Abstract
Background: Direct renin inhibition (DRI) is clinically inferior to other blockers of the renin–angiotensin system (RAS). Thus far, the underlying molecular causes of this finding remain unknown.
Methods: Twenty four patients with non-diabetic chronic kidney disease (CKD) stages III–IV and albuminuria were randomized to DRI or angiotensin receptor blocker (ARB). Employing a novel mass-spectrometry method, the concentrations of renin, aldosterone and plasma angiotensin peptides [Ang I, Ang II, Ang-(1-7), Ang-(1-5), Ang-(2-8), Ang-(3-8)] were quantified before and after an 8-week treatment.
Results: While blood pressure, renal function and albuminuria decreased comparably in both groups, profound RAS component differences were observed: DRI led to a massive renin increase, while suppressing both vasoconstrictive (Ang I and Ang II) and vasodilatory RAS metabolites (Ang-(1-7) and Ang-(1-5)). In contrast, ARB led to a four-fold increase of Ang I and Ang II, while Ang-(1-7) and Ang-(1-5) increased moderately but significantly. With ARB treatment, a decreased aldosterone-to-Ang II ratio suggested efficacy in blocking AT1 receptor.
Conclusions: DRI therapy abolishes all RAS effector peptides. ARB increases both vasoconstrictive and vasodilative angiotensins, while this is accompanied by efficient blockade of vasoconstrictive effects. These differential molecular regulations should be considered when selecting optimal antihypertensive and disease-modifying therapy in CKD patients.
Direct renin inhibition leads to a complete and lasting abolition of both classical and alternative RAS components.
Angiotensin receptor blockade leads to effective receptor blockade and up-regulation of alternative RAS components.
Differential molecular regulations of the RAS should be considered when selecting optimal antihypertensive and disease-modifying therapy in CKD patients.
Key messages
Introduction
The renin–angiotensin system (RAS) is a complex regulatory network with widespread effects on blood pressure regulation, salt metabolism and fluid homeostasis [Citation1–3]. Blockade of key RAS components through angiotensin receptor blockers (ARB) is used to treat hypertension and to delay the progression of chronic kidney disease (CKD), as an additional reduction of proteinuria in both diabetic and non-diabetic renal disease ensues [Citation4–8].
Several years ago, another mode of RAS blockade – direct renin inhibition (DRI) – was designed as an alternative or add-on to conventional RAS inhibitors to block a compensatory rise in RAS activity by recurring angiotensin II (Ang II) levels and aldosterone breakthrough [Citation9–11]. Yet, several large-scale clinical trials showing non-superior or even inferior outcomes of the DRI aliskiren compared to other RAS blockers have led to a meanwhile infrequent use of this agent [Citation12–17]. While typical side effects such as hyperkalemia and worsening of renal function are thought to be a consequence of reduced glomerular perfusion pressure, potential involved mechanisms on a RAS peptide level remain unclear to this date [Citation18].
The lack of follow-up analyses in humans investigating the molecular effects of aliskiren on both the classical RAS pathway composed of renin, Ang I, Ang II and aldosterone as well as the alternative RAS axis constituted by Ang-(1-7) and Ang-(1-5) makes conclusions about its effects on RAS metabolism challenging (). Apart from functional consequences DRI might fundamentally affect the RAS at the molecular level [Citation18,Citation19]. Hence, ablation of distinct beneficial RAS components might eliminate positive effects exerted by an inhibition of the classical RAS.
Figure 1. Basic model of the RAS, its main enzymes and the interfering substances used in the study.
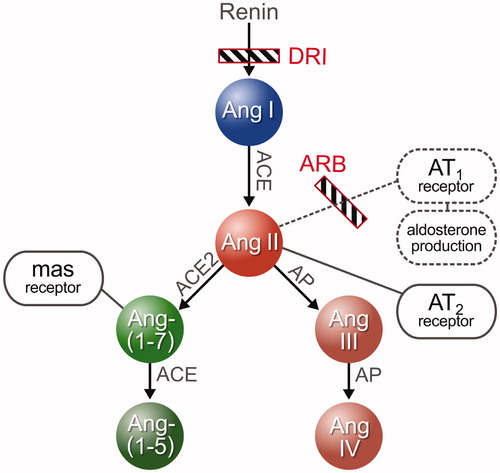
Specifically, the alternative RAS composed of Ang-(1-7) generated by ACE-2 and subsequent MAS receptor activation may confer beneficial vasodilatory, anti-inflammatory and anti-fibrotic effects opposing the pro-fibrotic actions of Ang II and the AT1 receptor as the central members of the classical RAS [Citation20–22]. Similarly, activation of the AT2 receptor has also been described as potentially conferring beneficial vasodilatory effects [Citation23,Citation24]. Other small peptides such as Ang-(2-8) and Ang-(3-8) currently remain of unclear function and importance. While Ang-(2-8) is known to have capacities similar to Ang II [Citation25], Ang-(3-8) is thought to be involved in memory processes [Citation26].
We hypothesize that – concomitant to an Ang II blockade – ARB treatment activates the alternative RAS cascade via MAS or AT2 receptors, while DRI prevents alternative RAS activation by completely blocking effector peptide formation.
We therefore aimed at elucidating the molecular effects of the DRI aliskiren on the RAS in CKD patients and compared it with the widely applied standard-of-care ARB candesartan in a prospective randomized trial.
Patients and methods
Ethical approval
The study was approved by the Ethics Committee of the Medical University of Vienna. Furthermore, it was registered at the European Clinical Trials Database (EUDRACT No.: 2012-000250-05) and at a publicly available clinical trial database (NCT01827202, http://clinicaltrials.gov). The study was carried out according to good clinical and scientific practice guidelines. All participants provided their written informed consent prior to participation.
Patient recruitment
This was a 10-week prospective, randomized controlled exploratory trial in 24 patients with non-diabetic, proteinuric CKD. Patients were eligible for inclusion if they were suffering from CKD stage III or IV (defined by an eGFR (MDRD) of 15–59 ml/min). Furthermore, patients had to have proteinuria defined by an albumin excretion rate >300 mg/24 hours or a urinary albumin/creatinine ratio >300 mg/g on a spot urine sample or >200 mg/g if they were already receiving any form of RAS blockade.
Patients with previously diagnosed diabetes mellitus were excluded in order to increase study population homogeneity as the RAS can be strongly influenced by a disturbed glucose metabolism [Citation27].
Additionally, patients had to be neither hypo- nor severely hypertensive at the time of inclusion (systolic blood pressure >120 mmHg and <180 mmHg on ambulatory measurement). Thus, according to the JNC7 classification, all patients classified as having high normal blood pressure or stage 1 or 2 hypertension [Citation28]. Pregnant patients and those planning on pregnancy were excluded. All patients were recruited from the outpatient Nephrology Clinic of the Medical University of Vienna between 2012 and 2015.
Study procedures
After inclusion, patients were unified regarding their antihypertensive therapy regimens. This included the elimination of all RAS-blocking agents for 2 weeks; if severely hypertensive blood pressure values were elicited through this measure, additional antihypertensive treatment – preferably with calcium channel or alpha-blockers – was introduced. Additional antihypertensive medication including beta-blockers and diuretics was allowed as needed, aiming at a systolic blood pressure value <150 mmHg at all times.
Following the 2-week run-in phase without RAS-blocking agents, the 8-week active study phase started. Patients were subjected to blood collection for the first RAS equilibrium analysis, measurement of renin and aldosterone as well as urinary analysis (including UACR).
Subsequently, patients were randomized to either receive aliskiren for 2 months (4 weeks 150 mg daily followed by 4 weeks 300 mg daily) or candesartan for 2 months (4 weeks 8 mg daily followed by 4 weeks 16 mg daily). Patients were instructed to ingest the study medication two hours before blood collection. A laboratory control of electrolytes and creatinine was performed after the first week of drug intake and again after dosage increase to exclude hyperkalemia and/or acute renal worsening elicited by the newly introduced RAS blockade.
At the end of the active study phase, determination of all RAS components as well as a full laboratory check-up was performed again.
Blood collection and processing
For the quantification of angiotensin metabolites, a blood collection via cubital vein was conducted. Six millilitres of heparinized peripheral blood were collected and chilled on ice immediately. Plasma was obtained by centrifugation (4 °C) at 2000×g for 10 min and 2 ml aliquots were stored at −80 °C until analysis. Previous results of our research group have shown similar qualitative outcomes when comparing the quantification of circulating and equilibrium angiotensin peptide levels [Citation29]. Thus, plasma conditioning was performed at 37 °C followed by stabilization by addition of an enzyme inhibitor cocktail (Attoquant Diagnostics) [Citation30]. Stable isotope-labelled internal standards for each Ang metabolite [Ang I, Ang II, Ang-(1-7), Ang-(1-5), Ang-(2-8), Ang-(3-8)] were added to stabilized plasma samples at a concentration of 200 pg/mL. Samples were then subjected to ultra performance liquid chromatography tandem-mass spectrometry (UPLC–MS/MS) based angiotensin quantification by Attoquant Diagnostics GmbH (Vienna, Austria) as described previously [Citation31–33]. Both renin and aldosterone concentration were measured with a clinical chemiluminescence immunoassay (DiaSorin LIAISON analyzer).
Statistical analysis
Demographic data are presented as mean values with standard deviation (SD) if normally distributed (verified by Shapiro–Wilk’s test) and unpaired Student’s t-test was performed to determine differences between the two medication groups. Non-normally distributed values are described as medians [interquartile range] and Mann–Whitney’s U-test was applied.
Angiotensin values below the lower limit of quantification (LLOQ) were substituted by LLOQ/Sqrt(2) [Citation34]. In , calculated median values, which were below the LLOQ, were specified as such. For the analysis of angiotensins before and after medical intervention, Wilcoxon’s signed-rank test was used. A p value ≤.05 was considered statistically significant.
The IBM SPSS System for Mac version 22.0.0 (SPSS, Inc., 2010, Chicago, IL) and GraphPad Prism Version 6 were used for all analyses (San Diego, CA).
Results
Baseline clinical characteristics
The respective study groups were homogeneous regarding demographic and baseline laboratory values at the time of study inclusion ( and suppl. Table 1). Notably, potassium levels of the candesartan group were significantly higher at inclusion; however, they decreased during the two-week run-in phase, during which previously ingested RAS blockers were stopped.
Table 1. Demographic and baseline values at study medication start (=two weeks after RAS blockade termination).
The included patients’ underlying renal disease was of mixed etiology: 12 patients had glomerular disease, five patients had vascular nephropathy, three patients had autosomal dominant polycystic kidney disease and four had other etiologies. No patient had any clinical or laboratory signs or a history of diabetes mellitus. The antihypertensive regimens were heterogeneous at the time of inclusion. Before unification of therapy, six patients ingested an ACEi and eight took an ARB. Overall, 13 patients were on multiple antihypertensive agents. All patients had previously received dietary counselling addressing the requirements of CKD stages III–IV.
DRI leads to complete angiotensin suppression; ARB elicits increase of classical and alternative angiotensins
RAS blockade with aliskiren significantly suppressed the entire classical RAS axis with decreased Ang I and Ang II levels (). Components of the alternative RAS axis, such as Ang-(1-7) and Ang-(1-5) remained at very low levels or even decreased to values close to or below the LLOQ ().
Figure 2. Classical RAS metabolites: Ang I and Ang II levels before and after medication intake. Concentrations are given in pg/mL. *p < .05; **p < .01.
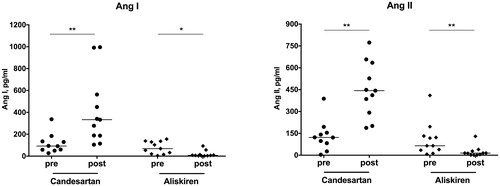
Figure 3. Alternative RAS metabolites: Ang-(1-7) and Ang-(1-5) levels before and after medication intake. Concentrations are given in pg/mL. *p < .05; **p < .01.
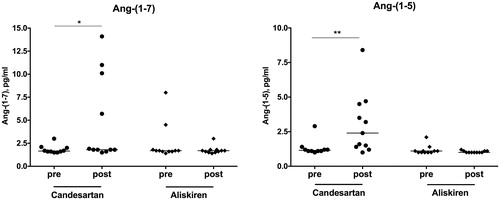
In clear contrast, we observed a considerable, approximately four-fold increase of both Ang I and Ang II in patients treated with an ARB. In further opposition to DRI therapy, ARB treatment also up-regulated alternative RAS peptides, characterized by elevated Ang-(1-7) and Ang-(1-5) concentrations ( and ).
Table 2. Angiotensin concentrations before and after medication. p-Values < .05 are marked bold.
Similarly, the end-products of the classical RAS, Ang-(2-8) and Ang-(3-8), were also up-regulated after ARB therapy but remained in the range of the LLOQ after DRI therapy (suppl. Figure 1, ).
In both treatment groups, the Ang II/Ang I ratio, which is a surrogate for plasma ACE activity, was not significantly altered by either medication: while it increased from a median of 1.2 [IQR 0.9–1.7] to 1.4 [0.7–1.9] (p = .959) in the aliskiren group, it also rose from 1.4 [0.9–1.7] to 1.6 [1.2–1.9] (p = .859) in candesartan-treated patients.
Differential regulation of renin and aldosterone
Both the most proximal and distal effectors of the RAS, renin and aldosterone, were prominently and oppositely affected by the study medications: while aliskiren massively up-regulated the peripheral renin concentration reflected by a 10-fold increase, only a modest rise was observed with candesartan (). Of note, aldosterone concentrations were lower in the ARB group than in the DRI group. However, this did not reach statistical significance (p = .104).
Table 3. Secondary outcome parameters p-Values < .05 are marked bold.
The aldosterone-to-renin ratio (ARR) was comparable between ARB and DRI therapy both before and after the medication phase and decreased in a similar manner ( and ). The aldosterone-to-Ang II ratio (AA2-Ratio), which was also comparable before medication intake, significantly decreased only in ARB-treated patients (p = .007).
Blood pressure, renal function and proteinuria
Blood pressure decreased slightly in the whole cohort from a mean of 137 (±11)/86 (±13) mmHg to a mean of 128 (±10)/81 (±9) mmHg (p for RRsys = 0.043, p for RRdiast=0.642). There were no significant differences in the drop of blood pressure between the aliskiren and the candesartan group ().
Similarly, eGFR and proteinuria significantly decreased during the study period across the entire cohort (p for eGFR = .053, p for UPCR=.005, p for UACR = .001), while no statistical differences between the study groups could be observed.
As measures of patient safety, serum potassium and eGFR were measured additionally after medication initiation and after dosage increase. After one week on study medication, overall potassium levels increased from 4.4 ± 0.4 mmol/L to 4.6 ± 0.4 mmol/L (p = .007) and eGFR remained stable (32 ± 12 ml/min and 31 ± 12 ml/min (p = .508)). After the medication increase, serum potassium levels showed a minimal and non-significant overall increase from 4.8 ± 0.5 mmol/L after one month on study drug to 4.9 ± 0.4 mmol/L after the increase (p = .603). Similarly, the eGFR remained stable as well (29 ± 10 ml/min to 29 ± 11 ml/min (p = .745)).
Discussion
In the present study, we found a differential molecular response of the RAS in patients with both CKD stages III–IV and proteinuria treated with the DRI aliskiren or the ARB candesartan over eight weeks, while the overall antihypertensive and antiproteinuric effect was comparable. The substantial RAS profile differences determined by a novel mass spectrometry method indicate considerably different pathomechanistic consequences of these distinct RAS-blocking treatments on angiotensin (Ang) metabolism (): Aliskiren treatment resulted in a profound suppression of all angiotensin metabolites, while treatment with candesartan elicited a distinct increase of both classical and alternative angiotensins.
Figure 4. Group median Ang levels. The illustration on the left contains the median Ang values of all 24 patients after the run-in phase, during which all RAS blockers were eliminated. The right upper illustration shows median Ang values after aliskiren intake; the right lower illustration shows median values after candesartan intake. Size of spheres and numbers aside represent median Ang concentrations in pg/mL analysed by mass spectrometry directly out of patient’s plasma. Numbers in brackets describe Ang sequence. Median values, which were below the LLOQ, were specified as such.
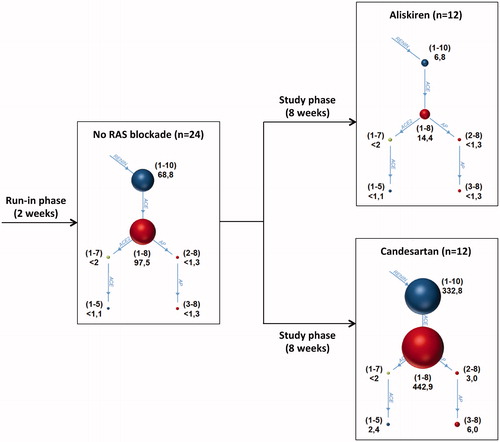
After DRI intake, effectors of the classical RAS axis such as Ang I and Ang II were substantially suppressed. Central peptides of the alternative RAS axis, namely Ang-(1-7) and Ang-(1-5), remained at minimal levels. This effective RAS blockade results in a compensatory up-regulation of renin levels as a consequence of an intact negative feedback loop of the RAS [Citation35]. Traditionally, it has been perceived that down-regulation of Ang II, which otherwise potently mediates pro-inflammatory effects, vascular damage and fibrosis, directly yields positive clinical effects. However, it has to be taken into account that the RAS also confers direct beneficial effects through ACE2-generated Ang-(1-7) [Citation36–38]. Similar nephron-protective effects have also been described for Ang II-mediated activation of AT2 receptors, which might be particularly relevant under pathologic conditions [Citation39]. Thus, the rationale to fully block detrimental Ang II clearly needs to be reconsidered with respect to beneficial RAS effects.
Further, no reduction of the so-called aldosterone breakthrough could be observed with DRI therapy, corroborating results from Bomback et al., who have previously shown an aldosterone breakthrough rate of at least 30% with aliskiren [Citation40]. Comparably, in our study three out of 12 patients randomized to aliskiren even showed a substantial aldosterone increase indicating an Ang-independent mechanism of aldosterone production.
In contrast, the widely used ARB candesartan generally elicited remarkably different molecular effects compared to aliskiren: first, peripheral renin concentrations only approximately doubled, which is potentially due to a smaller feedback effect, given that the RAS cascade can exert its full effect towards the most distal part – the angiotensin receptor I (AT1R).
Second, concentrations of the classical RAS effectors Ang I and Ang II quadrupled, similarly to peptides comprising the final path of the alternative RAS and other peptides, such as Ang-(1-5), Ang-(2-8) and Ang-(3-8).
After eight weeks of ARB intake, we observed a substantial decline of aldosterone concentrations. Putting these results into perspective, the Aldosterone-to-Ang II ratio (AA2-Ratio), which has recently been described and used as an improved method to diagnose primary aldosteronism [Citation41], enables a new perspective of RAS shifts upon ARB therapy. We observed a strong decrease of the AA2-Ratio with ARB therapy in all patients on the medication. ARB therapy had a considerably stronger impact on the AA2-Ratio than on aldosterone levels, which is possibly due to significant treatment-induced increases of renin and Ang II. This might then result in dominance of Ang II signalling at the receptor level, where it competes with the ARB for AT1R binding.
As ACE remained active in patients treated with an ARB, reflected by a stable Ang II/Ang I ratio, a rapid metabolic conversion from Ang-(1-7) to Ang-(1-5) via ACE perpetuated [Citation30]. Simultaneously, increased renin secretion stimulated by decreased Ang II signalling contributed to elevated Ang levels. Accordingly, both the Ang-(1-7) and even more the Ang-(1-5) concentration increased. From these results, it can be concluded that the beneficial effects associated with ARB use could not solely be mediated by blood pressure reduction and a decrease of glomerular pressure, but also by a fundamental change of RAS effector peptide profiles.
In summary, detrimental Ang II effects conferred by the AT1R, including vasoconstriction are effectively inhibited by candesartan [Citation42]. Yet, beneficial vasodilatory and antihypertrophic effects can still proceed via both AT2R activation and the ACE2-driven generation of alternative RAS peptides acting on the MAS receptor [Citation36,Citation39,Citation43,Citation44]. It appears evident that a functional shift towards the alternative RAS takes place upon ARB treatment that might be dominantly prevented by DRIs.
Our study has some limitations. Of the 24 included patients, only six were women, precluding the potential to make a statement on the influence of gender on RAS regulation. Further, we used a standard plasma renin concentration immunoassay. Campbell described that aliskiren use might lead to an overestimation of the renin concentration by assay interference [Citation45]. Yet, as the same author considered the direct measurement of angiotensins as the most reliable method to assess DRI effects and as we are able to provide detailed and sensitive Ang concentration data, we are confident to deliver solid information on the RAS with DRI therapy. Further, a cross-over study design might have increased the strength of the results with regard to information on an individual’s capability of RAS modulation. Last, three out of 12 future candesartan patients and five out of 12 aliskiren patients also ingested a beta blocker at the time of inclusion and throughout the study phase. Beta-blockers are known to suppress renin levels [Citation46,Citation47]. Yet, as we did not discontinue this therapy during the study phase and carried out paired analysis of angiotensins, interference with the attained results should be of negligible importance. Strengths of the study are its prospective nature and examination of human subjects as well as the simultaneous quantification of metabolites both of the classical and the alternative RAS and the high selectivity and specificity of the applied assay (internal standardization, UPLC–MS/MS analysis).
It would now be of high importance to analyse other forms of RAS blockade (e.g. ACEi, combined ARB and neprilysin inhibitors, aldosterone antagonists) to establish their potency regarding effective blockade of the classical RAS and activation of the alternative RAS. It might be anticipated that administration of an ACEi might result in strongly elevated Ang-(1-7) levels, while Ang II levels decrease significantly; studies analysing this form of RAS blockade are currently underway.
In conclusion, this is the first trial in humans studying the molecular changes of both the classical and alternative RAS resulting from antihypertensive therapy with the DRI aliskiren and the ARB candesartan in patients with hypertension and non-diabetic CKD. We demonstrate that aliskiren results in a profound and robust suppression of all measurable angiotensins, while ARB therapy not only leads to an increase of Ang I and Ang II but concurrently promotes an up-regulation of alternative RAS pathways. As the alternative RAS potentially confers beneficial clinical effects, these findings might be taken into consideration in the treatment of CKD patients.
Supplementary_Table_1_Ann_Med.docx
Download MS Word (15 KB)Suppl_Figure_1.tiff
Download TIFF Image (1.5 MB)Acknowledgements
The study was supported by the ‘Medical Scientific Fund of the Mayor of the City of Vienna’, project number 13002.
Disclosure statement
The authors declare no conflict of interest.
Additional information
Funding
References
- Peach MJ. Renin–angiotensin system: biochemistry and mechanisms of action. Physiol Rev. 1977;57:313–370.
- Ito M, Oliverio MI, Mannon PJ, et al. Regulation of blood pressure by the type 1A angiotensin II receptor gene. Proc Natl Acad Sci USA. 1995;92:3521–3525.
- Lavoie JL, Sigmund CD. Minireview: overview of the renin–angiotensin system—an endocrine and paracrine system. Endocrinology. 2003;144:2179–2183.
- Heeg JE, de Zeeuw D, de Jong PE. Antiproteinuric effect of ACE inhibitors. Lancet. 1988;2:1251–1252.
- Taguma Y, Kitamoto Y, Futaki G, et al. Effect of captopril on heavy proteinuria in azotemic diabetics. N Engl J Med. 1985;313:1617–1620.
- Gradman AH, Arcuri KE, Goldberg AI, et al. A randomized, placebo-controlled, double-blind, parallel study of various doses of losartan potassium compared with enalapril maleate in patients with essential hypertension. Hypertension. 1995;25:1345–1350.
- Remuzzi G, Ruggenenti P, Perico N. Chronic renal diseases: renoprotective benefits of renin–angiotensin system inhibition. Ann Intern Med. 2002;136:604–615.
- Márquez DF, Ruiz-Hurtado G, Ruilope LM, et al. An update of the blockade of the renin angiotensin aldosterone system in clinical practice. Expert Opin Pharmacother. 2015;16:2283–2292.
- Rahuel J, Rasetti V, Maibaum J, et al. Structure-based drug design: the discovery of novel nonpeptide orally active inhibitors of human renin. Chem Biol. 2000;7:493–504.
- Wood JM, Maibaum J, Rahuel J, et al. Structure-based design of aliskiren, a novel orally effective renin inhibitor. Biochem Biophys Res Commun. 2003;308:698–705.
- Azizi M, Ménard J, Bissery A, et al. Pharmacologic demonstration of the synergistic effects of a combination of the renin inhibitor aliskiren and the AT1 receptor antagonist valsartan on the angiotensin II-renin feedback interruption. J Am Soc Nephrol. 2004;15:3126–3133.
- McMurray JJ, Krum H, Abraham WT, et al. Aliskiren, enalapril, or aliskiren and enalapril in heart failure. N Engl J Med. 2016;374:1521–1532.
- Woo KT, Choong HL, Wong KS, et al. Aliskiren and losartan trial in non-diabetic chronic kidney disease. Renin–Angiotensin–Aldosterone Syst. 2014;15:515–522.
- Mihai G, Varghese J, Kampfrath T, et al. Aliskiren effect on plaque progression in established atherosclerosis using high resolution 3D MRI (ALPINE): a double-blind placebo-controlled trial. J Am Heart Assoc. 2013;2:e004879.
- Heerspink HJ, Persson F, Brenner BM, et al. Renal outcomes with aliskiren in patients with type 2 diabetes: a prespecified secondary analysis of the ALTITUDE randomised controlled trial. Lancet Diabetes Endocrinol. 2016;4:309–317.
- Soji K, Doi S, Nakashima A, et al. Efficacy of add-on therapy of aliskiren to an angiotensin II receptor blocker on renal outcomes in advanced-stage chronic kidney disease: a prospective, randomized, open-label study. Clin Exp Nephrol. 2015;19:631–638.
- Parving HH, Brenner BM, McMurray JJ, et al. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med. 2012;367:2204–2213.
- Rajagopalan S, Bakris GL, Abraham WT, et al. Complete renin–angiotensin–aldosterone system (RAAS) blockade in high-risk patients: recent insights from renin blockade studies. Hypertension. 2013;62:444–449.
- Moniwa N, Varagic J, Ahmad S, et al. Hemodynamic and hormonal changes to dual renin–angiotensin system inhibition in experimental hypertension. Hypertension. 2013;61:417–424.
- Oudit GY, Liu GC, Zhong J, et al. Human recombinant ACE2 reduces the progression of diabetic nephropathy. Diabetes. 2010;59:529–538.
- Sasaki S, Higashi Y, Nakagawa K, et al. Effects of angiotensin-(1-7) on forearm circulation in normotensive subjects and patients with essential hypertension. Hypertension. 2001;38:90–94.
- Carver KA, Smith TL, Gallagher PE, et al. Angiotensin-(1-7) prevents angiotensin II-induced fibrosis in cremaster microvessels. Microcirculation. 2015;22:19–27.
- Porrello ER, Delbridge LM, Thomas WG. The angiotensin II type 2 (AT2) receptor: an enigmatic seven transmembrane receptor. Front Biosci. 2009;14:958–972.
- Ewert S, Laesser M, Johansson B, et al. The angiotensin II receptor type 2 agonist CGP 42112A stimulates NO production in the porcine jejunal mucosa. BMC Pharmacol. 2003;3:2.
- Yugandhar VG, Clark MA. Angiotensin III: a physiological relevant peptide of the renin angiotensin system. Peptides. 2013;46:26–32.
- Gard PR. Cognitive-enhancing effects of angiotensin IV. BMC Neurosci. 2008;9 Suppl 2:S15.
- Lim HS, MacFadyen RJ, Lip GY. Diabetes mellitus, the renin–angiotensin–aldosterone system, and the heart. Arch Intern Med. 2004;164:1737–1748.
- Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252.
- Kovarik JJ, Antlanger M, Domenig O, et al. Molecular regulation of the renin–angiotensin system in haemodialysis patients. Nephrol Dial Transplant. 2015;30:115–123.
- Sharp S, Poglitsch M, Zilla P, et al. Pharmacodynamic effects of C-domain-specific ACE inhibitors on the renin–angiotensin system in myocardial infarcted rats. Renin–Angiotensin–Aldosterone Syst. 2015;16:1149–1158.
- Poglitsch M, Domenig O, Schwager C, et al. Recombinant expression and characterization of human and murine ACE2: species-specific activation of the alternative renin–angiotensin-system. Int J Hypertens. 2012;2012:428950.
- Ye M, Wysocki J, Gonzalez-Pacheco FR, et al. Murine recombinant angiotensin-converting enzyme 2: effect on angiotensin II-dependent hypertension and distinctive angiotensin-converting enzyme 2 inhibitor characteristics on rodent and human angiotensin-converting enzyme 2. Hypertension. 2012;60:730–740.
- Haber PK, Ye M, Wysocki J, et al. Angiotensin-converting enzyme 2-independent action of presumed angiotensin-converting enzyme 2 activators: studies in vivo, ex vivo, and in vitro. Hypertension. 2014;63:774–782.
- Croghan CW, Egeghy PP. Methods of dealing with values below the limit of detection using SAS. 2003. Available from: analytics.ncsu.edu/sesug/2003/SD08-Croghan.pdf2003.
- Fisher ND, Jan Danser AH, Nussberger J, et al. Renal and hormonal responses to direct renin inhibition with aliskiren in healthy humans. Circulation. 2008;117:3199–3205.
- Simões e Silva AC, Silveira KD, et al. ACE2, angiotensin-(1-7) and Mas receptor axis in inflammation and fibrosis. Br J Pharmacol. 2013;169:477–492.
- Crackower MA, Sarao R, Oudit GY, et al. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature. 2002;417:822–828.
- Santos RA, Ferreira AJ, Verano-Braga T, et al. Angiotensin-converting enzyme 2, angiotensin-(1-7) and Mas: new players of the renin–angiotensin system. J Endocrinol. 2013;216:R1–R17.
- Wenzel UO, Krebs C, Benndorf R. The angiotensin II type 2 receptor in renal disease. J Renin Angiotensin Aldosterone Syst. 2010;11:37–41.
- Bomback AS, Rekhtman Y, Klemmer PJ, et al. Aldosterone breakthrough during aliskiren, valsartan, and combination (aliskiren + valsartan) therapy. J Am Soc Hypertens. 2012;6:338–345.
- Poglitsch M, Ahmed AH, Resl M, et al. OS 35-04 the AA2-ratio: towards improved screening for primary aldosteronism in hypertension. J Hypertens. 2016;34 Suppl 1:e400.
- Goodfriend TL, Elliott ME, Catt KJ. Angiotensin receptors and their antagonists. N Engl J Med. 1996;334:1649–1654.
- Iyer SN, Chappell MC, Averill DB, et al. Vasodepressor actions of angiotensin-(1-7) unmasked during combined treatment with lisinopril and losartan. Hypertension. 1998;31:699–705.
- Aulakh GK, Sodhi RK, Singh M. An update on non-peptide angiotensin receptor antagonists and related RAAS modulators. Life Sci. 2007;81:615–639.
- Campbell DJ. Interpretation of plasma renin concentration in patients receiving aliskiren therapy. Hypertension. 2008;51:15–18.
- Holmer SR, Hense HW, Danser AH, et al. Beta adrenergic blockers lower renin in patients treated with ACE inhibitors and diuretics. Heart. 1998;80:45–48.
- Holmer SR, Hengstenberg C, Mayer B, et al. Marked suppression of renin levels by beta-receptor blocker in patients treated with standard heart failure therapy: a potential mechanism of benefit from beta-blockade. J Intern Med. 2001;249:167–172.
