?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Sleep regularity and chronotype can affect health, performance, and overall well-being. This observational study examines how sleep regularity and chronotype affect sleep quality and cardiorespiratory metrics. Data was collected from 1 January 2019 through 30 December 2019 from over 330 000 Sleep Number smart bed users across the United States who opted into this at-home study. A pressure signal from the smart bed reflected bed presence, movements, heart rate (HR), and breathing rate (BR). Participants (mean age: 55.69 years [SD: 14.0]; 51.2% female) were categorized by chronotype (16.8% early; 62.2% intermediate, 20.9% late) and regularity of sleep timing. Participants who were regular sleepers (66.1%) experienced higher percent restful sleep and lower mean HR and BR compared to the 4.8% categorized as irregular sleepers. Regular early-chronotype participants displayed better sleep and cardiorespiratory parameters compared to those with regular late-chronotypes. Significant variations were noted in sleep duration (Cohen’s d = 1.54 and 0.88, respectively) and restful sleep (Cohen’s d = 1.46 and 0.82, respectively) between early and late chronotypes, particularly within regular and irregular sleep patterns. This study highlights how sleep regularity and chronotype influence sleep quality and cardiorespiratory metrics. Irrespective of chronotype, sleep regularity demonstrated a substantial effect. Further research is necessary to confirm these findings.
Introduction
Sleep is a reversible state of perceptual disengagement and reduced responsiveness to the environment; in humans, it is usually accompanied by recumbence and quiescence (Carskadon and Dement Citation2011). Normal human sleep comprises two states, non-rapid eye movement and rapid eye movement, and these states alternate cyclically throughout a sleep session (Carskadon and Dement Citation2011).
Characteristics of sleep that promote physical and mental wellbeing have been mapped into a multidimensional space (Ru-SATED) to characterize sleep health (Buysse Citation2014). The dimensions of sleep health include consistency of sleep timing (regularity, R), the subjective quality of sleep (satisfaction, S), the ability to maintain attentive wakefulness (alertness, A), placement of sleep in the 24-h cycle (timing, T), ease of falling asleep and returning to sleep (sleep efficiency, E) and sleep duration (D). The dimensions of regularity, timing, and duration in the Ru-SATED model are attractive targets for intervention, as they may be voluntarily modified.
Sleep chronotype is a concept that represents the behavioral manifestation of one’s circadian rhythm (Randler Citation2016). A chronotype indicates the internal underlying sleep/wake rhythm which characterizes individual differences in rest and is defined in two ways: 1) individual preference for either morning or evening hours, or 2) the phase of entrainment of the sleep-wake cycle, which can be quantified by sleep midpoint on free days (Taylor and Hasler Citation2018). Chronotypes can be classified as early, intermediate, or late depending on individual differences in sleep-wake cycles. Early chronotypes rise early and feel most alert during the morning, while late chronotypes rise later and are most alert in the late afternoon or early evening (Lack et al. Citation2009).
Daily variations in sleep and wake are common. A validation study of a sleep regularity index in older adults found that 20% of the study cohort presented a pattern of sleep/wake irregularity (Lunsford-Avery et al. Citation2018). Regularity of sleep timing may provide both physical and cognitive benefits. Regular sleep reduces the risk of cardiovascular disease and is associated with a lower prevalence of metabolic syndrome (Lunsford-Avery et al. Citation2018) and can contribute to better academic performance among students (Medeiros et al. Citation2010). Conversely, irregular sleep has been associated with multiple adverse outcomes, including inflammation (Okun et al. Citation2011), obesity [in men], diabetes and heart failure (Patel et al. Citation2014), and impaired cognitive functioning (Kuula et al. Citation2018; Patel et al. Citation2014).
Interventions to regularize sleep and wake schedules independently from sleep duration can reduce daytime sleepiness (Manber et al. Citation1996). A study of the associations between chronotype and sleep regularity on subjective sleep quality and quantity in daytime working adults reported that a morning chronotype was associated with longer sleep duration, better subjective sleep quality, and shorter sleep onset latency (Soehner et al. Citation2011). Stable weekday rise time correlated with better self-reported sleep quality and shorter sleep onset latency; a more regular weekend bedtime was also associated with a shorter sleep onset latency (Soehner et al. Citation2011).
The study presented below is a non-interventional, retrospective, observational study funded by Sleep Number Corporation that utilized data collected from Sleep Number’s smart bed users who opted into data collection. The smart bed uses a pressure sensor-based full body ballistocardiography (BCG) to evaluate a sleeper’s bed presence, body movements, heart rate (HR), and breathing rate (BR). These data are used to detect sleep versus wake states and to derive summary sleep variables such as sleep duration and latency (Siyahjani et al. Citation2022). This system has been validated against polysomnography and provides reliable sleep metrics to unobtrusively measure ecologically valid human sleep under real-world conditions (Siyahjani et al. Citation2022). The objective of the current study was to investigate the influence of regularity and chronotype, which were quantified for each participant using the time of sleep onset, on sleep metrics and cardiorespiratory parameters, including sleep duration, (percent) restful sleep, time to fall asleep (TTFA), HR, and BR.
Methods
Study design
An out institutional review board (IRB)-approved study (Protocol: P2022-OO4) utilized data from smart bed users who opted into data collection. The study protocol and consent to participate were reviewed and approved by the IRB in accordance with the United States Food and Drug Administration regulations (21 code of federal regulation 50:1–48, 56101–124). Prospective participants in the study from the Sleep Number user population located across all 50 United States were contacted via the smart bed mobile application. Opting-in participants provided their consent through the mobile application.
The data collected from 1 January 2019 to 30 December 2019 were used in these analyses. Data were processed to determine mean HR and BR. Sleep and wake states were determined to assess sleep metrics (sleep duration, [percent] restful sleep, and TTFA). Data were analyzed depending on participant chronotype and regularity cluster as detailed below.
Analysis of chronotype and regularity
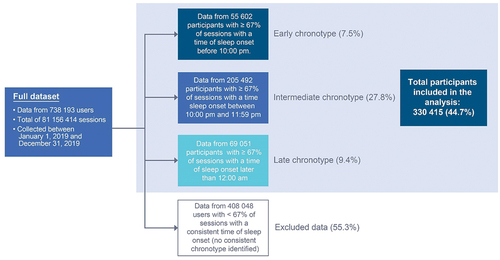
Participants were included in this study if their time of sleep onset was consistent in at least 67% of their recorded sleep sessions. Participants without consistent chronotypes in ≥67% of the recorded sleep sessions were excluded from this study. The time of sleep onset was used to determine chronotype instead of bed-entry time since entry into bed does not necessarily correspond to immediate sleep onset. It is common for sleepers to perform a variety of activities in bed before sleep (ie, reading, watching television, using smartphones, etc). Time of sleep onset is determined from the smart bed deep neural network which uses BCG signals to derive sleep and wake states (Siyahjani et al. Citation2022). Sleep onset latency measurements from the smart bed correlate with the gold standard for sleep monitoring, polysomnography (Siyahjani et al. Citation2022). Thus, the time of sleep onset was a consistent metric to determine chronotype using the smart bed. The ≥67% cut-off for time of sleep onset was chosen to ensure that the chronotypes we characterized were strong and consistent, as we were not able to administer a validated chronotype assessment or survey to participants.
Participants were classified into 1 of 3 chronotypes (i.e. the identified chronotype) by time of sleep onset across at least 67% of their recorded sleep sessions. Participant chronotypes were categorized as follows: early chronotype was defined as time of sleep onset before 10:00 pm; intermediate chronotype was defined as time of sleep onset between 10:00 pm and 11:59 pm; and late chronotype was defined as time of sleep onset of 12:00 am or later. We analyzed sleep metrics for 2 datasets, based on whether the sleep sessions were “in chronotype” (sessions were consistent with the participant’s identified chronotype) or “out of chronotype” (sessions were inconsistent with the participant’s identified chronotype).
Regularity was quantified using a method inspired by Lundsford-Avery et al (Lunsford-Avery et al. Citation2018). In brief, data from each 24-h day when sleep session data from the smart bed was available was divided into 96 fifteen-min epochs, and participants’ sleep/wake states were determined for each epoch. Epochs occurring at the same timepoint 24 h apart were compared and were categorized as either having the same or different states (sleep or wake).
Regularity was calculated as follows, given N days of analysis divided into M (M = 96) daily 15-min epochs, we assigned the value of the variable si,j = 1 if the participant was sleeping on day i in epoch j, and si,j = 0 if the participant was awake. Then the percentage of deviating epochs was calculated using the following equation:
where δ(si,j , si +1,j) = 1 if si,j = si +1,j, and 0 otherwise. The resulting percentage was converted to mean deviation time by considering that each epoch lasts for 15 min.
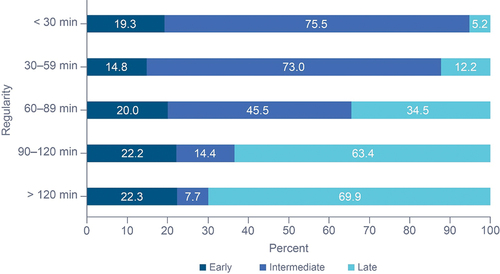
Participant regularity was divided into five categories based on the mean time of deviation from midpoint between sleep onset and wake up time: (1) <30 min deviation; (2) 30–59 min deviation; (3) 60–89 min deviation; (4) 90–120 min deviation; and (5) >120 min deviation. We identified two regularity clusters based on similarity: regular, with <60 min deviation in time of sleep onset (included 2 subcategories: <30 min deviation and 30 to 59 min deviation); and irregular: ≥90 min deviation in time of sleep onset (included 2 subcategories: 90 to 120 min deviation and >120-min deviation). We assessed the impact of regularity cluster on sleep duration, (percent) restful sleep, TTFA, HR, and BR. Additionally, interactions between chronotype and regularity cluster, and their impact on sleep metrics, were assessed.
Statistical analysis
The associations for all possible pair combinations of chronotype and sleep regularity categories were evaluated by calculating odds ratios (ORs) and corresponding 95% confidence intervals (CIs; Szumilas Citation2010). Group comparisons were made using Cohen’s d effect size which was calculated as the ratio between the difference of the mean values and the pooled standard deviation (Fritz et al. Citation2012). Cohen’s d = 0.01 was considered a very small effect size, d = 0.2 was considered a small effect size, d = 0.5 was considered a medium effect size, d = 0.8 was considered a large effect size, d = 1.2 was considered a very large effect size, and d = 2.0 was considered a huge effect size (Sawilowsky Citation2009). A two-way ANOVA was performed to analyze the effects of chronotype and regularity on each sleep metric; the distribution of the dependent variables is shown in Supplemental Figure S1.
Results
Participant demographics
For the analysis period of 1 January 2019 through 30 December 2019, 738,193 participants contributed 81,156,414 sleep sessions; of these, 330,145 participants had consistent chronotypes (as defined as consistent time of sleep onset in ≥67% of all sleep sessions, ) and contributed 64,137,854 sleep sessions for analysis. Participants without consistent chronotypes in ≥67% of all sleep sessions were not included in this study. Of the included participants, 16.8% (n = 55,602) had an early chronotype, 62.2% (n = 205,492) had an intermediate chronotype, and 20.9% (n = 69,051) had a late chronotype (). The regularity distribution showed that 66.1% of participants fell into the regular cluster (<60 min variability), while 4.7% of participants were irregular (≥90 min variability); this corresponds to 218 203 regular participants and 15,677 irregular participants (). Comparison of time of sleep onset between weekdays and weekends did not suggest a meaningful difference in sleep behaviors or participant schedules during the weekend (Supplemental Table S1).
Table 1. Participant demographics and baseline sleep characteristics.
In-chronotype vs out-of-chronotype sleep sessions
The first set of analyses investigated the impact of regularity on sleep and cardiorespiratory metrics. Participants who were more regular had higher percent restful sleep, lower HR, and lower BR regardless of sleep duration (). Regular early-chronotype participants had longer restful sleep and sleep duration, and lower HR and BR than regular late-chronotype participants. Within the irregular cluster, early-chronotype participants had longer percent restful sleep and sleep duration than late-chronotype participants; HR and BR were similar in early- and late-chronotype irregular sleepers. There was less differentiation between early- and late-chronotype sleep metrics in the irregular participants compared with the regular participants.
Figure 2. Impact of regularity on percent restful sleep (a), heart rate (b), and breathing rate (c) in sessions matching identified chronotype.
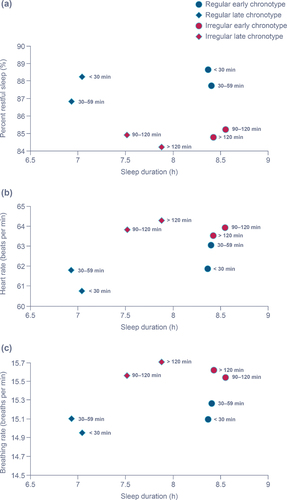
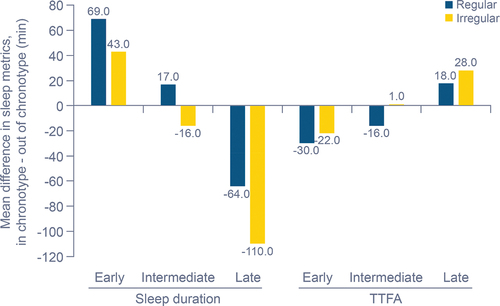
The second set of analyses compared nights in- and out-of-chronotype. We investigated sleep metrics and cardiorespiratory parameters from sessions when participants did not match their identified chronotype (out-of-chronotype sessions). Sleep metrics from sessions where participants were out-of-chronotype had varying changes depending on the identified chronotype and regularity cluster (). For out-of-chronotype sessions in the early-chronotype group, sleep duration decreased (by 32–69 min depending on regularity cluster) and TTFA increased (20–32 min, depending on regularity cluster). In contrast, late-chronotype participants had increased sleep duration (52–111 min, depending on regularity cluster) and decreased TTFA (15–29 min, depending on regularity cluster) in out-of-chronotype sessions. Percent restful sleep was not obviously impacted by in-chronotype versus out-of-chronotype sleep sessions for either early or late chronotypes. Intermediate-chronotype participants had no clear changes in sleep duration and percent restful sleep from in-chronotype to out-of-chronotype sessions; TTFA increased for regular participants (by 10–23 min) in out-of-chronotype sessions but was not obviously changed in irregular participants.
Table 2. Sleep metrics outside of assigned chronotype by regularity category.
In the early-chronotype group, regular participants had greater changes in sleep duration (mean: 69 min) and TTFA (mean: −30 min) compared with irregular participants (mean: 43 min and −22 min, respectively) between in-chronotype and out-of-chronotype sessions (). Regardless of regularity cluster, late-chronotype participants had longer sleep duration during out-of-chronotype sessions than early- and intermediate-chronotype participants, but shorter TTFA (). Intermediate-chronotype participants had longer sleep duration (+17 min) and shorter TTFA (−16 min) in the regular cluster compared with the irregular intermediate-chronotype cluster (−16 min and +1 min, respectively; ).
Interaction between regularity and chronotype
We investigated whether chronotype was distributed across regularity clusters. Regular participants were more likely to be identified as intermediate chronotype than irregular participants (OR: 17.25 [95% CI: 16.47, 18.08]) while irregular participants were more likely to be identified as late-chronotype than regular participants (OR: 13.36 [95% CI: 12.90, 13.84]; ). Only 5.2% of the most regular participants were late-chronotype, while 69.9% of the most irregular participants were identified as late-chronotype.
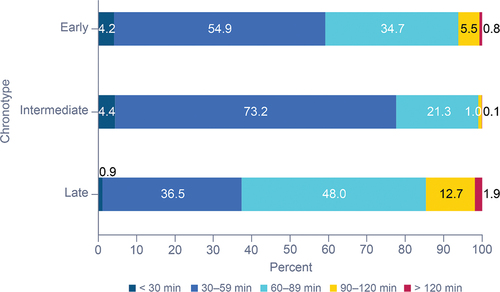
Early- and intermediate-chronotype participants were more likely to be regular than late-chronotype participants (). Early-chronotype participants were 2.4 times more likely to be regular than late-chronotype participants (OR: 2.42 [95% CI: 2.36, 2.47]), and 0.4 times more likely to be regular than intermediate-chronotype participants (OR: 0.416 [95% CI: 0.408, 0.424]). Overall, regular participants had shorter sleep duration (mean [SD]: 7:41 [0:37]), restful sleep (6:46 [0:34]), and TTFA (0:30 [0.06]) compared with irregular participants (8:13 [0:28], 7:00 [0.25] and 0:45 [0.06], respectively). Regular participants had slightly higher percent restful sleep than irregular participants (88.03% [0.70] vs 85.36% [1.03]). Overall, regular participants also had lower HR and BR, with a mean HR of 61.77 beats per min compared with 63.57 beats per min for irregular participants; mean BRs were 15.06 breaths per min vs 15.55 breaths per min, respectively, for regular and irregular participants.
Regularity impacted sleep metrics for the three chronotypes in in-chronotype sleep sessions (). Sleep duration was significantly different between early and late chronotypes (Cohen’s d = 1.54 for regular participants; Cohen’s d = 0.88 for irregular participants). Restful sleep was also significantly different between early and late chronotypes (Cohen’s d = 1.46 for regular participants; Cohen’s d = 0.82 for irregular participants). There was a moderate impact of chronotype on TTFA (Cohen’s d = 0.47 for regular participants; Cohen’s d = 0.68 for irregular participants). Effect sizes were low for percent restful sleep, HR, and BR (Cohen’s d = 0.01–0.21). Chronotype also influenced the impact of regularity on sleep metrics (); regular participants had a higher percent restful sleep (Cohen’s d = 0.60 for early chronotype; Cohen’s d = 0.43 for late chronotype) and shorter TTFA (Cohen’s d = 0.42 for early chronotype; Cohen’s d = 0.59 for late chronotype) compared with irregular participants; regular late chronotype participants also had shorter mean sleep duration (Cohen’s d = 0.68) and restful sleep duration (Cohen’s d = 0.43). Regularity had a smaller impact on HR and BR for early vs late chronotypes (Cohen’s d = 0.13–0.32). These differences were still present if we analyzed all sleep sessions (in chronotype and out of chronotype; Supplemental Table S2). Two-way ANOVA analysis was performed to analyze the effect of regularity cluster and chronotype on sleep metrics (Supplemental Table S3); there was a significant interaction between regularity and chronotype for all sleep metrics as well as a significant effect of both variables on each sleep metric.
Table 3. Sleep metrics across chronotypes (in-chronotype sessions).
Discussion
Chronotypes are a recognized behavioral manifestation of human sleep patterns, referring to an individual’s phase of entrainment and reflecting the interaction between the circadian system and the 24-h day (Fischer et al. Citation2017). Chronotypes (defined as the midpoint of sleep on non-workdays) are normally distributed in the US, but vary with age, with adolescents and young adults having the latest chronotypes (Fischer et al. Citation2017). There are significant impacts of chronotype on health, as seen in shift workers whose work schedules are not aligned with their natural chronotypes (Hittle and Gillespie Citation2018). Shift workers with chronotype mismatch demonstrate higher rates of obesity, metabolic abnormalities, and type 2 diabetes (Vetter et al. Citation2015; Wong et al. Citation2015) and an increased risk for some cancers (Dickerman et al. Citation2016; Lozano-Lorca et al. Citation2020; Ramin et al. Citation2013). Chronotype can also influence protein markers of cardiovascular disease (Baldanzi et al. Citation2022) and impact heart rate variability (Honkalampi et al. Citation2021). Matching chronotype to shift timing can improve sleep characteristics and cardiovascular metrics (Juda et al. Citation2013a; Yadav et al. Citation2016).
The chronotype breakdown of our participants was 16.8% early chronotype, 62.2% intermediate chronotype, and 20.9% late chronotype, a similar pattern of distribution as seen in other studies (Roenneberg et al. Citation2003, Citation2019). The analysis of chronotype assignment based on sleep onset time appeared to be consistent across the week and weekends which resulted in minor differences within each identified chronotype (Supplemental Table S1). Since weekend sleep onset times are less likely to be subject to a strict schedule due to work, school, or other obligations, and may be more representative of individual preference, they may closely align with the participant’s true chronotype.
The majority of regular participants in our study were early- or intermediate-chronotype sleepers, while irregular participants tended to fall into the late-chronotype group. This may be due to social constraints favoring an early chronotype such as work, school, or other obligations. Regularity, regardless of chronotype, was associated with higher percent restful sleep, lower HR, and lower BR. In a previous study, lower HR was associated with higher subjective sleep quality (Sajjadieh et al. Citation2020). Additionally, respiratory rate was shown to be significantly lower for all sleep stages compared to wakefulness (Gutierrez et al. Citation2016). Thus, a lower BR was correlated with lower wake after sleep onset which is in turn associated with higher sleep quality. Aligning with these findings, a study involving adolescents and young adults highlighted the correlation between later bedtimes and shorter sleep durations, as well as less healthy daily habits (Grummon et al. Citation2021). Additionally, our findings indicate that more regular sleepers exhibited better sleep metrics across diverse sleep durations and faster sleep initiation compared to irregular sleepers, emphasizing the advantages of maintaining consistent sleep patterns.
Previous studies have found that evening (late) chronotype shift workers had decreased sleep duration and quality compared to other chronotypes (Martin et al. Citation2015; Yazdi et al. Citation2014). We found similar results in our study population, with late-chronotype participants having reduced sleep duration and less restful sleep compared to early-chronotype participants, although the differences in percent restful sleep between early- and late-chronotype groups were small (Cohen’s d = 0.07–0.21).
A 2020 study assessed sleep regularity and the association of cardiac events and found that variations in sleep duration and timing may be risk factors for cardiovascular disease, independent of other risk factors (Huang et al. Citation2020). In our study, there appeared to be an interaction between regularity and chronotype (); regular early-chronotype participants had longer restful sleep and sleep duration and shorter TTFA compared with regular late-chronotype participants. However, regular late-chronotype participants had higher percent restful sleep and fell asleep faster than irregular late-chronotype participants, suggesting an impact of regularity on sleep quality regardless of chronotype. Regular participants also had lower HR and BR compared with irregular participants regardless of chronotype, indicating that these cardiorespiratory parameters may be influenced by regularity.
Figure 6. Summary of interactions between regularity and chronotype.
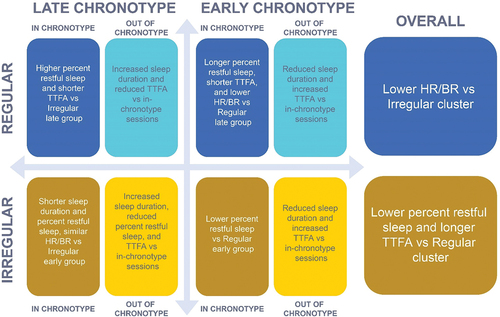
The difference in percent restful sleep between regular and irregular participants was greater for the early-chronotype group compared with the late-chronotype group (early: Cohen’s d = 0.60; late: Cohen’s d = 0.43). Differences in sleep duration and restful sleep were significantly higher among regular participants compared with irregular participants. These data suggest a strong association between regularity and objective aspects of sleep quality as assessed by sleep duration, restful sleep duration, and percent restful sleep duration, as well as an association between chronotype and sleep duration.
We investigated how sleep metrics might change in out-of-chronotype sleep sessions where participants did not match their identified chronotype. Analysis of in-chronotype and out-of-chronotype sleep metrics for each group indicated that late-chronotype participants had longer sleep duration and lower percent restful sleep than early or intermediate chronotype participants in their out-of-chronotype sleep sessions, regardless of regularity. Late-chronotype participants also fell asleep faster than early-chronotype participants in out-of-chronotype sessions. Early-chronotype participants had shorter sleep duration and similar percent restful sleep in out-of-chronotype sleep sessions, indicating worse objective sleep quality, in contrast to late-chronotype participants who had better objective sleep quality in out-of-chronotype sessions. The analysis of in-chronotype sessions versus out-of-chronotype sessions found that results within the late chronotype group were consistent with the results comparing the early versus late chronotype. This reinforces the idea that an early chronotype positively influences objective aspects of sleep quality.
Interactions between chronotype and regularity have been previously investigated. Similar to our data, Soehner et al. found that early chronotype was associated with better sleep quality, longer sleep duration, shorter sleep-onset latency, and greater regularity (Soehner et al. Citation2011). This previous study also found that a more stable rise-time was associated with better sleep quality and shorter sleep onset latency. Irregularity was associated with later bedtimes, perhaps indicating a late chronotype (similar to our finding that late-chronotype participants were more likely to have irregular sleep schedules), lower sleep quality and longer sleep onset latency. In agreement with our data, this previous study found that early-chronotype participants had greater regularity (Soehner et al. Citation2011). The current observational study helps to build a connection between chronotype and regularity and confirms previous results indicating that regularity is a key contributor to sleep quality.
This study was limited by its observational nature and the limited demographics of the participant population. The smart bed is primarily used in the United States by participants with a moderate-to-high socioeconomic status that may have a specific motivation to monitor their sleep patterns. Although participants with potential sleep disorders were not excluded from this study, we do believe that the study population has limited generalizability. The smart bed is sold only in the United States, and we understand that users of smart beds may not be representative of the global population. However, the ability of the system to gather ecologically valid data from thousands of individuals provides a large dataset from which to identify sleep patterns for future targeted investigations.
This study was also limited by the methods of chronotype and regularity categorization. Chronotype categorization for a given user was based on time of sleep onset for the majority of the user’s sleep sessions rather than a validated assessment tool such as the Munich ChronoType Questionnaire (Juda et al. Citation2013b; Roenneberg et al. Citation2003). While time to sleep onset measured using the smart bed has previously been shown to correlate to polysomnography (Siyahjani et al. Citation2022), systematic errors may have been introduced into our dataset. However, chronotypes determined by time to sleep onset remained consistent during the week versus weekend, indicating that potential biases introduced by using time to sleep onset measurements are small. Additionally, since the bed-entry time does not necessarily coincide with sleep intent, using time of sleep onset alone to measure chronotype may have overlooked the influence of individual bedtime and/or sleep behaviors. Further, participants who did not meet the cut-off for consistency in chronotype in ≥67% of sleep sessions were excluded, potentially introducing a bias toward regularity. Future work should consider the administration of validated questionnaires to formally quantify the chronotype and to identify additional dimensions of sleep, such as subjective alertness and subjective time of peak-performance, to more precisely map an individual’s chronotype.
Additionally, the quantification of sleep regularity was limited by our methods. In a 2018 study, 24-h actigraphy data were used to determine sleep regularity (Lunsford-Avery et al. Citation2018). Our study relied on the data collected by the smart bed and assumed that the bed-absence periods corresponded to the wake state. Our approach may overestimate regularity because napping periods outside the bed cannot be considered.
Our data suggest that maintaining a regular sleep schedule, regardless of chronotype, will maximize the proportion of restful sleep. Additionally, early chronotype was associated with increased sleep quality as assessed by sleep duration, restful sleep duration, and percent restful sleep. These data support recommendations for regular and early sleep schedules; however, these hypotheses need to be tested in an interventional study, and the interactions between chronotype, regularity, and additional dimensions of the Ru-SATED framework should be further investigated.
Supplemental Material
Download PDF (406.8 KB)Acknowledgments
Medical writing support was provided by Rachel C. Brown, PhD, of Oxford PharmaGenesis Inc., Newtown, PA, USA, and was funded by Sleep Number Corporation.
Disclosure statement
GGM, VC, BL, MA and MW are employees of Sleep Number Labs. RM is an employee of Sleep Number Corporation, the manufacturer of the Sleep Number smart bed. All authors are responsible for the design, execution, analysis, and conclusions of the study presented in this article, as well as the decision to publish the results.
Data availability statement
Due to the nature of this research, participants in this study did not agree for their data to be shared publicly, so supporting data is not available.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/07420528.2023.2298267.
Additional information
Funding
References
- Baldanzi G, Hammar U, Fall T, Lindberg E, Lind L, Elmstahl S, Theorell-Haglow J. 2022. Evening chronotype is associated with elevated biomarkers of cardiometabolic risk in the epihealth cohort: a cross-sectional study. Sleep. 45:zsab226. doi: 10.1093/sleep/zsab226.
- Buysse DJ. 2014. Sleep health: can we define it? Does it matter? Sleep. 37:9–17. doi: 10.5665/sleep.3298.
- Carskadon M, Dement W. 2011. Normal human sleep: an overview. In: Kryger M, Roth T Dement W, editors. Principles and practice of sleep medicine. St Louis: Elsevier Saunders. p. 16–26.
- Dickerman BA, Markt SC, Koskenvuo M, Hublin C, Pukkala E, Mucci LA, Kaprio J. 2016. Sleep disruption, chronotype, shift work, and prostate cancer risk and mortality: a 30-year prospective cohort study of finnish twins. Cancer Causes Control. 27:1361–1370. doi: 10.1007/s10552-016-0815-5.
- Fischer D, Lombardi DA, Marucci-Wellman H, Roenneberg T. 2017. Chronotypes in the us - influence of age and sex. PLoS One. 12:e0178782. doi: 10.1371/journal.pone.0178782.
- Fritz CO, Morris PE, Richler JJ. 2012. Effect size estimates: current use, calculations, and interpretation. J Exp Psychol Gen. 141:2–18. doi: 10.1037/a0024338. Erratum in 2012: J Exp Psychol Gen. 141(1):30. PMID: 21823805.
- Grummon AH, Sokol RL, Lytle LA. 2021. Is late bedtime an overlooked sleep behaviour? Investigating associations between sleep timing, sleep duration and eating behaviours in adolescence and adulthood. Public Health Nutr. 24(7):1671–1677. doi: 10.1017/S1368980020002050. Epub 2020 Aug 10.
- Gutierrez G, Williams J, Alrehaili GA, McLean A, Pirouz R, Amdur R, Jain V, Ahari J, Bawa A, Kimbro S, et al. 2016. Respiratory rate variability in sleeping adults without obstructive sleep apnea. Physiol Rep. 4:e12949.
- Hittle B, Gillespie G. 2018. Identifying shift worker chronotype: implications for health. Ind Health. 56:512–523. doi: 10.2486/indhealth.2018-0018.
- Honkalampi K, Jarvelin-Pasanen S, Tarvainen MP, Saaranen T, Vauhkonen A, Kupari S, Perkio-Makela M, Rasanen K, Oksanen T. 2021. Heart rate variability and chronotype - a systematic review. Chronobiol Int. 38:1786–1796. doi: 10.1080/07420528.2021.1939363.
- Huang T, Mariani S, Redline S. 2020. Sleep irregularity and risk of cardiovascular events: the multi-ethnic study of atherosclerosis. J Am Coll Cardiol. 75:991–999. doi: 10.1016/j.jacc.2019.12.054.
- Juda M, Vetter C, Roenneberg T. 2013a. Chronotype modulates sleep duration, sleep quality, and social jet lag in shift-workers. J Biol Rhythms. 28:141–151. doi: 10.1177/0748730412475042.
- Juda M, Vetter C, Roenneberg T. 2013b. The munich chronotype questionnaire for shift-workers (mctqshift). J Biol Rhythms. 28:130–140. doi: 10.1177/0748730412475041.
- Kuula L, Pesonen AK, Heinonen K, Kajantie E, Eriksson JG, Andersson S, Lano A, Lahti J, Wolke D, Räikkönen K, et al. 2018. Naturally occurring circadian rhythm and sleep duration are related to executive functions in early adulthood. J Sleep Res. 27:113–119.
- Lack L, Bailey M, Lovato N, Wright H. 2009. Chronotype differences in circadian rhythms of temperature, melatonin, and sleepiness as measured in a modified constant routine protocol. Nat Sci Sleep. 1:1–8. doi: 10.2147/nss.s6234.
- Lozano-Lorca M, Olmedo-Requena R, Vega-Galindo MV, Vazquez-Alonso F, Jimenez-Pacheco A, Salcedo-Bellido I, Sanchez MJ, Jimenez-Moleon JJ. 2020. Night shift work, chronotype, sleep duration, and prostate cancer risk: caplife study. Int J Environ Res Public Health. 17:6300. doi: 10.3390/ijerph17176300.
- Lunsford-Avery JR, Engelhard MM, Navar AM, Kollins SH. 2018. Validation of the sleep regularity index in older adults and associations with cardiometabolic risk. Sci Rep. 8:14158. doi: 10.1038/s41598-018-32402-5.
- Manber R, Bootzin R, Acebo C, Carskadon M. 1996. The effects of regularizing sleep-wake schedules on daytime sleepiness. Sleep. 19:432–441. doi: 10.1093/sleep/19.5.432.
- Martin JS, Laberge L, Sasseville A, Berube M, Alain S, Houle J, Hebert M. 2015. Day and night shift schedules are associated with lower sleep quality in evening-types. Chronobiol Int. 32:627–636. doi: 10.3109/07420528.2015.1033425.
- Medeiros ALD, Mendes DBF, Lima PF, Araujo JF. 2010. The relationships between sleep-wake cycle and academic performance in medical students. Biol Rhythm Res. 32:263–270. doi: 10.1076/brhm.32.2.263.1359.
- Okun ML, Reynolds CF, Buysse DJ, Monk TH, Mazumdar S, Begley A, Hall M. 2011. Sleep variability, health-related practices, and inflammatory markers in a community dwelling sample of older adults. Psychosom Med. 73:142–150. doi: 10.1097/PSY.0b013e3182020d08.
- Patel SR, Hayes AL, Blackwell T, Evans DS, Ancoli-Israel S, Wing YK, Stone KL, Osteoporotic Fractures in M, Study of Osteoporotic Fractures Research G. 2014. The association between sleep patterns and obesity in older adults. Int J Obes (Lond). 38:1159–1164. doi: 10.1038/ijo.2014.13.
- Ramin C, Devore EE, Pierre-Paul J, Duffy JF, Hankinson SE, Schernhammer ES. 2013. Chronotype and breast cancer risk in a cohort of us nurses. Chronobiol Int. 30:1181–1186. doi: 10.3109/07420528.2013.809359.
- Randler C. 2016. Chronotype in children and adolescents. Somnologie. 20:166–171. doi: 10.1007/s11818-016-0073-5.
- Roenneberg T, Pilz LK, Zerbini G, Winnebeck EC. 2019. Chronotype and social jetlag: a (self-) critical review. Biology (Basel). 8:54. doi: 10.3390/biology8030054.
- Roenneberg T, Wirz-Justice A, Merrow M. 2003. Life between clocks: daily temporal patterns of human chronotypes. J Biol Rhythms. 18:80–90. doi: 10.1177/0748730402239679.
- Sajjadieh A, Shahsavari A, Safaei A, Penzel T, Schoebel C, Fietze I, Mozafarian N, Amra B, Kelishadi R. 2020. The association of sleep duration and quality with heart tate variability and blood pressure. Tanaffos. 19:135–143.
- Sawilowsky S. 2009. New effect size rules of thumb. J Mod App Stat Meth. 8:597–599. doi: 10.22237/jmasm/1257035100.
- Siyahjani F, Garcia Molina G, Barr S, Mushtaq F. 2022. Performance evaluation of a smart bed technology against polysomnography. Sensors (Basel). 22:2605. doi: 10.3390/s22072605.
- Soehner AM, Kennedy KS, Monk TH. 2011. Circadian preference and sleep-wake regularity: associations with self-report sleep parameters in daytime-working adults. Chronobiol Int. 28:802–809. doi: 10.3109/07420528.2011.613137.
- Szumilas M. 2010. Explaining odds ratios. J Can Acad Child Adolesc Psychiatry. 19:227–229. Erratum in: J Can Acad Child Adolesc Psychiatry. 2015 Winter;24(1):58. PMID: 20842279; PMCID: PMC2938757.
- Taylor BJ, Hasler BP. 2018. Chronotype and mental health: recent advances. Curr Psychiatry Rep. 20:59. doi: 10.1007/s11920-018-0925-8.
- Vetter C, Devore EE, Ramin CA, Speizer FE, Willett WC, Schernhammer ES. 2015. Mismatch of sleep and work timing and risk of type 2 diabetes. Diab Care. 38:1707–1713. doi: 10.2337/dc15-0302.
- Wong PM, Hasler BP, Kamarck TW, Muldoon MF, Manuck SB. 2015. Social jetlag, chronotype, and cardiometabolic risk. J Clin Endocrinol Metab. 100:4612–4620. doi: 10.1210/jc.2015-2923.
- Yadav A, Rani S, Singh S. 2016. Working “out-of-phase” with reference to chronotype compromises sleep quality in police officers. Chronobiol Int. 33:151–160. doi: 10.3109/07420528.2015.1121876.
- Yazdi Z, Sadeghniiat-Haghighi K, Javadi AR, Rikhtegar G. 2014. Sleep quality and insomnia in nurses with different circadian chronotypes: morningness and eveningness orientation. Work. 47:561–567. doi: 10.3233/WOR-131664.