ± 34 min and over LIGHT weekends by 38 ± 28 min, but not significantly. DLMO phase did not differ between the 30% and 100% light intensity groups, and the data from these 2 groups were combined. It was concluded that the circadian phase of young people is delayed after keeping a commonly observed weekend sleep schedule. Morning exposure to short-wavelength light on weekends did not stabilize circadian timing in the subjects of the study.
Gooley et al. (Citation2010) tested whether cone photoreceptors contribute to the circadian regulation of light responses. About 48 subjects participated to a 9-day protocol in a laboratory environment free from time cues. First 3 days they maintained their regular sleep-wake schedules (8 h sleep, 16 h wake) in <190 lx after which the light were dimmed to <3 lx. This was followed by a 50-h constant routine of forced wakefulness and subsequent 8-h sleep opportunity. Then, subjects with dilated pupils were exposed to 6.5 h of narrow-bandwidth light exposure of either of the two wavelengths (blue 460 or green 555 nm) at 16 different irradiance levels in random order. The subjects stared at the light continuously for 90 min after which free gazing was allowed in the otherwise dark room for 10 min. This light exposure at night was to determine the dose-response curves for melatonin suppression and circadian phase resetting. Melatonin levels were assessed from saliva samples during constant routine and light exposure and from blood during both sleep and awake. At the beginning of the nocturnal light exposure, green and blue light were equally effective at suppressing melatonin, but later during the light exposure, the spectral sensitivity to green light was exponentially reduced relative to blue light. This suggests that cone photoreceptors (λmax = 555 nm) contribute substantially to nonvisual responses at the beginning of a light exposure and at low irradiances but not during long-term or high-intensity light exposure. Melanopsin was suggested to be responsible for the high-intensity and long-term circadian responses. The results indicate that short-duration (<90 min) exposure to narrow-bandwidth green (555 nm) light (≤ 24 lux) may be as effective as similar photon flux of blue (460 nm) light (≤ 2 lux).
Sharkey et al. (Citation2011) assigned 25 subjects randomly to receive blue (peak 470 nm, 225 lx) or dim (<1 lx) light for 1 h in the morning after wake-up. After a baseline week, subjects maintained an individualized, fixed, advanced 7.5-h sleep schedule for 6 days. Light exposures were measured by head-worn photometers. Saliva was collected to assess DLMO. No significant difference in circadian phase was found between the two groups after 6 days. Both groups resulted to circadian phase advance: the average DLMO was advanced in dim light group by (mean± SD) 1.5 ± 1.1 h and by 1.4 ± 0.7 h in the blue light group. Even though the blue-light group received more short-wavelength light during the first 90 min after waking, their phase shift was not enhanced compared to dim light group. No difference was found in circadian light exposures (short-wavelength weighted illuminance) or photopic illuminance across the entire wake episodes between the dim- and blue-light groups. The authors suggested that the timing of blue light exposure may not have been optimal for inducing a phase advance, since blue light was not found to elicit phase advances greater than the dim light.
Appleman et al. (Citation2013) conducted a 12-day study where 21 subjects, randomly divided into two groups, received either short-wavelength (blue, peak 476 nm) light for 2 h in the morning and light filtered (<535 nm) with orange-tinted glasses for 3 h in the evening (advance group), or at opposite times, that is orange-tinted glasses in the morning and blue light in the evening (delay group). Subjects kept their normal schedule for the first 5 days and received morning and evening light exposures the following 7 days in addition to a fixed sleep schedule (advanced by 90 min from the baseline). The study showed that the direction of circadian phase change is determined by the light-dark exposure, not by the fixed sleep schedule, and that both morning and evening light exposures need to be controlled to shift circadian phase. After 7 days on the fixed 90-min advanced sleep schedule, circadian phase advanced by 132 ± 19 min in advance group and delayed by 59 ± 7.5 min in delay group. No statistical differences occurred between the two groups in the mean light exposure values measured (circadian stimulus (CS), circadian illuminance (CLA) and photopic illuminance expressed in log lux).
Chellappa et al. (Citation2013) studied the impact of evening-time blue-enriched, polychromatic light on sleep. They measured EEG of 30 subjects to provide REM % data. The subjects were kept in dim light (<8 lx) from 18 to 19:30 h, in darkness (0 lx) from 19:30 to 21:30 h, and in light (approximately 28 lx) from 21:30 to 23:30 h. The light exposure was provided by compact fluorescent lamps of either 6500 K or 2500 K or incandescent lamp of 3000 K. However, they also provided more accurate CCTs for the three lamps: 5984 K, 2481 K and 2581 K, respectively, which are assumingly measured. This shows a rather notable difference between the rated and measured CCT values. For a more accurate representation of spectral characteristics, they provided SPDs of the lamps in a prior study (Chellappa et al. Citation2011), which shows a difference between a spiky SPD of 2500 K compact fluorescent lamp and a smooth SPD of 3000 K incandescent lamp. No significant differences were found in sleep structure across the first three NREM-REM sleep cycles with respect to the light conditions. The REM % was 18.2 ± 0.9% in both 6500 K and 3000 K lights, and 17.9 ± 1.0% in 2500 K light but no significant difference was found among the light conditions.
Figueiro et al. (Citation2014) studied whether morning-chronotypes and evening-chronotypes respond differently to light exposure while on a fixed, advanced sleep-wake schedule. They conducted a study of 13 days (6 days of baseline assessment and 7 days of light exposure and designed sleep schedule) of advanced sleep/wake protocol twice, once with advancing and once with delaying light exposure. Advancing light exposure included short-wavelength light in the morning and orange-tinted glasses restricting short wavelength in the evening, while delaying light exposure included orange-tinted glasses in the morning and short-wavelength light in the evening. No significant difference was found in the responses to light exposure between the two types of chronotypes. DLMO was significantly delayed after the delaying light exposure and significantly advanced after the advancing light exposure in both groups compared to the baseline week. The light-induced circadian phase changes were consistent with those predicted by previously published PRCs for both chronotypes. It was noted that light exposures of the subjects during the baseline weeks were significantly less than those during the weeks of light exposure.
In a between-subjects study, Ho Mien et al. (Citation2014) experimented with 24 subjects, divided into 3 groups of 8 by type of exposure, the impact of continuous red light (631 nm, 13 log photons/cm2/s), intermittent red light (1 min on/off) and white light (2500 lx) in laboratory facility free from time cues. The experiment started with a 26-h constant routine period (awake) and an 8-h sleep period, after which the light exposure started in the evening at 1 h before the habitual bedtime. The light exposure lasted 6 h. Salivary melatonin was assessed before and after the light exposure. They found that continuous and intermittent red light elicited similar circadian resetting responses, an average phase delay of almost an hour. Red light did not suppress melatonin secretion but it elicited prolonged pupillary constriction. However, individual differences were found: the circadian responses of two subjects exposed to red light were similar in magnitude to those exposed to bright white light, suggesting that cone photoreceptors may contribute to circadian phase resetting in some individuals. This is an interesting finding, as it suggests that visual photoreceptors may be partially responsible for light-induced melatonin suppression. However, it is admitted that sample size was small, 8 per type of light exposure.
Sander et al. (Citation2015) exposed 29 elderly (>65 a) subjects to two 3-week light experiments of either blue-enriched (280 lux) or blue-suppressed (240 lux) light in the morning and day (08:00–13:00 h) at home in a randomized cross-over design. After 1 pm, the light level was decreased to approximately 140 lx in all cases. The exposure periods were performed in Copenhagen area during late autumn (October–November) with 1 week in between exposures. Saliva was collected after each light exposure period in the evening (seven samples every 45 min). Saliva melatonin profile did not show significant changes between morning/daytime blue-enriched and blue-suppressed exposures. The only significant difference in measured blue light exposures of the subjects was because of the blue-enriched light from the experiment at 8:00–9:00 am due to the contributions from daylight. Large individual differences were found in the melatonin concentrations of the subjects: the concentrations ranged from <2 pg/mL at all times with no detectable nightly rise to a distinct increase during late evening and early night to approximately 50 pg/mL.
Geerdink et al. (Citation2016) investigated the circadian phase-shifting impact of blue and amber light therapy in a placebo-controlled study at home. About 42 subjects suffering from “social jetlag” on workdays were randomly assigned to either high-intensity blue light or amber light exposure (placebo) with similar photopic illuminance both given with 30-min pulses. The protocol lasted for 30 days: 14 baseline days without sleep restrictions, 9 consecutive treatment days with either 30-min blue or amber light pulses in the morning with a sleep advancing scheme, and 7 post-treatment days without sleep restrictions. Salivary melatonin was collected at the beginning, middle and end of baseline period, after light exposure period, and after post-treatment period. Light exposure was continuously recorded. Blue light exposure in the morning elicited a significantly larger (84 ± 51 min (SD)) phase advance of the melatonin rhythm during the light treatment period than morning amber light (48 ± 47 min). Wake-up time during the post-treatment days was slightly earlier (−21 ± 33 min) in the blue light and slightly later (+ 12 ± 33 min) in the amber light group compared to baseline of the subjects. This indicates that morning blue light at home can be used to advance the circadian phase.
Najjar and Zeitzer (Citation2016) conducted a 16-day experimental study of 39 subjects, exposing them for 60 min either to continuous light (n = 8) or sequences of 2-ms light flashes (n = 31) with inter-stimulus intervals ranging from 2.5 to 240 s. Melatonin phase shift and suppression were assessed in saliva samples before and after exposure periods. Subjects maintained a regular sleep/wake rhythm at home for the first 14 days of the 16-day protocol, and stayed in laboratory for testing for the night between days 15 and 16. Intermittent light was found to be more effective at eliciting circadian changes than continuous light. Continuous light for 1 h suppressed melatonin secretion by 51%± 40% and shifted it by –0.60 ± 0.34 h (delay). It was demonstrated that the human circadian system has the capacity to detect light at millisecond time scale in contrast to image-forming (visual) photoreception. The length of the interval between the flashes was found to affect the phase shift (delay) but not the melatonin concentrations. Flashing light at maximum inter-stimulus interval resulted to at least two-fold more effective phase delay in the circadian system compared to continuous exposure of same intensity and 3800 times the duration. Flashing light changed circadian timing by –2.99 to 0.30 hours depending on the inter-stimulus interval.
Cho et al. (Citation2016) investigated the impact of dim light at night on 23 subjects. The subjects were randomly divided into two groups, one receiving 5 lx (n = 11) and the other 10 lx (n = 12) at night from 23 to 07 h. Subjects were sleeping in supine position during the dim light condition. Subjects were asked to maintain regular sleep-wake cycle for 1 week before the study period and to refrain from medicine, coffee and alcohol consumption. The protocol lasted for three nights with the first two nights without light exposure (nights 0 and 1) and the third night (night 2) with either of the dim light exposures. REM sleep % and latency were measured. No differences between the two groups were found in sleep structure but altogether significant differences in sleep structure were found between nights 1 and 2, that is without and with nocturnal dim light exposure, including a significant difference in REM % between the nights. REM sleep latency showed significant night-group interaction but no difference between nights. Nocturnal exposure to dim light was significantly associated with an increased amount of REM sleep. The finding is interesting, as the nocturnal light levels were relatively dim (either 5 or 10 lx) and the subjects were sleeping with eyes closed during the exposure. This suggests that avoiding any light exposure during night may improve sleep quality.
Summary of qualified papers
There are two pioneering works (Brainard et al. Citation2001; Thapan et al. Citation2001) on the assessment of melatonin suppression by monochromatic light exposure. Light exposure of intense enough, the irradiance thresholds depending on the wavelength (Thapan et al. Citation2001), inhibited melatonin secretion and reduced the circulating melatonin levels. Melatonin levels remained maximally suppressed for about 15 min after cessation of the exposure and thereafter recovered in about 15 min (Thapan et al. Citation2001).
These two aforementioned works stimulated pragmatic comparisons between the exposures to blue light and green light (Cajochen et al. Citation2006; Gooley et al. Citation2010; Sletten et al. Citation2009). Exposure to blue light suppressed melatonin levels more than green light (Cajochen et al. Citation2006), this effect emerging in magnitude as a function of time (Gooley et al. Citation2010), whereas blue and green light exposure produced a phase shift of equal degree in the circadian rhythm of melatonin, this effect being independent of the age (Sletten et al. Citation2009).
At night, intermittent exposures either to red light (Ho Mien et al. Citation2014) or to blue light (Najjar and Zeitzer Citation2016) did not inhibit melatonin secretion, but they shifted the phase the circadian rhythm of melatonin. To a surprise, after wake-up in the morning, exposure to blue light (Crowley and Carskadon Citation2010; Sharkey et al. Citation2011) or blue-enriched light (Sander et al. Citation2015) for one hour was not effective in shifting the phase the circadian rhythm of melatonin. However, exposure to blue light, if administered with a sleep-advancing scheme (Geerdink et al. Citation2016) or with a control of conflicting light exposure (Appleman et al. Citation2013; Figueiro et al. Citation2014), produced a phase shift of the circadian rhythm of melatonin as anticipated.
Exposure to light for two hours before bedtime did not change the subsequent REM sleep (Chellappa et al. Citation2013). On the other hand, exposure to light for eight hours increased the proportion of REM sleep (Cho et al. Citation2016) which however remained within a range (20%–30% of total sleep time) judged to represent good sleep quality.
Discussion
In modern societies and gradually in developing areas, humans are increasingly exposed to artificial light that differs from the natural light exposure patterns in terms of SPD, timing, duration and intensity. Several scientific studies have shed light to the potential health impacts of light exposures transforming from natural to artificial light sources. In order to synthesize individual studies and their findings on the light-induced impacts on human circadian rhythm, a systematic literature review was conducted. In addition to deepening the understanding of the circadian rhythm disruption caused by artificial light exposure, the review will also serve as a basis for developing a human health-related metrics of light pollution to evaluate the environmental impacts of lighting products by the LCA method.
Critical comparison of qualified papers
The literature search resulted to 128 papers presenting experimental studies on human circadian rhythm affected by light exposure, measured either in terms of melatonin secretion or REM sleep parameters. The 15 papers fulfilling the quality criteria were analysed in detail to increase scientific understanding of the impacts of light on human circadian rhythm. It was found that a 2-h exposure to light (460 nm) in the evening suppresses melatonin, the maximum melatonin-suppressing effect of light exposure being achieved at the shortest wavelengths (424 nm), but the melatonin concentration recovers rather rapidly, within 15 min from cessation of the exposure. This suggests a short-term or simultaneous impact of the light exposure. In addition, melatonin secretion as well as melatonin suppression were found to reduce with age, but the light-induced phase advance of melatonin circadian rhythm is not impaired with age. The circadian rhythm was found to respond to both morning and evening light exposure (blue-enriched or blue-depleted) without significant differences between morning-types and evening-types of persons (chronotypes).
Observations on the non-qualified papers
With regard to the non-qualified papers in the SM 1, parameters worth noting are related to experiments with closed eyes. In his first study with light flashes, Zeitzer et al. (Citation2014) exposed a small sample of participants to either darkness or a sequence of 2-msec light flashes during sleep given every 30 sec from hours 2 to 3 after bedtime, eyelids closed. This shifted the timing of the circadian clock without major alterations to sleep itself. Similarly, Figueiro and Rea (Citation2012) showed how light delivered through eyelids during one hour suppressed melatonin and phase shift DLMO. Both studies suggested that phototherapy may also be given with closed eyes, and even while asleep (Zeitzer et al. Citation2014). However, from the light pollution perspective, it can be concluded that exposure to light after sleep onset may be equally harmful than evening exposure.
As for the individual sensitivity to evening light exposures, Canton et al. (Citation2009) did not find differences between blue and brown iris colour in the magnitude of the effect, but Higuchi et al. (Citation2008) showed that inter-individual difference in pupil area positively correlates with melatonin suppression. Also, in the early pubertal and prepubertal phase, adolescents were significantly more sensitive to evening light that in the late pubertal or postpubertal phase, even at a low, 150 lux exposure level (Crowley et al. Citation2015).
In contrast to experimental settings, the SM 2 draws the attention to naturally occurring and individually chosen conditions for circadian rhythm. Darker winter season at high latitudes associated with highest maximal melatonin level during the night compared to other seasons (Adamsson et al. Citation2016). The studies also highlight the interplay of the habitual bedtime and light exposure in determining the circadian phase shift (e.g. Akacem et al. Citation2016; Burgess and Eastman Citation2004). Other behavioral factors include the voluntary exposure to morning light. For example, active avoidance of morning light was associated with better adaptation of circadian rhythms to night shift work (Koller et al. Citation1994).
With regard to studies using sleep EEG as listed in the SM 3 but not among the selected publications, light exposure in the evening is reported to change the sleep EEG structure the following night (see SM 3), except if the exposure is a sequence of flashes of light after bedtime (Zeitzer et al. Citation2014). However, the effects seem to be small. For example, experimentally induced phase shifts of about one and a half hour had only a minimal effect on subsequent sleep propensity and architecture in healthy individuals (Carrier and Dumont Citation1995). The induced change is often associated with an altered course of sleep over the night. For example, blue in contrast to green light exposure in the evening shortened REM sleep duration and suppressed slow-wave sleep in the first hours of sleep, with a rebound toward the early morning hours (Munch et al. Citation2005). Noteworthy, individual melatonin levels were also found to respond very differently to light, as some individuals had suppressed melatonin at a typical room light conditions, whereas some individuals did not react to a moderate light exposure (Santhi et al. Citation2012).
Indications on intensity and spectral characteristics in relation to circadian rhythm
To avoid unwanted changes in the circadian phase or night-time sleep, light exposure in the evening and at night as well as in the morning needs to be controlled, as even the longest wavelengths (631 nm) or intermittent light exposures do induce circadian resetting responses. Circadian responses were also found when sleeping in home setting with low night-time light level with eyes closed, indicating that even low light levels are relevant for human circadian rhythms.
Study designs
The reviewed studies had a very varied methodology with regard to study designs and to providing light exposure details. Part of the experimental studies included a control group which was exposed to dark, dim or other control-level light, while other studies performed repeated measure experiments where the same subjects were exposed to both light and control conditions. The studies also indicated and controlled for prior light exposure in various manners. Prior light exposure was controlled and/or measured with a portable light measuring device for few weeks prior to the experiment at best, but some studies did not measure the prior light exposure at all, which is a shortcoming. In addition to specifically measuring the light exposure for a short period of time, the subjects’ adaptation to light environment, resulting from natural seasonal or behavioral patterns, may affect the sensitivity to light exposure (Wehr Citation1991).
Measurement of melatonin
The studies evaluated melatonin levels from blood, saliva and urine samples. Aoki et al. (Citation1998) preferred the collection of saliva over plasma (blood), as saliva collection is less invasive for the subject, and salivary melatonin has been validated for circadian phase assessment (Voultsios et al. Citation1997). The primary urinary metabolite of melatonin, 6-sulphatoxymelatonin, can be used to estimate the melatonin production over a longer time, but saliva collection is recommended for a more accurate and frequent evaluation of melatonin level. DLMO can only be estimated from blood and saliva samples.
Many studies found individual differences in melatonin patterns among subjects (e.g. Ho Mien et al. Citation2014; Kozaki et al. Citation2016; Sander et al. Citation2015), suggesting that subjects have individual melatonin levels and responses to light exposure. In addition to different melatonin levels, Higuchi et al. (Citation2008) found large inter-individual differences in pupil sizes. It is logical that age affects the sensitivity to light, as the lens properties change by age (e.g. Benloucif et al. Citation2006; Münch et al. Citation2011; Sletten et al. Citation2009).
Common methodological limitations
Given the scope of collected data from the 128 studies in the literature review, several limitations can be identified among the studies. First, the sample size was small in many studies: 39 studies out of 128 used sample size of 10 or less subjects. Second, the pupil size was controlled or measured only in very few studies. Pupil dilation is a way to control the pupil size. When the interest is in the impacts of light exposure in typical living environments, the pupil should not be dilated, as it is likely to exaggerate the results. Third, many studies did not indicate where the light level was measured. The light level should be measured at the eye level, that is cornea, when eyes are open. Fourth, part of the studies did not clearly state the status of the eyes during the exposure. The eyelid acts as a red-pass filter (Zeitzer et al. Citation2014) and transmits only approximately 3%–14% of light (Robinson et al. Citation1991) in a wavelength-dependent manner. Thus, the retinal exposure of light depends on the status of the eyes (open, closed). Fifth, many studies failed to indicate the spectral characteristics of the exposure in an accurate manner. This would require a presentation of SPD, the wavelength, or the peak wavelength with bandwidth. Overall, it is necessary that the intensity, spectral and temporal characteristics of the exposure are accurately and clearly expressed together with the qualities of the subjects, including age, status of eyes, pupil size, length and pattern of baseline period prior to exposure, and sleep pattern (sleep deprivation or habitual sleeping rhythm).
Limitations of the current review
Several limitations were identified in the current data collection because many pieces of information were very inconsistently reported in the reviewed studies. First, the information on posture of the subject during melatonin sample collection was not collected, although the posture has been suggested to affect melatonin levels (Deacon and Arendt Citation1994). Second, the timing of the experiments was not collected. This may affect the adaptation of subjects to light level, as seasonal variation of light has been reported to affect plasma melatonin pattern (Arendt et al. Citation1979; Touitou et al. Citation1984). Third, the information on menstrual cycle and potential intake of oral contraceptives among female subjects were not collected. Use of oral contraceptives has been found to increase night-time melatonin levels and the menstrual phase may affect the melatonin level (Webley and Leidenberger Citation1986; Wright and Badia Citation1999). Fourth, the length of baseline or control period was not collected. This relates to the prior light exposure.
Further research
The results of this systematic literature review are recommended to be used in developing a human-health related impact category of light pollution in the LCA methodology. In parallel with the environmental impacts of energy-related and material-related flows evaluated in conventional LCA method, this would enable assessing a wider sustainability context of lighting products. The light pollution category should first focus on the light-induced impacts on circadian rhythm. However, as the disruption of circadian rhythm may have further impacts on health, these further health impacts should be evaluated. Most importantly, light at night and night shift work have been associated with increased cancer risk (Straif et al. Citation2007). The physiological mechanisms remain unknown but few are suggested, including melatonin suppression, circadian disruption and chronic sleep deprivation (Haus and Smolensky Citation2013). In addition to potential increased cancer risk, lower melatonin secretion is associated with a higher risk for type 2 diabetes (McMullan et al. Citation2013).
Given the limitations of the studies listed in this review, future studies on light-induced impacts of circadian rhythm should clearly indicate the details of the experimental protocol and the subjects. Special attention shall be paid to the sample size and repeated measure design, as individual differences in melatonin levels were found in many studies. Several reviewed studies confirmed the role of a non-visual photoreceptor due to the spectral sensitivity of circadian responses of light, but the potential role of visual photoreceptors (cones) cannot be ignored, suggesting a need for further research. In addition, systematic reviews on other light-induced health concerns are needed, as well as meta-analyses of any negative health impacts, including circadian rhythm disruption, to further clarify the scientific evidence on impacts of light on human health.
Disclosure statement
The authors report no conflicts of interest.
Additional information
Funding
References
- Adamsson M, Laike T, Morita T. 2016. Annual variation in daily light exposure and circadian change of melatonin and cortisol concentrations at a northern latitude with large seasonal differences in photoperiod length. J Physiol Anthropol. 36:6.
- Akacem LD, Wright KP, LeBourgeois MK. 2016. Bedtime and evening light exposure influence circadian timing in preschool-age children: A field study. Neurobiol Sleep Circadian Rhythm. 1:27–31.
- Aoki H, Yamada N, Ozeki Y, Yamane H, Kato N. 1998. Minimum light intensity required to suppress nocturnal melatonin concentration in human saliva. Neurosci Lett. 252:91–94.
- Appleman K, Figueiro MG, Rea MS. 2013. Controlling light-dark exposure patterns rather than sleep schedules determines circadian phase. Sleep Med. 14:456–61.
- Arendt J, Wirz-Justice A, Bradtke J, Kornemark M. 1979. Long-term studies on immunoreactive human melatonin. Ann Clin Biochem. 16:307–12.
- Bedrosian TA, Nelson RJ. 2013. Bedrosian and Nelson 2013 influence of the modern light 3671_001.pdf. Mol Psychiatry. 18:751–57.
- Benloucif S, Burgess H, Klerman E, Lewy A, Middleton B, Murphy P, Parry B, Revell V. 2008. Measuring melatonin in humans. J Clin Sleep Med. 4:66–69.
- Benloucif S, Green K, L’Hermite-Balériaux M, Weintraub S, Wolfe L, Zee P. 2006. Responsiveness of the aging circadian clock to light. Neurobiol Aging. 27:1870–79.
- Brainard GC, Hanifin JP, Greeson JM, Byrne B, Glickman G, Gerner E, Rollag MD. 2001. Action spectrum for melatonin regulation in humans: Evidence for a novel circadian photoreceptor. J Neurosci. 21:6405–12.
- Brainard GC, Lewy AJ, Menaker M, Fredrickson RH, Miller LS, Weleber RG, Cassone V, Hudson D. 1988. Dose-response relationship between irradiance and the suppression of plasma meltonin in human volonteers. Brain Res. 454:212–18.
- Brocke J vom, Simons A, Niehaves B, Reimer K, Plattfaut R, Cleven A. 2009. Reconstructing the Giant: On the Importance of Rigour in Documenting the Literature Search Process. In: Eur Conf Inf Syst 2009 Proc. Verona, Italy.
- Burgess HJ, Eastman CI. 2004. Early versus late bedtimes phase shift the human dim light melatonin rhythm despite a fixed morning lights on time. Neurosci Lett. 356:115–18.
- Cajochen C, Jud C, Münch M, Kobialka S, Wirz-Justice A, Albrecht U. 2006. Evening exposure to blue light stimulates the expression of the clock gene PER2 in humans. Eur J Neurosci. 23:1082–86.
- Canton JL, Smith MR, Choi HS, Eastman CI. 2009. Phase delaying the human circadian clock with a single light pulse and moderate delay of the sleep/dark episode: No influence of iris color. J Circadian Rhythms. 7:8.
- Carrier J, Dumont M 1995. Sleep propensity and sleep architecture after bright light exposure at three different times of day. pp. 202–11.
- Chang A-M, Aeschbach D, Duffy JF, Czeisler CA. 2015. Evening use of light-emitting eReaders negatively affects sleep, circadian timing, and next-morning alertness. Proc Natl Acad Sci. 112:1232–37.
- Chellappa SL, Steiner R, Blattner P, Oelhafen P, Götz T, Cajochen C. 2011. Non-visual effects of light on melatonin, alertness and cognitive performance: Can blue-enriched light keep us alert? PLoS One. 6:e16429.
- Chellappa SL, Steiner R, Oelhafen P, Lang D, Götz T, Krebs J, Cajochen C. 2013. Acute exposure to evening blue-enriched light impacts on human sleep. J Sleep Res. 22:573–80.
- Cho C, Lee H, Yoon H, Kang S, Bok K, Jung K, Kim L, Lee E. 2016. Exposure to dim artificial light at night increases REM sleep and awakenings in humans. Chronobiol Int. 33:117–23.
- Cho Y, Ryu S-H, Lee BR, Kim KH, Lee E, Choi J. 2015. Effects of artificial light at night on human health: A literature review of observational and experimental studies applied to exposure assessment. Chronobiol Int. 0528:1–17.
- Commission International de l’Eclairage. 2014. Termlist – light pollution. Termlist - Comm Int l’Eclairage [Internet]. [accessed 2017 Jun 27]. Available from: http://eilv.cie.co.at/term/669
- Crowley S, Carskadon M. 2010. Modifications to weekend recovery sleep delay circadian phase in older adolescents. Chronobiol Int. 27:1469–92.
- Crowley SJ, Cain SW, Burns AC, Acebo C, Carskadon MA. 2015. Increased sensitivity of the circadian system to light in early/mid-puberty. J Clin Endocrinol Metab. 100:4067–73.
- Cucurachi S, Heijungs R, Peijnenburg WJGM, Bolte JFB, De Snoo GR. 2014. A framework for deciding on the inclusion of emerging impacts in life cycle impact assessment. J Clean Prod. 78:152–63.
- Deacon S, Arendt J. 1994. Posture influences melatonin concentrations in plasma and saliva in humans. Neurosci Lett. 167:191–94.
- Dominoni DM, Borniger JC, Nelson RJ. 2016. Light at night, clocks and health: From humans to wild organisms. Biol Lett. 12:20160015.
- European Commission - Joint Research Centre. 2011. International Reference Life Cycle Data System (ILCD) Handbook: Recommendations for Life Cycle Impact Assessment in the European context. Luxemburg.
- Figueiro MG, Plitnick B, Rea MS. 2014. The effects of chronotype, sleep schedule and light/dark pattern exposures on circadian phase. Sleep Med. 15:1554–64.
- Figueiro MG, Rea MS. 2012. Preliminary evidence that light through the eyelids can suppress melatonin and phase shift dim light melatonin onset. In: BMC Res Notes. 5(1). p. 221.
- Follenius M, Weibel L, Brandenberger G. 1995. Distinct modes of melatonin secretion in normal men. J Pineal Res. 18:135–40.
- Franz M, Wenzl FP. 2017. Critical review on life cycle inventories and environmental assessments of LED-lamps. Crit Rev Environ Sci Technol. 47:2017–78.
- Geerdink M, Walbeek TJ, Beersma DGM, Hommes V, Gordijn MCM. 2016. Short blue light pulses (30 min) in the morning support a sleep-advancing protocol in a home setting. J Biol Rhythms. 31:483–97.
- Gooley JJ, Rajaratnam SM, Brainard GC, Czeisler CA, Lockley SW. 2010. Spectral responses of the human circadian system depend on the irradiance and duration of exposure to light. sci transl med. 2(31).
- Hannibal J, Hindersson P, Østergaard J, Georg B, Heegaard S, Larsen PJ, Fabrenkrug J. 2004. Melanopsin is expressed in PACAP-containing retinal ganglion cells of the human retinohypothalamic tract. Investig Ophthalmol Vis Sci. 45:4202–09.
- Haus E, Smolensky M. 2013. Shift work and cancer risk: Potential mechanistic roles of circadian disruption, light at night, and sleep deprivation. Sleep Med Rev. 17:273–84.
- Higuchi S, Ishibashi K, Aritake S, Enomoto M, Hida A, Tamura M, Kozaki T, Motohashi Y, Mishima K. 2008. Inter-individual difference in pupil size correlates to suppression of melatonin by exposure to light. Neurosci Lett. 440:23–26.
- Ho Mien I, Chua ECP, Lau P, Tan LC, Lee ITG, Yeo SC, Tan SS, Gooley JJ. 2014. Effects of exposure to intermittent versus continuous red light on human circadian rhythms, melatonin suppression, and pupillary constriction. PLoS One. 9:e96532.
- Hölker F, Moss T, Griefahn B, Kloas W, Voigt CC. 2010. The dark side of light: A transdisciplinary research agenda for light. Ecol Soc. 15(4):13.
- Irwin A. 2018. The dark side of light. Nature. 553:268–70.
- Khalsa SBS, Jewett ME, Cajochen C, Czeisler CA. 2003. A phase response curve to single bright light pulses in human subjects. J Physiol. 549:945–952.
- Koller M, Härma M, Laitinen JT, Kundi M, Piegler B, Haider M. 1994. Different patterns of light exposure in relation to melatonin and Cortisol rhythms and sleep of night workers. J Pineal Res. 16:127–35.
- Kozaki T, Kubokawa A, Taketomi R, Hatae K. 2016. Light-induced melatonin suppression at night after exposure to different wavelength composition of morning light. Neurosci Lett. 616:1–4.
- Kripke D, Elliot J, Youngstedt S, Rex K. 2007. Circadian phase response curves to light in older and young women and men. J Circadian Rhythm. 5:1–13.
- Kyba CCM, Kuester T, A SDM, Baugh K, Jechow A, Hölker F, Bennie J, Cd E, Luis G, Kj G. 2017. Artificially lit surface of Earth at night increasing in radiance and extent. Sci Adv. 3:1–9.
- Lee M, Swanson B, de la Iglesia H. 2009. Circadian timing of REM sleep is coupled to an oscillator within the dorsomedial suprachiasmatic nucleus. Curr Biol. 19:848–52.
- McMullan C, Schernhammer E, Rimm E, Hu F, Forman J. 2013. Melatonin secretion and the incidence of type 2 diabetes. JAMA. 309:1388–96.
- Munch M, Kobialka S, Steiner R, Oelhafen P, Wirz-Justice A, Cajochen C. 2005. Wavelength-dependent effects of evening light exposure on sleep architecture and sleep EEG power density in men. AJP Regul Integr Comp Physiol. 290:R1421–R1428.
- Münch M, Scheuermaier KD, Zhang R, Dunne SP, Guzik AM, Silva EJ, Ronda JM, Duffy JF. 2011. Effects on subjective and objective alertness and sleep in response to evening light exposure in older subjects. Behav Brain Res. 224:272–78.
- Najjar RP, Zeitzer JM. 2016. Temporal integration of light flashes by the human circadian system. J Clin Invest. 126:938–47.
- Obayashi K, Saeki K, Kurumatani N. 2014. Association between light exposure at night and insomnia in the general elderly population: The HEIJO-KYO cohort. Chronobiol Int. 31:976–82.
- Reiter RJ, Tan D-X, Korkmaz A, Erren TC, Piekarski C, Tamura H, Manchester LC. 2007. Light at night, chronodisrup – tion, melatonin suppression, and cancer risk: a review. Crit Rev TM Oncog. 13:303–28.
- Revell V, Molina T, Eastman C. 2012. Human phase response curve to intermittent blue light using a commercially available device. J Physiol. 590:4859–68.
- Robinson J, Bayliss S, Fielder A. 1991. Transmission of light across the adult and neonatal eyelid in vivo. Vision Res. 31:1837–40.
- Rüger M, St Hilaire MA, Brainard GC, Khalsa SBS, Kronauer RE, Czeisler CA, Lockley SW. 2013. Human phase response curve to a single 6.5 h pulse of short-wavelength light. J Physiol. 591:353–363.
- Sander B, Markvart J, Kessel L, Argyraki A, Johnsen K. 2015. Can sleep quality and wellbeing be improved by changing the indoor lighting in the homes of healthy, elderly citizens? Chronobiol Int. 32:1049–60.
- Santhi N, Thorne HC, Van Der Veen DR, Johnsen S, Mills SL, Hommes V, Schlangen LJM, Archer SN, Dijk DJ. 2012. The spectral composition of evening light and individual differences in the suppression of melatonin and delay of sleep in humans. J Pineal Res. 53:47–59.
- Scholand MJ, Dillon HE. 2012. Life-cycle assessment of energy and environmental impacts of LED lighting products. Part 2: LED Manufacturing and Performance. U.S Department of Energy.
- Sharkey KM, Carskadon MA, Figueiro MG, Zhu Y, Rea MS. 2011. Effects of an advanced sleep schedule and morning short wavelength light exposure on circadian phase in young adults with late sleep schedules. Sleep Med. 12:685–92.
- Sletten TL, Revell VL, Middleton B, Lederle KA, Skene DJ. 2009. Age-related changes in acute and phase-advancing responses to monochromatic light. J Biol Rhythms. 24:73–84.
- Souman JL, Tinga AM, Te Pas SF, van Ee R, Bns V. 2018. Acute alerting effects of light: A systematic literature review. Behav Brain Res. 337:228–39.
- St Hilaire MA, Gooley JJ, Khalsa SBS, Kronauer RE, Czeisler CA, Lockley SW. 2012. Human phase response curve to a 1 h pulse of bright white light. J Physiol. 590:3035–3045.
- Straif K, Baan R, Grosse Y, Secretan B, El GF, Bouvard V, Altieri A, Benbrahim-Tallaa L, Cogliano V. 2007. Carcinogenicity of shift-work, painting, and fire-fighting. Lancet Oncol. 8:1065–66.
- Tähkämö L. 2017. Sustainable residential lighting practises and light pollution. In: Bertoldi P, editor. Proc 9th Int Conf Energy Effic Domest Appliances Light (EEDAL ’17). Irvine, CA, USA: Publications Office of the European Union; p. 1147–1160..
- Tähkämö L, Halonen L. 2015. Life cycle assessment of road lighting luminaires – comparison of light-emitting diode and high-pressure sodium technologies. J Clean Prod. 93:234–42.
- Tähkämö L, Martinsons C, Ravel P, Grannec F, Zissis G 2014. Solid State Lighting Annex : Life Cycle Assessment of Solid State Lighting Final Report. [place unknown].
- Thapan K, Arendt J, Skene DJ. 2001. An action spectrum for melatonin suppression: Evidence for a novel non-rod, non-cone photoreceptor system in humans. J Physiol. 535:261–67.
- Touitou Y, Fevre M, Bogdan A, Al E. 1984. Patterns of plasma melatonin with ageing and mental condition: Stability of nyctohemeral rhythms and differences in seasonal variations. Acta Endocrinol. 106:145–51.
- Touitou Y, Reinberg A, Touitou D. 2017. Association between light at night, melatonin secretion, sleep deprivation, and the internal clock: Health impacts and mechanisms of circadian disruption. Life Sci. 173:94–106.
- Voultsios A, Kennaway D, Dawson D. 1997. Salivary melatonin as a circadian phase marker: Validation and comparison to plasma melatonin. J Biol Rhythms. 12:457–66.
- Webley G, Leidenberger F. 1986. The circadian pattern of melatonin and its positive relationship with progesterone in women. J Clin Endocrinol Metab. 63:323–28.
- Wehr T. 1991. The durations of human melatonin secretion and sleep respond to changes in daylength (photoperiod). J Clin Endocrinol Metab. 73:1276–80.
- Wright KJ, Badia P. 1999. Effects of menstrual cycle phase and oral contraceptives on alertness, cognitive performance, and circadian rhythms during sleep deprivation. Behav Brain Res. 103:185–94.
- Zeitzer JM, Dijk D-J, Kronauer RE, Brown EN, Czeisler CA. 2000. Sensitivity of the human circadian pacemaker to nocturnal light: Melatonin phase resetting and suppression. J Physiol. 526:695–702.
- Zeitzer JM, Fisicaro RA, Ruby NF, Heller HC. 2014. Millisecond flashes of light phase delay the human circadian clock during sleep. J Biol Rhythms. 29:370–76.