Abstract
Background: Few studies investigated the combined effects of night-shift work, daytime napping, and nighttime sleep on cancer incidence and mortality.
Methods: A total of 25,377 participants were included in this study. Information on sleep habits, cancer incidences, and mortalities were collected. Cox proportional hazards models were used to calculate the adjusted hazard ratios and 95% confidence intervals (HRs, 95%CIs).
Results: Male subjects experienced ≥20 years of night-shift work, or without daytime napping had an increased risk of cancer, when compared with males who did not have night-shift work or napped for 1–30 min [HR (95%CI) = 1.27 (1.01–1.59) and 2.03 (1.01–4.13), respectively]. Nighttime sleep for ≥10 h was associated with a separate 40% and 59% increased risk of cancer [HR (95%CI) = 1.40 (1.04–1.88)] and cancer-caused mortality [HR (95%CI) = 1.59 (1.01–2.49)] than sleep for 7–8 h/night. Combined effects of three sleep habits were further identified. Male participants with at least two above risk sleep habits had a 43% increased risk of cancer [HR (95%CI) = 1.43 (1.07–2.01)] and a 2.07-fold increased cancer-caused mortality [HR (95%CI) = 2.07 (1.25–3.29)] than those who did not have any above risk sleep habits. However, no significant associations were observed among women.
Conclusions: Long night-shift work history, without daytime napping, and long nighttime sleep duration were independently and jointly associated with higher cancer incidence among males.
Night-shift work of ≥20 years, without napping, and nighttime sleep of ≥10 h were associated with increased cancer incidence.
Nighttime sleep ≥10 h was associated with a 2.07-fold increased cancer-caused mortality among males.
Combined effects of night-shift work ≥20 years, without napping, and nighttime sleep ≥10 h on increasing cancer incidence were existed among males.
KEY MESSAGES
Introduction
Cancer is the leading causes of death over the world, while more than 30% of cancer deaths could be prevented by modifying or avoiding risk factors, such as unhealthy living habits, including lack of physical activity, tobacco, and alcohol use (Citation1). Some studies have found that sleep habits, including night-shift work, daytime napping, and nighttime sleep, are associated with a series of adverse health outcomes, including metabolic syndrome (Citation2), cancer incidence (Citation3), and total mortality (Citation4). Night-shift work-related circadian disruption has been classified as the possibly carcinogenic to humans (Citation5). Exposure to light at night is associated with the suppressed pineal hormone melatonin, and impaired immune system by sleep deprivation (Citation6). Several epidemiologic studies reported increased risk for breast, colon, and prostate cancers among longtime night-shift workers (Citation7).
Daytime napping habits can reflect different concepts of lifestyle and health (Citation8), and it is regarded as a common life behavior among habitual nappers, since daytime napping might be a part of good lifestyle with stress relieving effects (Citation9). Although studies observed that excessive daytime napping is associated with increased risk of all-cause mortality and breast cancer risk (Citation10,Citation11), because excessively daytime napping may indicate an unhealthy condition, poorer quality of nighttime sleep (Citation12), the mechanisms for the relationship are not fully understood.
Both short and long nighttime sleep duration have been reported to be associated with total mortality and cancer incidence, suggesting a U-shaped relationship (Citation4,Citation13,Citation14). Several mechanisms of the association may attribute to sleep fragmentation, depression, fatigue, and decreased exercise (Citation15). However, various cut-off values for the definitions of short and long nighttime sleep duration were observed (e.g. 6 versus 9 h, 5 versus 10 h) (Citation16,Citation17), which complicated the interpretation of the relationships. In addition, since night-shift work, daytime napping, and nighttime sleep are closely linked with each other, it is necessary to assess the combined effects of these sleep habits on cancer incidence and cancer-caused mortality. However, only one case–control study reported the three sleep habits simultaneously with breast cancer risk (Citation18).
In this study, we aimed to investigate the independent and combined effects of three sleep habits, including night-shift work, daytime napping, and nighttime sleep, on cancer incidence and cancer-caused mortality among middle-aged and older Chinese in the Dongfeng-Tongji Cohort Study.
Material and methods
Study subjects
The Dongfeng-Tongji Cohort Study (DFTJ) was established in 2008 in Shiyan City, Hubei province of China. The detailed information of this cohort study has been reported previously (Citation19). Briefly, the retired workers were recruited from Dong-Feng Motor Corporation (DMC), and a total of 27,009 subjects finally completed the baseline questionnaires and medical examinations from September 2008 to June 2010. Among the above subjects, 25,978 participants (96.2%) subjects were successfully followed-up during the first follow-up period from June 2013 to October 2013. We excluded the 601 (2.2%) cancer patients who were diagnosed with cancer at the baseline study, and the left 25,377 respondents were included in the final data analyses. All participants provided written informed consent after explanation of the study, and this study was approved by the Ethics and Human Subject committee of the School of Public Health, Tongji Medical College, and Dong-Feng Motor Corporation.
Data collection
Assessment of sleep habits
In the baseline study, to acquire the information of sleep habits, participants were asked about the following questions in the questionnaire interview: (Citation1) Have you ever engaged in night-shift work during your lifetime? If “yes”, they were further asked the total length of their night-shift work duration, which was then categorized to 0.1–9.9, 1 0–19.9, ≥20 years. (Citation2) Did you have a habit of taking daytime napping over the past 6 months? If “yes”, they were further asked the average duration of their napping, which was categorized to 1–30, 3 1–60, ≥61 minutes. (Citation3) When did you usually go to sleep at night and wake up in the morning over the past six months? As a continuous variable, the average sleep duration per day was quantitative assessed.
The ascertainment of new cancer incidences and cancer-caused deaths
The endpoints of our analysis were total cancer incidence and cancer-caused mortality. Based on the follow-up survey, the self-reported cancer incidences and cancer-caused deaths were confirmed from DMC’s health-care service system, which consists of five DMC-owned hospitals that covers all retired employees. Cancer incidences and cancer-caused deaths were determined by physician review based on medical records and death certificates. International Classification of Diseases, 10th Revision (ICD-10) was used to classify the cancer incidences and cancer-caused deaths (ICD codes C00-C79).
Covariates assessment
The baseline demographic characteristics of age, gender, education level; the lifestyle factors, including physical activity, smoking, and alcohol drinking status, diet were gathered from DFTJ questionnaire. Subjects that smoked less than one cigarette per day for <1 year over their lifetime were defined as nonsmokers; otherwise, they were defined as smokers; smokers were further asked for age at starting or quitting smoking, number of cigarettes per day, and number of years of smoking. Alcohol drinkers were defined as those who had drunk alcohol at least once a week for more than six months over their lifetime; otherwise, they were defined as non-alcohol drinkers. Detailed records on occupational history include the kinds of work and number of working years. Medical histories include diagnosed medical conditions (e.g. chronic disease, including heart disease, diabetes, and stroke). Family history of cancer was defined as first-degree familial history of cancer. We also recorded their anthropometric data, such as weight, height, and waist circumference. Additional information was obtained from women, including number of children (full-term pregnancies), menopausal status (premenopausal or postmenopausal), use of hormone replacement therapy (yes or no), and contraception status (yes or no).
Follow-up
Person-years of the follow-up period were calculated as the number of years between the time when subjects jointed the cohort (i.e. the date participants completed their baseline questionnaire or baseline physical examination, which ever came first; or using 1 November 2008 for subjects with missing data on above dates) and the earliest of four dates: the date participants completed their follow-up questionnaire or follow-up physical examination; the date of primary cancer diagnosis, the date of death, or using 1 May 2013 for subjects with missing data on above dates. 1 November 2008 and 1 May 2013 are the median dates of all workers jointed into the cohort and completed the follow-up study, respectively.
Statistical analysis
The differences in distributions of baseline demographic characteristics and suspected risk factors between groups according to cancer incidence and cancer-caused mortality were tested by χ2 test. Since there was no deviation from proportionality in our study, we used the Cox proportional hazards regression models to examine the associations of sleep habits (night-shift work, daytime napping, and nighttime sleep duration) with cancer incidence and cancer-caused mortality among all, male, and female participants, respectively. The restricted cubic splines link to proportional hazards regression models were used to estimate the non-linear dose–response relationships between nighttime sleep duration and total cancer incidence (Citation20). Participants never engaged in night-shift work, had a daytime napping duration of 1–30 min (Citation21), and slept 7–8 h per night (Citation4), were used as the reference groups, respectively. The variables, including age at baseline (continuous), gender, BMI (continuous), family history of cancer (yes versus no), alcohol drinking (ever versus never), and smoking status (ever versus never), were treated as covariates in the final multivariable analysis models because they had the smallest value of AIC. Pack-years were additionally adjusted in models for men. For women, number of children, menopausal status (premenopausal or postmenopausal), hormone replacement therapy (yes or no), and contraception status (yes or no) were additionally adjusted in the Cox regression models.
In case that the chronic illness and preclinical disease may confound the relationships between sleep habits and cancer events, the sensitive analyses were conducted after exclusion of subjects reporting histories of chronic diseases (e.g., heart disease, diabetes, and stroke). In order to eliminate the role of reverse causality in relation to sleep and napping, we also performed analysis by excluding cancer events that occurred within 2 years of follow-up (266 new cancer cases and 108 deaths).
In addition, to evaluate the combined associations of night-shift work duration, daytime napping, and nighttime sleep with cancer incidence and mortality, participants were cross-classified by these three categorized sleep variables. Test for linear trend was performed by modeling a numerical value for each sleep category. We also calculated the total risk factor score (RFS) for each participant by combing all of the numerical scores assigned to each category (1 for ≥20 years and 0 for <20 years of night-shift work duration; 1 for no daytime napping and 0 for >0 min of daytime napping; 1 for ≥10 h and 0 for <10 h of nighttime sleep duration), and examined the associations of RFS with cumulative incidence of cancer and cumulative mortality of cancer by Kaplan–Meier failure probability curves. All analyses in this study were performed with SAS program (version 9.4; SAS Institute, Carry, NC), and statistical tests were two-sided with p < 0.05 as the level to reject the null hypothesis.
Results
General characteristic for study participants
During 114,162 person-years of follow up, we identified a total of 1251 cancer cases and 379 cancer-caused deaths. Higher cancer incidence and cancer-caused mortality were more likely to be observed among males, elders, smokers, and alcohol drinkers (all p < 0.01). Most of the participants (>67%) had no night-shift work history, while the most frequent self-reported daytime sleep duration and nighttime sleep duration was ≥61 min and 7–8 h, respectively. Participants with higher cancer incidence and mortality tend to had longer nighttime sleep duration (p = 0.013 and 0.004, respectively). There were also significant differences in the distribution of nighttime sleep duration between survivors and cancer-caused death group (p = 0.023). In contrast, the distributions of other covariates and sleep habits, including BMI, family history of cancer, night-shift work duration, and daytime napping were comparable both in cancer incidence and cancer-caused mortality groups (all p > 0.05, ). In addition, the baseline characteristics were also presented according to night-shift work duration, daytime napping, and nighttime sleep duration (Supplementary Tables 1, 2, and 3).
Table 1. General characteristics stratified by cancer status and survival conditions.
Associations of baseline sleep habits with cancer incidence and cancer-caused mortality
presents the associations between sleep habits and cancer incidence and cancer-caused mortality, after multivariable adjustment for the potential confounders. Compared with male participants who never had night-shift work history, those who experienced a ≥20 years of night-shift work duration had a 27% increased risk of cancer incidence (HR = 1.27, 95%CI: 1.01–1.59). For men who did not nap in the daytime, the HR for cancer risk was 2.03 (95%CI: 1.01–4.13) than those who took a nap for 1–30 min in the daytime.
Table 2. Multivariate adjusted hazard ratios for cancer incidence and cancer mortality by stratified sleep habits.
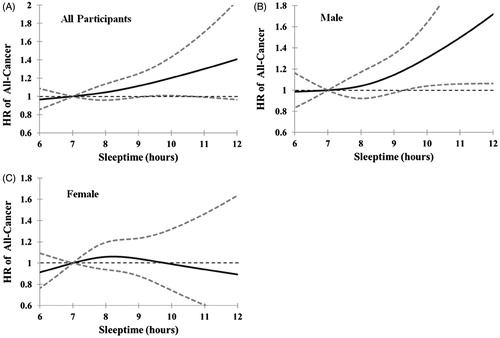
In the dose–response analysis, compared with subjects with nighttime sleep duration of 7 h, those who slept ≥10 h had a marginally higher risk of cancer incidence among all participants (). This non-liner dose–response relationship between nighttime sleep of ≥10 h and increased cancer incidence was significantly shown among male subjects (), but not seen among female subjects (). Based on the above findings, cut-off value of 10 h/night was added as one stratification criterion for the nighttime sleep duration. Finally, nighttime sleep duration was divided into <7, 7–8, 8.1–9.9, and ≥10 h groups. Those who slept ≥10 h/night had a 40% increased risk of cancer incidence and a 59% increased risk in cancer-caused mortality [HR (95%CI) = 1.40 (1.04–1.88) and 1.59 (1.01–2.49), respectively], when compared with those who slept 7–8 h/night. However, there were no significant associations of night-shift work duration and daytime napping duration with cancer-caused mortality among the male subjects. The above associations were essentially unchanged when we excluded these subjects with history of chronic diseases, including heart disease, diabetes, and stroke (Supplementary Table 4). Excluding the cancer events that occurred in the first 2 years of follow-up also had little impact on the results (Supplementary Table 5). We did not find any significant associations between these three sleep habits and cancer incidence and cancer-caused mortality among female participants (all p > 0.05).
Figure 1. Dose–response relations between nighttime sleep duration and all cancer risk. Data were modeled with restricted cubic splines that link to proportional hazards regression models, while adjusting for age, BMI, family history of cancer (yes/no), alcohol drinking (ever/never), and smoking status (ever/never), to examine the potential non-linear relationship between nighttime sleep duration and all cancer incidence. 7 h sleep per night was used as the reference. The solid line and the dash line represent the estimated hazard ratios (HRs) and their 95% confidence intervals (95%CIs) (A, B, and C are corresponding to all, male, and female participants, respectively).
Combined effects of sleep habits on cancer incidence and cancer-caused mortality among male participants
Since the above findings were mainly observed among male subjects, we further analyzed the combined effects of above three sleep habits on cancer incidence and cancer-caused mortality among male participants. As shown in , when using participants who never engaged in night-shift work and slept 7–8 h/night as the reference group, participants who slept 8.1–9.9 h/night and had night-shift work duration of ≥20 years had a 56% increased risk of cancer incidence (HR =1.56, 95%CI: 1.11–2.20), participants who slept ≥10 h/night and had night-shift work duration of 1 0–19.9 years or ≥20 years had a 2.30-fold (95%CI: 1.08–4.89) or 2.67-fold (95%CI: 1.44–3.08) cancer incidence, respectively. In addition, significant increasing trends in cancer risk were found from 0, 0.1–9.9, 1.0–19.9 to ≥20 years of night-shift work duration among men who slept 8.1–9.9 and ≥10 h/night (ptrend = 0.002 and ptrend < 0.001, respectively).
Table 3. Combined effects (hazard ratios with 95% CI) of night-shift work, daytime napping, and nighttime sleep duration on cancer incidence and mortality among male participants.
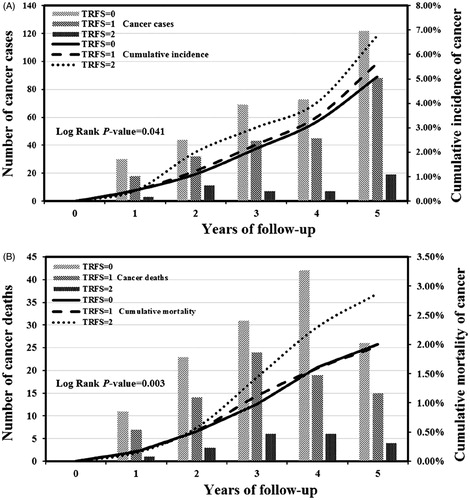
After calculating the risk factor score based on the risk sleep habits, we found that there were significant differences in cumulative cancer incidence (Log Rank p-value = 0.041) () and cancer-caused mortality (Log Rank p-value = 0.003) among subjects with RFS = 0, 1, and 2 subgroups (). Male subjects with a higher RFS had an increasing risk of cancer incidence and mortality (ptrend = 0.015 and 0.045, respectively), while participants with at least two risk factors (RFS ≥2) had the highest risk of cancer incidence and mortality [HR (95%CI) = 1.43 (1.07, 2.01), and 2.07 (1.25, 3.29), respectively].
Figure 2. The combined effects of three sleep habits on cumulative incidence and mortality of cancer among males during 5 years of follow-up (Kaplan–Meier failure probability plot). The total risk factor score (RFS) was calculated for each participant by combing all of the numerical scores assigned to each category of night-shiftwork (1 for ≥20 years; 0 for <20 years), daytime napping duration (1 for no daytime napping; 0 for >0 min/day), nighttime sleep duration (1 for ≥10 h/night; 0 for <10 h/night). Vertical bars represent the number of cancer cases (A) and cancer deaths (B) in each follow-up year, while indicative trend curves showed cumulative cancer incidence (A) and cancer-caused mortality (B).
Discussion
This study found that long night-shift work duration of ≥20 years and no daytime napping were associated with a 1.27- and 2.03-fold increased risk of cancer incidence among male participants, respectively. Compared with those who had normal sleep duration of 7–8 h/night, male subjects with nighttime sleep duration of ≥10 h per night had a 40% increase in cancer risk and 59% increase in cancer-caused mortality. In addition, participants with at least two risk factors of night-shift work duration ≥20 years, no daytime napping, and nighttime sleep duration ≥10 h had a 43% increased risk of cancer incidence and a 2.07-fold increased cancer-caused mortality than those who did not have any above risk sleep habits.
Night-shift work can disrupt the normal circadian clock and rhythms. Repeated sleep deprivation and disruption of the circadian rhythms and endogenous clock due to long-term exposure to artificial light at night can result in impairment of the immune system, which contribute the development of cancer (Citation6,Citation22). Both laboratory and observational studies have reported the impaired immune function in NK cell activity, IL-6 levels, and soluble TNF-α receptor 1 levels among poor sleepers due to inadequate or frequently disturbed sleep (Citation23). Previous cohort studies had reported that the significant associations of long period night-shift work with elevated risks of prostate (Citation24) and breast cancer incidence (Citation25). In this study, we also found that male participants with working history of night-shift work for ≥20 years had a significant increased risk of cancer. However, we did not found a significant association between night-shift work and cancer-caused mortality, which may due to shorter follow-up period (an average 4.5 years) of the present cohort. However, no comparable studies that focused on the association between night-shift work and cancer-specific mortality had been reported previously.
Compared with those who had a short daytime napping (1–30 min), we observed an increased risk of cancer incidence among male participants who never take a nap during daytime. The biological mechanisms underline the beneficial effects of moderate daily napping in lowering risk of cancer are unclear. There were studies reported moderate daytime napping may supplement sleep duration at night, reducing the performance deficits and sleepiness (Citation26). Although increased risk of cancer was found to change from those men without a nap to those who napped for 3.1–60 and >60 min, when cancer events that occurred in the first 2 years of follow-up were excluded, the protective effect of short nap (1–30 min) on cancer incidence remained unchanged. Similarly, Tanaka et al. (Citation27) found that the combination of a 30-min napping and moderate intensity exercise improved sleep quality and mental health. But a prospective study reported daytime napping (sometimes/usually versus rarely/never) was associated with slightly increased risk of cancer, while this association decreased significantly with the increased follow-up time (>4 years) (Citation11). The inconsistency may be attributed to differences in daytime napping assessment (quantitative versus frequency). In this cohort study, we did not observed a significant association between daytime napping and total cancer-caused mortality, and the same results were found in a meta-analysis of prospective cohort studies (Citation28), and a Japanese cohort study (Citation29). However, an older community population based study and a 13-year follow-up study found excessive daytime (>60 min) napping were associated with increased risk of all-cause mortality (Citation10,Citation30). It was suspected that excessive napping is associated with physical activity deficits and functional impairment, and what’s worse, they could also diminish the quality of subsequent sleep at night (Citation31). These findings raise the possibility that reasonable restriction of napping duration play import roles in the disease outcome.
Melatonin plays important roles in tumor cells apoptosis, anti-proliferation, anti-angiogenesis (Citation32), and anti-oxidative effect, protecting against the damage from carcinogenic substance (Citation33). Long nighttime sleep duration is suggested to be positively related to melatonin concentration (Citation34), thereby affecting cancer risk (Citation35). However, there were studies reported that longer sleep duration ≥8, 9, and 10 h/night were associated with an increased risk of colorectal cancer (Citation3,Citation17), lung cancer (Citation36), and a group of estrogen-mediated cancers, respectively (Citation37). In the current cohort study, male subjects with nighttime sleep duration ≥10 h had increased total cancer risk. Since longer sleepers tended to have elevated serum cortisol levels and decreased killer cell ability, both of which may promote the carcinogenesis and increased cancer risks (Citation34); while longer sleepers also tend to have higher levels of somnogenic cytokines, which may have direct pro-inflammatory effects on tumorigenesis (Citation38). One may suspect that this association may be attributed to an early symptom of disease and precede clinical diagnoses. However, in the present study, this association did not change substantially after exclusion of the subjects whose events occurred within 2-year follow-up or exclusion of people reporting histories of chronic diseases (heart disease, diabetes, and stroke) at baseline, suggesting long sleep duration itself may be an independent predictor (Citation39). In addition, a U-shaped association between sleep duration (both >8 and <7 h/night) and all-cause mortality was observed in a large American cohort and a community-based Japanese cohort (Citation13,Citation40). While in this study, the association between nighttime sleep duration and an increased risk of cancer-caused mortality was only seen among subjects who slept ≥10 h/night, but not in those with sleep duration of <7 h/night. Similar results were reported in Chinese elderly men in Taiwan (Citation41). Possible explanations for the discrepant results may attribute to the intrinsic differences of study populations. The participants in this cohort are middle-aged and older adults with only a few subjects (n = 1522, less than 6%) slept <7 h at night, and there was evidence shown that mean sleep duration steadily increased with aging (Citation42).
Night-shift work leads to sleep deprivation, longer nighttime sleep after night-shift work leads to sleep-wake cycle alterations, and no daytime napping may not supplement the sleep deprivation that caused by night-shift work and poor sleep quality, suggesting that three sleep habits are closely related to each other. We found that the combined effects of night-shift work of ≥20 years, no daytime napping, and nighttime sleep for ≥10 h/night were greater than their independent effects on the cumulative incidence and mortality of cancer. Although the underlying mechanisms of these associations are complicated and not fully understood, there were studies reported sleep habits involve in a number of biological processes and mechanisms, including circadian disruption, altered melatonin secretion patterns (Citation43), and deregulated clock-gene, which can induce tumorigenesis and progression of cancer (Citation44).
Since cancers are not uniformly associated with sleep habits, there were studies assessed the associations between sleep habits and cancer incidence by cancer type (e.g. shift work with breast cancer, and sleep disruption with prostate cancer) (Citation35,Citation45). However, we did not found significant associations between night-shift work of ≥20 years and risk of incident breast or prostate cancer [HR (95%CI) = 0.67 (0.29–1.55) and 0.92 (0.27–3.12), respectively, data not shown], probably due to the limited incident breast and prostate cancer cases (104 and 34, respectively). Further investigations of long-term cohort studies with more cancer type-specific cases are needed to verify the associations. In addition, the associations of sleep habits with cancer incidence and mortality were only found among men, but not for women. Although the underlying biological mechanisms are unclear, there were studies reported the sex difference in neuroendocrine context, in which sex hormones have long-lasting effects on circadian timing system (Citation46), while leading to sex differences in the circadian modulation (Citation47).
Strengths of our study include the prospective design with a high follow-up rate of 96.2%, and robust verification system for diseases (DMC’s health-care service system), which guarantee the authenticity and reliability of cancer incidences and cancer-caused mortality ascertainment. In addition, this is one of the few studies to present in detail exploring the independent and combined effects of sleep habits simultaneously. Some limitations also need to be noted in the present study. First, the self-reported lifestyle data in the baseline study may result in error of misclassification, we must be cautious when drawing inference. Second, the ageing retired workers tend to more susceptible to cancer and cancer-related death, which will bring selection bias and overestimate on the sleep-cancer associations when generalized to other younger groups. Finally, the short-term follow-up period along with fewer cancer cases may limit the statistical power to investigate the associations between sleep habits and cancer incidence by cancer type, while the unhealthy effects of sleep habits on overall cancer events in our study may lack of specificity. Further investigations on larger population-based long-term cohort studies and additional basic science research are needed to verify the associations found in our study.
Conclusions
In this cohort study, we observed that night-shift work of ≥20 years, without daytime napping, and nighttime sleep duration for ≥10 h were associated with an increased risk of cancer incidence among male participants, and nighttime sleep duration for ≥10 h were also associated with increasing cancer-caused mortality. In addition, male participants with at least two above risk sleep habits had a 43% increased risk of cancer incidence and a 2.07-fold increased cancer-caused mortality than male subjects who did not have any above risk sleep habits. These findings need to be verified in further larger population based long-term investigations.
Annals_of_medicien_supplement-2016-06-26.docx
Download MS Word (32.8 KB)Acknowledgements
The authors would like to appreciate all participants in this cohort study as well as all volunteers for collecting the samples and questionnaires. The authors also acknowledge all the staff in the hospitals for their medical assistants in collecting the clinic data.
Disclosure statement
No potential conflicts of interest exist or are disclosed.
Additional information
Funding
References
- World Health Organization [Internet]. Cancer. Available from: http://www.WHO.int/cancer/en/.
- Kim JY, Yadav D, Ahn SV, Koh S-B, Park JT, Yoon J, et al. A prospective study of total sleep duration and incident metabolic syndrome: the ARIRANG study. Sleep Med. 2015;16:1511–15.
- Zhang X, Giovannucci EL, Wu K, Gao X, Hu F, Ogino S, et al. Associations of self-reported sleep duration and snoring with colorectal cancer risk in men and women. Sleep 2013;36:681–8.
- Cappuccio FP, D'Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep 2010;33:585–92.
- Straif K, Baan R, Grosse Y, Secretan B, El Ghissassi F, Bouvard V, et al. Carcinogenicity of shift-work, painting, and fire-fighting. Lancet Oncol. 2007;8:1065–6.
- Haus EL, Smolensky MH. Shift work and cancer risk: potential mechanistic roles of circadian disruption, light at night, and sleep deprivation. Sleep Med Rev. 2013;17:273–84.
- Davis S, Mirick DK. Circadian disruption, shift work and the risk of cancer: a summary of the evidence and studies in Seattle. Cancer Causes Control. 2006;17:539–45.
- Dhand R, Sohal H. Good sleep, bad sleep! The role of daytime naps in healthy adults. Curr Opin Pulm Med. 2006;12:379–82.
- Trichopoulos D, Tzonou A, Christopoulos C, Havatzoglou S, Trichopoulou A. Does a siesta protect from coronary heart disease? Lancet 1987;2:269–70.
- Hays JC, Blazer DG, Foley DJ. Risk of napping: excessive daytime sleepiness and mortality in an older community population. J Am Geriatr Soc. 1996;44:693–8.
- Cairns BJ, Travis RC, Wang XS, Reeves GK, Green J, Beral V. A short-term increase in cancer risk associated with daytime napping is likely to reflect pre-clinical disease: prospective cohort study. Br J Cancer. 2012;107:527–30.
- Von Ruesten A, Weikert C, Fietze I, Boeing H. Association of sleep duration with chronic diseases in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam study. PLoS One 2012;7:e30972.
- Xiao Q, Keadle SK, Hollenbeck AR, Matthews CE. Sleep duration and total and cause-specific mortality in a large US cohort: interrelationships with physical activity, sedentary behavior, and body mass index. Am J Epidemiol. 2014;180:997–1006.
- Kakizaki M, Kuriyama S, Sone T, Ohmori-Matsuda K, Hozawa A, Nakaya N, et al. Sleep duration and the risk of breast cancer: the Ohsaki Cohort Study. Br J Cancer. 2008;99:1502–5.
- Grandner MA, Drummond SP. Who are the long sleepers? Towards an understanding of the mortality relationship. Sleep Med Rev. 2007;11:341–60.
- Thompson CL, Larkin EK, Patel S, Berger NA, Redline S, Li L. Short duration of sleep increases risk of colorectal adenoma. Cancer 2011;117:841–7.
- Zhao H, Yin JY, Yang WS, Qin Q, Li TT, Shi Y, et al. Sleep duration and cancer risk: a systematic review and meta-analysis of prospective studies. Asian Pac J Cancer Prev. 2013;14:7509–15.
- Wang P, Ren FM, Lin Y, Su FX, Jia WH, Su XF, et al. Night-shift work, sleep duration, daytime napping, and breast cancer risk. Sleep Med. 2015;16:462–8.
- Wang F, Zhu J, Yao P, Li X, He M, Liu Y, et al. Cohort profile: the Dongfeng-Tongji cohort study of retired workers. Int J Epidemiol. 2013;42:731–40.
- Greenland S, Michels KB, Robins JM, Poole C, Willett WC. Presenting statistical uncertainty in trends and dose–response relations. Am J Epidemiol. 1999;149:1077–86.
- Tamaki M, Shirota A, Hayashi M, Hori T. Restorative effects of a short afternoon nap (<30 min) in the elderly on subjective mood, performance and eeg activity. Sleep Res Online 2000;3:131–9.
- Brudnowska J, Peplonska B. Night shift work and cancer risk: a literature review. Med Pr. 2011;62:323–38.
- Bovbjerg DH. Circadian disruption and cancer: sleep and immune regulation. Brain Behav Immun. 2003;17:S48–S50.
- Kubo T, Ozasa K, Mikami K, Wakai K, Fujino Y, Watanabe Y, et al. Prospective cohort study of the risk of prostate cancer among rotating-shift workers: findings from the Japan collaborative cohort study. Am J Epidemiol. 2006;164:549–55.
- Akerstedt T, Knutsson A, Narusyte J, Svedberg P, Kecklund G, Alexanderson K. Night work and breast cancer in women: a Swedish cohort study. BMJ Open 2015;5:e008127.
- Ruggiero JS, Redeker NS. Effects of napping on sleepiness and sleep-related performance deficits in night-shift workers: a systematic review. Biol Res Nurs. 2014;16:134–42.
- Tanaka H, Taira K, Arakawa M, Urasaki C, Yamamoto Y, Okuma H, et al. Short naps and exercise improve sleep quality and mental health in the elderly. Psychiatry Clin Neurosci. 2002;56:233–4.
- Zhong G, Wang Y, Tao T, Ying J, Zhao Y. Daytime napping and mortality from all causes, cardiovascular disease, and cancer: a meta-analysis of prospective cohort studies. Sleep Med. 2015;16:811–19.
- Tanabe N, Iso H, Seki N, Suzuki H, Yatsuya H, Toyoshima H, et al. Daytime napping and mortality, with a special reference to cardiovascular disease: the JACC study. Int J Epidemiol. 2010;39:233–43.
- Leng Y, Wainwright NW, Cappuccio FP, Surtees PG, Hayat S, Luben R, et al. Daytime napping and the risk of all-cause and cause-specific mortality: a 13-year follow-up of a British population. Am J Epidemiol. 2014;179:1115–24.
- Stepanski EJ, Wyatt JK. Use of sleep hygiene in the treatment of insomnia. Sleep Med Rev. 2003;7:215–25.
- Di Bella G, Mascia F, Gualano L, Di Bella L. Melatonin anticancer effects: review. Int J Mol Sci. 2013;14:2410–30.
- Fischer T, Wigger-Alberti W, Elsner P. Melatonin in dermatology. Experimental and clinical aspects. Hautarzt 1999;50:5–11.
- Aeschbach D, Sher L, Postolache TT, Matthews JR, Jackson MA, Wehr TA. A longer biological night in long sleepers than in short sleepers. J Clin Endocrinol Metab. 2003;88:26–30.
- Pinheiro SP, Schernhammer ES, Tworoger SS, Michels KB. A prospective study on habitual duration of sleep and incidence of breast cancer in a large cohort of women. Cancer Res. 2006;66:5521–5.
- Luojus MK, Lehto SM, Tolmunen T, Erkkila AT, Kauhanen J. Sleep duration and incidence of lung cancer in ageing men. BMC Public Health 2014;14:295.
- Hurley S, Goldberg D, Bernstein L, Reynolds P. Sleep duration and cancer risk in women. Cancer Causes Control 2015;26:1037–45.
- Krueger JM, Obal FJ, Fang J, Kubota T, Taishi P. The role of cytokines in physiological sleep regulation. Ann N Y Acad Sci. 2001;933:211–21.
- Da Silva AA, de Mello RG, Schaan CW, Fuchs FD, Redline S, Fuchs SC. Sleep duration and mortality in the elderly: a systematic review with meta-analysis. BMJ Open 2016;6:e008119.
- Ikehara S, Iso H, Date C, Kikuchi S, Watanabe Y, Wada Y, et al. Association of sleep duration with mortality from cardiovascular disease and other causes for Japanese men and women: the JACC study. Sleep 2009;32:295–301.
- Lan TY, Lan TH, Wen CP, Lin YH, Chuang YL. Nighttime sleep, Chinese afternoon nap, and mortality in the elderly. Sleep 2007;30:1105–10.
- Tamakoshi A, Ohno Y. Self-reported sleep duration as a predictor of all-cause mortality: results from the JACC study, Japan. Sleep 2004;27:51–4.
- Claustrat B, Brun J, Chazot G. The basic physiology and pathophysiology of melatonin. Sleep Med Rev. 2005;9:11–24.
- Bollinger T, Bollinger A, Oster H, Solbach W. Sleep, immunity, and circadian clocks: a mechanistic model. Gerontology 2010;56:574–80.
- Markt SC, Flynn-Evans EE, Valdimarsdottir UA, Sigurdardottir LG, Tamimi RM, Batista JL, et al. Sleep duration and disruption and prostate cancer risk: a 23-year prospective study. Cancer Epidemiol Biomarkers Prev. 2016;25:302–8.
- Yan L, Silver R. Neuroendocrine underpinnings of sex differences in circadian timing systems. J Steroid Biochem Mol Biol. 2016;160:118–26.
- Santhi N, Lazar AS, McCabe PJ, Lo JC, Groeger JA, Dijk DJ. Sex differences in the circadian regulation of sleep and waking cognition in humans. Proc Natl Acad Sci USA. 2016;113:E2730–9.
