Abstract
Objective: To determine the value of early- or mid-trimester amniotic fluid levels of interleukin-6 (IL-6), matrix metalloproteinase-8 (MMP-8), and glucose for predicting preterm delivery.
Methods: Randomized controlled trials and two-arm prospective, retrospective, cohorts, and case-controlled studies in which patients received early- or mid-trimester amniocentesis for karyotyping, and biomarker testing of the amniotic fluid was performed and delivery data were available were included in the analysis.
Results: Outcome measures were the associations of amniotic fluid IL-6, MMP-8, and glucose levels with preterm delivery. Differences in means with 95% confidence intervals (CIs) were calculated. Of 288 articles identified, 14 were included in the meta-analysis with a total of 675 patients who had preterm birth and 2518 patients who had term births. The preterm-delivery group had significantly higher amniotic fluid IL-6 and MMP-8 levels, and a significantly lower glucose level than the term delivery group (IL-6: difference in means = 0.32, 95% CI: 0.22–0.43, p < 0.001; MMP-8: difference in means = 4.47, 95% CI: 0.83–8.11), p = 0.016; glucose: difference in means = −5.22, 95% CI: −8.19 to −2.26, p = 0.001)
Conclusion: Early- or mid-trimester amniotic fluid IL-6, MMP-8, and glucose levels are useful for predicting the risk of preterm delivery.
Median amniotic fluid ferritin and IL-6 levels, and mean amniotic fluid ALP levels were higher in the preterm group.
The preterm-delivery group had significantly higher amniotic fluid IL-6 and MMP-8 levels, and a significantly lower glucose level than the term-delivery group.
KEY MESSAGES
Introduction
Preterm birth is defined as a birth that occurs before the 37th week of gestation, and is estimated to occur in up to 10% of all pregnancies (Citation1). As a leading cause of neonatal morbidity and mortality, preterm births account for around 70% of neonatal deaths and 50% of long-term neurological disabilities (Citation2). Thus, preterm births place a large burden on healthcare systems worldwide (Citation1,Citation3). The etiology of preterm birth remains largely unknown despite years of research, and is likely the result of multifactorial causes (Citation1). Intraamniotic infection and/or inflammation, however, have been strongly correlated with the risk of preterm birth (Citation4). The ability to predict preterm birth and patients at risk of delivering prematurely is important as it may allow interventions to decrease the risk and thus lower preterm birth rates, and associated neonatal morbidity and mortality.
Numerous biomarkers, defined as parameters that can be measured in a biological sample that can provide information on the potential effects of exposure, have been examined for their potential to determine an increased risk of preterm birth (Citation5,Citation6). The wide range of biomarkers examined includes serum, amniotic fluid, and/or cervicovaginal fluid levels of various cytokines (e.g., interleukins (IL), angiogenin, transforming growth factor alpha (TGF-α), macrophage inflammatory protein 1-alpha (MIP-1-alpha), and intracellular adhesion molecule 1(ICAM-1) (Citation5,Citation7–9). The majority of those examined, however, have not been shown to have value in predicting preterm birth.
Early- and mid-trimester amniocentesis remains a common method for the prenatal diagnosis of chromosomal anomalies and other genetic disorders. As intraamniotic inflammation/infection has been linked to the risk of preterm delivery, attention has been given to the evaluation of amniotic fluid biomarkers that may indicate intraamniotic inflammation/infection that can be obtained at the time of amniocentesis. Although a number of amniotic fluid biomarkers have been studied, those that have shown the most promise include interleukin-6 (IL-6), matrix metalloproteinase-8 (MMP-8), and glucose, though studies have not been entirely consistent with respect to their predictive value (Citation5,Citation7,Citation10–22).
Thus, the purpose of this study was to perform a systematic review of the literature and meta-analysis to examine the value of amniotic fluid levels of IL-6, MMP-8, and glucose obtained at early- or mid-trimester amniocentesis performed for prenatal diagnosis for predicting subsequent preterm delivery.
Methods
Sources
This systematic review and meta-analysis was conducted in accordance with PRISMA guidelines (Citation23). Medline, PubMed, Cochrane, EMBASE, and Google Scholar databases were searched until 22 December 2015 using combinations of the keywords: amniocentesis, amniotic fluid, biomarkers, predictive biomarkers, preterm birth, spontaneous preterm delivery, interleukin-6, matrix metalloproteinase-8 (MMP-8), glucose, and neonatal complications. Reference lists of relevant studies were hand-searched to identify additional studies. Studies were identified by the search strategy by two independent reviewers, and when there was uncertainty regarding eligibility, a third reviewer was consulted.
Study selection
Inclusion criteria for the meta-analysis were: (Citation1) Randomized controlled trials and two-arm prospective, retrospective, cohorts, and case-controlled studies; (Citation2) Patients received early- or mid-trimester amniocentesis for karyotyping and biomarker testing of the amniotic fluid was performed; (Citation3) Delivery data were available. Letters, comments, editorials, case reports, and personal communications were excluded, as were studies in which no primary outcome data was presented.
The following information/data were extracted from studies that met the inclusion criteria: the name of the first author, year of publication, study design, number of participants in each group, participants’ age and gender, and outcome data. Data were extracted by two reviewers, and when there was uncertainty, a third reviewer was consulted.
Quality assessment
The Newcastle-Ottawa scale was used to assess the quality of cohort and case-controlled studies (Citation24). The Newcastle-Ottawa scale is a validated tool that evaluates the quality of nonrandomized studies in three broad areas: enrollment criteria, comparability of study groups, and assessment of outcomes. The quality of each study is scored on the basis of the areas mentioned above, with 4 of the 9 total points indicating the quality of the patient selection; two points the quality of between-group comparability, and three points the quality of the outcome assessment. A score of 9 indicates the highest quality, a score of 0, the lowest. Two independent reviewers performed the quality assessment, and a third was consulted for resolution of any disagreements.
Outcome measures and data analysis
Outcome measures were the associations of amniotic fluid levels of IL-6, MMP-8, and glucose with preterm delivery. Means with standard deviations were calculated and were compared between patients with and without preterm delivery. If means and standard deviations were not reported, then the median, range, and the size of the sample were used to estimate the mean and variance (Citation25). If the median and interquartile range (IQR) were reported, it was assumed that the median of the outcome variable was equal to the mean response, and the width of the interquartile range was ∼1.35 standard deviation (Citation26).
An effect size, difference in means with 95% confidence interval (CI), was calculated for each individual study and for studies combined. A difference in means >0 indicated the effect size was higher in the preterm than in the term-delivery group, and a difference in means <0 indicated the effect size was lower in the preterm than in the term-delivery group. A difference in means = 0 indicated the effect size was similar between the preterm- and term-delivery groups. A χ2-based test of homogeneity was performed and the inconsistency index (I2) and Q statistics were determined. If I2 was >50% or >75%, the study was considered to be heterogeneous or highly heterogeneous, respectively. If I2 was <25%, the study was considered to be homogeneous. If the I2 statistic was >50% or the Q statistic value of p was <0.05, a random-effects model of analysis was used because significant heterogeneity was present. Otherwise, fixed-effects models were employed. Combined effects were calculated, and a two-sided p value <0.05 was considered to indicate statistical significance. Sensitivity analysis was carried out using the leave-one-out approach. Publication bias was assessed by constructing funnel plots and Egger’s test. The absence of publication bias was indicated by the data points forming a symmetric funnel-shaped distribution, and a one-tailed significance level p > 0.05 (Egger’s test) (Citation27). All analyses were performed using Comprehensive Meta-Analysis statistical software, version 2.0 (Biostat, Englewood, NJ).
Results
Literature search
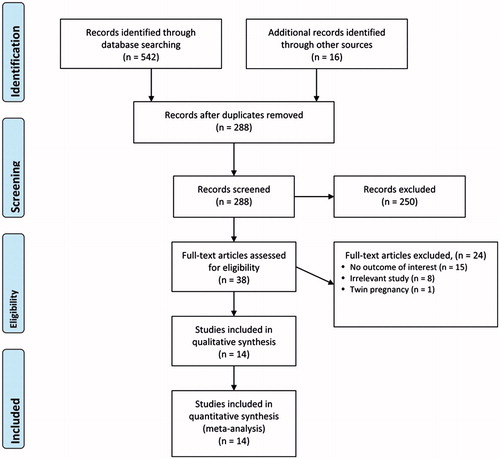
A flow diagram of study selection is shown in . A total of 288 articles were identified in the database searches after duplicates were removed. After screening by abstracts and titles, 250 articles were excluded and the full texts of 38 articles were assessed for eligibility. Of these, 24 were excluded, the reasons for which are shown in . Thus, 14 studies were included in the meta-analysis (Citation7,Citation10–22).
Basic characteristics of the studies are summarized in , and amniotic fluid levels of IL-6, MMP-8, and glucose are shown in . The majority of the studies were either prospective cohort or case-controlled. The 14 studies included a total of 675 patients who delivered preterm, and 2518 patients who had term births. The mean maternal age ranged from 31 to 40 years.
Table 1. Characteristics of studies included in the meta-analysis.
Table 2. Amniotic fluid IL-6, MMP-8, and glucose levels.
Outcome measures and meta-analyses
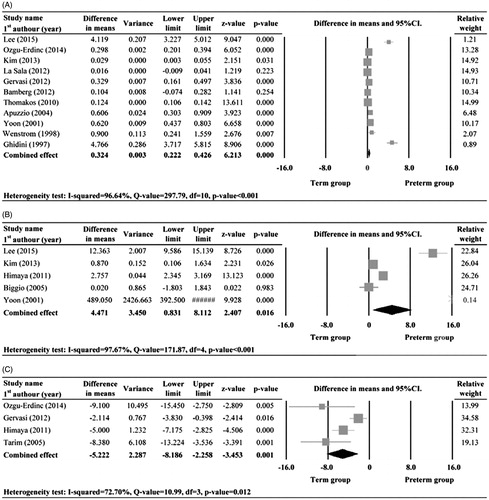
There were eleven (Citation7,Citation10–15,Citation17,Citation20–22), five (Citation10,Citation12,Citation16,Citation19,Citation21), and four (Citation11,Citation14,Citation16,Citation18), studies with complete data of amniotic fluid IL-6, MMP-8, and glucose levels for the preterm- and term-delivery groups, respectively, that were included in the meta-analyses. There was evidence of heterogeneity in the studies with respect to all three measures, and thus random-effects models of analysis were used (IL-6: Q statistic = 297.79, I2 = 96.64%, p < 0.001; MMP-8: Q statistic = 171.87, I2 = 97.67%, p < 0.001; glucose: Q statistic = 10.99, I2 = 72.70%, p = 0.012).
The combined difference in means of the analyses indicated that the preterm-delivery group had significantly higher amniotic fluid IL-6 and MMP-8 levels, and a significantly lower glucose level than the term-delivery group (IL-6: difference in means = 0.32, 95%CI: 0.22–0.43, p < 0.001; MMP-8: difference in means = 4.47, 95%CI: 0.83–8.11), p = 0.016; glucose: difference in means = −5.22, 95%CI: −8.19 to −2.26, p = 0.001) ().
Sensitivity analysis and publication bias
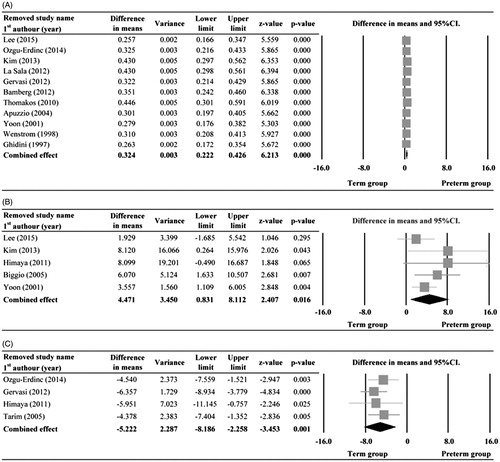
Sensitivity analysis was performed using the leave-one-out approach in which the meta-analyses of IL-6, MMP-8, and glucose were performed with each study removed in turn (). The direction and magnitude of combined estimates did not vary markedly with the removal of the studies, indicating that each of the meta-analyses had good reliability and the data was not overly influenced by each study.
Figure 3. Sensitivity-analysis for IL-6 (A), MMP-8 (B), and glucose (C) between preterm and term delivery groups. Abbreviations: SE: standard error; CI: confidence interval, lower limit and upper limit; lower limit and upper limit of 95% CI, respectively.
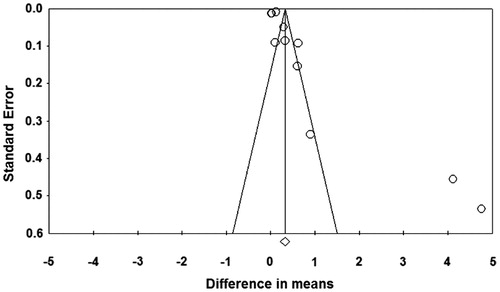
However, the results of Egger’s test showed the publication bias might be present in the analysis of IL-6 (t = 3.03, one-tailed p = 0.007, ). Publication bias was not assessed for MMP-9 and glucose as there were not enough studies to detect funnel plot asymmetry (Citation27).
Quality assessment
The Newcastle-Ottawa quality assessment of the included studies is shown in . All studies had a quality score of 7 or more, indicating moderate to high quality. The risk of bias of individual studies was low.
Discussion
Preterm delivery is a significant cause of neonatal morbidity and mortality worldwide, and utilizes a large portion of healthcare resources. Identifying patients at risk of preterm delivery at early stage of pregnancy may allow interventions that result in lower preterm birth rates and associated decrease in neonatal morbidity and mortality. The results of this meta-analysis indicated that elevated early or mid-trimester amniotic fluid IL-6 and MMP-8 levels, and decreased glucose levels are associated with preterm delivery.
A large amount of research has been devoted to determining risk factors and predictors of preterm delivery; and while advances have been made, no single method has been identified that can consistently predict which patients will delivery prematurely (Citation3). As intraamniotic infection/inflammation has been clearly shown to be associated with and increased risk of preterm delivery, attention has been given to examining the predictive value of biomarkers associated with inflammation (Citation5,Citation8,Citation10). Although many biomarkers have been examined, including levels determined in maternal serum, amniotic fluid, and cervicovaginal fluid, amniotic fluid levels of IL-6, MMP-8, and glucose have shown a great deal of promise.
IL-6 is an interleukin that has a broad range of functions and acts as both a pro-inflammatory cytokine and an anti-inflammatory myokine (Citation28). A number of studies have reported an association of elevated amniotic fluid IL-6 levels and preterm delivery (Citation9,Citation12,Citation29). Interestingly, however, although elevated levels are considered to indicate intraamniotic infection and/or inflammation, Weissenbacher et al. (Citation30) reported similar amniotic fluid levels in patients with clinical signs of intrauterine infection or inflammation and those without any evidence of infection or inflammation. On the other hand, the study showed IL-10 and IL-8 levels were significantly elevated in patients with evidence of infection and IL-4 levels were significantly lower in those with evidence of infection as compared to those without infection. While most evidence suggests elevated IL-6 levels are associated with infection/inflammation and predictive of preterm delivery, few studies have examined the predictive value of specific cutoff points. Hus et al. (Citation31) examined amniotic fluid levels of IL-6 in 350 women who had an amniocentesis at 16–20 weeks for genetic indications. Of the 350 women, 58 delivered at <37 weeks’ gestation and receiver operating characteristic analysis revealed that an IL-16 cutoff value of 105 pg/mL had a sensitivity of 90.2%, specificity of 52.7%, and a negative predictive value of 84.3% for predicting a preterm birth.
MMPs are a large family of endopeptidases, which are capable of degrading extracellular matrix proteins, processing various bioactive molecules, and are involved in chemokine/cytokine inactivation and cell behaviors including proliferation, migration, differentiation, and apoptosis (Citation32). Elevated cervical tissue MMP-8 levels are associated with cervical ripening at term (Citation33), and increased levels in amniotic fluid have been associated with premature rupture of the membranes, and amniotic infection (Citation19,Citation34,Citation35). Similar to IL-6, a number of studies have reported that elevated amniotic fluid levels are predictive of preterm delivery (Citation9,Citation10,Citation12,Citation36). Little data, however, is available as to specific values and their sensitivity and specificity for predicting preterm birth. Interestingly, Rohkonen et al. (Citation37) reported that cervical fluid MMP-8 concentrations in early and mid-pregnancy were not different between women who delivered at term or preterm, but were lower in mid-pregnancy in women with preterm delivery preceded by premature preterm rupture of membranes <37 weeks as compared with women who delivered at term and women who had preterm delivery initiated by spontaneous onset of labor.
Prior study has clearly shown that a decreased amniotic fluid glucose level is associated with microorganisms in the amniotic cavity and/or an inflammatory response (Citation38–40). Studies indicating a decreased amniotic fluid glucose level is predictive of preterm delivery are consistent with these findings and support the hypothesis that intraamniotic infection/inflammation is a causative factor in preterm delivery.
Two prior systematic reviews have examined a wide range of biomarkers for predicting preterm delivery. In 2011, Conde-Agudelo et al. (Citation5) evaluated 30 novel biomarkers by reviewing 72 studies including 89,786 women. The results showed that while proteome profile and prolactin in cervicovaginal fluid and MMP-8 in amniotic fluid had good predictive value, they were only examined in a small number of studies. IL-6 and angiogenin in amniotic fluid, and human chorionic gonadotrophin and phosphorylated insulin-like growth factor binding protein-1 in cervicovaginal fluid had moderate predictive accuracy; and the remaining biomarkers had low predictive accuracies. The authors concluded that none of the evaluated biomarkers were clinically useful for predicting preterm birth. Another review in 2011 by Hee et al. (Citation9) found that the value of specific biomarkers for predicting preterm delivery varied with risk preterm delivery (high vs. low risk), and whether or not symptoms of preterm delivery were present. There are some differences between the two prior analyses and the current investigation. The two prior studies primarily focused on specimens collected at a later stage of pregnancy or immediately prior to delivery. The current study, however, focused on specimens collected during early or midtrimester amniocentesis and indicated there was a predictive value to samples collected early in pregnancy, which may have value in providing early intervention to patients at increased risk of preterm delivery.
There are limitations to this study that should be considered with respect to the interpretation of the results. Significant heterogeneity was present among the studies; however, the sensitivity analysis using leave-one-out approach did not find any marked variation, hence the analysis can be considered robust. Publication bias could only be assessed for IL-6 due to the limited numbers of studies for the other markers, and the assessment indicated possible publication bias due to the extreme values found in Lee et al. (Citation10) and Ghidini et al. (Citation7) studies. The predictive value of combinations of the biomarkers was not examined (Citation41), nor did we examine the value of a number of other biomarkers that studies have suggested may be useful for predicting preterm delivery. We did not examine the effect of complications occurring later in pregnancy, for example, premature rupture of the membranes or neonatal complications. Lastly, race and ethnicity were not taken into account and some studies have suggested that biomarker levels and their association with preterm delivery vary with race (Citation6,Citation42).
In summary, early- or mid-trimester amniotic fluid IL-6, MMP-8, and glucose levels are useful for predicting the risk of preterm delivery. Though the sensitivity, specificity, and cutoff points are yet to be determined, levels determined at the time of genetic amniocentesis may allow categorization of a patient as increased risk of preterm delivery and closer monitoring and early intervention may lead to improved outcomes. Further studies of the predictive value of biomarkers collected early in pregnancy for preterm delivery are warranted.
Disclosure statement
The authors declare no conflict of interest.
Funding
The present study was funded by a Research Project of Science and Technology grant from Guangdong Provincial Science and Technology Department [2013B022000015]; the China Preventive Medicine Research Project; and the National Natural Science Foundation of China [81470067].
References
- Frey HA, Klebanoff MA. The epidemiology, etiology, and costs of preterm birth. Semin Fetal Neonatal Med. 2016;21:68–73.
- Matthews TJ, MacDorman MF. Infant mortality statistics from the 2010 period linked birth/infant death data set. Natl Vital Stat Rep. 2013;62:1–26.
- Georgiou HM, Di Quinzio MK, Permezel M, Brennecke SP. Predicting preterm labour: current status and future prospects. Dis Markers. 2015;2015:435014.
- Vinturache AE, Gyamfi-Bannerman C, Hwang J, Mysorekar IU, Jacobsson B, Preterm Birth International Collaborative (PREBIC). Maternal microbiome - A pathway to preterm birth. Semin Fetal Neonatal Med. 2016;21:94–9.
- Conde-Agudelo A, Papageorghiou AT, Kennedy SH, Villar J. Novel biomarkers for the prediction of the spontaneous preterm birth phenotype: a systematic review and meta-analysis. BJOG. 2011;118:1042–54.
- Brou L, Almli LM, Pearce BD, Bhat G, Drobek CO, Fortunato S, et al. Dysregulated biomarkers induce distinct pathways in preterm birth. BJOG. 2012;119:458–73.
- Ghidini A, Jenkins CB, Spong CY, Pezzullo JC, Salafia CM, Eglinton GS. Elevated amniotic fluid interleukin-6 levels during the early second trimester are associated with greater risk of subsequent preterm delivery. Am J Reprod Immunol. 1997;37:227–31.
- Cháfer-Pericás C, Stefanovic V, Sánchez-Illana Á, Escobar J, Cernada M, et al. Novel biomarkers in amniotic fluid for early assessment of intraamniotic infection. Free Radic Biol Med. 2015;89:734–40.
- Hee L. Likelihood ratios for the prediction of preterm delivery with biomarkers. Acta Obstet Gynecol Scand. 2011;90:1189–99.
- Lee SM, Park JS, Norwitz ER, Oh S, Kim EJ, Kim SM, et al. Mid-trimester amniotic fluid pro-inflammatory biomarkers predict the risk of spontaneous preterm delivery intwins: a retrospective cohort study. J Perinatol. 2015;35:542–6.
- Ozgu-Erdinc AS, Cavkaytar S, Aktulay A, Buyukkagnici U, Erkaya S, Danisman N. Mid-trimester maternal serum and amniotic fluid biomarkers for the prediction of preterm delivery and intrauterine growth retardation. J Obstet Gynaecol Res. 2014;40:1540–6.
- Kim A, Lee ES, Shin JC, Kim HY. Identification of biomarkers for preterm delivery in mid-trimester amniotic fluid. Placenta. 2013;34:873–8.
- La Sala GB, Ardizzoni A, Capodanno F, Manca L, Baschieri MC, Soncini E, et al. Protein microarrays on midtrimester amniotic fluids: a novel approach for the diagnosis of early intrauterine inflammation related to preterm delivery. Int J Immunopathol Pharmacol. 2012;25:1029–40.
- Gervasi MT, Romero R, Bracalente G, Erez O, Dong Z, Hassan SS, et al. Midtrimester amniotic fluid concentrations of interleukin-6 and interferon-gamma-inducible protein-10: evidence for heterogeneity of intra-amniotic inflammation and associations with spontaneous early (<32 weeks) and late (>32 weeks) preterm delivery. J Perinat Med. 2012;40:329–43.
- Bamberg C, Fotopoulou C, Thiem D, Roehr CC, Dudenhausen JW, Kalache KD. Correlation of midtrimester amniotic fluid cytokine concentrations with adverse pregnancy outcome in terms of spontaneous abortion, preterm birth, and preeclampsia. J Matern Fetal Neonatal Med. 2012;25:812–17.
- Himaya E, Rhalmi N, Girard M, Tétu A, Desgagné J, Abdous B, et al. Midtrimester intra-amniotic sludge and the risk of spontaneous preterm birth. Am J Perinatol. 2011;28:815–20.
- Thomakos N, Daskalakis G, Papapanagiotou A, Papantoniou N, Mesogitis S, Antsaklis A. Amniotic fluid interleukin-6 and tumor necrosis factor-alpha at mid-trimester genetic amniocentesis: relationship to intra-amniotic microbial invasion and preterm delivery. Eur J Obstet Gynecol Reprod Biol. 2010;148:147–51.
- Tarim E, Bağiş T, Kiliçdağ EB, Sezgin N, Yanik F. Are amniotic fluid C-reactive protein and glucose levels, and white blood cell counts at the time of genetic amniocentesis related with preterm delivery? J Perinat Med. 2005;33:524–9.
- Biggio JR, Jr., Ramsey PS, Cliver SP, Lyon MD, Goldenberg RL, Wenstrom KD. Midtrimester amniotic fluid matrix metalloproteinase-8 (MMP-8) levels above the 90th percentile are a marker for subsequent preterm premature rupture of membranes. Am J Obstet Gynecol. 2005;192:109–13.
- Apuzzio J, Chan Y, Al-Khan A, Illsley N, Kim PL, Vonhaggen S. Second-trimester amniotic fluid interleukin-10 concentration predicts preterm delivery. J Matern Fetal Neonatal Med. 2004;15:313–17.
- Yoon BH, Oh SY, Romero R, Shim SS, Han SY, Park JS, et al. An elevated amniotic fluid matrix metalloproteinase-8 level at the time of mid-trimester genetic amniocentesis is a risk factor for spontaneous preterm delivery. Am J Obstet Gynecol. 2001;185:1162–7.
- Wenstrom KD, Andrews WW, Hauth JC, Goldenberg RL, DuBard MB, Cliver SP. Elevated second-trimester amniotic fluid interleukin-6 levels predict preterm delivery. Am J Obstet Gynecol. 1998;178:546–50.
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151:W65–94.
- Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses; 2002. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm.
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13.
- Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration; 2011. Available from: http://www.cochrane-handbook.org. Updated March, 2011.
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002.
- Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813:878–88.
- Chaemsaithong P, Romero R, Korzeniewski SJ, Martinez-Varea A, Dong Z, Yoon BH, et al. A point of care test for interleukin-6 in amniotic fluid in preterm prelabor rupture of membranes: a step toward the early treatment of acute intra-amniotic inflammation/infection. J Matern Fetal Neonatal Med. 2016;29:360–7.
- Weissenbacher T, Laubender RP, Witkin SS, Gingelmaier A, Schiessl B, Kainer F, et al. Diagnostic biomarkers of pro-inflammatory immune-mediated preterm birth. Arch Gynecol Obstet. 2013;287:673–85.
- Hsu TY, Lin H, Lan KC, Ou CY, Tsai CC, Cheng BH, et al. High interleukin-16 concentrations in the early second trimester amniotic fluid: an independent predictive marker for preterm birth. J Matern Fetal Neonatal Med. 2013;26:285–9.
- Verma RP, Hansch C. Matrix metalloproteinases (MMPs): chemical-biological functions and (Q)SARs. Bioorg Med Chem. 2007;15:2223–68.
- Sennstrom MB, Brauner A, Bystrom B, Malmstrom A, Ekman G. Matrix metalloproteinase-8 correlates with the cervical ripening process in humans. Acta Obstet Gynecol Scand. 2003;82:904–11.
- Lee SE, Romero R, Jung H, Park CW, Park JS, Yoon BH. The intensity of the fetal inflammatory response in intraamniotic inflammation with and without microbial invasion of the amniotic cavity. Am J Obstet Gynecol. 2007;197:294.e1–e6.
- Maymon E, Romero R, Pacora P, Gomez R, Athayde N, Edwin S, et al. Human neutrophil collagenase (matrix metalloproteinase 8) in parturition, premature rupture of the membranes, and intrauterine infection. Am J Obstet Gynecol. 2000;183:94–9.
- Kim SM, Romero R, Lee J, Chaemsaithong P, Lee MW, Chaiyasit N, et al. About one-half of early spontaneous preterm deliveries can be identified by a rapid matrix metalloproteinase-8 (MMP-8) bedside test at the time of mid-trimester genetic amniocentesis. J Matern Fetal Neonatal Med. 2015;7:1–9.
- Rahkonen L, Rutanen EM, Nuutila M, Sainio S, Sorsa T, Paavonen J. Matrix metalloproteinase-8 in cervical fluid in early and mid pregnancy: relation to spontaneous preterm delivery. Prenat Diagn. 2010;30:1079–85.
- Romero R, Jimenez C, Lohda AK, Nores J, Hanaoka S, Avila C, et al. Amniotic fluid glucose concentration: a rapid and simple method for the detection of intraamniotic infection in preterm labor. Am J Obstet Gynecol. 1990;163:968–74.
- Kirshon B, Rosenfeld B, Mari G, Belfort M. Amniotic fluid glucose and intraamniotic infection. Am J Obstet Gynecol. 1991;164:818–20.
- Gauthier DW, Meyer WJ, Bieniarz A. Correlation of amniotic fluid glucose concentration and intraamniotic infection in patients with preterm labor or premature rupture of membranes. Am J Obstet Gynecol. 1991;165:1105–10.
- Jia X. Value of amniotic fluid IL-8 and Annexin A2 in prediction of preterm delivery in preterm labor and preterm premature rupture of membranes. J Reprod Med. 2014;59:154–60.
- Menon R, Bhat G, Saade GR, Spratt H. Multivariate adaptive regression splines analysis to predict biomarkers of spontaneous preterm birth. Acta Obstet Gynecol Scand. 2014;93:382–91.