Abstract
The recovery of Moses Lake from hypereutrophy to mesotrophy over a 25-year period resulted from the addition of large quantities of low nitrogen (N) and phosphorus (P) dilution water and a change in irrigation practices. Throughout the recovery, the in-lake ratio of nitrate-N to soluble reactive P (SRP) remained well below the Redfield ratio, indicating short-term N limitation. Nevertheless, the disproportionately greater reduction in inflow P than N, relative to the Redfield ratio, caused the long-term reduction of total P (TP), SRP and chlorophyll. Lake TN:TP ratios consistently remained slightly above the Redfield ratio, apparently through N fixation, despite continued N limitation. Cyanobacteria (primarily Aphanizomenon) dominated the plankton algae during the recovery period until the lake became mesotrophic and calculated net internal loading of P was undetectable. These results demonstrate that usually inflow P, not N, should be reduced to effect long-term recovery of eutrophic lakes, despite observed short-term limitation by N.
Nitrogen (N) has received increased interest lately, not only as a limiting nutrient, but also as one that needs to be reduced, along with phosphorus (P), to control eutrophication (CitationPaerl 2007, Citation2008, CitationLewis et al. 2008, CitationLewis and Wurtsbaugh 2008, CitationConley et al. 2009). The many results from bioassays showing N to be limiting as often as P, and flaws in the evidence for over emphasis on P, support a new paradigm of N and P having more or less equal importance, according to CitationLewis and Wurtsbaugh (2008). However, the limiting nutrient in eutrophic lakes is usually N because N:P ratios decline with eutrophication (CitationDowning and McCauley 1992), and nitrate-N reaches very low, often undetectable levels in summer. So, would the limiting nutrient in the lake be the appropriate choice for reduction in the inflow to reduce the lake's trophic state? CitationLewis and Wurtsbaugh (2008) separated the question of which nutrient controls growth in the lake from which nutrient should be controlled in the input to manage lakes, and they concluded for management purposes the answer is P.
Evidence from lake responses to manipulations of input nutrients also points to P as the nutrient to reduce to reduce trophic state. While most of the management experience in recovering eutrophic lakes is from wastewater diversion in which inputs of both N and P were reduced, the low N:P ratio in wastewater (∼ 3:1) usually resulted in much more P removed than N, relative to the Redfield ratio (7:1). Therefore, most of the recovery has been accorded to P removal, especially when slow recovery was attributed to continued internal loading of P from sediments (CitationCooke et al. 2005). Further, an investigation of the response of 18 European lakes to wastewater diversion concluded that algal biomass began to decline only after soluble reactive phosphorus (SRP) reached < 10 μ g/L (CitationSas et al. 1989); therefore, controlling N would provide minimum benefit due to N fixation because N fixation operates to restore the N:P ratio to the Redfield level (CitationHutchinson 1970). Moreover, reducing the external N supply relative to P may actually promote dominance by cyanobacteria (Schindler 1977, CitationNürnberg 2007, CitationSchindler et al. 2008, CitationVrede et al. 2009).
The reduction of input N as well as P may be necessary to reduce algal biomass if N fixation by cyanobacteria is considered incapable of utilizing the available P in some lakes with low N:P ratios (CitationLewis et al. 2008). Others have advocated the need to target both N and P to reduce blooms of cyanobacteria in N-limited estuarine areas, or in lakes where watersheds are connected to estuaries (CitationPaerl, 2007, Citation2008, Conely et al. 2009). Nevertheless, the pertinent question for management of most eutrophic lakes and reservoirs is which nutrient should be targeted in the external input to reduce eutrophication and its symptoms?
This question assumes that water residence time is adequate for N-fixing cyanobacteria because growth is slow if dependent on N fixation. In lakes with N-fixing cyanobacteria, or where conditions are conducive to their growth, ratios of N:P and/or algal bioassays in the lake may not provide the correct answer. Long-term, whole-lake experiments in which the nutrient inputs are manipulated and the lake response in situ is observed are the only reliable way to judge the relative importance of each nutrient (CitationCarpenter 2008, CitationSchindler et al. 2008). Results from Lake 227 clearly show that not only is P the nutrient to target, but N reduction promoted blooms of N-fixing cyanobacteria (CitationSchindler et al. 2008). This effect supports the caution against N reduction, as did the results from 18 European lakes (CitationSas et al. 1989). Therefore, targeting both N and P may not only be much costlier than necessary, but may even promote blooms of N-fixing cyanobacteria, especially in cases of high internal P loading.
These results from Moses Lake before and after dilution water addition and a shift from rill (ditch) to spray irrigation demonstrate the importance of P control in the inflow despite N limitation. Those treatments reduced inflow P preferentially to N and moved the lake from hypereutrophy to low eutrophy and finally to mesotrophy over a 25-year period. Dilution water from the Columbia River was low in both P and N, but high background concentration of inflow N in Crab Creek caused a much greater reduction in P than N in the combined total inflow. The lake response to the reduction in inflow P concentration, relative to N, was determined by comparing pretreatment with three subsequent sets of post-treatment data from the central part of the lake. This case demonstrates that short-term indicators such as the N:P ratio and bioassays are not a direct indicator of long-term management effectiveness.
Description of moses lake
Moses Lake, in east-central Washington, was originally a natural lake created by wind blown sand dunes that had historically dammed Crab Creek. The lake level was stabilized with two dams, one constructed in 1929 and another in 1963.
The lake has an area of 2900 ha with a mean depth of 5.6 m and a maximum depth of 11.5 m at South Lake (). The lake is mostly polymictic because 80% of the area is too shallow to stratify. Parker Horn (site 7; ) and South Lake (site 9) together represent 26% of the lake's area and have been used to illustrate the trends in water quality (CitationWelch et al. 1992) because most of the dilution water entering via Crab Creek passed through those sections. However, the lower part of Rocky Ford Arm (Cascade, site 8), representing another 14% of the lake's area, also received dilution water and had similar water quality as Parker Horn. Even one-third of the water farther up Rocky Ford Arm (site 12) was composed of dilution water (as traced by conductivity; CitationWelch and Patmont 1980).
Figure 1 Moses Lake showing inflow of Columbia River dilution water from the East Low Canal (1) entering via Rocky Coulee Wasteway (2) into Crab Creek (3) and then into Parker Horn (5). Upper Parker Horn (5) water was pumped at times into Upper Pelican Horn (19). Water sampling sites are shown by number and solid lines for transects.
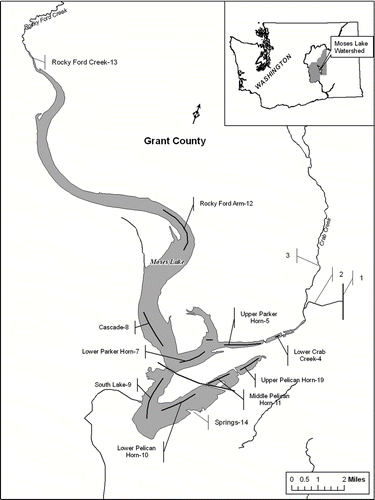
The two tributaries have similar flow volume (): Crab Creek drains 80% of the 5265 km2 watershed, which is mostly range land and dry land agriculture, but also contains some irrigated land (112 km2); and Rocky Ford Creek is largely spring fed. The two streams have typically contributed similar inflows during April–September (CitationJones and Welch 1990). If the combined flow rates represented the whole year, water residence time in the lake would be about 1 year (CitationJones and Welch 1990, CitationWelch et al. 1992).
Manipulations
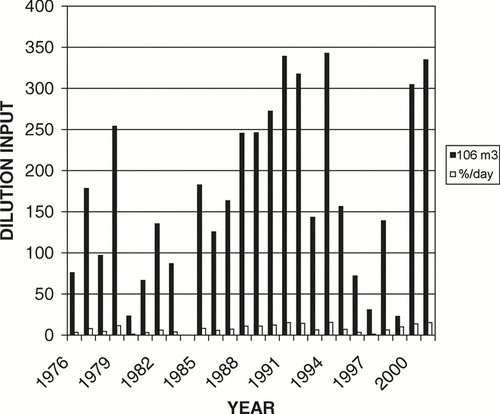
Starting in spring 1977, large volumes of low-nutrient Columbia River water were systematically diverted from the East Low Canal (site 1; ) through Rocky Coulee Wasteway (site 2) and into Crab Creek (site 3). Dilution water inputs from 1977 through 2001 averaged 170 × 106 m3 during April through early and sometimes late summer (). That quantity represents about 1.1 lake volumes, which would reduce water residence time to about 0.4 years if the April–September flows represented the whole year (CitationWelch et al. 1992). The lake has continued to receive dilution water each year, except in 1984. Dilution water averaged, in μ g/L: TP = 22, SRP = 4, and NO3-N = 34 (CitationWelch et al. 1989).
Figure 2 The quantity of Columbia River dilution water in 106 m3 (solid bars) added during spring and early summer to Parker Horn of Moses Lake from the East Low Canal through Rocky Coulee Wasteway and Crab Creek. Clear bars are water exchange rates for the Parker Horn volume in %/day for the 6-month dilution period, and whole-lake volume is 154 × 106 m3.
In addition to dilution water, sewage effluent from the City of Moses Lake was diverted from Middle Pelican Horn (site 11, ) in 1984, and irrigation practices gradually switched during the 1970s from about two-thirds rill to three-fourths spray type (CitationWelch and Weiher 1987). That switch may have caused the observed gradual decrease in SRP from 32 to 7 μ g/L and TP from 119 to 47 μ g/L in the background surface inflow entering via Crab Creek (site 3) during the 1970s and 1980s (CitationWelch et al. 1992). Wastewater diversion had a major effect in Middle Pelican Horn, a minor effect in South Lake and no effect in Parker Horn (CitationWelch et al. 1992).
Sampling procedures
The lake was sampled twice monthly at eight sites from April through September during 1969–1970 and 1977–1988 (CitationBush et al. 1972, CitationWelch et al. 1992). Water was collected at a depth of 0.5 m across a series of transects (). Discrete samples were also collected monthly at 0.5 m at the same sites during the spring–summer of 2001 by the Washington Deptartment of Ecology (CitationCarroll 2006). On the same occasions, samples were collected from Rocky Ford Creek (site 13), the inflow to the Rocky Ford Arm, and from Lower Crab Creek (site 4), the inflow to Parker Horn that combined Upper Crab Creek (site 3) flow with Columbia River dilution water entering from the East Low irrigation canal (site 1) via Rock Coulee Wasteway (site 2).
Sample analysis
Samples for soluble nutrients were filtered through 0.45-μ m Millipores at the site. The SRP was determined by the acid molybdate heteropoly blue method and NO3 by cadmium reduction (CitationEPA 1979). The TP and TN were determined on previously frozen samples: TP after persulfate digestion and TN by ultraviolet light oxidation through the mid-1980s (CitationStrickland and Parsons 1972) and afterward by persulfate oxidation (Solorzano and Sharp 1990). Oxidized samples were analyzed for NO2+ NO3-N.
Chlorophyll a (chl) was determined by the flurometric method, corrected for phaeophytin, through 1986 and spectrophometrically afterward. Due to instrument problems, values for 1984 were determined from a regression of algal biovolume on chl using past data. Methods for chl and nutrients are described in more detail in CitationWelch et al. (1992).
Data from Lower Parker Horn (site 7; ) and South Lake (site 9) are presented as averages of the spring–summer means within four groupings: pre-treatment (1969–1970), post-dilution (1977–1984), post-dilution and diversion (1986–1988), and most recent (2001). Data from 1985 are omitted from the period mean because of exceptionally high internal P loading (2 × average), caused by high wind mixing during August that produced low water column stability and high lake TP and chl. The other groups of years had rather consistent in-lake conditions, despite low dilution input in 1980 after the Mount St. Helens ash fall, and no dilution water addition in 1984, the year sewage was diverted. For example, the means and standard errors (± SE) for summer TP were 74 ± 3 and 44 ± 2 μ g/L for 1977–1984 and 1986–1988, respectively. Data from Lower Parker Horn and South Lake were used to indicate lake conditions during May–September because they were most affected by dilution water that began in April and by irrigation return, although Rocky Ford Arm (site 12) and Cascade (site 8) were also affected. Lower Crab Creek (site 4) during April–September represented inflow to Parker Horn of dilution water mixed with normal flow containing background nutrient levels.
Results
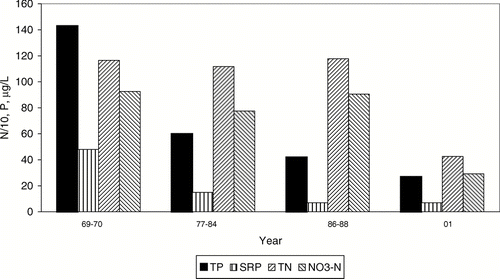
The effect of dilution water addition and changed irrigation practices was a 4-fold decrease in the spring–summer mean P concentrations in lower Crab Creek (site 4; ). The trend of decreasing inflow TP concentration persisted through 2001, while SRP changed little after the mid 1980s. In contrast, TN and NO3-N concentrations showed little change through the 1980s, remaining at levels of 1200 and 800 μ g/L, respectively. Not until 2001 did TN and NO3-N decrease by more than one-half (). The lower inflow TN and NO3-N concentrations in 2001 may have been due in part to larger dilution water addition, which was about double the average during 1986–1988 (). However, dilution water addition in 1986–1988 was already about 50% greater than during 1977–1984, yet inflow TN and NO3-N concentrations were similar for those two periods.
Figure 3 Average April–September in-flow concentrations of P and N to Parker Horn of Moses Lake before (1969–1970) and after the start (1977) of systematic dilution. N concentrations were divided by 10 to conform to scale for comparison.
Concentrations of TP and SRP in the lake (mean of Lower Parker Horn and South Lake) decreased in proportion to the decrease in inflow concentrations (). The TP decreased from a pre-treatment mean of > 150 μ g/L (hypereutrophy) to 17 μ g/L (mesotrophy) by 2001. Chlorophyll also declined in proportion to TP, from a pretreatment mean of > 50 to 11 μ g/L in 2001.
Figure 4 Average May–September chlorophyll and nutrients in Parker Horn and South Lake of Moses Lake before (1969–1970) and after the start (1977) of systematic dilution. N concentrations were divided by 10 to conform to scale for comparison.
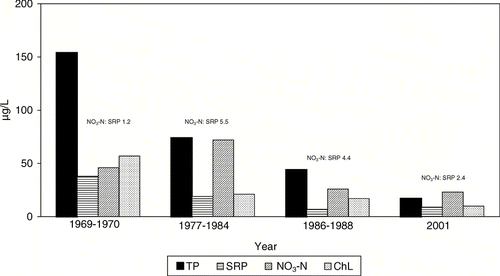
Total P still varies from year to year. Parker Horn was sampled on five occasions in 2005 and averaged 33 μ g/L (WQE 2005). Although South Lake was not sampled, TP probably would have been lower, judging from past years. Therefore, the two-area mean was probably around the 30 μ g/L meso-eutrophic boundary (CitationNürnberg 1996).
Mean spring–summer NO3-N concentration in the lake actually increased during 1977–1984 with dilution water addition before declining in the mid-1980s (). Nevertheless, low inlake NO3-N:SRP ratios persisted after treatment, indicating that N continued to be limiting. During periods of high algal biomass, NO3-N reached very low, often nondetectable levels.
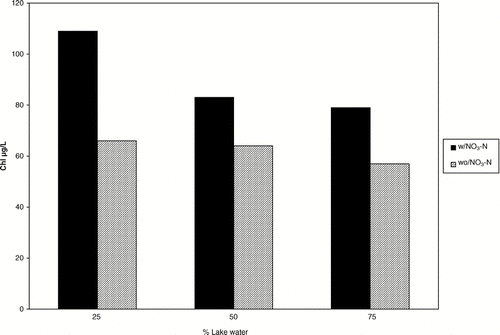
Nitrogen limitation was also demonstrated by in-lake bioassays conducted in 100 L plastic bags 1970 (CitationBuckley 1971, CitationWelch et al. 1972). Chlorophyll increased in proportion to the concentration of NO3-N added (). The NO3-N and SRP concentrations in all bags and the ambient lake averaged 9 and 3 μ g/L, respectively, at the start of the 13-day bioassay. Because low-nutrient dilution water with 9 μ g/L NO3-N and 16 μ g/L SRP was added to the bags at 25, 50 and 75%, there was dilution of the 1 mg/L NO3-N that was previously added to lake water in half the bags. Thus, the highest initial NO3-N concentration was in the 25% dilution bags, which produced the highest chl (). Colony counts of cyanobacteria, which represented nearly all the biomass, also increased much more with NO3-N added, but the increase in colonies was not proportional to NO3-N concentration added, as was the case for chl (; ).
Table 1 Net yield of cyanobacteria (Anabaena, Aphanizomenon and Microcystis) in colonies/mL in 100 L plastic bags suspended in Moses Lake in August 1970 diluted with low-nutrient Columbia River water with and without NO3-N added to lake water at 1 mg/L. Bag water was exchanged at 10%/day with water of the initial compositions during the 13-day experiment (from CitationBuckley, 1971).
Figure 5 Response of algae, as mean chl of four consecutive highest values, to low-nutrient Columbia River water addition to 100-L plastic bags in situ at 25, 50 and 75% lake water, with NO3-N added to lake water at 1 mg/L, resulting in initial NO3-N of 751, 502 and 232 μg/L, respectively. Controls (cross hatched) were with dilution water but no NO3-N added. NO3-N concentration decreased to an averaged of 4 μ g/L in all bags after 13 days of the experiment (CitationBuckley 1971).
While dilution water was low in N as well as P, the high NO3-N background levels in Crab Creek caused the dilution water addition to greatly raise the mixed inflow NO3-N:SRP ratio to nearly 60 from the 1969–1970 ratio of about 20 (). This amounted to a preferential input reduction of P over N; therefore, the reduction of chl and improvement in lake quality resulted from a reduction of in-flow P, despite the continued, lower-than Redfield, inlake NO3-N:SRP ratio () and continued short-term N limitation when those ratios were low in the lake (; ).
Figure 6 Average N:P ratios in the inflow (April–September) and in Parker Horn and South Lake of Moses Lake (May–September) before (1969–1970) and after the start (1977) of systematic dilution. The dashed line (RR) is the Redfield ratio.
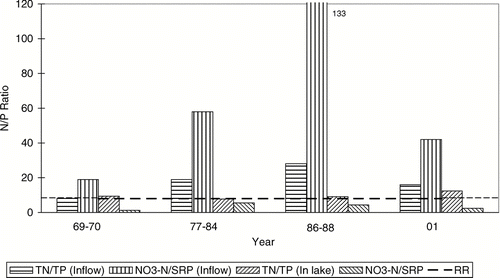
In contrast to the low NO3-N:SRP ratio, TN:TP in the lake (8–12) was consistently near the Redfield ratio, even though it was much higher in the inflow (). Again, the high background NO3-N in Crab Creek caused the high inflow TN:TP ratio. The inlake TN:TP ratio probably remained near the Redfield ratio due to N fixation; the N-fixer Aphanizomenon was usually the dominant alga. With the lake TN:TP ratio tending to be lowered by the high internal P loading, as well as probable denitrification, a high N-fixation rate was obviously necessary to maintain the in-lake TN:TP near the Redfield ratio (). Nitrogen fixation was measured in Moses Lake during July–August 1981–1982 (CitationBrenner 1983). The fixation rate in the top 2 m averaged 8 mg/m2 per day (n = 15), which was about equal (90%) to the summer loading rate of N. Nitrogen fixation by Aphanizomenon in another hypereutrophic lake (Clear Lake, CA), accounted for nearly half the total N input (CitationHorne and Goldman 1972). Mesocosm experiments in a eutrophic lake have shown similar contributions from N fixation in response to low inflow N:P ratio, with lake TN:TP remaining rather constant (CitationVrede et al. 2009).
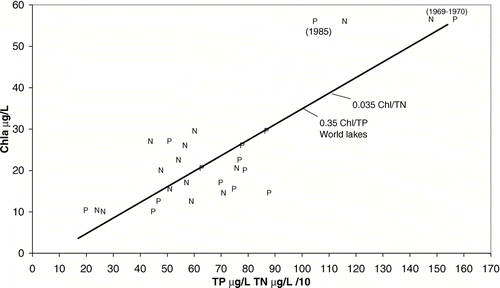
Maintenance of TN:TP near the Redfield ratio in the lake is illustrated by a comparison of chl with TN and TP (). The data fall reasonably close to a line that represents a chl:TP ratio of 0.35, which is the average for world lakes (CitationNürnberg 1996). The same line also represents a ratio for chl:TN that was set at 0.1 of the chl:TP ratio. The exceptionally high chl in 1985 (), despite dilution, was due to the high net internal P loading (computed by mass balance) from high wind mixing that summer, a rate of 4.6 mg/m2 per day versus the 10-year average of 1.9 mg/m2 per day. Net internal loading was shown to depend strongly on wind mixing, as indicated by relative thermal resistance to mixing (RTRM; Jones and Welch 1990).
Figure 7 Conformance of May–September means for chl, TP (P) and TN (N X 1/10 for scaling) to a line with slopes of 0.35 for chl:TP and 0.035 for chl:TN in Parker Horn and South Lake of Moses Lake before (1969–1970) and after the start (1977) of systematic dilution. High internal P loading accounts for nonconformance in 1985.
Discussion
The long-term dilution of Moses Lake with low-nutrient Columbia River water disproportionately lowered P more than N, despite similarly low N and P in dilution water. Dilution was more effective at lowering inflow P than N concentration because background NO3-N was very high. Also, background P concentrations in Crab Creek decreased substantially accompanying a switch in irrigation practiced during the 1970s–1980s. Decreased P, therefore, must have been the cause for the decrease in lake chl and change in lake trophic state from hypereutrophic to mesotrophic over a 25-year period. This is strong evidence that decreased P was the cause for the decrease in lake chl and the change in lake trophic state from hypereutrophic to mesotrophic over a 25-year period. This observation is consistent with current experience and rationale for emphasizing control of P rather than N to reduce eutrophication and its effects in lakes (CitationCarpenter 2008, CitationSchindler et al. 2008, CitationVrede et al. 2009).
Improvement of the lake's trophic state to mesotrophy in 2001 was probably due, in large part, to the lack of net internal P loading, computed from mass balance (CitationCarroll 2006). As long as internal loading of P continued to be one-third of the total (external plus internal), as was the average for 10 of 12 years during 1977–1988, increasing the volume of dilution water beyond 180 × 106 m3 (only ∼ 5% greater than the 25-year average) was expected to be only minimally effective at further reducing lake TP (CitationJones and Welch 1990). Declining internal P loading over that 25-year period following a reduction in inflow concentration was not unexpected, however, based on histories of lake recovery (CitationCooke et al. 2005).
Also consistent with the decrease in TP to a mesotrophic level (< 30 μ g/L), was the decreased dominance by cyanobacteria. This group of algae, consisting of primarily Aphanizomenon but also Anabaena and Microcystis, decreased from nearly 100% of the biomass in 1969–1970, when the lake was hypereutrophic, to an average of 65 ± 22% after dilution began in the 1970s–1980s, to < 5% in 2001 (CitationWelch et al. 1992, CitationCarroll 2006). That change was also not unexpected, because reducing TP to < 30 μ g/L and chl to < 10 μ g/L has been shown to greatly decrease the probability of cyanobacteria being 50% of the biomass (CitationDowning et al. 2001).
The lower inflow of TN and NO3-N concentrations in 2001 compared to previous years probably did not account for the further decrease in trophic state (). Concentration of TP was also lower in the inflow and the lake in 2001 than during 1986–1988, but in-lake NO3-N was not appreciably lower in 2001. Although in-lake TN was lower in 2001 (211 μ g/L) than during 1986–1988 (400 μ g/L), the TN:TP ratio in the lake was not substantially higher in 2001 (12.4) than in 1986–1988 (9.1).
The disproportionately greater reduction in P than N to Moses Lake was probably similar to that for most lakes recovering from the diversion of wastewater with a low TN:TP ratio. Wastewater diversion projects have not usually evaluated results in terms of change in inflow N:P ratio, but rather have emphasized P reduction in both inflow and lake water (CitationSas et al. 1989, CitationWilander and Persson 2001, CitationCooke et al. 2005). Consequently, the reduction of in-lake P has been considered the key to lake recovery. For example, in Lake Washington, where in-lake P and chl decreased markedly soon after diversion of wastewater, in-lake NO3-N remained relatively unchanged (CitationEdmondson 1970). As in Lake Washington, average spring–summer NO3-N in Moses Lake did not decline consistently with chl as did TP and SRP.
There may be situations where inflow N reduction would recover eutrophic lakes without resulting in increased dominance by cyanobacteria and N fixation, which would be unlike the results from Lake 227 (CitationSchindler et al. 2008). While N-fixation is apparently adequate to make up inlake N deficiencies in most eutrophic lakes, the energy demanding process may be too slow in reservoirs with short water retention times of 1–2 weeks. Retention times less than about 10 days have been found to reduce cyanobacteria biomass accumulation (CitationPersson 1981). Reduction of in-flow N in such circumstances would probably be cost-effective only if background P were naturally high and uncontrollable.
These results demonstrate that a management plan to reduce eutrophication should not be based solely on simple, short-term indicators, such as lake N:P ratio and bioassays. These indicators, while instructive, cannot represent the long-term response to whole-lake manipulations, as do the ELA experiments (CitationSchindler 2008) and histories of lake recovery from nutrient load reductions (CitationEdmondson 1970, CitationSas et al. 1989, CitationWilander and Persson 2001, CitationCooke et al. 2005). The results of these observations clearly show that P reduction, either from external or internal sources, most cost-effectively controls eutrophication in fresh water lakes. The author is unaware of any published case that demonstrates the effectiveness of N-only reduction, or for the necessity of N reduction in addition to P reduction.
Acknowledgments
Moses Lake was sampled through 1988, and data described here were produced by graduate students in the Department of Civil and Environmental Engineering, University of Washington. In chronological order they were: R. Bush, J. Buckley, C. Patmont, M. Tomasek, M. Brenner, K. Carlson, S. Marquis, E. Weiher, V. Okereke, C. Jones, and R. Barbiero. Funding for the work came from the Washington Department of Ecology (WDOE), USEPA and the Moses Lake Irrigation and Rehabilitation District (MLIRD) to the University of Washington.
References
- Buckley , J. A. 1971 . Effects of low nutrient dilution water and mixing on the growth of nuisance algae , MS thesis 116 University of Washington .
- Brenner , M. V. 1983 . The cause for the effect of dilution water in Moses Lake , MS thesis University of Washington .
- Bush , R. M. , Welch , E. B. and Buchanan , R. J. 1972 . Plankton associations and related factors in a hypereutrophic lake . Water Air Soil Pollut. , 1 : 257 – 274 .
- Carpenter , S. R. 2008 . Phosphorus control is critical to mitigating eutrophication . Proc. Natl. Acad. Sci. , 105 : 11039 – 11040 .
- Carroll , J. 2006 . Moses Lake phosphorus-response model and recommendations to reduce phosphorus loading , Olympia , WA : Dept. of Ecology, State of Washington . Pub. No. 06-03-011
- Conley , D. J. , Paerl , H. W. , Howarth , R. W. , Boesch , D. F. , Seitzinger , S. P. , Havens , K. E. , Lancelot , C. and Likens , G. E. 2009 . Controlling eutrophication: nitrogen and phosphorus . Science , 323 : 1014 – 1015 .
- Cooke , G. D. , Welch , E. B. , Peterson , S. A. and Nichols , S. A. 2005 . Restoration and management of lakes and reservoirs. , 3rd ed. , Boca Raton , FL : CRC Press, Inc. .
- Downing , J. A. and McCauley , E. 1992 . The nitrogen phosphorus relationship in lakes . Limnol. Oceanogr. , 37 : 936 – 945 .
- Downing , J. A. , Watson , S. B. and McCauley , E. 2001 . Predicting cyanobacteria dominance in lakes . Can. J. Fish. Aquat. Sci. , 58 : 1905 – 1908 .
- Edmondson , W. T. 1970 . Phosphorus, nitrogen, and algae in Lake Washington after diversion of sewage . Science , 169 : 690 – 691 .
- EPA . 1979 . Methods for the chemical analysis of water and wastes , Cincinnati , OH : Environmental Protection Agency, Office of Water Quality . EPA 600/4-79-020
- Horne , A. J. and Goldman , C. R. 1972 . Nitrogen fixation in Clear Lake, Calif. I. Seasonal variation and the role of heterocysts . Limnol. Oceanogr. , 17 : 678 – 692 .
- Hutchinson , G. E. 1970 . The Biosphere . Sci. Am. , 223 : 44 – 53 .
- Jones , C. A. and Welch , E. B. 1990 . Internal phosphorus loading related to mixing and dilution in a dentritic, shallow prairie lake . J. Water Pollut. Cont. Fed. , 62 : 847 – 852 .
- Lewis , W. M. Jr. , Saunders , J. F. III and McCutchan , J. H. Jr. 2008 . Application of a nutrient-saturation concept to the control of algal growth in lakes . Lake Reserv. Manage. , 24 : 41 – 46 .
- Lewis , W. M. Jr. and Wurtsbaugh , W. A. 2008 . Control of lacustrine phytoplankton by nutrients: Erosion of the phosphorus paradigm . Int. Rev. Hydrobiol. , 93 : 446 – 465 .
- Nürnberg , G. K. 1996 . Trophic state of clear and colored, soft- and hardwater lakes with special consideration of nutrients, anoxia, phytoplankton and fish . Lake Reserv. Manage. , 12 : 432 – 447 .
- Nürnberg , G. K. 2007 . Low-nirate days (LND), a potential indicator of cyanobacteria blooms in a eutrophic hardwater reservoir . Water Qual. Res. J. Can. , 42 : 269 – 283 .
- Paerl , H. Nutrient and other environmental controls of harmful cyanobacterial blooms along the fresh water—marine continuum . Proceedings of the Interagency, International Symposium on Cyanobacterial Harmful Algal Blooms, Advances in Experimental Medicine & Biology . Edited by: Hudnell , H. K. pp. 215 – 241 .
- Paerl , H. 2008 . Cyano HABs and climate change . Lakeline , 28 : 29 – 33 .
- Persson , P. E. 1981 . Growth of Oscillatoria agardhii in a hypereutrophic brackish-water bay . Anr. Bot. Finn. , 18 : 1 – 12 .
- Sas , H. , Ahlgren , I. , Bernhardt , H. , Bostrom , B. , Clasen , J. , Forsberg , C. , Imboden , D. , Kamp-Nielson , L. , Mur , L. , de Oude , N. , Reynolds , C. , Schreurs , H. , Seip , K. , Sommer , U. and Vermij , S. 1989 . Lake restoration by reduction of nutrient loading: expectations, experiences and extrapolations , St. Augustine , , Germany : Academia-Verlag Richarz .
- Schlindler , D. W. 1977 . Evolution of phosphorus limitation in lakes . Science , 195 : 260 – 262 .
- Schindler , D. W. , Hecky , R. E. , Findlay , D. L. , Stainton , M. P. , Parker , B. R. , Paterson , M. J. , Beaty , K. G. , Lyng , M. and Kasian , S. E. M. 2008 . Eutrophication of lakes cannot be controlled by reducing nitrogen input: Results of a 37-year whole-ecosystem experiment . Proc. Natl. Acad. Sci. , 105 : 11254 – 11258 .
- Solorzano , L. and Sharp , J. H. 1980 . Determination of total dissolved nitrogen in natural waters . Limnol. Oceanogr. , 25 : 751 – 754 .
- Strickland , J. D. H. and Parsons , T. R. 1972 . A practical handbook of seawater analysis. Bull. Fish. Res. Board Can. No. 167
- Vrede , T. , Ballantyne , A. , Mille-Lindblom , C. , Algesten , G. , Gudasz , C. , Lindahl , S. and Brunberg , A. K. 2009 . Effects of N:P loading ratios on phytoplankton community composition, primary production and N fixation in a eutrophic lake . Freshwater Biol. , 54 : 331 – 334 .
- Welch , E. B. , Barbiero , R. P. , Bouchard , D. and Jones , C. A. 1992 . Lake trophic state change and constant algal composition following dilution and diversion . Ecol. Eng. , 1 : 173 – 197 .
- Welch , E. B. , Buckley , J. A. and Bush , R. M. 1972 . Dilution as an algal bloom control . J. Water Poll. Cont. Fed. , 44 : 2245 – 2265 .
- Welch , E. B. , Jones , C. A. and Barbiero , R. P. 1989 . Moses Lake quality: results of dilution, sewage diversion and BMPs – 1977 through 1988 , Water Res. Ser. Tech. Rep. 118 65 Seattle , WA : Dept. Civil & Environ. Eng., University of Washington .
- Welch , E. B. and Patmont , C. R. 1980 . Lake restoration by dilution: Moses Lake, Washington . Water Res. , 14 : 1317 – 1325 .
- Welch , E. B. and Weiher , E. R. 1987 . Improvement in Moses Lake quality from dilution and sewage diversion . Lake Reserv. Manage. , 3 : 58 – 65 .
- Wilander , A. and Persson , G. 2001 . Recovery from eutrophication: Experiences of reduced phosphorus input to the four largest lakes of Sweden . Ambio , 30 : 475 – 485 .