Abstract
Background: Chronic obstructive pulmonary disease (COPD) patients have an increased cardiovascular (CV) morbidity and mortality. Common carotid intima-media thickness (CCA-IMT) and carotid plaques are surrogate markers of subclinical atherosclerosis and predictors of CV events.
Methods and results: We performed a meta-analysis to evaluate the association between COPD and subclinical atherosclerosis. Studies evaluating the impact of COPD on CCA-IMT and on the prevalence of carotid plaques were systematically searched.
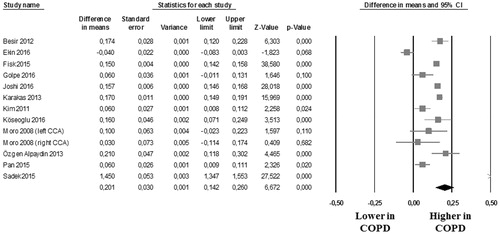
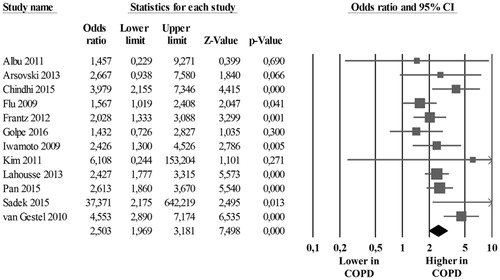
Results: Twenty studies (2082 COPD patients and 4844 controls) were included, 12 studies with data on CCA-IMT (13 data-sets on 1180 COPD patients and 2312 controls) and 12 studies reporting on the prevalence of carotid plaques (1231 COPD patients and 4222 controls). Compared to controls, COPD patients showed a significantly higher CCA-IMT (mean difference [MD]: 0.201 mm; 95%CI: 0.142, 0.260; p < .001), and an increased prevalence of carotid plaques (Odds Ratio [OR]: 2.503; 95%CI: 1.333, 2.175; p < .0001). Meta-regression models showed a direct association between disease severity [as expressed by Global Initiative for Chronic Obstructive Lung Disease (GOLD) class] and the difference in the risk of carotid plaques presence between COPD patients and controls.
Conclusions: COPD is significantly associated with subclinical atherosclerosis. These findings may be useful to plan adequate CV prevention strategies.
COPD patients show a higher CCA-IMT and an increased prevalence of carotid plaques compared with controls.
A more severe pulmonary disease is associated with a higher prevalence of carotid plaques in COPD patients.
Screening for subclinical atherosclerosis may be worthy in COPD patients to plan specific prevention strategies.
Key messages
Introduction
Chronic obstructive pulmonary disease (COPD) is a chronic, progressive lung disease and an increasing cause of global morbidity and mortality [Citation1]. In contrast to the trend for cancer and cardiovascular (CV) diseases, death rates from COPD have been progressively rising over the last decade [Citation2] and, according to estimates of the World Health Organization (WHO), by 2030 this condition may become the third leading cause of death worldwide [Citation3].
Over the last few years, there has been an increased awareness about the systemic nature of COPD [Citation4] and the frequent and important chronic comorbidities that may contribute significantly to its severity and mortality [Citation5]. In particular, numerous reviews, cross-sectional and longitudinal studies indicated that CV disease is more frequent in COPD patients than in the general population, representing a burden even greater than that of the lung disease itself in this clinical setting [Citation6]. It has been estimated that for every 10% decrease in lung function [as expressed by forced expiratory volume in 1 second (FEV1)], CV mortality increases by nearly 30% [Citation7].
COPD and CV disease share some major risk factors such as smoking habit, diabetes mellitus, hypertension and obesity [Citation8,Citation9]. However, some evidence confirmed that COPD is associated with CV manifestations independently from concomitant risk factors [Citation7,Citation10], thus suggesting that COPD itself may be considered an independent CV risk factor. To further address this issue, a growing attention has been given to the assessment of the association between COPD and subclinical atherosclerosis, a recognized marker of CV disease [Citation11].
Carotid intima-media thickness (IMT) assessment is a non-invasive imaging test for subclinical atherosclerosis [Citation12,Citation13], and it has been widely accepted as one of the strongest predictors of major CV events (stroke, myocardial infarction, heart failure or CV death) [Citation14,Citation15]. The presence of carotid plaques is considered an even more reliable predictor of CV events than IMT [Citation16]. Thus, these surrogate markers of subclinical atherosclerosis provide important prognostic information over and above traditional CV risk factors.
Both prospective [Citation17,Citation18] and retrospective studies [Citation19,Citation20] documented accelerated atherosclerosis in COPD patients. However, the evidence is limited by small sample size and potential confounding factors, and no meta-analytical data providing an overall information about this issue are currently available.
In order to provide a comprehensive overview of the relationship between COPD and subclinical atherosclerosis, we performed a systematic review with meta-analysis of literature studies evaluating the impact of COPD on IMT and on the prevalence of carotid plaques.
Methods
A protocol for this review was prospectively developed, detailing the specific objectives, the criteria for study selection, the approach to assess study quality, the outcomes, and the statistical methods.
Search strategy
To identify all available studies, a detailed search pertaining to COPD and the markers of CV risk (i.e. carotid IMT and plaques) was conducted according to PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) guidelines [Citation21]. A systematic search was performed in the electronic databases (PubMed, Web of Science, Scopus, EMBASE), using the following search terms in all possible combinations: chronic obstructive pulmonary disease, carotid intima-media thickness, carotid plaques, atherosclerosis, atheroma. The last search was performed on 20 December 2016. The search strategy was developed without any language restriction (Supplemental Table 1).
In addition, the reference lists of all retrieved articles were manually reviewed. In case of missing data, study authors were contacted by e-mail to try to retrieve original data. Two independent authors (PA and MNDDM) analysed each article and performed the data extraction independently. In case of disagreement, a third investigator was consulted (RL). Discrepancies were resolved by consensus. Selection results showed a high inter-reader agreement (κ = 0.98) and have been reported according to PRISMA flowchart (Supplemental Figure 1).
Data extraction and quality assessment
According to the pre-specified protocol, all studies evaluating the impact of COPD on the markers of CV risk were included. Case-reports, case-series without a control group, reviews and animal studies were excluded. To be included in the analysis, a study had to provide values (means with standard deviation or standard error) of common carotid artery IMT (CCA-IMT) and/or the prevalence of carotid plaques among COPD patients and controls. The included studies were classified as having a prospective or a retrospective design.
In each study, data regarding sample size, major clinical and demographic variables, values of IMT, and prevalence of carotid plaques in COPD patients and controls were extracted.
Patients with COPD were diagnosed and classified according to 2010 Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines (http://www.goldcopd.org) [Citation1]. In brief, these recommendations state that a post-bronchodilator FEV1/FVC value <0.7 is diagnostic for COPD. For staging purposes, post-bronchodilator FEV1 is used: mild/GOLD I (≥80%), moderate/GOLD II (50–79%), severe/GOLD III (30–49%) and very severe/GOLD IV (<30%).
Given the characteristics of the included studies, the evaluation of methodological quality of each study was performed with the Newcastle–Ottawa Scale (NOS), which is specifically developed to assess quality of non-randomized observational studies [Citation22]. The scoring system encompasses three major domains (selection, comparability, exposure) and a resulting score range between 0 and 8, a higher score representing a better methodological quality. Results of the NOS quality assessment are reported in Supplemental Table 2.
Statistical analysis and risk of bias assessment
Statistical analysis was carried out using Comprehensive Meta-analysis [Version 2, Biostat, Englewood, NJ (2005)].
Differences among cases and controls were expressed as mean difference (MD) with pertinent 95% confidence intervals (95%CI) for continuous variables, and as Odds Ratio (OR) with pertinent 95%CI for dichotomous variables. IMT has been expressed in millimeters (mm).
The overall effect was tested using Z scores and significance was set at p < .05. Statistical heterogeneity between studies was assessed with chi square Cochran’s Q test and with I2 statistic, which measures the inconsistency across study results and describes the proportion of total variation in study estimates, that is due to heterogeneity rather than sampling error. In detail, I2 values of 0% indicates no heterogeneity, 25% low, 25–50% moderate and 50% high heterogeneity [Citation23].
Absolute risk of carotid plaques presence in COPD patients and controls was calculated as (number of subjects with carotid plaques)/(total number of subjects) in each group. The attributable risk was defined as (risk of carotid plaques presence in COPD patients – risk of carotid plaques presence in controls)/(risk of carotid plaques presence in COPD patients) [Citation24].
Publication bias was assessed by Egger’s test and represented graphically by funnel plots of the standard difference in means versus the standard error. Visual inspection of funnel plot asymmetry was performed to address for possible small-study effect, as well as Egger’s test to address publication bias, over and above any subjective evaluation. A p < .10 was considered statistically significant [Citation25]. In case of a significant publication bias, Duval and Tweedie’s trim and fill method was used to allow for the estimation of an adjusted effect size [Citation26]. In order to be as conservative as possible, the random-effect method was used to take into account the variability among included studies.
Sensitivity analyses
We repeated analyses by including only the studies judged as “high quality” according to NOS (i.e. NOS ≥ to the median value found among included studies).
In order to avoid the risk of data overlap, a sensitivity analysis was performed after excluding studies involving the same recruitment centres and enrolling patients in the same period time as other included studies.
A further analysis was performed after excluding studies providing a composite IMT, calculated by averaging the CCA with the internal carotid artery (ICA) and/or the carotid bifurcation (BIF) measurements.
Finally, we repeated analyses after stratifying studies according to design (prospective and retrospective), and after excluding studies with only abstract available.
Subgroup analysis
In order to evaluate the impact of disease severity on the atherosclerotic burden, we performed separate analyses for patients with mild/moderate COPD (i.e. GOLD class I and II) and those with severe/very severe disease (i.e. GOLD class III and IV).
Meta regression analyses
We hypothesized that differences among included studies may be affected by demographic variables [mean age, male gender], COPD treatment [therapy with β-agonists, anticholinergic agents, corticosteroids (CCS), supplemental oxygen], clinical data from patient evaluation questionnaires [St George’s Respiratory Questionnaire (SGRQ), Modified Medical Research Council (MMRC) questionnaire], pulmonary functional measures [FEV1, forced expiratory volume in 1 second/forced vital capacity (FEV1/FVC), diffusing capacity for carbon monoxide (DLCO), GOLD class], six-minutes walking test [6MWT], CT parameters [relative area with attenuation values below −950 HU (RA950), wall area per cent (WA%)], hyperpolarized helium-3 (3 He) MRI parameters [apparent diffusion coefficients (ADC), ventilation defect percent (VDP)], laboratory tests [hemogasanalysis parameters, C-reactive protein (CRP) levels] and coexistence of traditional CV risk factors [hypertension, smoking habit, smoking pack-years, diabetes mellitus, obesity, altered levels of total cholesterol (TC), LDL-cholesterol (LDLc), HDL-cholesterol (HDLc) and/or triglycerides (TGs)]. To assess the possible effect of such variables in explaining different results observed across studies, we planned to perform meta-regression analyses after implementing a regression model with changes in CCA-IMT or presence of carotid plaques as dependent variables (y) and the above mentioned co-variates as independent variables (x). This analysis was performed with Comprehensive Meta-analysis [Version 2, Biostat, Englewood, NJ (2005)].
Results
After excluding duplicate results (n = 598), the search retrieved 912 articles. Of these studies, 585 were excluded because they were off the topic after scanning the title and/or the abstract, 304 because they were reviews/comments/case reports or they lacked of data of interest. Of three studies, the on-line full-length version was not available but data could be extracted from the abstract. Other three studies were excluded after full-length paper evaluation. Overall, a total of 1490 articles were excluded.
Thus, 20 articles (2082 COPD patients and 4844 controls) were included in the final analysis [Citation17–20,Citation27–42] (Supplemental Figure 1). Twelve studies (13 data-sets) reported data on CCA-IMT (1180 COPD patients and 2312 controls) and 12 studies on the prevalence of carotid plaques (1231 COPD patients and 4222 controls).
Study characteristics
With the only exception of two retrospective studies [Citation19,Citation20], all included studies had a prospective design. Major characteristics of study populations are shown in and further data about COPD severity are reported in .
Table 1. Demographic and clinical data of patients with chronic obstructive pulmonary disease (COPD) and controls in included studies.
Table 2. Measures of disease severity in patients with chronic obstructive pulmonary disease (COPD) in included studies.
The number of patients varied from 38 to 432, the mean age from 59.2 to 76.7 years, and the prevalence of male gender from 36.1 to 100%.
The presence of diabetes mellitus was reported by 0–44% of patients, hypertension by 0–72.7%, smoking habit by 17.4–73.6%, past smoking history by 25.9–62.8% and hyperlipidaemia by 0–39%.
Mean body mass index (BMI) varied from 22.5 to 28 kg/m2. Mean values of TC ranged from 4.44 to 5.69 mmol/l, of LDLc from 2.57 to 3.26 mmol/l, of HDLc from 1 to 1.60 mmol/l and of TGs from 1.02 to 1.72 mmol/l.
Mean values of FEV1 varied from 46.3 to 90.5% predicted, of FEV1/FVC from 51.3 to 65.6, and of CRP from 1.02 to 5.50 mg/dl. GOLD class I was reported by 0–66.9%, class II by 14–44.8%, class III by 29–60% and class IV by 6–33.3%.
Only one study [Citation17] reported data on the prevalence of obesity (21%). One study [Citation33] provided information on MMRC class (class 0 in 14.9%, class I in 43.2%, class II in 35.8%, class III in 35.9%, class IV in 0%), one [Citation37] on COPD treatment (β-agonists in 73%, anticholinergic agents in 56%, CCS in 57%) and one [Citation33] on hemogasanalysis parameters (mean night-time oxygen saturation, 89%). No study reported data on 6MWT, SGQR, DLCO, CT R950 or WA%, 3 He MRI ADC or VDP.
One study [Citation42] reported separate data for left and right CCA-IMT, thus two different data-sets were considered for this study.
Common carotid artery IMT
In 12 studies (13 data-sets) [Citation19,Citation20,Citation27,Citation29,Citation30,Citation33,Citation35–37,Citation39, Citation40,Citation42], we found that the 1180 COPD patients showed a significantly higher CCA-IMT than the 2312 controls (MD: 0.201 mm; 95%CI: 0.142, 0.260; p < .001, ). Heterogeneity among these studies was statistically significant (I2 = 98.3%; p < .001) and no reduction in the overall heterogeneity was found after excluding one study at time.
Carotid plaques
The analysis of the 12 studies [Citation17,Citation18,Citation20,Citation28,Citation31–34,Citation36,Citation38, Citation40,Citation41] showed that the absolute risk of carotid plaques presence was 43.4% (95%CI: 3.2, 5.5) in 1231 COPD patients and 25.7% (95%CI: 17.2, 36.4) in 4222 controls with a corresponding OR of 2.503 (95%CI: 1.333, 2.175; p < .001, ) and with an attributable risk of 41.8%. The heterogeneity among studies was significant (I2 = 48%; p = .032), but after excluding the study by van Gestel et al. [Citation41], results were confirmed without significant heterogeneity (OR: 2,292; 95%CI: 1866, 2815; p < .001, I2 = 25.3%; p = .203).
Sensitivity analyses
The median value of NOS quality assessment was 5. Thus, the analyses were repeated by including only the 14 studies classified as “high quality” (NOS ≥5) [Citation5,Citation19,Citation20,Citation32–39,Citation41,Citation42] (Supplemental Table 2). Of interest, after excluding studies classified as “low quality” [Citation29,Citation31,Citation40] and those with only abstract available [Citation18,Citation28,Citation30], our results were substantially confirmed ().
Table 3. Sensitivity analyses. Panel a: “high quality” studies (i.e. Newcastle–Ottawa Scale ≥5); Panel B: exclusion of studies potentially reporting on the same population as other included studies; Panel C: exclusion of studies providing a composite IMT; panel D: prospective studies; panel E: exclusion of studies with only abstract available.
The significant difference in CCA-IMT and prevalence of carotid plaques between COPD patients and controls was confirmed after excluding studies [Citation31,Citation35] potentially reporting on the same population as other included studies [Citation29,Citation41] ().
Similar results were confirmed also after excluding two studies [Citation19,Citation20] providing a composite IMT, calculated by averaging the CCA-IMT with the ICA-IMT and/or the IMT at the level of the carotid BIF ().
With the exception of two studies [Citation19,Citation20] all studies were prospective. When the analyses were repeated by including only prospective studies, we confirmed our results for all outcomes (). Due to the low number of studies, it was not possible to perform a sensitivity analysis for retrospective studies.
Similar results were confirmed also after excluding studies [Citation18,Citation29,Citation30] with only abstract available ().
Subgroup analysis
A total of three studies [Citation20,Citation38,Citation41] stratified data on carotid plaques prevalence according to disease severity. Of interest, the significant difference in the prevalence of carotid plaques between COPD patients and controls was substantially confirmed when separately considering patients with mild/moderate COPD (i.e. GOLD classes I and II) () and those with severe/very severe disease (i.e. GOLD classes III and IV) (). Due to the low number of studies stratifying data on CCA-IMT according to GOLD class, no further sensitivity analysis was performed for this outcome.
Table 4. Subgroup analysis. Panel A: studies on mild/moderate COPD (i.e. GOLD class I and II); Panel B: studies on severe/very severe COPD (GOLD class III and IV).
Publication bias
As it is recognized that publication bias can affect the results of meta-analyses, we attempted to assess this potential bias using funnel plots analysis.
Visual inspection of funnel plots for studies evaluating CCA-IMT and the prevalence of carotid plaques suggested the absence of publication bias and of small-study effect (Supplemental Figure 2), confirmed by Egger’s test (p = .704 and p = .499, respectively).
Meta-regression analyses
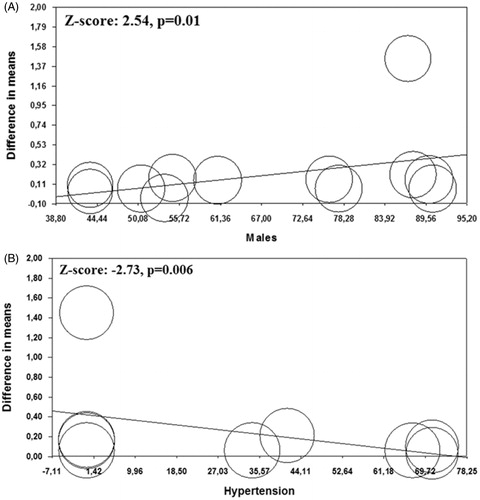
Regression models showed that male gender and hypertension significantly impacted on CCA-IMT (Z = 2.54; p = .010, , and Z = –2.73; p = .006, , respectively), while all the other demographic and clinical variables did not impact on the observed results (Supplemental Table 3).
Figure 3. Meta-regression analyses. Impact of male gender (Panel A) and hypertension (Panel B) on common carotid artery intima-media thickness (CCA-IMT) in chronic obstructive pulmonary disease patients (COPD) patients and controls.
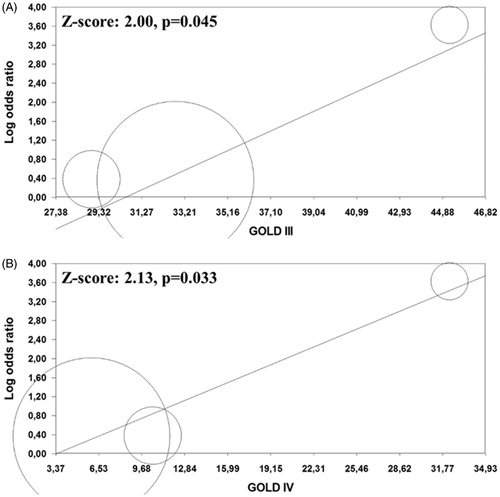
Meta-regression analyses for carotid plaques prevalence showed that GOLD classes III and IV were associated with a high effect size (Z = 2.00; p = .045, , and Z = 2.13; p = .033, , respectively). All the other co-variates tested did not impact on the difference in the risk of carotid plaques presence between COPD patients and controls (Supplemental Table 3).
Discussion
Results of the present meta-analysis consistently show that COPD is associated with subclinical atherosclerosis. In particular, we reported an increased CCA-IMT, accompanied by higher prevalence of carotid plaques in COPD patients. Our findings are strengthened by the sensitivity analyses. Moreover, regression models were able to further refine results, showing a direct association between COPD severity (i.e. GOLD classes III and IV) and the difference in the risk of carotid plaques presence between COPD patients and controls.
Overall, these data suggest an increased CV risk in patients with COPD and suggest the need for a strict monitoring of CV risk factors and of subclinical signs of atherosclerosis in COPD patients. The relevance of subclinical atherosclerosis lies in its ability to predict the CV risk and to further contribute to the morbidity of COPD patients. This is in line with previous published studies reporting an increased risk of major CV events [Citation7] and increased CV mortality in patients with COPD [Citation6].
Many inherited and acquired risk factors are thought to have a causal role in the atherosclerotic process. Although most COPD patients have a smoking history, the relationship between subclinical atherosclerosis and COPD seems to be more complex and the smoking habit might not entirely explain the accelerated atherosclerotic process in this clinical setting [Citation10]. Accordingly, our regression models showed that the association between COPD and carotid atherosclerosis was independent of baseline smoking status and most traditional CV risk factors. Thus, other mechanisms should be considered to explain such an association.
Inflammatory and immunological mechanisms have been suggested to independently impact on atherosclerosis progression in COPD patients [Citation43]. Immune-mediated inflammation seems to play a pivotal role in the pathogenesis of atherosclerosis [Citation44–46], being involved in endothelial dysfunction, plaque rupture and thrombosis [Citation47,Citation48]. Patients with COPD have elevated levels of CRP [Citation49], a marker of inflammation associated with the atherosclerotic burden in the general population [Citation50,Citation51] and poor prognosis in patients with COPD [Citation52]. Previous studies have shown that severe COPD patients also have elevated circulating levels of other markers of inflammation, such as tumour necrosis factor, endothelin-1, and interleukin-6 compared with healthy controls [Citation53–55]. More recently, the presence of systemic inflammation has been documented also in moderate COPD [Citation43]. This may explain why even a modest decline in FEV1 (from a mean of 109% of predicted to 88% of predicted) is associated with a five-fold increase in CV morbidity and mortality [Citation6]. In keeping with this, our sensitivity analyses confirmed an increased prevalence of carotid plaques also when specifically considering patients with mild/moderate COPD (i.e. GOLD classes I and II). However, a pooled analysis of large cohort studies (>500 patients) reporting on the relationship between FEV1 and CV mortality demonstrated that individuals in the lowest FEV1 quintile have a 75% increase in the risk for CV mortality compared with those in the highest FEV1 quintile [Citation43]. Overall, these data suggest that CV risk progressively increases as the lung function decreases. This is consistent with the results of our regression analyses, showing that a more severe COPD (as expressed by GOLD class III and IV) significantly and directly impacted on the difference in the risk of carotid plaques presence between COPD patients and controls. Thus, on the basis of available data in included studies, we were not able to identify a specific COPD phenotype with an increased CV risk but we are able to identify a high-risk patient subset, represented by patients with severe/very severe disease.
Overall, these findings are in line with all available experimental and clinical evidences, supporting the hypothesis that premature atherosclerosis may be one of the main features of COPD. The clinical relevance of our results can be better understood when we consider that the risk of myocardial infarction increases of 43% every 0.163 mm increase in carotid IMT [Citation47] and that the prevalence of carotid plaques is an even more reliable predictor of CV events than IMT [Citation16].
These data clearly suggest the need for a strict monitoring of CV risk factors in COPD patients, with particular regard to those with severe disease. A careful risk stratification may orientate the choice on which COPD patients could benefit for a very strict monitoring of CV risk factors and of subclinical signs of atherosclerosis. In keeping with this, we potentially identified a subset of COPD patients who are at higher risk for carotid atherosclerosis. Indeed, our regression models for CCA-IMT showed that an increasing percentage of males is associated with a higher MD between COPD patients and controls. Thus, we might hypothesize an interaction between male gender and COPD for the progression of the atherosclerotic process, and male patients with COPD could be regarded as a high-risk patient subset. In contrast, being a recognized CV risk factor for both COPD patients and non-COPD subjects, the presence of hypertension was associated with a low effect-size in our meta-regression analyses on CCA-IMT, thus reducing the difference between cases and controls for this outcome.
Our results support the need for large long-term interventional trials with CV end-points to investigate whether disease treatment obtained with appropriate medical and pulmonary rehabilitation approaches may modify the CV risk in COPD patients.
Some potential limitations of our study need to be discussed. First, studies included in our meta-analysis have different inclusion and exclusion criteria and most of patients included in the analysis had concomitant CV risk factors (hypertension, smoking, obesity, diabetes mellitus, hyperlipidaemia) and different disease activity status. Since meta-analysis is performed on aggregate data and some missing information is present in each study, the multivariate approach allowed for the adjustment for some (but not all) potential confounders. In particular, it should be considered that most studies did not provide some important information allowing for a better definition and characterization of COPD severity and phenotype (e.g. 6MWT, SGQR, DLCO, CT R950 or WA%, 3 He MRI ADC or VDP). Thus, although results of meta-regression analyses were able to refine analyses by assessing the influence of most clinical and demographic variables on the observed results, caution is necessary in overall results interpretation.
Second, heterogeneity among the studies was generally significant. Although it was not possible to conclusively ascertain sources of heterogeneity, all results were confirmed after adjustment for potential publication bias.
As a further limitation of our study, it should be considered that there are many other 2D (e.g. total plaque area) and 3D (e.g. total plaque volume, vessel wall volume) carotid atherosclerosis measurements that were not considered in this meta-analysis, which are debatably superior to CCA-IMT and carotid plaques at predicting CV events. However, given the lack of studies evaluating these markers of atherosclerosis, our meta-analysis only focused on CCA-IMT and prevalence of carotid plaques.
Finally, we have to consider that the definition of carotid plaques widely varied among different studies. However, the presence of atherosclerotic plaques was identified as an IMT >1.2–1.5 mm in the larger part of included studies and this might partially offset the above limitation.
In conclusion, in our meta-analysis, COPD was significantly associated with subclinical atherosclerosis, and, in turn, with an increased CV risk. Thus, patients with COPD may benefit from a more meticulous screening for CV risk factors and more specific CV prevention strategies, even in an early stage of the lung disease. However, additional and specifically designed studies are needed in order to establish the optimal management of these patients.
Supplementary_material.doc
Download MS Word (232 KB)Disclosure statement
All authors have nothing to report.
Funding
No funding or economic support have been received for this study.
References
- Global Initiative for Chronic Obstructive Lung Disease (GOLD): global strategy for the diagnosis, management and prevention of COPD; 2009. Available from: http://www.goldcopd.org
- Hurd SS, Lenfant C. COPD: good lung health is the key. Lancet. 2005;366:1832–1834.
- Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet. 1997;349:1498–1504.
- Evans RA, Morgan MD. The systemic nature of chronic lung disease. Clin Chest Med. 2014;35:283–293.
- Hillas G, Perlikos F, Tsiligianni I, et al. Managing comorbidities in COPD. Int J Chron Obstruct Pulmon Dis. 2015;10:95–109.
- Sin DD, Wu L, Man SF. The relationship between reduced lung function and cardiovascular mortality: a population-based study and a systematic review of the literature. Chest. 2005;127:1952e9.
- Sin DD, Man SF. Chronic obstructive pulmonary disease as a risk factor for cardiovascular morbidity and mortality. Proc Am Thorac Soc. 2005;2:8–11.
- Yanbaeva DG, Dentener MA, Creutzberg EC, et al. Systemic effects of smoking. Chest. 2007;131:1557–1566.
- Cebron Lipovec N, Beijers RJ, van den Borst B, et al. The prevalence of metabolic syndrome in chronic obstructive pulmonary disease: a systematic review. COPD. 2016;13:399–406.
- Stone IS, Barnes NC, Petersen SE. Chronic obstructive pulmonary disease: a modifiable risk factor for cardiovascular disease? Heart. 2012; 98:1055–1062.
- Simon A, Chironi G, Levenson J. Performance of subclinical arterial disease detection as a screening test for coronary heart disease. Hypertension. 2006;48:392–396.
- Bots ML, Grobbee DE. Intima media thickness as a surrogate marker for generalised atherosclerosis. Cardiovasc Drugs Ther. 2002;16:341–351.
- de Groot E, Hovingh GK, Wiegman A, et al. Measurement of arterial wall thickness as a surrogate marker for atherosclerosis. Circulation. 2004;109:III33–III38.
- O’Leary DH, Polak JF, Kronmal RA, et al. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. N Engl J Med. 1999;340:14–22.
- Chambless LE, Heiss G, Folsom AR, et al. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: the Atherosclerosis Risk in Communities (ARIC) Study, 1987–1993. Am J Epidemiol. 1997;146:483–494.
- Belcaro G, Nicolaides AN, Ramaswami G, et al. Carotid and femoral ultrasound morphology screening and cardiovascular events in low risk subjects: a 10-year follow-up study (the CAFES-CAVE study). Atherosclerosis. 2011;156:379–387.
- Albu A, Fodor D, Bondor C, et al. Carotid arterial stiffness in patients with chronic obstructive pulmonary disease. Acta Physiol Hung. 2011;98:117–127.
- Arsovski Z, Gavrilovski M, Kaeva B, et al. Carotid wall intima-media thickness (IMT) in COPD patients. Eur Res J. 2013;42:3667.
- Joshi RW, Agrawal R, Pandharipande MS, et al. Carotid intima media thickness in chronic obstructive pulmonary disease. VJIM. 2016;20:19–23.
- Sadek SH, Hassan AA, AbdElrahman G, et al. Subclinical cardiovascular changes in chronic obstructive pulmonary disease patients: Doppler ultrasound evaluation. Egypt J Bronchol. 2015;9:140–145.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Med. 2009;6:e1000097.
- Wells GA, Shea B, O’Connell D, et al. Ottawa Hospital Research Institute. The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560.
- Walter SD. The estimation and interpretation of attributable risk in health research. Biometrics. 1976;32:829–849.
- Sterne JA, Egger M, Smith GD. Systematic reviews in health care: investigating and dealing with publication and other biases in meta-analysis. BMJ. 2001;323:101–105.
- Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463.
- Beşir FH, Yılmaz Aydın L, Yazgan Ö, et al. Evaluation of carotid intima media thickness in chronic obstructive pulmonary disease patients. Tuberk Toraks. 2012;60:238–245.
- Chindhi S, Thakur S, Sarkar M, et al. Subclinical atherosclerotic vascular disease in chronic obstructive pulmonary disease: prospective hospital-based case control study. Lung India. 2015;32:137–141.
- Ekin S, Arısoy A, Gunbatar H, et al. The relationships among the levels of oxidative and antioxidative parameters, FEV1 and prolidase activity in COPD. Redox Rep. 2016;15:1–4.
- Fisk M, Mäki Petäjä K, Gale N, et al. Carotid intima-media thickness and aortic stiffness are elevated in chronic obstructive pulmonary disease (COPD) subjects compared to controls and not associated with systemic inflammation. Circulation. 2015;132:A12457.
- Flu WJ, van Kuijk JP, Hoeks SE, et al. Intima media thickness of the common carotid artery in vascular surgery patients: a predictor of postoperative cardiovascular events. Am Heart J. 2009;158:202–208.
- Frantz S, Nihlén U, Dencker M, et al. Atherosclerotic plaques in the internal carotid artery and associations with lung function assessed by different methods. Clin Physiol Funct Imaging. 2012;32:120–125.
- Golpe R, Mateos-Colino A, Testa-Fernández A, et al. Blood pressure profile and hypertensive organ damage in COPD patients and matched controls. The RETAPOC study. PLoS ONE. 2016;11:e0157932.
- Iwamoto H, Yokoyama A, Kitahara Y, et al. Airflow limitation in smokers is associated with subclinical atherosclerosis. Am J Respir Crit Care Med. 2009;179:35–40.
- Karakas O, Cullu N, Karakas E, et al. Evaluation of carotid intima-media thickness in the patients with chronic obstructive pulmonary disease. Acta Med Mediterran. 2013;29:265–267.
- Kim SJ, Yoon DW, Lee EJ, et al. Carotid atherosclerosis in patients with untreated chronic obstructive pulmonary disease. Int J Tuberc Lung Dis. 2011;15:1265–1270.
- Köseoğlu C, Kurmuş Ö, Ertem AG, et al. Association between carotid intima-media thickness and presence of coronary artery disease in chronic obstructive pulmonary disease patients. Anatol J Cardiol. 2016;16:601–607.
- Lahousse L, van den Bouwhuijsen QJ, Loth DW, et al. Chronic obstructive pulmonary disease and lipid core carotid artery plaques in the elderly: the Rotterdam Study. Am J Respir Crit Care Med. 2013;187:58–64.
- Ozgen Alpaydin A, Konyar Arslan I, Serter S, et al. Metabolic syndrome and carotid intima-media thickness in chronic obstructive pulmonary disease. Multidiscip Respir Med. 2013;8:61.
- Pan J, Xu L, Cai SX, et al. The association of pulmonary function with carotid atherosclerosis in older Chinese: Guangzhou Biobank Cohort Study-CVD Subcohort. Atherosclerosis. 2015;243:469–476.
- van Gestel YR, Flu WJ, van Kuijk JP, et al. Association of COPD with carotid wall intima-media thickness in vascular surgery patients. Respir Med. 2010;104:712–716.
- Moro L, Pedone C, Scarlata S, et al. Endothelial dysfunction in chronic obstructive pulmonary disease. Angiology. 2008;59:357–364.
- Sin DD, Man SF. Why are patients with chronic obstructive pulmonary disease at increased risk of cardiovascular diseases? The potential role of systemic inflammation in chronic obstructive pulmonary disease. Circulation. 2003;107:1514–1519.
- Ambrosino P, Lupoli R, Spadarella G, et al. Autoimmune liver diseases and antiphospholipid antibodies positivity: a meta-analysis of literature studies. J Gastrointestin Liver Dis. 2015;24:25–34.
- Ambrosino P, Lupoli R, Tarantino P, et al. Viral hepatitis and anti-phospholipid antibodies positivity: a systematic review and meta-analysis. Dig Liver Dis. 2015;47:478–487.
- Ambrosino P, Tasso M, Lupoli R, et al. Non-invasive assessment of arterial stiffness in patients with rheumatoid arthritis: a systematic review and meta-analysis of literature studies. Ann Med. 2015;47:457–467.
- van der Meer IM, Bots ML, Hofman A, et al. Predictive value of noninvasive measures of atherosclerosis for incident myocardial infarction: the Rotterdam Study. Circulation. 2004;109:1089–1094.
- Ambrosino P, Tarantino L, Di Minno G, et al. The risk of venous thromboembolism in patients with cirrhosis. A systematic review and meta-analysis. Thromb Haemost. 2017;117:139–148.
- Sin DD, Man SF. Systemic inflammation and mortality in chronic obstructive pulmonary disease. Can J Physiol Pharmacol. 2007;85:141–147.
- Quaglia LA, Freitas W, Soares AA, et al. C-reactive protein is independently associated with coronary atherosclerosis burden among octogenarians. Aging Clin Exp Res. 2014;26:19–23.
- Ridker PM, Hennekens CH, Buring JE, et al. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843.
- Man SF, Connett JE, Anthonisen NR, et al. C-reactive protein and mortality in mild to moderate chronic obstructive pulmonary disease. Thorax. 2006;61:849–853.
- Schols AM, Creutzberg EC, Buurman WA, et al. Plasma leptin is related to proinflammatory status and dietary intake in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160:1220–1226.
- Takabatake N, Nakamura H, Abe S, et al. The relationship between chronic hypoxemia and activation of the tumor necrosis factor-alpha system in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;161:1179–1184.
- Eid AA, Ionescu AA, Nixon LS, et al. Inflammatory response and body composition in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164:1414–1418.