 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Numerous functional measures related to anaerobic performance display daily variation. The diversity of tests and protocols used to assess anaerobic performance related to diurnal effects and the lack of a standardized approach have hindered agreement in the literature. Therefore, the aim of the present study was to investigate and systematically review the evidence relating to time-of-day differences in anaerobic performance measures. The entire content of PubMed (MEDLINE), Scopus, SPORTDiscus® (via EBSCOhost) and Web of Science and multiple electronic libraries were searched. Only experimental research studies conducted in male adult participants aged ≥ 18 yrs before May 2021 were included. Studies assessing tests related to anaerobic capacity or anaerobic power between a minimum of two time-points during the day (morning vs evening) were deemed eligible. The primary search revealed that a total of 55 out of 145 articles were considered eligible and subsequently included. Thirty-nine studies assessed anaerobic power and twenty-five anaerobic capacity using different modes of exercise and test protocols. Forty-eight studies found several of their performance variables to display time-of-day effects, with higher values in the evening than the morning, while seven studies did not find any time-of-day significance in any variables which were assessed. The magnitude of difference is dependent on the modality and the exercise protocol used. Performance measures for anaerobic power found jump tests displayed 2.7 to 12.3% differences, force velocity tests ~8% differences, sprint tests 2.7 to 11.3% differences and 5-m multiple shuttle run tests 3.7 to 13.1% differences in favour of the evening. Performance measures for anaerobic capacity found Wingate test to display 1.8 to 11.7% differences and repeated sprint tests to display 3.4 to 10.2% differences. The only test not to display time-of-day differences was the running based anaerobic sprint test (RAST). Time-of-day variations in anaerobic performance has previously been partially explained by higher core-body and/or muscle temperature and better muscle contractile properties in the afternoon, although recent findings suggest that differences in methodology, motivation/arousal, habitual training times and chronotypes could provide additional explanations. There is a clear demand for a rigorous, standardised approach to be adopted by future investigations which control factors that specifically relate to investigations of time-of-day.
Introduction
A large body of research has shown that physical and physiological variables display a diurnal variation in a temperate environment (around 17–22°C) in males (Atkinson and Reilly Citation1996; Drust et al. Citation2005). In the absence of external cues, cortisol levels, melatonin levels and core/muscle temperature levels are believed to play a role in the circadian regulation through signals directed by the suprachiasmatic nucleus (Reilly and Waterhouse Citation2009a, b). It has long been established that both cortisol levels (Reilly and Waterhouse Citation2009b) and body temperature (Atkinson and Reilly Citation1996, Pullinger et al. Citation2019) are higher in the mid-afternoon/evening, while levels of melatonin display higher values during the nocturnal period (Edwards et al. Citation2000). Muscle force production and power output also display an evening superiority, regardless of the muscle group measured (Atkinson and Reilly Citation1996; Drust et al. Citation2005). Anaerobic performance, such as anaerobic power in activities lasting less than 6 seconds (short-term, maximal power output) have previously shown to peak between 17:00 to 19:00 h (Bernard et al. Citation1998; Racinais et al. Citation2004). Similarly, anaerobic capacity, further defined as activities lasting between 30-s to 2-min in duration show a parallel peak in performance with greater values detected during evening hours between 16:00 to 19:00 h (Chtourou et al. Citation2012b; Souissi et al. 2013 c). The diurnal variation in peak power has previously shown to have an amplitude of ~7% and an acrophase around 17:00 h, while average power has displayed a higher amplitude of ~11% with an acrophase occurring slightly later at 18:00 h (Dergaa et al. Citation2019).
Time-of-day differences in anaerobic performance have previously been investigated using an array of tests and equipment (Aloui et al. Citation2013; Kin-Isler Citation2006; Melhim Citation1993; Souissi et al. Citation2010, Citation2002). Research concerning diurnal variation in anaerobic power has involved using sprint tests on a cycle ergometer (Souissi et al. Citation2010, Citation2004), swimming (Zarrouk et al. Citation2012b) or overground running (Chtourou et al. Citation2018), jump tests (Bernard et al. Citation1998; Heishman et al. Citation2017), force-velocity tests (Falgairette et al. Citation2003). In order to assess anaerobic capacity, many tests ranging from the Wingate test (Hill and Chtourou Citation2020), repeated sprint performance/ability (Chtourou et al. Citation2018; Pullinger et al. Citation2018a, Citation2018b), running based anaerobic sprint test (RAST; Dergaa et al. Citation2019) have previously been used in the literature to assess time-of-day variation. Classical Wingate tests have shown differences in mean and peak power by 11% and 14% during evening time (Drust et al. Citation2005). Variables related to repeated sprint performance have shown to peak between 17:00 h and 19:00 h with differences ranging from 3.4% to 10.2%. Ranges observed in daily variation of performance are dependent on numerous characteristics related to the performance variable measured, the mode of exercise (running vs. cycling) and the protocol used (duration of sprint, duration of recovery, the number of sprints), the fitness level of the athlete and the motivation of the subject (Giacomoni et al. Citation2006; Pullinger et al. Citation2014). Although most variables related to anaerobic performance have been thoroughly investigated, with diurnal variation evident; a diurnal variation in the variable “fatigue index” is not always reported in the literature and this inconsistency is attributed to the mode of exercise used and the type of protocol. Bishop et al. (Citation2011), found that fatigue index was a valid measure of performance, however, fatigue index did not display time-of-day differences in repeated sprint performance (Pullinger et al. Citation2018a, Citation2018b).
The observation of notable changes in diurnal variation are still unknown but involve several potential contributing factors (Edwards et al. Citation2013; Pullinger et al. Citation2018a, Citation2018b). The evening superiority in muscle force production and power output has been attributed to a causal link between core body/muscle temperatures and performance and has previously at least partially been linked to diurnal changes in core and muscle temperatures (Robinson et al. Citation2013), although the exact mechanism(s) between performance and central temperature require additional research. Further, peripheral or muscle-related variables (contractibility, metabolism, and morphology of muscle fibres) influenced by hormonal and ionic muscle process variations (Reilly and Waterhouse 2009; Tamm et al. Citation2009), central/neurological factors (central nervous system command, alertness, motivation, and mood: Castaingts et al. Citation2004; Giacomoni et al. Citation2005; Racinais Citation2010; Racinais et al. Citation2005a) and/or greater phosphorylation of M-band-associated proteins (Ab Malik et al., Citation2020) have also been put forward as potential explanations that affect diurnal variation in muscle performance. Finally, aspects related to research design deemed specifically important for studies of a chronobiological (time-of-day) nature can influence potential findings. The lack of standardisation of these methods and adherence to these aspects hinder agreement on time-of-day effects and performance. Therefore, considering the large differences between findings and methodologies currently used to assess time-of-day and anaerobic performance measures, providing a clear and comprehensive review on this topic will help identify the current research gaps in our understanding within the area. In addition, highlighting the methodological concerns and other findings will help improve future studies related to anaerobic performance measures and time-of-day.
Given the amount of research and the equivocal evidence presented in the literature, the aim of the present paper was to examine the following research question: “In healthy adolescent males, what is the magnitude of time-of-day differences in performance variables related to anaerobic power and capacity?” In addition, information will be provided in relation to aspects related to research design deemed specifically important for studies of a chronobiological (time-of-day) nature.
Methods
Reporting standard
This systematic review conforms to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Page et al. Citation2021). The PRISMA checklist is presented in Appendix 1, indicating the page numbers where items of information are present in the current manuscript.
Eligibility criteria
The inclusion criteria were based on the Cochrane guidelines for conducting systematic reviews (Higgins et al. Citation2021). The criteria for inclusion and exclusion were set and agreed by all six authors. Following the initial selection process of studies, three authors (AR, SP & TB) independently completed the eligibility assessment in a blinded standardized way by screening the titles and abstracts. To be considered eligible, the manuscript had to meet the following inclusion criteria:
Population – healthy males and adult participants (18+ years of age) only. Females were excluded due to the impact of hormonal fluctuations on performance parameters thereby rendering it difficult to interpret findings. Female sex hormones have displayed substantial physiological effects related to altering fluid regulation, and modifications in thermoregulatory, muscular and metabolic responses all of which have been shown to affect anaerobic performance (Meignié et al. Citation2021).
Time-of-day – compared the effects of morning versus evening in performance variables related to anaerobic power and/or anaerobic capacity (a minimum of two time-points).
Anaerobic power – force-velocity test, crictical power test, jump tests (e.g. squat jump, counter movement jump test), Margaria Kalamen test, sprint test (cycling, running or swimming) and multiple shuttle run test
and/or
Anaerobic capacity – Repeated sprint testing, Running Based Anaerobic Sprint Test (RAST), 60 to 800-meter run, Cunningham Faulkner test and Wingate test
Design – Randomised and/or counterbalanced trials
Literature search strategy and information sources
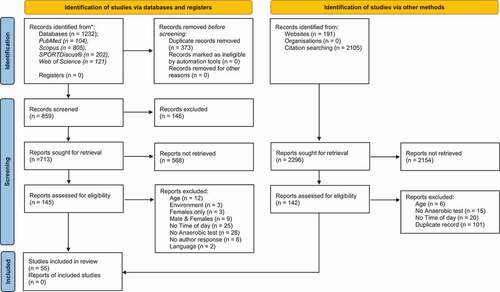
A computerised English-language literature search of the grey literature (TB & SP): Liverpool John Moores University electronic library, Manipal Academy of Higher Education electronic library, Qatar National Library; and electronic databases: PubMed (MEDLINE), Scopus, SPORTDiscus® (via EBSCOhost) and Web of Science were conducted (October 2020 – April 2021). A search for relevant content related to anaerobic power and/or anaerobic capacity and time-of-day variation using the following search syntax using Boolean operators in titles, abstracts, and keywords of indexed documents: (“circadian variation” OR “diurnal variation” OR “time-of-day” OR “circadian fluctuation”) AND (“anaerobic capacity” OR “anaerobic power” OR “short-term power output” OR “anaerobic performance”) was conducted (Appendix 2). Additional advanced search techniques using wildcards, truncation and proximity searching were incorporated. Secondary searches consisting of the reference lists of all papers included were screened manually for additional relevant papers, as part of the secondary search (AR & TB). In addition, forward reference searching was conducted to explore potential follow-up studies through citations and authors. One author (SP) independently carried out the searches for study selection to minimise potential selection bias. presents the flow of papers through the study selection process using the PRISMA 2020 flow diagram (Page et al. Citation2021).
Figure 1. PRISMA 2020 flow diagram (Page et al. Citation2021) of the study selection process.
Study selection
Where both male and female participants took part in a research study, the article was included if the data from male participants could be independently identified. In instances where the title and abstract did not contain enough detail to indicate whether an article was relevant to the review, the complete article was obtained and read. This enabled the authors to determine whether the paper met the primary inclusion criteria. In instances where the primary purpose of the article was not an investigation looking at the effects of time-of-day, meaning a minimum of two time-points were not assessed (morning and evening), the papers were excluded from the review. Letters to the editor, conference abstracts and literature reviews were excluded as these studies were not found to be methodologically-quality-assessable and/or critically appraisable.
Data extraction
Data extraction was performed by two authors (AR & TB) independently and a data check performed by a third author (SP) with the following data extracted from the included studies: (1) the study authors and date; (2) the number of participants and participant’s characteristics (e.g. age, body mass, stature); (3) the circadian chronotype questionnaire used to assess the participants (and their scores); (4) the time-of-day testing sessions took place (e.g. morning, afternoon, evening); (5) anaerobic power or anaerobic capacity test used; (6) equipment used (e.g. cycle ergometer, non-motorised treadmill, Ergojump); (7) performance variables assessed (e.g. jump height, peak power, fatigue index, time); (8) the significance established with P values; and (9) % difference between testing time-points (if results were provided) and information as to whether diurnal variation was established. In addition, analysis regarding aspects relating to research design and factors deemed specifically important in investigations of chronobiological nature were quantified; randomisation, counterbalancing, record of light intensity, control of meals, control of room temperature, control of sleep and fitness of participants, as previously used by Pullinger et al. (Citation2019). In most instances, a simple ‘yes’ or ‘no’ was recorded against each of the included studies, other than ‘fitness’ (when the studies were classified as having ‘trained’ or ‘untrained’ participants). All articles that made no specific reference to any of these primary areas were considered to indicate a negative response and ‘no’ was marked against the area in question.
Quality assessment
A modified 26-item methodological quality assessment checklist on each included article using the Downs and Black scale (Downs and Black Citation1998). was conducted. The checklist consisted of 26 “yes”-or- “no” questions which were scored totalling up to a possible 27 points. The questions were categorized under 4 sections: Reporting (10 items; 1–10), External validity (3 items; 11–13), Internal validity study bias (7 items; 14–20) and internal validity confounding selection bias (7 items; 21–26). The quality assessment of the articles was conducted by two reviewers (AR and TB) independently with disagreement on 5 manuscripts (9.1%). The observed differences were resolved by a third reviewer (SP).
Results
Search results
The literature search ended on April 2021 and the primary database search revealed 1232 articles and 2296 articles via other methods. presents the number of articles found in each electronic database and a detailed flow chart of the literature search, including all the steps performed. Once duplicates were removed, 859 titles remained in the reference manager (Mendeley, Elsevier, Amsterdam, The Netherlands). Following the examination of titles, abstracts and keywords of all these manuscripts, 145 academic studies were deemed eligible and retained for full text-analysis. After additional full-text analysis, 55 studies were deemed eligible and included in the systematic review. Reasons for exclusion can be found in . Upon further inspection of all articles in their bibliographical references, none of these studies met the inclusion criteria and hence were deemed ineligible.
Study characteristics
The detailed participant characteristics are shown in . A total of 813 participants were included across the 55 studies (average number of participants per study = 15), ranging from a total of 8 to 31 participants. Forty-three studies (78%) assessed circadian chronotype of participants using the morningness-eveningness questionnaire (Horne and Ostberg Citation1976). The majority of participants belonged to the intermediate chronotype (73.0%), 12.8% to the moderately morning chronotype and 3.1% to the moderately evening chronotype. A further 6.4% of participants belonged to either the “extreme” morning (5.4%) or “extreme” evening (0.6%) chronotype. Two studies did not provide detailed information in regard to circadian chronotype of its participants, stating that they belonged to either moderately morning or intermediate chronotypes (5.1%; see ). A total of twelve studies failed to report any information related to chronotype for their participants.
Table 1. Summary of the articles reviewed for anaerobic power (n = 16) with an overview of the participants, the experimental protocols with the time-of-day, exercise mode, performance test, the variables examined, and the main findings related to time-of-day in relation to each variable
Table 2. Summary of the articles reviewed for anaerobic capacity (n = 30) with an overview of the participants, the experimental protocols with the time-of-day, exercise mode, performance test, the variables examined, and the main findings related to time-of-day in relation to each variable
Table 3. Summary of the articles reviewed which conducted an anaerobic power and anaerobic capacity test (n = 9) with an overview of the participants, the experimental protocols with the time-of-day, exercise mode, performance test, the variables examined, and the main findings related to time-of-day in relation to each variable
The time-of-day during which morning sessions took place ranged from 05:30 to 11:00 h and evening sessions between 16:00 and 19:30 h, in 51 studies. A total of 2 studies used different time-points to assess diurnal variation (Heishman et al. Citation2017; Hill and Smith Citation1991). In addition, 9 studies used additional time-points to assess diurnal variation; Aziz et al. (Citation2012) (21:00 h); Bernard et al. (Citation1998) (14:00 h); Falgairette et al. (Citation2003) (14:00 h); Heishman et al. (Citation2017) (13:45 h); Hill and Smith (Citation1991) (03:00 h and 21:00 h); Kin-Isler (Citation2006) (13:00 h); Souissi et al. (Citation2003) (02:00 h; 14:00 h and 22:00 h); Souissi et al. (Citation2019b) (13:00 h; 15:00 h), Unver and Atan (Citation2015) (14:00 h).
The total number of studies which performed an anaerobic capacity test was 39, while 25 performed an anaerobic power test. From these, 9 (16.4%) had a combination of both an anaerobic capacity and anaerobic power test, with 16 (29.1%) only conducting anaerobic power tests and 30 (54.5%) only anaerobic capacity tests. The mode of exercise varied across studies, with 37 studies using cycling, 14 using jumping, 10 using running and 2 using swimming. From these, 9 studies a combination of testing modes, such as: cycling, running and jumping (Aloui et al. Citation2017; Bernard et al. Citation1998); cycling and jumping (Chtourou et al. Citation2012a; Chtourou et al. 2012 c; Racinais et al. Citation2004; Souissi et al. 2013b; Souissi et al. Citation2019a); and running and jumping (López-Samanes et al. 2017; Pavlović et al. Citation2018). The type of anaerobic tests varied from Wingate tests, force velocity tests, sprint tests, repeated sprint tests, and multiple shuttle run tests using cycling, running or swimming as the mode of exercise. Different jump tests were also used ranging from squat jump, countermovement jump, static jump, continuous jump and long jump ().
Forty-eight studies found several of their performance variables to display time-of-day effects, with higher values in the evening than the morning, while seven studies (Aziz et al. Citation2012; Chtourou et al. Citation2018; Falgairette et al. Citation2003; Gholamhasan et al. Citation2013; Giacomoni et al. Citation2006; Nikolaidis et al. Citation2018; Unver and Atan Citation2015) did not find any time-of-day significance in any variables which were assessed. A further 18 studies also found at least one of their variables not displaying time-of-day variation (excluding the studies mentioned above). Variables assessed during the Wingate test (n = 28), found all measures of peak power (maximal power) and mean power to always be significantly in favour of evening performance compared to the morning with ranges from 3.4 to 7.5% and 1.6 to 11.7%, respectively. Only one study used a RAST protocol and found all variables to be significantly better in the evening compared to the morning session. Repeated sprint performance found 8 of the 10 studies to report time-of-day significance, with higher values in the evening, with ranges from 3.5 to 9.0% dependant on the mode of exercise, the protocol used, and the variable assessed (see Pullinger et al. Citation2019b). A total of 6 studies performed a force velocity test and found significant differences to be present in measures of maximal power (7.8%), cycling power, absolute power cycling, and peak velocity in favour of the evening. However, one study (Souissi et al. Citation2008) showed no significant difference in peak velocity, while one other study (Falgairette et al. Citation2003) showed no significant difference in peak power and total work. Most studies found measures of jump height or distance to be significantly higher in the evening compared to the morning in the squat jump (3.3 to 9.6%), counter movement jump (2.7 to 12.3%), Sargeant jump, static jump, long jump (3.6%), Abalakov jump test (6.9%), and 30-s continuous jump (11.4%). Singular sprints also showed a tendency for significant better performance in the afternoon compared to the morning, with overground running sprint times decreasing by 10.9% for 5-m sprint, 2.7 to 11.3% for a 10-m sprint, and 10.8% for a 20-m sprint. The 50-m sprint did not show any significance. Cycling sprint performance was also significantly higher for power output at half-pedal (3.7%) for a 5 to 6-s maximal sprint, and maximal power (4.5%) and maximal force (3.8%) for a 7-s maximal sprint. A 10-s sprint showed no significant difference for maximal sprint power, while variables of power output full pedal and maximal velocity showed no significant differences for a 5 to 6-s, and a 7-s sprint respectively. Swim times over 10-m and 25-m showed significantly better times in the evening up to 1.5% in two studies. The multiple shuttle run test found performance variables to be significantly different in both studies, with total distance (3.7 to 4.4%), and highest distance (4.4 to 13.1%) better in the afternoon. Fatigue index was assessed in a total of 21 studies, with the majority of studies displaying no significant difference. However, 7 of these studies did display a significant difference between morning and evening, with ranges from 2.2 to 62.2%, using multiple shuttle run test, repeated sprint ability test or Wingate test as their testing protocol. The majority of studies which used multiple time-points found majority of majority of measures in the late afternoon or evening (15:00–22:00 h) to be significantly higher than measures in the early morning (02:00–03:00 h), morning (07:00–10:00 h), or mid-afternoon (13:00–14:00 h).
The substantial differences in methodological and clinical heterogeneity among studies meant we were unable to conduct a meta-analysis and pool the observed data-sets to evaluate the evidence related to findings in anaerobic performance and therefore provided in-depth information related to unweighted results. Missing data information, differences in populations, metrics, outcomes and designs were the main reasons for a meta-analysis not to be pursued. Conducting a meta-analysis will simply compound the errors and produce an inappropriate set of results and summary.
Quality of work
provides detailed information related to randomisation, counterbalancing, record of light intensity, control of meals, control of room temperature, control of sleep and fitness, to quantify for the control of aspects relating to research design deemed specifically important in investigations of a chronobiological nature. None of the studies met all 7 criteria required for an investigation of chronobiological nature. Only one study failed to provide any information related to fitness of participants. A total of 27 counterbalanced the order of administration to minimise learning effects and 39 studies performed the time-of-day session in a randomised order. From these, 17 used counterbalancing and randomisation within their protocol. The majority of studies controlled for meals (n = 39) and sleep (n = 48) of their participants and controlled for room temperature (n = 32), but very few recorded light intensity (n = 7). Only 4 studies quantified all four of the 4 aforementioned criteria (Bougard and Davenne Citation2012; Pullinger et al. Citation2014, Citation2018a, Citation2018b).
Methodological quality control and publication bias
Based on a modified 26-item Downs and Black (Citation1998) checklist, the results of the methodological quality assessment of the included studies ranged from 19 to 26. Reporting (10 items; items 1–10) showed 6 items to be fully met by all studies (Items 1–4, 6 and 9), with 10 studies meeting full criteria for reporting. External validity (3 items; items 11–13) displayed all three items to be met by only 28 studies. Internal validity study bias (7 items; items 14–20) reported 5 items out of 7 items (items 16–20) to be fully met, with one study fully meeting all criteria for internal validity study bias (Souissi et al. Citation2019b). Confounding selection bias (6 items; items 21–26) reported 2 studies to meet all criteria (Chtourou et al. Citation2012a, 2012 c), with item 21 met by all studies. Detailed methodological quality assessment scores can be found in .
Discussion
The present study analysed data from studies that compared the effects of diurnal variation on anaerobic performance measures and determined the quality of evidence that reports a “peak” time for performance. The main finding of this review was that most research papers (n = 49; 89.1%) established time-of-day differences related to anaerobic performances, with significantly greater values observed in the afternoon (16:00 to 19:30 h) compared to the morning (05:30 to 11:00 h) dependent on the variable assessed.
Anaerobic power
Twenty-five papers were found to have investigated time-of-day effects on a measure of anaerobic power (). The majority of studies established found a diurnal variation in anaerobic power performance measures, consistently peaking in the afternoon or early evening (16:00 h – 19:30 h) compared to the morning (06:00 h – 10:00 h), in agreement with previously established research related to human performance. Only three studies (Falgairette et al. Citation2003; Nikolaidis et al. Citation2018; Unver and Atan Citation2015) failed to establish time-of-day variation (12%) in any of their measures.
Time-of-day differences in jump tests ranged from 2.7 to 12.3%, with the magnitude of difference highly dependent on the jump test used and performance variable measured. All seven different jump tests used to assess anaerobic power in the literature; counter movement jump, squat jump, static jump, Abalakov jump, long jump, 30-s continuous jump and the Sargent jump found jump height and/or flight time and/or distance and/or power to be significantly higher in the afternoon. The only measures which failed to establish any diurnal variation were fatigue index in a 30-s continuous jump test. It has been suggested that jump performance is closely related to peripheral mechanisms of muscular contraction (Castaingts et al. Citation2004). Findings suggest that the higher core temperature present in the afternoon ameliorates the peripheral mechanisms of the muscular contraction thus increasing short term maximal performance (Belkhir et al. 2020). The increase in body temperature could enhance the extensibility of connective tissue as well as the viscosity and conduction velocity of connective tissue (Racinais and Oksa Citation2010). Previous studies have also reported, a significant diurnal change in tendon stiffness (Pearson and Onambele Citation2005) and muscle architecture (pennation angle: fibre arrangement relative to the force generation axis; Pearson et al. Citation2004). The authors reported higher tendon stiffness and pennation angle in the morning compared to the afternoon. Onambele-Pearson and Pearson (Citation2007) reported that the tendon stiffness increased by 20% in the morning when compared to the afternoon and this suggest that the tendon is more compliant in the afternoon. Further, it has been suggested that increased body temperatures reduce muscle viscosity and increase the extensibility of connective tissues (Waterhouse et al. Citation2005), thus facilitating both neuromuscular and metabolic systems (Racinais and Oksa Citation2010), ultimately increasing jump performance.
Performance variables related to the force velocity test displayed contradictory findings. Mean power displayed time-of-day variation with values of maximal power showing an increase of ~ 8% in the evening compared to the morning. Falgairette et al. (Citation2003) however, failed to establish any diurnal variation in peak power. Peak velocity established time-of-day difference in one of the three studies which assessed this variable, while total force and total work found no significance between morning and evening measures (). Several factors have been put forward to explain the diurnal variation observed in maximal power in the force velocity test. As power is the product of force multiplied by velocity, fluctuations in power is dependent on diurnal fluctuations in force or velocity (Souissi et al. Citation2007b; Souissi et al. Citation2008). As velocity fails to establish time-of-day variation as opposed to (maximal) force, it has been suggested that force, which has previously shown to have a significant correlation with maximal isometric voluntary force and peak torques in isokinetic knee extensions (Driss et al. Citation2002), plays a significant role in time-of-day variation observations in maximal power. Diurnal fluctuations in maximal force have been attributed to variations in muscle contractile properties (Davenne and Gauthier Citation1997; Racinais et al. Citation2005a), which are affected by the intracellular variation present within the muscle (Martin et al. Citation1999), and to the circadian rhythm in central temperature (Racinais et al. Citation2005a).
Sprint tests also showed significant time-of-day differences between morning and evening, with faster sprint times in 5-m to 20-m overground running distance (range: 2.7–11.3%), in 25-m swim distance and for mean power output (half pedal) during a 5-s to 6-s cycling sprint (3.7%), and maximal power and maximal force during a 7-s cycling sprint (3.8–4.5%) observed in the evening condition. Overground sprints of 50-m only found a trend for significance, while a longer duration cycling sprint of 10-s found no differences in mean power between morning and evening. One study also established no differences in 25-m swim sprint between conditions. It has been suggested that both neuromuscular changes and fluctuations in core temperature present from morning to evening result in observed differences in overground running performance (Pavlović et al. Citation2018). The authors also mention that physical attributes, such as leg muscle qualities play a role in elucidating sprint performance (Young et al. Citation2015), and that sleeping patterns play a role in the presence of short-term maximal performance (Drust et al. Citation2005). The significant findings related to swim performance minimise the observation of diurnal variation solely related to fluctuations in core temperature and suggest that observed differences are due to a combination of both central and peripheral factors (Zarrouk et al. Citation2012b). Nikolaidis et al. (Citation2018) suggested that the lack of diurnal variation in 25-m swim performance was due to the use of a different exercise protocol and methodology compared to other studies. The observed differences in power output during cycling sprints is thought to be due to the simultaneous increases in local temperature, as opposed to solely core temperature, which has previously been suggested (Racinais et al. Citation2004). In addition, the diurnal rhythm of muscle contractile processes was responsible for the diurnal variation in muscular short-term cycling performance (Racinais et al. Citation2005a, Citation2004).
Both studies which performed a 5-m multiple shuttle run test found peak distance and total distance to display time-of-day variations, with higher values observed in the afternoon for total distance and peak distance (range: 3.7 to 13.1%). However, only one study found fatigue index to differ significantly. Belkhir et al. (2019) hypothesised that the total duration of the 5-m shuttle run test is about 6 min and this duration needs a high contribution of the aerobic pathway to maintain the maximal performance. Thus, it is possible that the better total distance during the 5-m shuttle run test observed in the afternoon was related to better energy production from the aerobic pathway. However, Souissi et al. (Citation2019b) suggested that the observed differences were due a possible link between the diurnal fluctuation of short-term maximal performance and the daily variation of core temperature, and daily fluctuations of attention and motivation (Reilly and Edwards Citation2007).
Anaerobic capacity
Thirty-nine papers were found to have investigated time-of-day effects on a measure of anaerobic capacity (). Similarly, to anaerobic power, measures of anaerobic capacity also consistently peaked in the afternoon or early evening (17:00 h – 19:00 h), when compared to the morning (06:00 h – 10:00 h). Four studies (Aziz et al. Citation2012; Chtourou et al. Citation2018; Gholamhasan et al. Citation2013; Giacomoni et al. Citation2006) did not find time-of-day variation in measures related to anaerobic capacity (10.3%)
All twenty-two studies conducting a 30-s Wingate test found both values for peak power (range: 2.4–9.5%) and mean power (1.8–11.7%) to display significantly better values in the afternoon compared to the morning. Only 3 out of 12 studies which assessed fatigue index during a 30-s Wingate test, found a significant difference between morning and evening measures (4.1%). A 60-s Wingate test also found significant differences in favour of evening performance for peak power (8.2%) and mean power over 30-s and 60-s (7.8%). The modified Wingate test found both peak power and measures of anaerobic capacity to be significantly higher in the afternoon. Only one study observed no differences between morning and evening values for anaerobic power during the Wingate test. Using an electromyographic activity registration, Chtourou et al. (Citation2011) showed a significant increase in muscle power and neuromuscular efficiency in the late afternoon compared to the morning during the first 20-s of a 30-s Wingate test. These findings could indicate that the diurnal variation in muscle power depends on peripheral mechanisms of muscular contraction. Further, the authors indicated a possible intervention of central mechanisms (e.g., central nervous system command, motivation) of the muscular contraction during last 10-s of the Wingate test. Lericollais et al. (Citation2011) reported that the range of motion of the ankle angle decreased in the afternoon compared to the morning indicating a possible relationship between the afternoon increase in muscle power during a 60-s Wingate test and the cycling kinematic parameters. The authors reported two phases of power evolution during a 60-s Wingate test: (i) rapid power decrease while value are higher in the afternoon compared to the morning during the first 20-s) and (ii) slower power reduction with no-significant time of day effect during the last 40-s of the exercise. In addition, Souissi et al. (Citation2007) reported higher aerobic contribution to the energy production in the afternoon compared to the morning. This aerobic contribution was important for maintaining the muscle power over the 30-s maximal cycling and, thus, the authors reported higher fatigue index in the morning compared to the afternoon.
One study performed a RAST test and found peak power, average power and minimal power to be significantly higher in the afternoon compared to the morning (Dergaa et al. Citation2019). The authors suggest that the increase in temperature in the afternoon compared to the morning could explain the diurnal variation of RAST performance and could also be related to sleep, although findings in the literature related to sleep are inconsistent.
Rhythms in repeated-sprint performance also display diurnal variation, with the majority of repeated-sprint performance variables consistently peaking between 17:00 and 19:00 h with lowest values observed between 06:00 and 10:00 h (range: 3.4 to 10.2%). It was found the magnitude of difference is highly dependent on aspects such as the performance variable measured, the mode of exercise, the sprint duration, the type of recovery, the number of sprint repetitions and the training status of subjects. Only one study out of the ten found no significant differences between morning and evening performance. The authors suggest that the occurrence of fatigue and recovery patterns from all-out intermittent exercise may be differentially affected by time-of-day and that non-significant findings were due to methodological differences with similar studies. Nevertheless, it has been suggested that superiority in repeated-sprint performance can be attributed to a causal link between performance and both core body and and local muscle temperatures. However, recent findings suggest this causal link is not as simple as has previously been suggested (Pullinger et al. Citation2014). As a result, other factors have been proposed and are determined by both endogenous (outputs from the body clock) and exogenous (environmental) components (Edwards et al. Citation2013; Zhang et al. Citation2009). These components are related to motivational aspects, subjective arousal, sleepiness, ionic changes and hormonal fluctuations (cortisol ratio, thyroid secretion and testosterone ratio) (Zhang et al. Citation2009). More recently, a study performed by Ab Malik et al. (Citation2020) reported a post-translational state of human muscle proteins after exercise in the morning (08:00 h) and the evening (18:00 h), with significant differences in the phosphorylation of proteins within or close to the muscle M-band that could relate the well-established morning versus evening differences in performance. The phosphorylation of these proteins may alter the M-band structure and disrupt force transmission, thus potentially explaining the lower force outputs observed in the morning compared to evening.
Methodological quality and control
With reference to methodological quality, the included studies all reached a quality assessment score of ≥70%, of which two reached values > 90% (; Chtourou et al. Citation2012a, 2012 c). As far as we are aware only one review has looked into the chronobiological study design perspectives (Pullinger et al., Citation2019), and in agreement with their findings, we also established an apparent lack of control for important factors which specifically relate to investigations of chronobiological nature. It has long been established that the periodicity of the body clock in human beings is affected by external environmental rhythmic cues which affect the continual adjustment of the body clock (zeitgebers). Rhythmic cues such as the light-dark cycle (recording of light intensity), the feeding-fasting cycle (control of meals) and the activity-inactivity cycle (Aschoff Citation1965; Aschoff and Wever Citation1980; Dunlap et al. Citation2004) are primary factors that require control in studies related to time-of-day. Only seven studies (12.7%) reported information for the consideration of light or dark exposure by recording light intensity (). However, thirty-eight studies (69.1%) did control for meals, either through calorific intake and/or timing of meals, a factor previously stressed to play a vital role in chronobiology studies (; Bougard et al. Citation2009). Further, forty-three studies (78%) used, the Horne and Ostberg (Citation1976) morningness-eveningness questionnaire as the chronotype questionnaire to assess their participant’s chronotype scores. Three studies reported participants to belong to either the “extreme” morning (5.4%) or “extreme” evening (0.6%) chronotype. It has previously been established that extreme chronotype influences RPE, fatigue and submaximal performance in the morning (Vitale and Weydahl Citation2017). In addition, evening-types have shown to reach higher values, increased cortical and spinal excitability levels and were able to generate more torque in the evening compared with the morning (Roden et al. Citation2017). Therefore, more research is required to gain a better understanding on the diurnal effects of anaerobic performance and extreme chronotypes.
Table 4. Detailed information related to randomization, counterbalancing, record of light intensity, control of meals, control of room temperature, control of sleep and fitness for articles related to chronobiology (time-of-day)
Table 5. Results of the detailed methodological quality assessment scores based on a modified 26-item Downs and Black (Citation1998) checklist
The evening superiority in muscle force production and power output has long been attributed and associated to the causal link present between core body/local muscle temperatures and performance (Zhang et al. Citation2009). The higher evening core body temperatures (~0.6 to 0.8°C in rectal and gut sites; Edwards et al. Citation2013, Citation2002) and local muscle temperature (~0.30°C in vastus lateralis; Edwards et al. Citation2013; Robinson et al. Citation2013) have shown to increase both neural function (Martin et al. Citation1999) and force-generating capacities of the muscles (Bernard et al. Citation1998; Coldwells et al. Citation1994; Giacomoni et al. Citation2005; Melhim Citation1993). Evidence in the literature has found muscle force development to increase by ~ 5% with every 1°C increase in resting core temperature (Bergh and Ekblom Citation1979) and through passive warming of the musculature (Asmussen and Bøje Citation1945; Ball et al. Citation1999), with recent findings suggesting that the diurnal variation in performance can be partially attributed to core and/or local muscle temperatures (Pullinger et al. Citation2018b; Robinson et al. Citation2013). However, the causal link between temperature and performance is complex with recent findings suggesting that different physiological mechanisms are involved when core body and local muscle temperatures are decreased or increased, respectively (Pullinger et al. Citation2018b). Nevertheless, changes in core body and/or local muscle temperatures potentially negate some of the diurnal variation present within anaerobic performance, further highlighting the importance for controlling room temperatures. In our cohort of studies, only slightly more than half of the studies (56.4%) reported on factors related to temperature, which is in agreement with Pullinger et al. (Citation2019), would seem to be more of an oversight rather than through choice.
The majority of studies (87.3%) within this systematic review controlled for factors related to sleep “control,” such as rising and waking times, keeping similar sleeping habits to “normal life,” not staying up late and no prevalence of insomnia or sleep deprivation. Sleep is not only essential for the brain and body to function, but a lack of sleep has previously shown to be closely associated with impairment in human performance (Walsh et al. Citation2020), with measures of anaerobic power and anaerobic capacity severely impaired (Souissi et al. Citation2003). A large body of research has previously investigated the effect of sleep and sleep deprivation on performance measures and central fatigue (Edwards and Waterhouse Citation2009; Kirschen et al. Citation2020; Waterhouse et al. Citation2011) and found performance to be negatively associated with disturbed sleeping patterns and lack of sleep. Considering increased levels of fatigue associated with time-since-last-sleep and the known restorative influences of sleep, measures of cognitive performance and central arousal are suggested to decline as time-awake increases (Ball et al. Citation1999). Given most studies provided some detail related to control of sleep, such as timings of retiring/waking times and the amount of rest allowed during testing days, this would suggest that sleep loss did not affect findings in the majority of studies.
All studies reported information related to background (e.g. students, cyclists, team-sport players) and/or fitness levels (e.g. active, trained, healthy) of their participants, thus unlikely to represent issues with the interpretation of findings (Guette et al. Citation2005; Häkkinen Citation1989). Nevertheless, training status has previously shown to affect cycling performance (Hopker et al. Citation2013), Wingate performance (de Salles Painelli et al. Citation2014) and measures of peak power (Bishop and Spencer Citation2004) when comparing elite, trained and untrained individuals. In addition, it is of great importance that participants taking part in time-of-day studies are fully familiarised with the procedures and that protocols used within each study, and that the mode of exercise used is specific to the sport/background of the individuals. It has previously been proposed that the observation of diurnal variation in performance are closely related to subject familiarisation, training status and mode of exercise specificity (Bambaeichi et al. Citation2005; Giacomoni et al. Citation2006; Reilly et al. Citation1997). A lack of control related to the number and timing of familiarisation sessions impacts subsequent experimental findings as a result of neuromuscular adaptations still taking place within the experimental time-of-day sessions. Very few studies provide detailed information related to the number of familiarisation sessions participants undertook or used statistics to show systematic and random bias. Therefore, it is of paramount importance to utilise an objective method to assess whether the cohort of participants are fully familiarised is (Pullinger et al. Citation2019). In addition, counterbalancing of sessions, to guarantee internal validity by controlling the potential confounds created by sequence and order effects, and randomisation, to eliminate selection bias and balance known and unknown confounding factors are equally imperative. In this systematic review, it was established that only half the studies (50.9%) randomised sessions, while approximately two-thirds (70.9%) used counterbalancing within their research. These findings suggest that a number of studies have potential biased results due to learning effects and time-of-day observations could in part be due to acute neuromuscular adaptations associated with a lack of familiarisation, randomisation, and counterbalancing through the initial learning of motor recruitment pathways as opposed to any endogenously driven diurnal rhythm. It must be noted that randomisation of sessions is highly dependent on the aims of the study, with studies merely looking at differences between morning and evening without the need of a control group, not requiring randomisation within the methodological set-up. Interestingly, only four studies (7.2%; Bougard and Davenne Citation2012; Pullinger et al. Citation2014, Citation2018a, Citation2018b) quantified all aspects relating to research design and factors deemed specifically important in investigations of chronobiological nature (), not taking into consideration randomisation due to the nature of the studies.
Finally, it must be highlighted that there is a clear diurnal type bias in the majority of studies where participants where free to live a normal life, and diurnal measures were taking in the morning (05:30 to 11:00 h) and evening (16:00 to 19:30 h), respectively. Nine studies (16.4%) performed a more circadian type study, ranging from 3 to 6 “equally” spaced time-points across a 24 h day period, although no cosinor analysis was undertaken. Timings of sessions are affected by factors such as opening times of the laboratory and the willingness of participants to take part in sessions scheduled early morning, thus finding large ranges in morning testing sessions. In addition, training habits of individuals potentially play a role in the range of established diurnal variation in values, due to habitual previous training times and preferences. Further, few authors align testing time-points in accordance to previously established findings related to the lows and highs of the rhythms of core body/local muscle temperatures, and anaerobic performance variables.
Strength and weaknesses
The primary limitation of the present review is associated to several methodological limitations. Considerable differences in methodological and clinical heterogeneity among the 55 studies meant we were unable to conduct a meta-analysis and pool the observed data-sets to evaluate the evidence related to findings in anaerobic power and anaerobic capacity performance (Borenstein et al. Citation2009). Our findings with reference to chronobiological study design perspectives of studies encountered considerable inconsistencies in the methods and scientific rigor of the past research, in addition to the relationship between time-of-day and anaerobic performance. Future studies ought to consider stricter protocols which take into account these factors to reduce external influences on anaerobic performance.
The primary strength of the present systematic review is that it was performed using a structured analysis according to the PRISMA guidelines (Page et al. Citation2021) and is the first to provide an in-depth overview of all the literature considering time-of-day and anaerobic performance. As far as we are aware, this is only the second review providing in-depth analysis relating chronobiological factors and how these factors may influence anaerobic performance. Another strength of this systematic review is the diversity of databases used within the search strategy and the strong method created to incorporate search terms that are specific and important. Importantly, the current review focused solely on the anaerobic performance paradigm and only included studies designed to assess diurnal variation.
Conclusion
The present systematic review confirms that most studies demonstrated that both anaerobic power (2.7 to 13.1%) and anaerobic capacity (1.8–11.7%) are time of day dependent with higher afternoon (16:00 and 19:30 h) values compared to the morning (05:30 to 11:00 h). These diurnal variations in performance are principally explained by higher core body and/or muscle temperature and better muscle contractile properties in the afternoon, although the recent literature would suggest it is more complex than this. Differences in methodology, motivation/arousal, habitual training times and chronotypes could provide an explanation as to why some studies/variables did not display time-of-day variation. Therefore, there is a strong requirement for more rigorous research to be conducted that control factors that specifically relate to investigations related to chronobiology (time-of-day), such as appropriate familiarization of participants with the performance test, randomization, counterbalancing, record of light intensity, control of meals, control of room temperature, control of sleep and fitness level. It is of paramount importance that future studies utilise appropriate testing times, as close to the time-points of the core body temperature minimum and maximum values as possible, whilst taking into account effects of sleep inertia and restriction.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Á L-S, Moreno-Pérez D, Maté-Muñoz JL, Domínguez R, Pallarés JG, Mora-Rodriguez R, Ortega JF. 2017. Circadian rhythm effect on physical tennis performance in trained male players. J Sports Sci. 35(21):2121–2128. doi:https://doi.org/10.1080/02640414.2016.1258481
- Ab Malik Z, Bowden Davies KA, Hall ECR, Barrett J, Pullinger SA, Erskine RM, Shepherd SO, Iqbal Z, Edwards BJ, Burniston JG. 2020. Diurnal differences in human muscle isometric force in vivo are associated with differential phosphorylation of sarcomeric m-band proteins. Proteomes. 8(3):1–22. doi:https://doi.org/10.3390/proteomes8030022
- Abedelmalek S, Chtourou H, Aloui A, Aouichaoui C, Souissi N, Tabka Z. 2013. Effect of time of day and partial sleep deprivation on plasma concentrations of IL-6 during a short-term maximal performance. Eur J Appl Physiol. 113(1):241–248. doi:https://doi.org/10.1007/s00421-012-2432-7
- Aloui A, Chtourou H, Hammouda O, Souissi H, Chaouachi A, Chamari K, Souissi N. 2013. Effects of Ramadan on the diurnal variations of physical performance and perceived exertion in adolescent soccer players. Biol Rhythm Res. 44(6):869–875. doi:https://doi.org/10.1080/09291016.2013.780697
- Aloui K, Abedelmalek S, Boussetta N, Shimi I, Chtourou H, Souissi N. 2017. Opuntia ficus-indica juice supplementation: What role it plays on diurnal variation of short-term maximal exercise? Biol Rhythm Res. 48(2):315–330. doi:https://doi.org/10.1080/09291016.2016.1263000
- Aschoff J. (1965). Circadian rhythms in man (author’s transl). Science. 148:1427–1432. doi:https://doi.org/10.1126/science.148.3676.1427. PMID: 14294139.
- Aschoff J, Wever R. (1980). On reproducibility of circadian rhythms in man (author’s transl). Klin Wochenschr. 58:323–335. doi:https://doi.org/10.1007/bf01477275. PMID: 6993775.
- Asmussen E, Bøje O. 1945. Body temperature and capacity for work. Acta Physiol Scand. 10(1):1–22. doi:https://doi.org/10.1111/j.1748-1716.1945.tb00287.x
- Atkinson G, Reilly T. 1996. Circadian variation in sports performance. Sports Med. 21(4):292–312. doi:https://doi.org/10.2165/00007256-199621040-00005
- Aziz AR, Chia MYH, Low CY, Slater GJ, Png W, Teh KC. 2012. Conducting an Acute Intense Interval Exercise Session During the Ramadan Fasting Month: What Is the Optimal Time of the Day? Chronobiol Int. 29(8):1139–1150. doi:https://doi.org/10.3109/07420528.2012.708375
- Ball D, Burrows C, Sargeant AJ. 1999. Human power output during repeated sprint cycle exercise: The influence of thermal stress. Eur J Appl Physiol. 79:360–366. doi:https://doi.org/10.1007/s004210050521. PMID: 10090637.
- Bambaeichi E, Reilly T, Cable NT, Giacomoni M. 2005. Influence of time of day and partial sleep loss on muscle strength in eumenorrheic females. Ergonomics. 48(11–14):1499–1511. doi:https://doi.org/10.1080/00140130500101437
- Bergh U, Ekblom B. 1979. Influence of muscle temperature on maximal muscle strength and power output in human skeletal muscles. Acta Physiol Scand. 107:33–37. doi:https://doi.org/10.1111/j.1748-1716.1979.tb06439.x. PMID: 525366.
- Bernard T, Giacomoni M, Gavarry O, Seymat M, Falgairette G. 1998. Time-of-day effects in maximal anaerobic leg exercise. Eur J Appl Physiol Occup Physiol. 77(1–2):133–138. doi:https://doi.org/10.1007/s004210050311
- Bishop D, Girard O, Mendez-Villanueva A. 2011. Repeated-sprint ability part II: Recommendations for training. Sports Med. 41(9):741–756. doi:https://doi.org/10.2165/11590560-000000000-00000
- Bishop D, Spencer M. 2004. Determinants of repeated-sprint ability in well-trained team-sport athletes and endurance-trained athletes. J Sports Med Phys Fitness. 44:1–7. PMID: 15181383.
- Belkhir Y, Rekik G, Chtourou H, Souissi N. 2019. Listening to neutral or self-selected motivational music during warm-up to improve short-term maximal performance in soccer players: Effect of time of day. Physiol Behav. 204:168–173. doi:https://doi.org/10.1016/j.physbeh.2019.02.033. PMID: 30817975.
- Belkhir Y, Rekik G, Chtourou H, Souissi N. 2020. Effect of listening to synchronous versus motivational music during warm-up on the diurnal variation of short-term maximal performance and subjective experiences. Chronobiol Int. 37(11):1611–1620. doi:https://doi.org/10.1080/07420528.2020.1797764. PMID: 32741226.
- Borenstein M, Hedges LV, Higgins JP, Rothstein HR. 2009. Introduction to meta-analysis. Cornwall (UK): John Wiley & Sons.
- Bougard C, Bessot N, Moussay S, Sesboue B, Gauthier A. 2009. Effects of waking time and breakfast intake prior to evaluation of physical performance in the early morning. Chronobiol Int. 26:307–323. doi:https://doi.org/10.1080/07420520902774532. PMID: 19212843.
- Bougard C, Davenne D. 2012. Effects of sleep deprivation and time-of-day on selected physical abilities in off-road motorcycle riders. Eur J Appl Physiol. 112(1):59–67. doi:https://doi.org/10.1007/s00421-011-1948-6
- Boussetta N, Abedelmalek S, Aloui K, Souissi N, Souissi N. 2017. The effect of strength training by electrostimulation at a specific time of day on immune response and anaerobic performances during short-term maximal exercise, Biological Rhythm Research. 48:1, 157–174, doi: https://doi.org/10.1080/09291016.2016.1245379
- Castaingts V, Martin A, Van Hoecke J, Pérot C. 2004. Neuromuscular efficiency of the triceps surae in induced and voluntary contractions: Morning and evening evaluations. Chronobiol Int. 21(4–5):631–643. doi:https://doi.org/10.1081/CBI-120039207
- Chaâri N, Frikha M, Elghoul Y, Mezghanni N, Masmoudi L, Souissi N. 2014a. Warm-up durations and time-of-day impacts on rate of perceived exertion after short-term maximal performance. Biological Rhythm Research. 45(2): 257–265. doi: https://doi.org/10.1080/09291016.2013.805910
- Chaâri N, Frikha M, Mezghanni M, Ayadi J, Chaouachi A, Souissi N. 2015 Does post-warm-up rest interval affect the diurnal variation of 30-s Wingate cycle ergometry? Biological Rhythm Research. 46(6):949–963, doi: https://doi.org/10.1080/09291016.2015.1073477
- Chaâri N, Frikha M, Mezghanni N, Masmoudi L, Souissi N. 2014b. Time-of-day and warm-up durations effects on thermoregulation and anaerobic performance in moderate conditions. Biological Rhythm Research. 45(4): 495–508. doi: https://doi.org/10.1080/09291016.2013.851904
- Chtourou H, Aloui A, Hammouda O, Chaouachi A, Chamari K, Souissi N. 2013. Effect of static and dynamic stretching on the diurnal variations of jump performance in soccer players. PLoS One. 8(8):e70534. doi: https://doi.org/10.1371/journal.pone.0070534. PMID: 23940589.
- Chtourou H, Chaouachi A, Driss T, Dogui M, Behm DG, Chamari K, Souissi N. 2012a. The effect of training at the same time of day and tapering period on the diurnal variation of short exercise performances. J Strength Cond Res. 26(3):697–708. doi:https://doi.org/10.1519/JSC.0b013e3182281c87
- Chtourou H, Driss T, Souissi S, Gam A, Chaouachi A, Souissi N. 2012b. The effect of strength training at the same time of the day on the diurnal fluctuations of muscular anaerobic performances. J Strength Cond Res. 26(1):217–225. doi:https://doi.org/10.1519/JSC.0b013e31821d5e8d
- Chtourou H, Driss T, Souissi S, Gam A, Chaouachi A, Souissi N. 2012c. The effect of strength training at the same time of the day on the diurnal fluctuations of muscular anaerobic performances. J Strength Cond Res. 26(1):217–25. doi: https://doi.org/10.1519/JSC.0b013e31821d5e8d. PMID: 21993020.
- Chtourou H, Engel FA, Fakhfakh H, Fakhfakh H, Hammouda O, Ammar A, Trabelsi K, Souissi N, Sperlich B. 2018. Diurnal variation of short-term repetitive maximal performance and psychological variables in elite judo athletes. Front Physiol. 9:1499. doi:https://doi.org/10.3389/fphys.2018.01499. PMID: 30416454.
- Chtourou H, Zarrouk N, Chaouachi A, Dogui M, Behm DG, Chamari K, Hug F, Souissi N. 2011. Diurnal variation in Wingate-test performance and associated electromyographic parameters. Chronobiol Int. 28(8):706–13. doi: https://doi.org/10.3109/07420528.2011.596295. PMID: 21793694.
- Coldwells A, Atkinson G, Reilly T. 1994. Sources of variation in back and leg dynamometry. Ergonomics. 37:79–86. doi:https://doi.org/10.1080/00140139408963625. PMID: 8112285.
- Davenne D, Gauthier A 1997. Location of the mechanisms involved in the circadian rhythm of muscle strength. Biological clocks: Mechanism and application, Y Touitou. Excerpta Medica, Amsterdam; 158–161
- de Salles Painelli V, Saunders B, Sale C, Harris RC, Solis MY, Roschel H, Gualano B, Artioli GG, Lancha AH Jr. 2014. Influence of training status on high-intensity intermittent performance in response to β-alanine supplementation. Amino Acids. 46(5):1207–1215. doi:https://doi.org/10.1007/s00726-014-1678-2
- Dergaa I, Fessi MS, Chaabane M, Souissi N, Hammouda O. 2019. The effects of lunar cycle on the diurnal variations of short-term maximal performance, mood state, and perceived exertion. Chronobiol Int. 36(9):1249–1257. doi:https://doi.org/10.1080/07420528.2019.1637346
- Downs SH, Black N. 1998. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 52(6):377–384. doi:https://doi.org/10.1136/jech.52.6.377
- Driss T, Vandewalle H, Le Chevalier JM, Monod H. 2002. Force-velocity relationship on a cycle ergometer and knee-extensor strength indices. Can J Appl Physiol. 27(3):250–262. doi:https://doi.org/10.1139/h02-015
- Drust B, Waterhouse J, Atkinson G, Edwards B, Reilly T. 2005. Circadian rhythms in sports performance - An update. Chronobiol Int. 22(1):21–44. doi:https://doi.org/10.1081/CBI-200041039
- Dunlap JC, Loros JJ, DeCoursey P. 2004. Chronobiology: Biological timekeeping. Sunderland (MA): Sinauer Associates.
- Edwards B, Waterhouse J, Reilly T, Atkinson GA. 2002. Comparison of the suitabilities of rectal, gut, and insulated axilla temperatures for measurement of the circadian rhythm of core temperature in field studies. Chronobiol Int. 19(3):579–597. doi:https://doi.org/10.1081/cbi-120004227
- Edwards BJ, Waterhouse J. 2009. Effects of one night of partial sleep deprivation upon diurnal rhythms of accuracy and consistency in throwing darts. Chronobiol Int. 26:756–768. doi:https://doi.org/10.1080/07420520902929037. PMID: 23281719.
- Edwards BJ, Atkinson G, Waterhouse J, Reilly T, Godfrey R, Budgett R. 2000. Use of melatonin in recovery from jet-lag following an eastward flight across 10 time-zones. Ergonomics. 43(10):1501–1513. doi:https://doi.org/10.1080/001401300750003934
- Edwards BJ, Pullinger SA, Kerry JW, Robinson WR, Reilly TP, Robertson CM, Waterhouse JM. 2013. Does raising morning rectal temperature to evening levels offset the diurnal variation in muscle force production? Chronobiol Int. 30(4):486–501. doi:https://doi.org/10.3109/07420528.2012.741174
- Falgairette G, Billaut F, Ramdani S. 2003. Recovery duration and maximal anaerobic power in the daytime. Can J Appl Physiol. 28(2):213–224. doi:https://doi.org/10.1139/h03-017
- Frikha M, Chaâri N, Souissi N. 2020. Warm-up durations in a hot-dry climate affect thermoregulation, mean power-output and fatigue, but not peak power in specific soccer repeated-sprint ability. BMC Sports Science, Medicine and Rehabilitation. 46(4):497–509. doi: https://doi.org/10.1080/09291016.2015.1020710
- Gholamhasan J, Sajad A, Mehdi RG, Javad MS. 2013. The effect of exercise in the morning and the evening times on aerobic and anaerobic power of the inactive subjects. World Appl Sci J. 22(8):1146–1150. doi:https://doi.org/10.5829/idosi.wasj.2013.22.08.2558
- Giacomoni M, Billaut F, Falgairette G. 2006. Effects of the time of day on repeated all-out cycle performance and short-term recovery patterns. Int J Sports Med. 27(6):468–474. doi:https://doi.org/10.1055/s-2005-865822
- Giacomoni M, Edwards B, Bambaeichi E. 2005. Gender differences in the circadian variations in muscle strength assessed with and without superimposed electrical twitches. Ergonomics. 48(11–14):1473–1487. doi:https://doi.org/10.1080/00140130500101452
- Guette M, Gondin J, Martin A. 2005. Time-of-day effect on the torque and neuromuscular properties of dominant and non-dominant quadriceps femoris. Chronobiol Int. 22:541–558. doi:https://doi.org/10.1081/CBI-200062407. PMID: 16076653.
- Häkkinen K. 1989. Neuromuscular and hormonal adaptations during strength and power training. A review. J Sports Med Phys Fitness. 29:9–26. PMID: 2671501.
- Heishman AD, Curtis MA, Saliba EN, Hornett RJ, Malin SK, Weltman AL. 2017. Comparing performance during morning vs. afternoon training sessions in intercollegiate basketball players. J Strength Cond Res. 31(6):1557–1562. doi:https://doi.org/10.1519/JSC.0000000000001882
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch V (editors). 2020. Cochrane handbook for systematic reviews of interventions version 6.2 (updated February 2021). Cochrane, 2021. www.training.cochrane:handbook.
- Hill DW, Chtourou H. 2020. The effect of time of day and chronotype on the relationships between mood state and performance in a Wingate test. Chronobiol Int. 37(11):1599–1610. doi:https://doi.org/10.1080/07420528.2020.1786394
- Hill DW, Smith JC. 1991. Effect of time of day on the relationship between mood state, anaerobic power, and capacity. Percept Mot Skills. 72(1):83–87. doi:https://doi.org/10.2466/PMS.72.1.83-87
- Hopker JG, Coleman DA, Gregson HC, Jobson SA, Von der Haar T, Wiles J, Passfield L. 1985. The influence of training status, age, and muscle fiber type on cycling efficiency and endurance performance. J Appl Physiol. 115(5):723–729. doi:https://doi.org/10.1152/japplphysiol.00361.2013
- Hopker JG, Coleman DA, Gregson HC, Jobson SA, Von der Haar T, Wiles J, Passfield L. 2013. The influence of training status, age, and muscle fiber type on cycling efficiency and endurance performance. J Appl Physiol. 115(5):723–9. doi:https://doi.org/10.1152/japplphysiol.00361.2013.
- Horne JA, Ostberg O. 1976. A self assessment questionnaire to determine morningness eveningness in human circadian rhythms. Int J Chronobiol. 4(2):97–110. PMID: 1027738.
- Kin-Isler A. 2006. Time-of-day effects in maximal anaerobic performance and blood lactate concentration during and after a supramaximal exercise. Isokinet Exerc Sci. 14(4):335–340. doi:https://doi.org/10.3233/ies-2006-0246
- Kirschen GW, Jones JJ, Hale L. 2020. The impact of sleep duration on performance among competitive athletes: A systematic literature review. Clin J Sport Med. 30(5):503–512. doi:https://doi.org/10.1097/JSM.0000000000000622
- Lericollais R, Gauthier A, Bessot N, Sesboüé B, Davenne D. 2009. Time-of-day effects on fatigue during a sustained anaerobic test in well-trained cyclists. Chronobiol Int. 26(8):1622–35. doi: https://doi.org/10.3109/07420520903534492. PMID: 20030545.
- Lericollais R, Gauthier A, Bessot N, Davenne D. 2011. Diurnal evolution of cycling biomechanical parameters during a 60-s Wingate test. Scand J Med Sci Sports. 21(6):e106–14. doi: https://doi.org/10.1111/j.1600-0838.2010.01172.x. PMID: 20807387.
- Lopes-Silva JP, Santos JFDS, Franchini E. 2019. Can caffeine supplementation reverse the effect of time of day on repeated-sprint exercise performance? Appl Physiol Nutr Metab. 44: 187–193. doi:https://doi.org/10.1139/apnm-2018-0373. PMID: 30058345.
- Martin A, Carpentier A, Guissard N, Van Hoecke J, Duchateau J. 1999. Effect of time of day on force variation in a human muscle. Muscle Nerve. 22(10):1380–1387. doi:https://doi.org/10.1002/(sici)1097-4598(199910)22:10<1380::aid-mus7>3.0.co;2-u
- Meignié A, Duclos M, Carling C, Orhant E, Provost P, Toussaint JF, Antero J. 2021. The effects of menstrual cycle phase on elite athlete performance: A critical and systematic review. Front Physiol. 12:654585. doi:https://doi.org/10.3389/fphys.2021.654585
- Melhim AF. 1993. Investigation of circadian rhythms in peak power and mean power of female physical education students. Int J Sports Med. 14(6):303–306. doi:https://doi.org/10.1055/s-2007-1021182
- Nikolaidis S, Kosmidis I, Sougioultzis M, Kabasakalis A, Mougios V. 2018. Diurnal variation and reliability of the urine lactate concentration after maximal exercise. Chronobiol Int. 35(1):24–34. doi: https://doi.org/10.1080/07420528.2017.1380037. PMID: 29172728.
- Onambele-Pearson NL, Pearson SJ. 2007. Time-of-day effect on patella tendon stiffness alters vastus lateralis fascicle length but not the quadriceps force-angle relationship. J Biomech. 40(5):1031–7. doi: https://doi.org/10.1016/j.jbiomech.2006.04.001. PMID: 16828102.
- Otani H, Kaya M, Tamaki A, Goto H, Goto T, Shirato M. 2018. Diurnal effects of prior heat stress exposure on sprint and endurance exercise capacity in the heat. Chronobiol Int. 35(7):982–995. doi: https://doi.org/10.1080/07420528.2018.1448855. PMID: 29561175.
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM. 2021. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLoS Med. 18(3):e1003583. doi:https://doi.org/10.1371/journal.pmed.1003583
- Pavlović L, Stojiljković N, Aksović N, Stojanović E, Valdevit Z, Scanlan AT, Milanović Z. 2018. Diurnal variations in physical performance: Are there morning-to-evening differences in elite male handball players? J Hum Kinet. 63(1):117–126. doi:https://doi.org/10.2478/hukin-2018-0012
- Pearson SJ, Cobbold M, Harridge SD. 2004. Power output of the lower limb during variable inertial loading: A comparison between methods using single and repeated contractions. Eur J Appl Physiol. 92(1–2):176–181. doi:https://doi.org/10.1007/s00421-004-1046-0
- Pearson SJ, Onambele GN. 2005. Acute changes in knee-extensors torque, fiber pennation, and tendon characteristics. Chronobiol Int. 22(6):1013–1027. doi:https://doi.org/10.1080/07420520500397900
- Pullinger S, Robertson CM, Oakley AJ, Hobbs R, Hughes M, Burniston JG, Edwards BJ. 2019a. Effects of an active warm-up on variation in bench press and back squat (upper and lower body measures). Chronobiol Int. 36(3):392–406. doi:https://doi.org/10.1080/07420528.2018.1552596
- Pullinger SA, Brocklehurst EL, Iveson RP, Burniston JG, Doran DA, Waterhouse JM, Edwards BJ. 2014. Is there a diurnal variation in repeated sprint ability on a non-motorised treadmill? Chronobiol Int. 31(3):421–432. doi:https://doi.org/10.3109/07420528.2013.865643
- Pullinger SA, Cocking S, Robertson CM, Tod D, Doran DA, Burniston JG, Varamenti E, Edwards BJ. 2019b. Time-of-day variation on performance measures in repeated-sprint tests: A systematic review. Chronobiol Int. 37(4):451–468. doi:https://doi.org/10.1080/07420528.2019.1703732
- Pullinger SA, Oksa J, Brocklehurst EL, Iveson RP, Newlove A, Burniston JG, Doran DA, Waterhouse JM, Edwards BJ. 2018a. Controlling rectal and muscle temperatures: Can we offset diurnal variation in repeated sprint performance? Chronobiol Int. 35(7):959–968. doi:https://doi.org/10.1080/07420528.2018.1444626
- Pullinger SA, Oksa J, Clark LF, Guyatt JWF, Newlove A, Burniston JG, Doran DA, Waterhouse JM, Edwards BJ. 2018b. Diurnal variation in repeated sprint performance cannot be offset when rectal and muscle temperatures are at optimal levels (38.5°C). Chronobiol Int. 35(8):1054–1065. doi:https://doi.org/10.1080/07420528.2018.1454938
- Racinais S. 2010. Different effects of heat exposure upon exercise performance in the morning and afternoon. Scand J Med Sci Sport. 20:80–89. doi:https://doi.org/10.1111/j.1600-0838.2010.01212.x. PMID: 21029194.
- Racinais S, Blonc S, Hue O. 2005. Effects of active warm-up and diurnal increase in temperature on muscular power. Med Sci Sports Exerc. 37(12):2134–2139. doi:https://doi.org/10.1249/01.mss.0000179099.81706.11
- Racinais S, Hue O, Blonc S. 2004. Time-of-day effects on anaerobic muscular power in a moderately warm environment. Chronobiol Int. 21(3):485–495. doi:https://doi.org/10.1081/CBI-120038632
- Racinais S, Blonc S, Hue O. 2005a. Effects of active warm-up and diurnal increase in temperature on muscular power. Med Sci Sports Exerc. 37(12):2134–9. doi: https://doi.org/10.1249/01.mss.0000179099.81706.11. PMID: 16331141.
- Racinais S, Connes P, Bishop D, Blonc S, Hue O. 2005b. Morning versus evening power output and repeated-sprint ability. Chronobiol Int. 22: 1029–1039. doi:https://doi.org/10.1080/07420520500397918. PMID: 16393706.
- Racinais S, Perrey S, Denis R, Bishop D. 2010. Maximal power, but not fatigability, is greater during repeated sprints performed in the afternoon. Chronobiol Int. 7:855–864. doi:https://doi.org/10.3109/07420521003668412. PMID: 20560715.
- Racinais S, Oksa J. 2010. Temperature and neuromuscular function. Scand J Med Sci Sports. 20:1–18. doi:https://doi.org/10.1111/j.1600-0838.2010.01204.x
- Reilly T, Atkinson G, Waterhouse J. 1997. Biological rhythms and exercise. Oxford: Oxford University Press.
- Reilly T, Edwards B. 2007. Altered sleep–wake cycles and physical performance in athletes. Physiol Behav. 90(2–3):274–284. doi:https://doi.org/10.1016/j.physbeh.2006.09.017
- Reilly T, Waterhouse J. 2009a. Circadian aspects of body temperature regulation in exercise. J Therm Biol. 34(4):161–170. doi:https://doi.org/10.1016/j.jtherbio.2009.01.005
- Reilly T, Waterhouse J. 2009b. Sports performance: Is there evidence that the body clock plays a role? Eur J Appl Physiol. 106(3):321–332. doi:https://doi.org/10.1007/s00421-009-1066-x
- Robinson, WR. Pullinger SA, Kerry JW, Giacomoni M, Robertson CM, Burniston JG, Waterhouse JM, Edwards BJ. 2013. Does lowering evening rectal temperature to morning levels offset the diurnal variation in muscle force production? Chronobiology International. 30:998–1010. doi:https://doi.org/10.3109/07420528.2013.793197
- Robinson WR, Pullinger SA, Kerry JW, Giacomoni M, Robertson CM, Burniston JG, Waterhouse JM, Edwards BJ. 2013. Does lowering evening rectal temperature to morning levels offset the diurnal variation in muscle force production? Chronobiol Int. 30(8):998–1010. doi:https://doi.org/10.3109/07420528.2013.793197
- Roden LC, Rudner TD, Rae DE. 2017. Impact of chronotype on athletic performance: Current perspectives. Physiol Ther. 7:1–6. doi:https://doi.org/10.2147/CPT.S99804
- Souissi M, Abedelmalek S, Chtourou H, Boussita A, Hakim A, Sahnoun Z. 2013. Effects of time-of-day and caffeine ingestion on mood states, simple reaction time, and short-term maximal performance in elite judoists. Biol Rhythm Res. 44(6):897–907. doi:https://doi.org/10.1080/09291016.2013.780700
- Souissi M, Abedelmalek S, Chtourou H, Boussita A, Hakim A, Sahnoun Z. 2013a. Effects of time-of-day and caffeine ingestion on mood states, simple reaction time, and short-term maximal performance in elite judoists, Biological Rhythm Research. 44(6):897–907, doi: https://doi.org/10.1080/09291016.2013.780700
- Souissi N, Chtourou H, Aloui A, Hammouda O, Dogui M, Chaouachi A, Chamari K. 2013c. Effects of time-of-day and partial sleep deprivation on short-term maximal performances of judo competitors. J Strength Cond Res. 27(9):2473-80. doi: https://doi.org/10.1519/JSC.0b013e31827f4792. PMID: 23974210.
- Souissi N, Bessot N, Chamari K, Gauthier A, Sesboüé B, Davenne D. 2007. Effect of time of day on aerobic contribution to the 30-s Wingate test performance. Chronobiol Int. 24(4):739–48. doi: https://doi.org/10.1080/07420520701535811. PMID: 17701684.
- Souissi M, Chtourou H, Hdidar R, Azaeiz R, Dogui M, Souissi N, Tabka Z. 2013. Effects of three types of chronobiotics on anaerobic performances and their diurnal variations. Biological Rhythm Research. 44:(2): 245-254, doi: https://doi.org/10.1080/09291016.2012.667981
- Souissi N, Bessot N, Chamari K, Gauthier A, Sesboüé B, Davenne D. 2007a. Effect of time of day on aerobic contribution to the 30-s Wingate test performance. Chronobiol Int.24 (4):739–48. doi: https://doi.org/10.1080/07420520701535811. PMID: 17701684.
- Souissi N, Driss T, Chamari K, Vandewalle H, Davenne D, Gam A, Fillard JR, Jousselin E. 2010. Diurnal variation in wingate test performances: Influence of active warm-up. Chronobiol Int. 27(3):640–652. doi:https://doi.org/10.3109/07420528.2010.483157
- Souissi N, Gauthier A, Sesboue B, Larue J, Davenne D. 2004. Circadian rhythms in two types of anaerobic cycle leg exercise: Force-velocity and 30-s Wingate tests. Int J Sports Med. 25(1):14–19. doi:https://doi.org/10.1055/s-2003-45226
- Souissi N, Gauthier A, Sesboüé B, Larue J, Davenne D. 2002. Effects of regular training at the same time of day on diurnal fluctuations in muscular performance. J Sports Sci. 20(11):929–937. doi:https://doi.org/10.1080/026404102320761813
- Souissi N, Sesboue B, Gauthier A, Larue J, Davenne D. 2003. Effects of one night’s sleep deprivation on anaerobic performance the following day. Eur J Appl Physiol. 89(3–4):359–366. doi:https://doi.org/10.1007/s00421-003-0793-7
- Souissi N, Souissi H, Sahli S, Tabka Z, Dogui M, Ati J, Davenne D. 2007b. Effect of Ramadan on the diurnal variation in short-term high power output. Chronobiol Int. 24(5):991–1007. doi: https://doi.org/10.1080/07420520701661914. PMID: 17994351.
- Souissi M, Souissi Y, Mseddi E, Sahnoun Z. 2019a. The effects of caffeine on the diurnal variation of the reaction time and short-term maximal performance after one night of sleep deprivation. Biol Rhythm Res. 20:1544–1559. doi:https://doi.org/10.1080/09291016.2019.1664794
- Souissi N, Souissi M, Souissi H, Chamari K, Tabka Z, Dogui M, Davenne D. 2008. Effect of time of day and partial sleep deprivation on short-term, high-power output. Chronobiol Int. 25(6):1062–1076. doi:https://doi.org/10.1080/07420520802551568
- Souissi Y, Souissi M, Chtourou H. 2019b. Effects of caffeine ingestion on the diurnal variation of cognitive and repeated high-intensity performances. Pharmacol Biochem Behav. 177:69–74. doi:https://doi.org/10.1016/j.pbb.2019.01.001. PMID: 30611752.
- Tamm AS, Lagerquist O, Ley AL, Collins DF. 2009. Chronotype influences diurnal variations in the excitability of the human motor cortex and the ability to generate torque during a maximum voluntary contraction. J Biol Rhythms. 24(3):211–224. doi:https://doi.org/10.1177/0748730409334135
- Unver S, Atan T. 2015. Investigation of the changes in performance measurements based on circadian rhythm. Anthropologist. 19(2):423–430. doi:https://doi.org/10.1080/09720073.2015.11891676
- Vitale JA, Weydahl A. 2017. Chronotype, physical activity, and sport performance: A systematic review. Sports Med. 47(9):1859–1868. doi:https://doi.org/10.1007/s40279-017-0741-z
- Walsh NP, Halson SL, Sargent C, Roach GD, Nédélec M, Gupta L, Leeder J, Fullagar HH, Coutts AJ, Edwards BJ, et al. 2020. Sleep and the athlete: Narrative review and 2021 expert consensus recommendations. Br J Sports Med. 55:356–368. doi:https://doi.org/10.1136/bjsports-2020-102025
- Walsh NP, Halson SL, Sargent C, Roach GD, Nédélec M, Gupta L, Leeder J, Fullagar HH, Coutts AJ, Edwards BJ, et al. 2021. Sleep and the athlete: narrative review and 2021 expert consensus recommendations. Br J Sports Med. 55:356–368. doi: https://doi.org/10.1136/bjsports-2020-102025.
- Waterhouse J, Drust B, Weinert D, Edwards B, Gregson W, Atkinson G, Kao S, Aizawa S, Reilly T. 2005. The circadian rhythm of core temperature: Origin and some implications for exercise performance. Chronobiol Int. 22(2):207–225. doi:https://doi.org/10.1081/CBI-200053477
- Waterhouse J, Folkard S, Van Dongen H, Minors D, Owens D, Kerkhof G, Weinert D, Nevill A, Macdonald I, Sytnik N. et al. (2011). Temperature profiles, and the effect of sleep on them, in relation to morningness-eveningness in healthy female subjects. Chronobiol Int. 18:227–247. doi:https://doi.org/10.1081/cbi-100103188. PMID: 11379664.
- Young WB, Dawson B, Henry GJ. 2015. Agility and change-of-direction speed are independent skills: Implications for training for agility in invasion sports. Int J Sports Sci Coach. 10(1):159–169. doi:https://doi.org/10.1260/1747-9541.10.1.159
- Zarrouk N, Chtourou H, Rebai H, Hammouda O, Souissi N, Dogui M, Hug F. 2012a. Time of day effects on repeated sprint ability. Int J Sports Med. 33(12):975–80. doi:https://doi.org/10.1055/s-0032-1312626. PMID: 22782387.
- Zarrouk N, Chtourou H, Zarrouk I, Rebai H, Tabka Z, Dogui M. 2012b. Diurnal Variations of swim performances: effect of water temperature. Science & Sports. 27:101–106. doi:https://doi.org/10.1016/j.scispo.2011.07.002
- Zhang X, Dube TJ, Esser KA. 2009. The role of clock genes in cardiometabolic disease: Working around the clock: Circadian rhythms and skeletal muscle. J. Appl Physiol. 107(5):1647–1654. doi:https://doi.org/10.1152/japplphysiol.00725.2009
APPENDIX
From: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(7): e1000097. doi:10.1371/journal.pmed1000097
For more information, visit: www.prisma-statement.org.
Appendix 1.
Literature search strategy example for PubMed (MEDLINE)
Search syntax:
(“time of day” OR “time-of-day” OR “daily rhythm” OR “daily variation” OR “daily fluctuation” OR “diurnal rhythm” OR “diurnal variation” OR “diurnal fluctuation” OR “circadian rhythm” OR “circadian variation” OR “circadian fluctuation”)
AND
(“anaerobic power” OR “anaerobic capacity” OR “force-velocity test” OR “vertical jump test” OR “Countermovement Jump Test” OR “standing long jump test” OR “Margaria Kalamen Power Test” OR “Ball throw test” OR “staircase test” OR “stair climb test” OR “Wingate test” OR “critical power test” OR “ Cycle ergometer test” OR “sprint test” OR “Running Based Anaerobic Sprint Test” OR “Maximal Anaerobic Running” Test OR “Cunningham Faulkner test” OR “Repeated sprint test” OR “Repeated sprint ability test” OR “Swim Test” OR “Row ergometer test” OR “Agility Test”)
Filters: Publication date to 2021/04/30
’
Records identified and screened for PubMed (MEDLINE):
N = 104
1. Abedelmalek S, Chtourou H, Aloui A, Aouichaoui C, Souissi N, Tabka Z. 2013. Effect of time of day and partial sleep deprivation on plasma concentrations of IL-6 during a short-term maximal performance. Eur J Appl Physiol. 113(1):241–248. doi: 10.1007/s00421-012-2432-7. PMID: 22677919.
2. Adamah-Biassi EB, Hudson RL, Dubocovich ML. 2014. Genetic deletion of MT1 melatonin receptors alters spontaneous behavioral rhythms in male and female C57BL/6 mice. Horm Behav. 66(4):619–27. doi: 10.1016/j.yhbeh.2014.08.012. PMID: 25200199.
3. Anyan J, Verwey M, Amir S. 2017. Individual differences in circadian locomotor parameters correlate with anxiety- and depression-like behavior. PLoS One. 12(8):e0181375. doi: 10.1371/journal.pone.0181375. PMID: 28763478.
4. Ashkenazy T, Einat H, Kronfeld-Schor N. 2009. Effects of bright light treatment on depression- and anxiety-like behaviors of diurnal rodents maintained on a short daylight schedule. Behav Brain Res. 201(2):343–6. doi: 10.1016/j.bbr.2009.03.005. PMID: 19428655.
5. Ashkenazy-Frolinger T, Einat H, Kronfeld-Schor N. 2015. Diurnal rodents as an advantageous model for affective disorders: novel data from diurnal degu (Octodon degus). J Neural Transm. 122(1):35–45. doi: 10.1007/s00702-013-1137-3. PMID: 24352409.
6. Aubrecht TG, Weil ZM, Magalang UJ, Nelson RJ. 2013. Dim light at night interacts with intermittent hypoxia to alter cognitive and affective responses. Am J Physiol Regul Integr Comp Physiol. 305(1):R78-86. doi: 10.1152/ajpregu.00100.2013. PMID: 23657638.
7. Bächli H, Steiner MA, Habersetzer U, Wotjak CT. 2008. Increased water temperature renders single-housed C57BL/6 J mice susceptible to antidepressant treatment in the forced swim test. Behav Brain Res. 187(1):67–71. doi: 10.1016/j.bbr.2007.08.029. PMID: 17923159.
8. Baklouti H, Chtourou H, Aloui A, Chaouachi A, Souissi N. 2015. Effect of active warm-up duration on morning short-term maximal performance during Ramadan. Libyan J Med. 10(1):26229. doi: 10.3402/ljm.v10.26229. PMID: 25676856.
9. Bardi M, Hampton JE, Lambert KG. 2010. Fecal Dehydroepiandrosterone (DHEA) Immunoreactivity as a Noninvasive Index of Circulating DHEA Activity in Young Male Laboratory Rats. Comp Med. 60(6):455–60. PMID: 21262132.
10. Bedrosian TA, Weil ZM, Nelson RJ. 2012. Chronic citalopram treatment ameliorates depressive behavior associated with light at night. Behav Neurosci. 126(5):654–8. doi: 10.1037/a0029699. PMID: 22889310.
11. Beĭer EV, Arushanian EB, Titenok AL, Alferov V V. 2003. The dorsal hippocampus injury influences chronobiological effects of depressants and antidepressants in rats. Eksp Klin Farmakol. 66(3):9–12. PMID: 12924224.
12. Ben-Hamo M, Tal K, Paz-Cohen R, Kronfeld-Schor N, Einat H. 2016. Differential effects of photoperiod length on depression- and anxiety-like behavior in female and male diurnal spiny mice. Physiol Behav. 165:1–6. doi: 10.1016/j.physbeh.2016.06.030. PMID: 27343805.
13. Bernard T, Giacomoni M, Gavarry O, Seymat M, Falgairette G. 1998. Time-of-day effects in maximal anaerobic leg exercise. Eur J Appl Physiol Occup Physiol. 77(1–2):133–8. doi: 10.1007/s004210050311. PMID: 9459533.
14. Bilski J, Jaworek J, Pokorski J, Nitecki J, Nitecka E, Pokorska J 2016. Effects of time of day and the wingate test on appetite perceptions, food intake and plasma levels of adipokines. J Physiol Pharmacol. 67(5):667–76. PMID: 28011947.
15. Bilu C, Einat H, Tal-Krivisky K, Mizrahi J, Vishnevskia-Dai V, Agam G 2019. Red white and blue – bright light effects in a diurnal rodent model for seasonal affective disorder. Chronobiol Int. 36(7):919–26. doi: 10.1080/07420528.2019.1595638. PMID: 30983429.
16. Bougard C, Bessot N, Moussay S, Sesbouee B, Gauthier A. 2009. Effects of Waking Time and Breakfast Intake Prior to Evaluation of Physical Performance in the Early Morning. Chronobiol Int. 26(2):307–23. doi: 10.1080/07420520902774532. PMID: 19212843.
17. Carnevali L, Vacondio F, Rossi S, Callegari S, Macchi E, Spadoni G 2015. Antidepressant-like activity and cardioprotective effects of fatty acid amide hydrolase inhibitor URB694 in socially stressed Wistar Kyoto rats. Eur Neuropsychopharmacol. 25(11):2157–69. doi: 10.1016/j.euroneuro.2015.07.015. PMID: 26391492.
18. Chtourou H, Chaouachi A, Hammouda O, Chamari K, Souissi N. 2012. Listening to Music Affects Diurnal Variation in Muscle Power Output. Int J Sports Med. 33(1):43–7. doi: 10.1055/s-0031-1284398. PMID: 22134883.
19. Chtourou H, Hammouda O, Chaouachi A, Chamari K, Souissi N. 2012. The Effect of Time-of-Day and Ramadan Fasting on Anaerobic Performances. Int J Sports Med. 33(2):142–7. doi: 10.1055/s-0031-1286251. PMID: 22318530.
20. Chtourou H, Hammouda O, Souissi H, Chamari K, Chaouachi A, Souissi N. Diurnal variations in physical performances related to football in young soccer players. Asian J Sports Med. 3(3):139–44. doi: 10.5812/asjsm.34604. PMID: 23012632.
21. Chtourou H, Zarrouk N, Chaouachi A, Dogui M, Behm DG, Chamari K 2011. Diurnal Variation in Wingate-Test Performance and Associated Electromyographic Parameters. Chronobiol Int. 28(8):706–13. doi: 10.3109/07420528.2011.596295. PMID: 21793694.
22. Cissé YM, Russart KLG, Nelson RJ. 2017. Depressive-like behavior is elevated among offspring of parents exposed to dim light at night prior to mating. Psychoneuroendocrinology. 83:182–6. doi: 10.1016/j.psyneuen.2017.06.004. PMID: 28644985.
23. Cleary-Gaffney M, Coogan AN. 2018. Limited evidence for affective and diurnal rhythm responses to dim light-at-night in male and female C57Bl/6 mice. Physiol Behav. 189:78–85. doi: 10.1016/j.physbeh.2018.03.010. PMID: 29540316.
24. Crossland RF, Balasa A, Ramakrishnan R, Mahadevan SK, Fiorotto ML, den Veyver IB. 2017. Chronic maternal low-protein diet in mice affects anxiety, night-time energy expenditure and sleep patterns, but not circadian rhythm in male offspring. PLoS One. 12(1). doi: 10.1371/journal.pone.0170127. PMID: 28099477.
25. De Bundel D, Gangarossa G, Biever A, Bonnefont X, Valjent E. 2013. Cognitive dysfunction, elevated anxiety, and reduced cocaine response in circadian clock-deficient cryptochrome knockout mice. Front Behav Neurosci. 7. doi: 10.3389/fnbeh.2013.00152. PMID: 24187535.
26. Deats SP, Adidharma W, Lonstein JS, Yan L. 2014. Attenuated orexinergic signaling underlies depression-like responses induced by daytime light deficiency. Neuroscience. 272:252–60. doi: 10.1016/j.neuroscience.2014.04.069. PMID: 24813431.
27. Dergaa I, Fessi MS, Chaabane M, Souissi N, Hammouda O. 2019. The effects of lunar cycle on the diurnal variations of short-term maximal performance, mood state, and perceived exertion. Chronobiol Int. 36(9):1249–57. doi: 10.1080/07420528.2019.1637346. PMID: 31368366.
28. Duangdao DM, Clark SD, Okamura N, Reinscheid RK. 2009. Behavioral phenotyping of neuropeptide S receptor knockout mice. Behav Brain Res. 205(1):1–9. doi: 10.1016/j.bbr.2009.07.024. PMID: 19646487.
29. Easton A, Arbuzova J, Turek FW. 2003. The circadian Clock mutation increases exploratory activity and escape-seeking behavior. Genes Brain Behav. 2(1):11–9. doi: 10.1034/j.1601–183x.2003.00002.x. PMID: 12882315.
30. Einat H, Kronfeld-Schor N, Eilam D. 2006. Sand rats see the light: short photoperiod induces a depression-like response in a diurnal rodent. Behav Brain Res. 173(1):153–7. doi: 10.1016/j.bbr.2006.06.006. PMID: 16831474.
31. El Yacoubi M, Bouali S, Popa D, Naudon L, Leroux-Nicollet I, Hamon M 2003. Behavioral, neurochemical, and electrophysiological characterization of a genetic mouse model of depression. Proc Natl Acad Sci U S A. 100(10):6227–32. doi: 10.1073/pnas.1034823100. PMID: 12732720.
32. Eliakim A, Brasel JA, Cooper DM. 1999. GH response to exercise: assessment of the pituitary refractory period, and relationship with circulating components of the GH-IGF-I axis in adolescent females. J Pediatr Endocrinol Metab. 12(1):47–55. doi: 10.1515/jpem.1999.12.1.47. PMID: 10392348.
33. Fernandez JW, Grizzell JA, Philpot RM, Wecker L. 2014. Postpartum depression in rats: differences in swim test immobility, sucrose preference and nurturing behaviors. Behav Brain Res. 272:75–82. doi: 10.1016/j.bbr.2014.06.041. PMID: 24983658.
34. Flaisher-Grinberg S, Gampetro DR, Kronfeld-Schor N, Einat H. 2011. Inconsistent effects of photoperiod manipulations in tests for affective-like changes in mice: implications for the selection of appropriate model animals. Behav Pharmacol. 22(1):23–30. doi: 10.1097/FBP.0b013e3283425012. PMID: 21169813.
35. Fonken LK, Kitsmiller E, Smale L, Nelson RJ. 2012. Dim nighttime light impairs cognition and provokes depressive-like responses in a diurnal rodent. J Biol Rhythms. 27(4):319–27. doi: 10.1177/0748730412448324. PMID: 22855576.
36. Fonken LK, Nelson RJ. 2013. Dim light at night increases depressive-like responses in male C3H/HeNHsd mice. Behav Brain Res. 243:74–8. doi: 10.1016/j.bbr.2012.12.046. PMID: 23291153.
37. Goda R, Otsuka T, Iwamoto A, Kawai M, Shibata S, Furuse M 2015. Serotonin levels in the dorsal raphe nuclei of both chipmunks and mice are enhanced by long photoperiod, but brain dopamine level response to photoperiod is species-specific. Neurosci Lett. 593:95–100. doi: 10.1016/j.neulet.2015.03.035. PMID: 25797183.
38. Guscott M, Bristow LJ, Hadingham K, Rosahl TW, Beer MS, Stanton JA 2005. Genetic knockout and pharmacological blockade studies of the 5-HT7 receptor suggest therapeutic potential in depression. Neuropharmacology. 48(4):492–502. doi: 10.1016/j.neuropharm.2004.11.015. PMID: 15755477.
39. Hammouda O, Chtourou H, Chahed H, Ferchichi S, Chaouachi A, Kallel C 2012 High Intensity Exercise Affects Diurnal Variation of Some Biological Markers in Trained Subjects. Int J Sports Med. 33(11):886–91. doi: 10.1055/s-0032-1301887. PMID: 22791622.
40. Hill DW, Chtourou H. 2020. The effect of time of day and chronotype on the relationships between mood state and performance in a Wingate test. Chronobiol Int. 37(11):1599–1610. doi: 10.1080/07420528.2020.1786394. PMID: 32924652.
41. Hill DW, Leiferman JA, Lynch NA, Dangelmaier BS, Burt SE. 1998. Temporal specificity in adaptations to high-intensity exercise training. Med Sci Sports Exerc. 30(3):450–5. doi: 10.1097/00005768-199803000-00017. PMID: 9526893.
42. Hill DW, Smith JC. 1991. Circadian rhythm in anaerobic power and capacity. Can J Sport Sci. 16(1):30–2. PMID: 1645212.
43. Hill DW, Smith JC. 1991. Effect of time of day on the relationship between mood state, anaerobic power, and capacity. Percept Mot Skills. 72(1):83–7. doi: 10.2466/pms.1991.72.1.83. PMID: 2038539.
44. Jalewa J, Wong-Lin K, McGinnity TM, Prasad G, Hölscher C. 2014. Increased number of orexin/hypocretin neurons with high and prolonged external stress-induced depression. Behav Brain Res. 272:196–204. doi: 10.1016/j.bbr.2014.05.030. PMID: 24867335.
45. Jørgensen MG. 2014. Assessment of postural balance in community-dwelling older adults – methodological aspects and effects of biofeedback-based Nintendo Wii training. Dan Med J. 61(1): B4775. PMID: 24393594
46. Kawai H, Machida M, Ishibashi T, Kudo N, Kawashima Y, Mitsumoto A. 2018. Chronopharmacological analysis of antidepressant activity of a dual-action serotonin noradrenaline reuptake inhibitor (SNRI), milnacipran, in rats. Biol Pharm Bull. 41(2):213–9. doi: 10.1248/bpb.b17-00733. PMID: 29386481.
47. Keers R, Pedroso I, Breen G, Aitchison KJ, Nolan PM, Cichon S 2012. Reduced anxiety and depression-like behaviours in the circadian period mutant mouse afterhours. PLoS One. 7(6):e38263. doi: 10.1371/journal.pone.0038263. PMID: 22719873.
48. Kelliher P, Connor TJ, Harkin A, Sanchez C, Kelly JP, Leonard BE. 2000. Varying responses to the rat forced-swim test under diurnal and nocturnal conditions. Physiol Behav. 69(4–5):531–9. doi: 10.1016/s0031-9384(00)00213–4. PMID: 10913793.
49. Koprdova R, Bogi E, Belovicova K, Sedlackova N, Okuliarova M, Ujhazy E 2016. Chronic unpredictable mild stress paradigm in male Wistar rats: effect on anxiety- and depressive-like behavior. Neuroendocrinol Lett. 37(1):103–110. PMID: 28263537.
50. Krivisky K, Ashkenazy T, Kronfeld-Schor N, Einat H. 2011. Antidepressants reverse short-photoperiod-induced, forced swim test depression-like behavior in the diurnal fat sand rat: further support for the utilization of diurnal rodents for modeling affective disorders. Neuropsychobiology. 63(3):191–6. doi: 10.1159/000321805. PMID: 21304227.
51. Küüsmaa M, Schumann M, Sedliak M, Kraemer WJ, Newton RU, Malinen J-P 2016. Effects of morning versus evening combined strength and endurance training on physical performance, muscle hypertrophy, and serum hormone concentrations. Appl Physiol Nutr Metab. 41(12):1285–94. doi: 10.1139/apnm-2016-0271. PMID: 27863207.
52. Lericollais R, Gauthier A, Bessot N, Davenne D. 2016. Diurnal evolution of cycling biomechanical parameters during a 60-s Wingate test. Scand J Med Sci Sports. 21(6):e106-14. doi: 10.1111/j.1600–0838.2010.01172.x. PMID: 20807387.
53. Lericollais R, Gauthier A, Bessot N, Sesboüé B, Davenne D. 2009. Time-of-day effects on fatigue during a sustained anaerobic test in well-trained cyclists. Chronobiol Int. 26(8):1622–35. doi: 10.3109/07420520903534492. PMID: 20030545.
54. Lericollais R, Gauthier A, Bessot N, Zouabi A, Davenne D. 2013. Morning Anaerobic Performance Is Not Altered by Vigilance Impairment. PLoS One. 8(3):e58638. doi: 10.1371/journal.pone.0058638. PMID: 23516522.
55. Li N, Xu Y, Chen X, Duan Q, Zhao M. 2015. Sex-specific diurnal immobility induced by forced swim test in wild type and clock gene deficient mice. Int J Mol Sci. 16(4):6831–41. doi: 10.3390/ijms16046831. PMID: 25815598.
56. Lopes-Silva JP, da Silva Santos JF, Franchini E. 2019. Can caffeine supplementation reverse the effect of time of day on repeated-sprint exercise performance? Appl Physiol Nutr Metab. 44(2):187–93. doi: 10.1139/apnm-2018-0373. PMID: 30058345.
57. López-Samanes Á, Moreno-Pérez D, Maté-Muñoz JL, Domínguez R, Pallarés JG, Mora-Rodriguez R 2017. Circadian rhythm effect on physical tennis performance in trained male players. J Sports Sci. 35(21):2121–8. doi: 10.1080/02640414.2016.1258481. PMID: 27918240.
58. Moon E, Choe B-M, Park J-M, Chung YI, Lee BD, Park J-H 2018. Protein kinase C activity and delayed recovery of sleep-wake cycle in mouse model of bipolar disorder. Psychiatry Investig. 15(9):907–13. doi: 10.30773/pi.2018.05.23. PMID: 30235919.
59. Moriya S, Tahara Y, Sasaki H, Ishigooka J, Shibata S. 2015. Housing under abnormal light-dark cycles attenuates day/night expression rhythms of the clock genes Per1, Per2, and Bmal1 in the amygdala and hippocampus of mice. Neurosci Res. 99:16–21. doi: 10.1016/j.neures.2015.05.005. PMID: 26026603.
60. Nagy AD, Iwamoto A, Kawai M, Goda R, Matsuo H, Otsuka T 2015. Melatonin adjusts the expression pattern of clock genes in the suprachiasmatic nucleus and induces antidepressant-like effect in a mouse model of seasonal affective disorder. Chronobiol Int. 32(4):447–57. doi: 10.3109/07420528.2014.992525. PMID: 25515595.
61. Orozco-Solis R, Montellier E, Aguilar-Arnal L, Sato S, Vawter MP, Bunney BG 2017. A circadian genomic signature common to ketamine and sleep deprivation in the anterior cingulate cortex. Biol Psychiatry. 82(5):351–60. doi: 10.1016/j.biopsych.2017.02.1176. PMID: 28395871
62. Otani H, Kaya M, Tamaki A, Goto H, Goto T, Shirato M. 2018. Diurnal effects of prior heat stress exposure on sprint and endurance exercise capacity in the heat. Chronobiol Int. 2018;35(7):982–95. doi: 10.1080/07420528.2018.1448855. PMID: 29561175.
63. Overstreet DH, Friedman E, Mathe AA, Yadid G. 2005. The Flinders Sensitive Line rat: A selectively bred putative animal model of depression. Neurosci Biobehav Rev. 29(4–5, SI):739–59. doi: 10.1016/j.neubiorev.2005.03.015. PMID: 15925699.
64. Petit E, Mougin F, Bourdin H, Tio G, Haffen E. 2014. Impact of 5-h phase advance on sleep architecture and physical performance in athletes. Appl Physiol Nutr Metab. 39(11):1230–6. doi: 10.1139/apnm-2013-0531. PMID: 25140762.
65. Pokk P, Väli M. 2001. Small platform stress increases exploratory activity of mice in staircase test. Prog Neuropsychopharmacol Biol Psychiatry. 25(7):1435–44. doi: 10.1016/s0278-5846(01)00195–6. PMID: 11513357.
66. Popoli M. 2009. Agomelatine: innovative pharmacological approach in depression. CNS Drugs. 23(2):27–34. doi: 10.2165/11318640-000000000-00000. PMID: 19708723.
67. Racinais S, Hue O, Hertogh C, Damiani M, Blonc S. 2004. Time-of-day effects in maximal anaerobic leg exercise in tropical environment: A first approach. Int J Sports Med. 25(3):186–90. doi: 10.1055/s-2003-45258. PMID: 15088242.
68. Racinais S, Perrey S, Denis R, Bishop D. 2010. Maximal power, but not fatigability, is greater during repeated sprints performed in the afternoon. Chronobiol Int. 27(4):855–64. doi: 10.1007/s40279-013-0066-5. PMID: 20560715.
69. Raghavendra V, Kaur G, Kulkarni SK. 2000. Anti-depressant action of melatonin in chronic forced swimming-induced behavioral despair in mice, role of peripheral benzodiazepine receptor modulation. Eur Neuropsychopharmacol. 10(6):473–81. doi: 10.1016/s0924-977x(00)00115–2. PMID: 11115737.
70. Ramírez-Rodríguez G, Klempin F, Babu H, Benítez-King G, Kempermann G. 2009. Melatonin modulates cell survival of new neurons in the hippocampus of adult mice. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 34(9):2180–91. doi: 10.1038/npp.2009.46. PMID: 19421166.
71. Rao SH, Diwan P V. 1995. A behavioral profile of bilateral anophthalmic mutant rats. Exp Anim. 44(1):67–9. doi: 10.1538/expanim.44.67. PMID: 7705482.
72. Reilly T, Brooks GA. 1990. Selective persistence of circadian rhythms in physiological responses to exercise. Chronobiol Int. 7(1):59–67. doi: 10.3109/07420529009056955. PMID: 2372852.
73. Reilly T, Down A. 1992. Investigation of circadian-rhythms in anaerobic power and capacity of the legs. J Sports Med Phys Fitness. 32(4):343–7. PMID: 1293415.
74. Reilly T, Garrett R. 1998. Investigation of diurnal variation in sustained exercise performance. Ergonomics. 41(8):1085–94. doi: 10.1080/001401398186397. PMID: 9715668.
75. Robertson A, Watt JM, Galloway SDR. 2004. Effects of leg massage on recovery from high intensity cycling exercise. Br J Sports Med. 38(2):173–6. doi: 10.1136/bjsm.2002.003186. PMID: 15039254.
76. Rosenwasser AM. 1989. Effects of chronic clonidine administration and withdrawal on free-running circadian activity rhythms. Pharmacol Biochem Behav. 33(2):291–7. doi: 10.1016/0091-3057(89)90502–9. PMID: 2813468.
77. Roveda E, Mule A, Galasso L, Castelli L, Scurati R, Michielon G 2020. Effect of chronotype on motor skills specific to soccer in adolescent players. Chronobiol Int. 37(4):552–63. doi: 10.1080/07420528.2020.1729787. PMID: 32093513.
78. Saidi O, Doré E, Maso F, Mack-Inocentio D, Walrand S, Pereira B 2019. Acute effect of an intensified exercise program on subsequent sleep, dietary intake and performance in junior rugby players. Eur J Appl Physiol. 119(9):2075–82. doi: 10.1007/s00421-019-04196-5. PMID: 31346707.
79. Schnell A, Sandrelli F, Ranc V, Ripperger JA, Brai E, Alberi L 2015. Mice lacking circadian clock components display different mood-related behaviors and do not respond uniformly to chronic lithium treatment. Chronobiol Int. 32(8):1075–89. doi: 10.3109/07420528.2015.1062024. PMID: 26317159.
80. Schulz D, Aksoy A, Canbeyli R. 2008. Behavioral despair is differentially affected by the length and timing of photic stimulation in the dark phase of an L/D cycle. Prog Neuropsychopharmacol Biol Psychiatry. 32(5):1257–62. doi: 10.1016/j.pnpbp.2008.03.019. PMID: 18485555.
81. Slattery DA, Uschold N, Magoni M, Bär J, Popoli M, Neumann ID, Reber S. Behavioural consequences of two chronic psychosocial stress paradigms: anxiety without depression. Psychoneuroendocrinology. 37(5):702–14. doi: 10.1016/j.psyneuen.2011.09.002. PMID: 21962377.
82. Sokolowska E, Viitanen R, Misiewicz Z, Mennesson M, Saarnio S, Kulesskaya N 2020. The circadian gene Cryptochrome 2 influences stress-induced brain activity and depressive-like behavior in mice. Genes Brain Behav. 20(4):e12708. doi: 10.1111/gbb.12708. PMID: 33070440.
83. Solberg LC, Horton TH, Turek FW. 1999. Circadian rhythms and depression: effects of exercise in an animal model. Am J Physiol. 276(1):R152-61. doi: 10.1152/ajpregu.1999.276.1.R152. PMID: 9887189.
84. Solomon MB, Loftspring M, de Kloet AD, Ghosal S, Jankord R, Flak JN 2015. Neuroendocrine Function After Hypothalamic Depletion of Glucocorticoid Receptors in Male and Female Mice. Endocrinology. 156(8):2843–53. doi: 10.1210/en.2015–1276. PMID: 26046806.
85.Soria M, Anson M, Escanero JF. 2016. Correlation Analysis of Exercise-Induced Changes in Plasma Trace Element and Hormone Levels During Incremental Exercise in Well-Trained Athletes, Biol Trace Elem Res. 170(1):55–64. doi: 10.1007/s12011-015-0466-5. PMID: 26271307
86. Soria M, Gonzalez-Haro C, Anson M, Lopez-Colon JL, Escanero JF. 2015. Plasma levels of trace elements and exercise induced stress hormones in well-trained athletes. J Trace Elem Med Biol. 31:113–9. doi: 10.1016/j.jtemb.2015.04.004. PMID: 26004901.
87. Souissi H, Chaouachi A, Chamari K, Dogui M, Amri M, Souissi N. 2010. Time-of-Day Effects on Short-Term Exercise Performances in 10-to 11-Year-Old Boys. Pediatr Exerc Sci. 22(4):613–23. doi: 10.1123/pes.22.4.613. PMID: 21242609.
88. Souissi H, Chtourou H, Chaouachi A, Chamari K, Souissi N, Amri M. 2012. Time-of-day effects on EMG parameters during the Wingate test in boys. J Sport Sci Med. 11(3):380–6. PMID: 24149343.
89. Souissi N, Bessot N, Chamari K, Gauthier A, Sesbouee B, Davenne D. 2007. Effect of time of day on aerobic contribution to the 30-s wingate test performance. Chronobiol Int. 24(4):739–48. doi: 10.1080/07420520701535811. PMID: 17701684
90. Souissi N, Driss T, Chamari K, Vandewalle H, Davenne D, Gam A 2010. Diurnal variation in Wingate test performances: influence of active warm-up. Chronobiol Int. 27(3):640–52. doi: 10.3109/07420528.2010.483157.
91. Souissi N, Gauthier A, Sesboue B, Larue J, Davenne D. 2004. Circadian rhythms in two types of anaerobic cycle leg exercise: Force-velocity and 30-s Wingate tests. Int J Sports Med. 25(1):14–19. doi: 10.1055/s-2003-45226. PMID: 14750007. PMID: 20524806.
92. Souissi N, Gauthier A, Sesboüé B, Larue J, Davenne D. 2002. Effects of regular training at the same time of day on diurnal fluctuations in muscular performance. J Sports Sci. 20(11):929–937. doi: 10.1080/026404102320761813. PMID: 12430993.
93. Souissi N, Sesboue B, Gauthier A, Larue J, Davenne D. 2003. Effects of one night’s sleep deprivation on anaerobic performance the following day. Eur J Appl Physiol. 89(3–4):359–366. doi: 10.1007/s00421-003-0793-7. PMID: 12736846.
94. Souissi N, Souissi H, Sahli S, Tabka Z, Dogui M, Ati J, Davenne D. 2007. Effect of ramadan on the diurnal variation in short-term high power output. Chronobiol Int. 24(5):991–1007. doi: 10.1080/07420520701661914. PMID: 17994351
95. Souissi N, Souissi M, Souissi H, Chamari K, Tabka Z, Dogui M, Davenne D. 2008. Effect of time of day and partial sleep deprivation on short-term,high-power output. Chronobiol Int. 25(6):1062–1076. doi: 10.1080/07420520802551568. PMID: 19005905.
96. Tal-Krivisky K, Kronfeld-Schor N, Einat H. 2015. Voluntary exercise enhances activity rhythms and ameliorates anxiety- and depression-like behaviors in the sand rat model of circadian rhythm-related mood changes. Physiol Behav. 151:441–7. doi:10.1016/j.physbeh.2015.08.002. PMID: 26253214.
97. Taylor AN, Rahman SU, Tio DL, Sanders MJ, Bando JK, Truong AH, Prolo P. 2006. Lasting neuroendocrine-immune effects of traumatic brain injury in rats. J Neurotrauma. 23(12):1802–13. doi: 10.1089/neu.2006.23.1802. PMID: 17184190.
98. Uz T, Manev H. 2001. Prolonged swim-test immobility of serotonin N-acetyltransferase (AANAT)-mutant mice. J Pineal Res. 30(3):166–70. doi: 10.1034/j.1600–079x.2001.300305.x. PMID: 11316327.
99. Vincent MY, Jacobson L. 2014. Glucocorticoid receptor deletion from the dorsal raphé nucleus of mice reduces dysphoria-like behavior and impairs hypothalamic-pituitary-adrenocortical axis feedback inhibition. Eur J Neurosci. 39(10):1671–81. doi: 10.1111/ejn.12538. PMID: 24684372.
100. Wang X-L, Wang D-Q, Jiao F-C, Ding K-M, Ji Y-B, Lu L 2020. Diurnal rhythm disruptions induced by chronic unpredictable stress relate to depression-like behaviors in rats. Pharmacol Biochem Behav. 194. doi: 10.1016/j.pbb.2020.172939. PMID: 32437704.
101. Workman JL, Weil ZM, Tuthill CR, Nelson RJ. 2008. Maternal pinealectomy increases depressive-like responses in Siberian hamster offspring. Behav Brain Res. 189(2):387–91. doi: 10.1016/j.bbr.2008.01.016. PMID: 18328579.
102. Yanai S, Endo S. 2016. Early onset of behavioral alterations in senescence-accelerated mouse prone 8 (SAMP8). Behav Brain Res. 308:187–95. doi: 10.1016/j.bbr.2016.04.026. PMID: 27093926
103. Yang S, Farias M, Kapfhamer D, Tobias J, Grant G, Abel T, Bucan M. 2007. Biochemical, molecular and behavioral phenotypes of Rab3A mutations in the mouse. Genes Brain Behav. 6(1):77–96. doi: 10.1111/j.1601–183X.2006.00235.x. PMID: 16734774.
104. Yoo C-H, Song K-H, Lim I S-, Woo D-C, Choe B-Y. 2018. Metabolic effects of light deprivation in the prefrontal cortex of rats with depression-like behavior: In vivo proton magnetic resonance spectroscopy at 7 T. Brain Res. 1687:95–103. doi: 10.1016/j.brainres.2018.02.047. PMID: 29501652.
’
Studies assessed for eligibility from PubMed (MEDLINE):
N = 34
1. Abedelmalek S, Chtourou H, Aloui A, Aouichaoui C, Souissi N, Tabka Z. 2013. Effect of time of day and partial sleep deprivation on plasma concentrations of IL-6 during a short-term maximal performance. Eur J Appl Physiol. 113(1):241–248. doi: 10.1007/s00421-012-2432-7. PMID: 22677919.
8. Baklouti H, Chtourou H, Aloui A, Chaouachi A, Souissi N. 2015. Effect of active warm-up duration on morning short-term maximal performance during Ramadan. Libyan J Med. 10(1):26229. doi: 10.3402/ljm.v10.26229. PMID: 25676856.
13. Bernard T, Giacomoni M, Gavarry O, Seymat M, Falgairette G. 1998. Time-of-day effects in maximal anaerobic leg exercise. Eur J Appl Physiol Occup Physiol. 77(1–2):133–8. doi: 10.1007/s004210050311. PMID: 9459533.
14. Bilski J, Jaworek J, Pokorski J, Nitecki J, Nitecka E, Pokorska J 2016. Effects of time of day and the wingate test on appetite perceptions, food intake and plasma levels of adipokines. J Physiol Pharmacol. 67(5):667–76. PMID: 28011947.
16. Bougard C, Bessot N, Moussay S, Sesbouee B, Gauthier A. 2009. Effects of Waking Time and Breakfast Intake Prior to Evaluation of Physical Performance in the Early Morning. Chronobiol Int. 26(2):307–23. doi: 10.1080/07420520902774532. PMID: 19212843.
18. Chtourou H, Chaouachi A, Hammouda O, Chamari K, Souissi N. 2012. Listening to Music Affects Diurnal Variation in Muscle Power Output. Int J Sports Med. 33(1):43–7. doi: 10.1055/s-0031-1284398. PMID: 22134883.
19. Chtourou H, Hammouda O, Chaouachi A, Chamari K, Souissi N. 2012. The Effect of Time-of-Day and Ramadan Fasting on Anaerobic Performances. Int J Sports Med. 33(2):142–7. doi: 10.1055/s-0031-1286251. PMID: 22318530.
20. Chtourou H, Hammouda O, Souissi H, Chamari K, Chaouachi A, Souissi N. Diurnal variations in physical performances related to football in young soccer players. Asian J Sports Med. 3(3):139–44. doi: 10.5812/asjsm.34604. PMID: 23012632.
21. Chtourou H, Zarrouk N, Chaouachi A, Dogui M, Behm DG, Chamari K 2011. Diurnal Variation in Wingate-Test Performance and Associated Electromyographic Parameters. Chronobiol Int. 28(8):706–13. doi: 10.3109/07420528.2011.596295. PMID: 21793694.
27. Dergaa I, Fessi MS, Chaabane M, Souissi N, Hammouda O. 2019. The effects of lunar cycle on the diurnal variations of short-term maximal performance, mood state, and perceived exertion. Chronobiol Int. 36(9):1249–57. doi: 10.1080/07420528.2019.1637346. PMID: 31368366.
39. Hammouda O, Chtourou H, Chahed H, Ferchichi S, Chaouachi A, Kallel C 2012 High Intensity Exercise Affects Diurnal Variation of Some Biological Markers in Trained Subjects. Int J Sports Med. 33(11):886–91. doi: 10.1055/s-0032-1301887. PMID: 22791622.
40. Hill DW, Chtourou H. 2020. The effect of time of day and chronotype on the relationships between mood state and performance in a Wingate test. Chronobiol Int. 37(11):1599–1610. doi: 10.1080/07420528.2020.1786394. PMID: 32924652.
41. Hill DW, Leiferman JA, Lynch NA, Dangelmaier BS, Burt SE. 1998. Temporal specificity in adaptations to high-intensity exercise training. Med Sci Sports Exerc. 30(3):450–5. doi: 10.1097/00005768-199803000-00017. PMID: 9526893.
42. Hill DW, Smith JC. 1991. Circadian rhythm in anaerobic power and capacity. Can J Sport Sci. 16(1):30–2. PMID: 1645212.
43. Hill DW, Smith JC. 1991. Effect of time of day on the relationship between mood state, anaerobic power, and capacity. Percept Mot Skills. 72(1):83–7. doi: 10.2466/pms.1991.72.1.83. PMID: 2038539.
51. Küüsmaa M, Schumann M, Sedliak M, Kraemer WJ, Newton RU, Malinen J-P 2016. Effects of morning versus evening combined strength and endurance training on physical performance, muscle hypertrophy, and serum hormone concentrations. Appl Physiol Nutr Metab. 41(12):1285–94. doi: 10.1139/apnm-2016-0271. PMID: 27863207.
52. Lericollais R, Gauthier A, Bessot N, Davenne D. 2016. Diurnal evolution of cycling biomechanical parameters during a 60-s Wingate test. Scand J Med Sci Sports. 21(6):e106-14. doi: 10.1111/j.1600–0838.2010.01172.x. PMID: 20807387.
53. Lericollais R, Gauthier A, Bessot N, Sesboüé B, Davenne D. 2009. Time-of-day effects on fatigue during a sustained anaerobic test in well-trained cyclists. Chronobiol Int. 26(8):1622–35. doi: 10.3109/07420520903534492. PMID: 20030545.
54. Lericollais R, Gauthier A, Bessot N, Zouabi A, Davenne D. 2013. Morning Anaerobic Performance Is Not Altered by Vigilance Impairment. PLoS One. 8(3):e58638. doi: 10.1371/journal.pone.0058638. PMID: 23516522.
56. Lopes-Silva JP, da Silva Santos JF, Franchini E. 2019. Can caffeine supplementation reverse the effect of time of day on repeated-sprint exercise performance? Appl Physiol Nutr Metab. 44(2):187–93. doi: 10.1139/apnm-2018-0373. PMID: 30058345.
57. López-Samanes Á, Moreno-Pérez D, Maté-Muñoz JL, Domínguez R, Pallarés JG, Mora-Rodriguez R 2017. Circadian rhythm effect on physical tennis performance in trained male players. J Sports Sci. 35(21):2121–8. doi: 10.1080/02640414.2016.1258481. PMID: 27918240.
62. Otani H, Kaya M, Tamaki A, Goto H, Goto T, Shirato M. 2018. Diurnal effects of prior heat stress exposure on sprint and endurance exercise capacity in the heat. Chronobiol Int. 2018;35(7):982–95. doi: 10.1080/07420528.2018.1448855. PMID: 29561175.
67. Racinais S, Hue O, Hertogh C, Damiani M, Blonc S. 2004. Time-of-day effects in maximal anaerobic leg exercise in tropical environment: A first approach. Int J Sports Med. 25(3):186–90. doi: 10.1055/s-2003-45258. PMID: 15088242.
68. Racinais S, Perrey S, Denis R, Bishop D. 2010. Maximal power, but not fatigability, is greater during repeated sprints performed in the afternoon. Chronobiol Int. 27(4):855–64. doi: 10.1007/s40279-013-0066-5. PMID: 20560715.
73. Reilly T, Down A. 1992. Investigation of circadian-rhythms in anaerobic power and capacity of the legs. J Sports Med Phys Fitness. 32(4):343–7. PMID: 1293415.
74. Reilly T, Garrett R. 1998. Investigation of diurnal variation in sustained exercise performance. Ergonomics. 41(8):1085–94. doi: 10.1080/001401398186397. PMID: 9715668.
88. Souissi H, Chtourou H, Chaouachi A, Chamari K, Souissi N, Amri M. 2012. Time-of-day effects on EMG parameters during the Wingate test in boys. J Sport Sci Med. 11(3):380–6. PMID: 24149343.
89. Souissi N, Bessot N, Chamari K, Gauthier A, Sesbouee B, Davenne D. 2007. Effect of time of day on aerobic contribution to the 30-s wingate test performance. Chronobiol Int. 24(4):739–48. doi: 10.1080/07420520701535811. PMID: 17701684
90. Souissi N, Driss T, Chamari K, Vandewalle H, Davenne D, Gam A 2010. Diurnal variation in Wingate test performances: influence of active warm-up. Chronobiol Int. 27(3):640–52. doi: 10.3109/07420528.2010.483157.
91. Souissi N, Gauthier A, Sesboue B, Larue J, Davenne D. 2004. Circadian rhythms in two types of anaerobic cycle leg exercise: Force-velocity and 30-s Wingate tests. Int J Sports Med. 25(1):14–19. doi: 10.1055/s-2003-45226. PMID: 14750007.
92. Souissi N, Gauthier A, Sesboüé B, Larue J, Davenne D. 2002. Effects of regular training at the same time of day on diurnal fluctuations in muscular performance. J Sports Sci. 20(11):929–937. doi: 10.1080/026404102320761813. PMID: 12430993.
93. Souissi N, Sesboue B, Gauthier A, Larue J, Davenne D. 2003. Effects of one night’s sleep deprivation on anaerobic performance the following day. Eur J Appl Physiol. 89(3–4):359–366. doi: 10.1007/s00421-003-0793-7. PMID: 12736846.
94. Souissi N, Souissi H, Sahli S, Tabka Z, Dogui M, Ati J, Davenne D. 2007. Effect of ramadan on the diurnal variation in short-term high power output. Chronobiol Int. 24(5):991–1007. DOI: 10.1080/07420520701661914. PMID: 17994351
95. Souissi N, Souissi M, Souissi H, Chamari K, Tabka Z, Dogui M, Davenne D. 2008. Effect of time of day and partial sleep deprivation on short-term,high-power output. Chronobiol Int. 25(6):1062–1076. doi: 10.1080/07420520802551568. PMID: 19005905.
