Abstract
Background: Despite current interest in the unfavourable impact of non-traditional lipid profiles on cardiovascular disease, information regarding its relations to reduced glomerular filtration rate (GFR) in H-type hypertension population has not been systemically elucidated.
Methods: Analyses were based upon a cross-sectional study of 3259 participants with H-type hypertension who underwent assessment of biochemical, anthropometric and blood pressure values. Reduced GFR was considered if meeting estimated GFR <60 ml/min/1.73 m2.
Results: A stepwise multivariate regression analysis indicated that non-traditional lipid parameters remained as independent determinants of estimated GFR (all p < .001). In multivariable models, we observed a 50%, 51%, 31%, and 24% higher risk for decreased GFR with each SD increment in TC/HDL-C, TG/HDL-C, LDL-C/HDL-C ratios and non-HDL-C levels, respectively. The highest quartile of TC/HDL-C, TG/HDL-C and LDL-C/HDL-C ratios carried reduced GFR odds (confidence intervals) of 5.50 (2.50 to 12.09), 6.63 (2.58 to 17.05) and 2.22 (1.15 to 4.29), respectively.
Conclusions: The relative independent contribution of non-traditional lipid profiles, as indexed by TC/HDL-C, TG/HDL-C, LDL-C/HDL-C ratios and non-HDL-C, towards reduced GFR putting research evidence at the very heart of lipoprotein-mediated renal injury set a vital example for applying a clinical and public health recommendation for reducing the burden of chronic kidney disease.
Non-traditional lipid profiles has been linked with the occurrence of cardiovascular disease, but none of the studies that address the effect of non-traditional lipid profiles on reduced GFR risk in H-type hypertension population has been specifically established.
A greater emphasis of this study resided in the intrinsic value of TC/HDL-C, TG/HDL-C, LDL-C/HDL-C ratios and non-HDL-C that integrate atherogenic and anti-atherogenic lipid molecules to predict the risk of reduced GFR among H-type hypertension population and provide insight into the pathophysiology of subsequent cardio-cerebrovascular outcomes.
In a large Chinese H-type hypertension adults, the relative independent contribution of non-traditional lipid profiles, as indexed by TC/HDL-C, TG/HDL-C, LDL-C/HDL-C ratios and non-HDL-C, towards reduced GFR putting research evidence at the very heart of lipoprotein-mediated renal injury set a vital example for applying a clinical and public health recommendation for reducing the burden of CKD.
KEY MESSAGES
1. Introduction
Hypertension is a leading global health challenge due to its high prevalence and contributing to cardiovascular disease (CVD) and chronic kidney disease [Citation1,Citation2]. In China, about one-third of the adult population had hypertension, particularly the most important causes of CVD mortality and premature death [Citation2,Citation3]. The concurrence of hypertension and hyper-homocysteinaemia (HHcy) by its concentration greater than 10 μmol/L, termed H-type hypertension, accounts for the current burden of hypertension in China [Citation4–6]. A large randomized clinical trial of efficacy of folic acid therapy in primary stroke prevention has demonstrated that H-type hypertension individuals represented 80.3% of 20,702 participants with hypertension [Citation7]. An increasing number of population studies established evidence of a positive and independent relationship of H-type hypertension with carotid atherosclerotic plaques and cardio-cerebrovascular events [Citation5,Citation7–9]. The prevalence of H-type hypertension is remarkably increasing, and it has been recognized as a serious clinical and public health problem resulting in poor outcomes and high costs in China. More importantly, it has become evident that HHcy substantially increased the risk of renal function decline in hypertensive adults, which has generated interest in a potential role for H-type hypertension as a risk factor for the progression of chronic kidney disease (CKD) [Citation4,Citation10,Citation11]. The combined effect of H-type hypertension with CKD might exert concerted efforts for the risk of vascular disease. Accordingly, the early screening and intervention of risk factors for the decline of kidney function in adults with H-type hypertension before the development of cardio-cerebrovascular implications would be of great clinical benefit.
Analyses of data from prior studies have pointed out development and progression of kidney damage in the setting of abnormal lipoprotein metabolism [Citation12–14]. The notion that renal vascular atherosclerosis, glomerulosclerosis and tubulointerstitial injury linked with a pattern of dyslipidaemia characterized by elevated plasma triglyceride (TG) and high-density lipoprotein cholesterol (HDL-C) deficiency has been a long-standing recognition in animal models [Citation15–17]. Interestingly, considerable evidence has raised uncertainties regarding the adverse impact of hyperlipidaemia on renal disease [Citation18–22]. In other words, the question of whether traditional lipid variables contributed to the initiation of reduced glomerular filtration rate (GFR) remained unanswered. Currently, there is an area of active investigations as non-traditional lipid profiles (have been shown to be strong and independent predictors of CVD and all-cause mortality [Citation23–27]. For instance, by inversely integrating HDL-C, a higher total cholesterol (TC)/HDL-C ratio has been acceptable for reflecting, to some extent, discordance between particle concentration and cholesterol content [Citation28,Citation29]. In this regard, TC/HDL-C ratio provided incremental improvement in CVD events prediction beyond measurements of low-density lipoprotein cholesterol (LDL-C) and non-HDL-C [Citation25,Citation26]. Likewise, the clinical relevance in quantifying atherogenic and cardiometabolic risk grows, as the effectiveness of TG/HDL-C ratio to identify insulin resistance and concentrations of small dense LDL particles has been well documented [Citation23,Citation24,Citation30,Citation31]. It was worthy of note that LDL-C/HDL-C ratio was considered as being more useful than isolated lipid values for atherosclerotic status and cardiovascular risk assessment, owing to better reflection of the interactions between lipid fractions [Citation27,Citation32].
Despite the research to date, expert opinions were divided regarding the need to recommend non-traditional lipid profiles measurements in the routine evaluation of reduced GFR risk [Citation33–36]. As previously outlined, an elevated TG/HDL-C ratio affected the incidence and progression of CKD, with an obvious effect on the decline in estimated GFR (eGFR) [Citation33,Citation34]. Conversely, a sample of 1843 subjects from Southern China revealed that controversies persisted about the merits of TC/HDL-C, TG/HDL-C and LDL-C/HDL-C ratios in detecting prevalent CKD [Citation35]. However, none of the studies that address the effect of non-traditional lipid profiles on reduced GFR risk in H-type hypertension population has been specifically established. To better understand lipoprotein metabolism abnormality and its prognosis on subjects with concomitant H-type hypertension and loss of kidney function, further research using more accurate measures of non-traditional lipid profiles is warranted. On these premises, the objective of this study aimed to add to previous studies by producing reliable estimates of the impact of non-traditional lipid profiles (TC/HDL-C, TG/HDL-C, LDL-C/HDL-C ratios and non-HDL-C) on prevalent reduced GFR in rural Chinese H-type hypertension adults.
2. Materials and methods
2.1. Study population
This study is part of a large population-based cross-sectional epidemiological investigation of 11,956 permanent residents (≥35 years of age) in rural areas of China. The full details regarding the design and rationale of the study were extensively described elsewhere [Citation37,Citation38]. Our eligible dataset (serum lipoproteins and kidney function data) consisted of 3259 H-type hypertension participants in the current analysis. The Ethics Committee of China Medical University (Shenyang, China) approved the study protocol. We obtained written informed consent from each participant before enrolment, and the whole data and procedures conformed to the principles of ethical standards.
2.2. Data collection and measurements
Prior reports have described the data collection and methods selection in detail [Citation37,Citation38]. Cardiologists and trained nurses administered a structured questionnaire to document specified data on demographic, health-related behaviours, indexes of lipids, history of CVD (coronary heart disease, arrhythmia and heart failure) and any self-reported medication used in the past two weeks.
Study participants waited for at least 5 min in a relaxed and sitting position. Then, blood pressure was measured by two randomly trained observers using automatic electronic sphygmomanometer (HEM-907; Omron, Kyoto, Japan) with the arm supported at the level of the heart. The mean readings of three replicate measurements were recorded for the present analysis.
Anthropometric indices were conducted when the subjects wore in light clothing without shoes. Briefly, their weight was quantified to the nearest 0.1 kg in the utility of a calibrated digital scale. A portable stadiometer was used for height measurement (rounded to nearest 0.1 cm) in a standing position. After full expiration, waist circumference (WC) was measured using a plastic measuring tape from the horizontal line at 1 cm above the belly button. Anthropometric indices were measured twice and then averaged. Body mass index (BMI) was calculated as weight per height squared (kg/m2).
Fasting antecubital vein blood specimens were designed to be obtained by each participant in the morning after an overnight fasting with 12 h for assessing TC, TG, LDL-C, HDL-C, fasting plasma glucose (FPG) and serum creatinine (SCr). Plasma homocysteine was determined by enzymatic cycling assay (Hitachi, Japan). Comprehensive storage process and standard laboratory measurement methods were available in the previous reports [Citation37,Citation38]. Non-HDL-C was calculated as the difference between TC and HDL-C. eGFR was estimated with an established method by virtue of the improved accuracy of the CKD-EPI equation [Citation39], which overcame some of the limitations of the MDRD Study equation across various study populations and clinical conditions.
2.3. Definition
HHcy was diagnosed if the subjects had homocysteine concentration greater than 10 μmol/L [Citation4–6]. HHcy coexisting with hypertension were classified as H-type hypertension [Citation4–6]. Reduced GFR was ascertained in subjects with eGFR <60 ml/min/1.73m2 [Citation40].
2.4. Statistical analyses
Descriptive statistics for all covariates were stratified by decreased GFR and summarized as mean (SD) or median (interquartile range), when appropriate, for continuous variables and frequency (percentage) for categorical variables. Demographic, biochemical and clinical characteristics of the H-type hypertension population by decreased GFR status were analysed using unpaired Student’s t-test to compare mean values and the chi-squared test to evaluate differences in prevalence rates. Data sets that did not have equal variances were examined using the nonparametric Mann–Whitney test. TG/HDL-C was logarithmically transformed to normalize their skewed distributions before entering into the regression analysis. The chi-square linear-by-linear association test was assessed to test for linear trends across the quartile groups of non-traditional lipid parameters for percentages of decreased GFR. Pearson’s correlation regarding the determinant factors for eGFR was conducted. For stepwise multivariate regression analysis involving examine the associations of non-traditional lipid profiles with eGFR, β-coefficients are displayed per 1 SD increase. Non-traditional lipid profiles were modelled both as continuous variables and as categorized into quartiles to evaluate their influence on stratification of reduced GFR. We started with the unadjusted analysis indicating the relationship of each of the non-traditional lipid profiles and prevalent reduced GFR in terms of odds ratios (ORs) per 1 SD increased of the variable, followed by the multivariable models adjusting age, sex, race, education level, family income, physical activity, current smoker and drinker, body mass index, waist circumference, systolic blood pressure, diastolic blood pressure, fasting plasma glucose, history of cardiovascular disease and medication used. Additionally, to help characterize shapes of associations, unadjusted and multivariable-adjusted ORs of the probability of reduced GFR were expressed comparing the highest quartile of non-traditional lipid profiles with the referent category. The area under the curve (AUC) by receiver operating characteristic analysis was calculated to evaluate the diagnostic ability of non-traditional lipid profiles for the prediction of reduced GFR and determine the cut-off point with a maximum combined sensitivity and specificity. All of the statistical analyses involved the application of SPSS 21.0 software (IBM Corp) and MedCalc version 12.1.4.0 (MedCalc software, Belgium), and a two-tailed p <.05 was adopted to be statistically significant.
3. Results
Demographics, laboratory and clinical characteristics of H-type hypertension subjects with and without reduced GFR are reported in . The mean (SD) age of the 3259 H-type hypertension population was 58.78 ± 10.20 years and 51.6% were males, including 112 (3.4%) individuals with diagnosed reduced GFR. Individuals with reduced GFR were older, had higher systolic blood pressure (SBP) and BMI and had worse SCr, FPG and lipids (all p < .05), but no difference was observed in diastolic blood pressure (DBP), WC and LDL-C. They also had higher measures of non-traditional lipid profiles, including TC/HDL-C, TG/HDL-C, LDL-C/HDL-C ratios and non-HDL-C (all p < .001). In addition, the proportions of reduced GFR patients with a history of CVD and medication used were substantially higher than in the group of eGFR ≥60 (all p < .001).
Table 1. Demographics, laboratory and clinical characteristics of H-type hypertension population according to the presence or absence of reduced GFR.
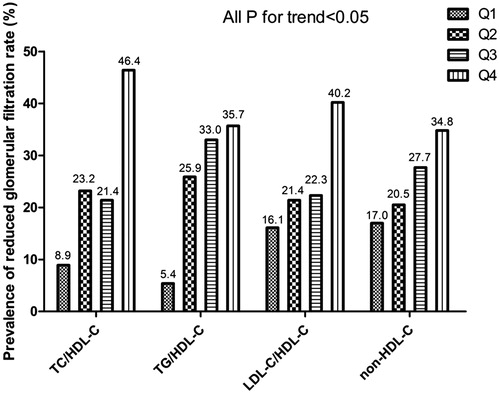
The prevalence of reduced GFR in hypertensive adults with HHcy was compared among the four quartiles of non-traditional lipid profiles (). The probability of declined renal function presented a proportional increase with elevated quartiles of non-traditional lipid parameters in a dose-response manner (all p < .005). The rates of decline in eGFR were 5.2-, 6.6-, 2.5- and 2.0-fold higher across quartiles of TC/HDL-C, TG/HDL-C, LDL-C/HDL-C ratios and non-HDL-C, respectively.
Figure 1. The prevalence of reduced GFR in H-type hypertension population by quartiles of TG/HDL-C, TC/HDL-C, LDL-C/HDL-C ratios and non-HDL-C. The proportion of prevalent reduced GFR increased across ascending quartiles of each nontraditional lipid profile (P for linear trend <0.05). Abbreviations: TC, total cholesterol; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; non-HDL-C, non-high-density lipoprotein cholesterol.
In univariate correlation analysis (), strong inverse associations of TC/HDL-C, TG/HDL-C, LDL-C/HDL-C ratios and non-HDL-C with eGFR were noted (all p < .001). Factors other than the non-traditional lipid profiles associated with reduced GFR included age, race, education level, family income, physical activity, current smoker and drinker, SBP, DBP, BMI, history of CVD and medication used. Subsequently, a comparison of standardized β coefficients for the independent relations between non-traditional lipid indexes and reduced GFR was obtained in the stepwise multivariate regression analysis. Even with inclusion of the potential confounders found in the univariate analyses into the models, TC/HDL-C, TG/HDL-C, LDL-C/HDL-C ratios and non-HDL-C remained as independent predictors of decreased GFR in H-type hypertension population (β = −0.185, −0.155, −0.065 and −0.109, respectively; p < .001 for each). In these models, the magnitude of the effect of TC/HDL-C was the strongest in the impact on an independent association with reduced GFR.
Table 2. Pearson’s correlation and stepwise multivariate regression analysis of nontraditional lipid profiles and estimated GFR in subjects with H-type hypertension.
Odd ratios of reduced GFR related to one SD differences and categorical estimates in the non-traditional lipid profiles of interest were presented in . Non-traditional lipid profiles were positively correlated with reduced GFR before and after adjustment for risk factors and biomarkers (Model 2, TC/HDL-C ratio, OR: 1.561, 95%CI: 1.295–1.882 per 1 SD increment, p < .001; TG/HDL-C ratio, OR: 1.585, 95%CI: 1.282–1.961 per 1 SD increment, p < .001; LDL-C/HDL-C ratio, OR: 1.346, 95%CI: 1.124–1.611 per 1 SD increment, p = .001; non-HDL-C, OR: 1.317, 95%CI: 1.103–1.573 per 1 SD increment, p = .002). Additional adjustment for clinical covariates attenuated, but did not abolish, the positive relationship of non-traditional lipid variables and the risk of reduced GFR, which revealed a 50%, 51%, 31% and 24% higher risk for decreased GFR with each SD increase in TC/HDL-C, TG/HDL-C, LDL-C/HDL-C ratios and non-HDL-C levels, respectively (Model 3). When non-traditional lipid profiles were categorized in quartiles, the fully adjusted odds of reduced GFR were 5.497 (2.499–12.093) for TC/HDL-C ratio, 6.632 (2.580–17.048) for TG/HDL-C ratio and 2.221 (1.149–4.292) for LDL-C/HDL-C ratio in participants with the top quartiles in comparison with the lower 25th percentile with a significant difference in the prevalence ratios by multivariable logistic regression analysis (Model 3), whereas participants in highest non-HDL-C quartiles had a modest and not statistically significant increase in the likelihood of decreased GFR (OR: 1.196, 95%CI: 0.638–2.241, p = .577). Moreover, the statistically significant exposure-response gradient of TC/HDL-C, TG/HDL-C, and LDL-C/HDL-C ratios to the prevalence of reduced GFR by the liner trend test was established. Graded ORs of decreased GFR across incremental quartiles of TC/HDL-C ratio (p for trend < .001), TG/HDL-C ratio (p for trend < .001), and LDL-C/HDL-C ratio (p for trend = .011) were noted.
Table 3. Unadjusted and adjusted odd ratios for the presence of reduced glomerular filtration rate according to continuous or quartiles of non-traditional lipid profiles.
As shown in , the AUC for the predictive value of each non-traditional lipid parameter for reduced GFR was statistically significant. A TC/HDL-C ratio of 4.48 was identified as an effective cut-off point for reduced GFR (AUC =0.646, 95% CI: 0.629–0.663, p < .001), and it had a sensitivity of 51.8% and specificity of 73.2%. Meanwhile, ROC curve analysis was applied to test the efficiency of TG/HDL-C ratio in discriminating the presence of decreased GFR with an AUC of 0.629 (0.612 to 0.646) and its value of 0.77 or more predicted decreased GFR (sensitivity 91.1% and specificity 36.1%). In particular, the AUC obtained with TC/HDL-C ratio for CKD was significantly greater than those obtained with LDL-C/HDL-C ratio (AUC 0.646 vs. 0.608, p = .021) and non-HDL-C (AUC 0.646 vs. 0.597, p = .0168) and was comparable with those obtained with TG/HDL-C ratio (AUC 0.646 vs. 0.629, p = .4026).
Table 4. Area under the ROC curve (AUC) for identification of reduced glomerular filtration rate by each nontraditional lipid index.
4. Discussion
Novel findings in our study highlighted the rationale for using the lipid ratio of TC/HDL-C, TG/HDL-C, LDL-C/HDL-C and non-HDL-C as easily obtained parameters in screening H-type hypertension population of reduced GFR risk in routine clinical practice. The relations of TC/HDL-C, TG/HDL-C and LDL-C/HDL-C ratios to decreased GFR were continuous and graded in models adjusting for a wide range of established risk factors. Dyslipidaemia (defined by non-traditional lipid profiles)-induced reduced GFR, might represent a progressive pre-clinical condition that contributes to dyslipidaemia-induced kidney disease progression and future cardiovascular events. This intriguing study buttressed the notion that therapy targeting at non-traditional lipid profiles may generate substantial additional benefit on mitigating the burden of high-risk cardio-cerebrovascular disease individuals.
Dyslipidaemia, characterized by abnormal lipid profile and alterations in lipoproteins, was a well-known cause of established CKD [Citation12–14]. Numerous previous analyses of lipoprotein-induced renal injury have been identified by utilizing animal models [Citation15–17]. Although there is agreement about the implication of abnormal lipid metabolism on risks of incident CKD, the extent to whether lower HDL-C levels leads to impaired kidney function is currently under debate [Citation18–22]. Specifically, drawing on data from the ARIC study, low HDL-C and hypertriglyceridaemia were shown to promote a rise in serum creatinine, a measure of poor renal outcomes [Citation19]. Investigators from the Physicians’ Health Study described elevated TC played a key role in developing renal dysfunction [Citation20]. Unexpectedly, in a prospective cohort CRIC study, none of the lipid indexes, of particular interest HDL-C, constituted a significant risk for the progression of kidney disease [Citation21]. It is now becoming evident that unfavourable lipid and lipoprotein abnormalities, quantified by atherogenic changes in LDL particle distribution, are present early in the course of renal insufficiency, which may not be directly appreciated with conventional lipid measurements [Citation41]. Studies examining the possible reno-protective effects of lipid-lowering agents on progression of kidney disease were contradictory [Citation42–44]. A recent systematic review of 27 randomized trials with greater statistical power has indicated that compared with placebo, treatment with statins delayed the risk of renal function decline in the context of reducing urinary protein excretion rate [Citation42]. At variance with above results, pharmacologic manipulation of the HDL-C level with statins failed to uniformly translate into prevention of clinical renal outcomes in some large, interventional studies [Citation43,Citation44]. The benefit on the progression of glomerular injury in randomized clinical trials of statins has been controversial in light of the uncertain epidemiological relationship reported between traditional lipid parameters and CKD, suggesting the need for more powerful and detailed estimates of blood lipids and kidney disease. These facts were even more relevant because conventional lipid measurements did not fully capture the differences in LDL particle number and size, a common hallmark of relevant changes in lipoprotein distribution.
Notably, non-traditional lipid profiles have been portended to be important pathophysiologic contributors to CVD and composite vascular end point [Citation23–27]. There was strong evidence reflecting that TC/HDL-C ratio, as an index of atherogenic particle burden, has given rise to a new paradigm of reflecting lipoprotein particle concentration and size not available in cholesterol-based measurements [Citation28,Citation29]. At present, the existence of lipoprotein abnormalities, as determined by TC/HDL-C ratio, offered potential additional information to LDL-C and was at least equivalent to non-HDL-C and apolipoprotein fractions in the prediction of cardiovascular events among several study cohorts [Citation25–27]. Obviously, TG/HDL-C ratio, a commonly used surrogate estimate of insulin resistance and cardiometabolic risk, represented a marker of the atherogenic dyslipidaemia characterized by small dense LDL and large very low-density lipoproteins (VLDL) [Citation30,Citation31], which served as a pragmatic way to identify a subset of general population that is at greatly increased risk for coronary heart disease (CHD) [Citation23,Citation24]. In the setting of coronary plaque progression and early stage atherosclerosis, there has been an increasing focus on LDL-C/HDL-C ratio for the identification of hyperlipidaemia as an alternative to the standard lipid profile [Citation27,Citation32]. Further, population studies have outlined the superiority of non-HDL-C, which encompassed all of the atherogenic apoB-containing lipoproteins, over LDL-C alone as the cornerstone of CHD risk evaluation [Citation25,Citation26].
As the process of glomerulosclerosis shares a highly similar pathogenesis with atherosclerosis, it is reasonable to infer that lipoprotein-mediated renal injury can be attributed to non-traditional lipid profiles. There has been a great interest in exploring the role of non-traditional lipid parameters in the prediction of CKD, but their results were inconclusive [Citation33–36,Citation45]. A nationally representative sample of Korean adults offered mechanistic insight into why TG/HDL-C ratio was a useful parameter to identify subjects susceptible to CKD [Citation45]. In an elegant and comprehensive research on hyperlipidaemia and CKD progression in a large Japanese population, Tsuruya K et al confirmed that a higher TG/HDL-C ratio was proved to be reflective of the decline in eGFR and adverse renal outcomes [Citation33,Citation34]. Partially diverse results emerged from two observational studies, where there were no meaningful relations of changes in eGFR with TC/HDL-C ratios and LDL-C/HDL-C ratio levels [Citation35,Citation36]. It is well acknowledged that CKD is highly prevalent among hypertensive patients with almost half the population probably affected [Citation46]. H-type hypertension, an established risk factor for cardio-cerebrovascular diseases, has increased at an alarming rate over recent years in China [Citation4–9]. Given that hypertension coexisting with HHcy appeared to have an additive or synergistic effect on the renal injury with resultantly increased glomerulosclerosis, individuals with H-type hypertension were prone to have the worst target organ damages, poorest renal function and most severe cardiovascular injuries [Citation4,Citation10,Citation11]. Reliable assessment of the joint correlations of H-type hypertension with the risk of CKD is critical for improving CVD stratification and developing a tailored screening and therapeutic strategy. Therefore, non-traditional lipid profiles, an approach integrating atherogenic and anti-atherogenic lipid molecules, are needed for a more complete and useful characterization of lipid abnormalities as initiators of renal disease. Under this scenario, this middle-aged, cross-sectional, population-based design is initiated to examine and validate the practicality of non-traditional lipid profiles as the key correlates of reduced GFR in a Chinese population sample with a high prevalence of H-type hypertension.
Our research described here expanded on the issue of the significant associations of non-traditional lipid profiles with the risk of cardiovascular events, highlighting lipid assessment in decreased GFR can be simplified by measurement of TC/HDL-C, TG/HDL-C, LDL-C/HDL-C ratios, and non-HDL-C. Collectively, these results provided further evidence in support of the emerging concept that CKD in patients with H-type hypertension and cardio-cerebrovascular disease may have similar pathophysiological backgrounds. Of note, the independent relation of TC/HDL-C, TG/HDL-C and LDL/HDL-C ratios with decreased GFR persisted with the inclusion of a wide range of comorbidities, history of CVD and medication used in the regression models, implying that these factors were unlikely to explain these effects. In the present study, we acquired that TG/HDL-C ratio was independently linked with decreased GFR and was at least equivalent to TC/HDL-C ratio in the magnitude of the effect. Although unclear, it may be that TC/HDL-C captured atherogenic lipid abnormalities other than LDL-C with a reflection of alterations in lipid particles such as lower HDL-C and its subfractions, higher small dense LDL particles and VLDL particles similarly to that of TG/HDL-C.
There is a panel of plausible pathomechanisms responsible for the increased risk of reduced GFR in those with poor non-traditional lipid profiles. First, significant alterations in apolipoproteins, probably as a consequence of dyslipidaemia in the early stages of CKD, could mediate changes in plasma lipid levels, characterizing a state of elevated triglyceride-rich lipoproteins and diminished HDL levels [Citation47]. Owing to genetic expression and biosynthesis of apoprotein (ApoA)-I and ApoA-II at sites of HDL production deficiency in liver, a decrease in HDL-C levels might be result of marked reductions in serum concentration of these apoproteins, which are the main protein constituents of the HDL particle [Citation48]. Furthermore, albumin that has been assumed to serve as a carrier of free cholesterol from the peripheral to HDL appears to be depressed in CKD patients. The phenomenon of hypoalbuminaemia in favouring declined HDL-C levels is well recognized in the context of chronic inflammation [Citation47]. It is clear that CKD is responsible for the pronounced decline in lipoprotein lipase activity compounded by elevated ApoC-III. ApoC-III, a potent inhibitor of lipoprotein lipase, plays a crucial part in higher levels of TG [Citation14]. Second, CKD results in profound dysregulation of lipid metabolism accompanied by generation of small dense LDL particles, accumulation of intermediate-density lipoprotein (IDL) and chylomicron remnants [Citation14]. Mechanisms by which lipid disorder may contribute to renal injury include the conversion of lipoproteins to oxidized lipoproteins by mesangial cells and macrophages [Citation13]. The circulating oxidized LDL and oxidation-prone, atherogenic remnants promote monocytes and macrophages to release inflammatory cytokines and chemokines, reactive oxygen species and vasoactive substances which, in turn, accelerate inflammation and lead to mesangial cell proliferation and extracellular matrix expansion [Citation49]. Third, small dense LDL-C particles, as measured by TC/HDL-C ratio and TG/HDL-C ratio, were considered to be a strong correlate of insulin resistance, which has been appreciated as a risk factor for the progression of renal dysfunction [Citation50].
The findings of the present study had important clinical and public health implications. As the basis for public health interventions, non-traditional lipid parameters provided a conceptual framework for understanding the importance of lipoprotein abnormalities in the pathogenesis of CKD with mechanistic information contributed from the H-type hypertension population, which was compatible with the results of previous studies. Thus, for the first time to our knowledge, we demonstrated that high-risk cardio-cerebrovascular disease individuals could be identified with sufficient diagnostic accuracy to justify close monitoring or even initiation prevention of abnormal lipoprotein metabolism in the evaluation of non-traditional lipid profiles. The simplicity and cost-effective of non-traditional lipid indices have made it possible for application to large-scale clinical and epidemiological studies. Despite the aforementioned strengths, certain limitations merit consideration. First, due to the cross-sectional nature of our study, we were unable to establish causality or evaluate long-term outcomes to determine the impact of non-traditional lipid profiles on the incidence and progression of CKD. Secondly, kidney disease in individuals with eGFR values above 60 mL/min/1.73 m2 may have been underestimated because quantitative measurements of proteinuria were not available in present study. Thirdly, the lack of information on vitamin B12, folic acid, and methylenetetrahydrofolate reductase (MTHFR) genotype in our H-type hypertension population may limit our ability to estimate precise relationships as to the mechanisms linking nontraditional lipid profiles to reduced GFR. Fourth, given that our results are predominantly applicable to Chinese adults, we cannot know to which extent the present findings apply to other racial or ethnic populations.
5. Conclusions
A greater emphasis of this study resided in the intrinsic value of TC/HDL-C, TG/HDL-C, LDL-C/HDL-C ratios and non-HDL-C that integrate atherogenic and anti-atherogenic lipid molecules to predict the risk of reduced GFR among H-type hypertension population and provide insight into the pathophysiology of subsequent cardio-cerebrovascular outcomes. These specific relationships may represent targets for novel management strategies and therapies aimed at lowering non-traditional lipid profiles to reduce future morbidity and mortality given that H-type hypertension patients carry a high burden of kidney disease and have poor prognosis.
Acknowledgements
Dr. Yingxian Sun was responsible for enabling the project completion.
Disclosure statement
The authors declare that they have no competing interests.
Additional information
Funding
References
- Lawes CM, Vander Hoorn S, Rodgers A. Global burden of blood-pressure-related disease, 2001. Lancet. 2008;371:1513–1518.
- Kearney PM, Whelton M, Reynolds K, et al. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223.
- Lewington S, Lacey B, Clarke R, et al. The burden of hypertension and associated risk for cardiovascular mortality in China. JAMA Intern Med. 2016;176:524–532.
- Ye Z, Wang C, Zhang Q, et al. Prevalence of homocysteine-related hypertension in patients with chronic kidney disease. J Clin Hypertens. 2017;19: 151–160.
- Chen Z, Wang F, Zheng Y, et al. H-type hypertension is an important risk factor of carotid atherosclerotic plaques. Clin Exp Hypertension (New York, NY: 1993). 2016;38:424–428.
- Qin X, Huo Y. H-Type hypertension, stroke and diabetes in China: Opportunities for primary prevention. J Diabetes. 2016;8:38–40.
- Huo Y, Li J, Qin X, et al. Efficacy of folic acid therapy in primary prevention of stroke among adults with hypertension in China: the CSPPT randomized clinical trial. JAMA. 2015;313:1325–1335.
- Towfighi A, Markovic D, Ovbiagele B. Pronounced association of elevated serum homocysteine with stroke in subgroups of individuals: a nationwide study. J Neurol Sci. 2010;298:153–157.
- Zhang Z, Fang X, Hua Y, et al. Combined effect of hyperhomocysteinemia and hypertension on the presence of early carotid artery atherosclerosis. J Stroke Cerebrovasc Dis. 2016;25:1254–1262.
- Xu X, Qin X, Li Y, et al. Efficacy of folic acid therapy on the progression of chronic kidney disease: the renal substudy of the China stroke primary prevention trial. JAMA Intern Med. 2016;176:1443–1450.
- Xie D, Yuan Y, Guo J, et al. Hyperhomocysteinemia predicts renal function decline: a prospective study in hypertensive adults. Sci Rep. 2015;5:16268.
- Vaziri ND. Dyslipidemia of chronic renal failure: the nature, mechanisms, and potential consequences. Am J Physiol Renal Physiol. 2006;290:F262–F272.
- Dalrymple LS, Kaysen GA. The effect of lipoproteins on the development and progression of renal disease. Am J Nephrol. 2008;28:723–731.
- Vaziri ND, Norris K. Lipid disorders and their relevance to outcomes in chronic kidney disease. Blood Purif. 2011;31:189–196.
- Kasiske BL, O'Donnell MP, Schmitz PG, et al. Renal injury of diet-induced hypercholesterolemia in rats. Kidney Int. 1990;37:880–891.
- Ganda A, Magnusson M, Yvan-Charvet L, et al. Mild renal dysfunction and metabolites tied to low HDL cholesterol are associated with monocytosis and atherosclerosis. Circulation. 2013;127:988–996.
- Vaziri ND, Navab M, Fogelman AM. HDL metabolism and activity in chronic kidney disease. Nat Rev Nephrol. 2010;6:287–296.
- Bowe B, Xie Y, Xian H, et al. Low levels of high-density lipoprotein cholesterol increase the risk of incident kidney disease and its progression. Kidney Int. 2016;89:886–896.
- Muntner P, Coresh J, Smith JC, et al. Plasma lipids and risk of developing renal dysfunction: the atherosclerosis risk in communities study. Kidney Int. 2000;58:293–301.
- Schaeffner ES, Kurth T, Curhan GC, et al. Cholesterol and the risk of renal dysfunction in apparently healthy men. J Am Soc Nephrol. 2003;14:2084–2091.
- Rahman M, Yang W, Akkina S, et al. Relation of serum lipids and lipoproteins with progression of CKD: the CRIC study. Clin J Am Soc Nephrol: CJASN. 2014;9:1190–1198.
- Chawla V, Greene T, Beck GJ, et al. Hyperlipidemia and long-term outcomes in nondiabetic chronic kidney disease. Clin J Am Soc Nephrol. CJASN. 2010;5:1582–1587.
- Barzi F, Patel A, Woodward M, et al. A comparison of lipid variables as predictors of cardiovascular disease in the Asia Pacific region. Ann Epidemiol. 2005;15:405–413.
- Bittner V, Johnson BD, Zineh I, et al. The triglyceride/high-density lipoprotein cholesterol ratio predicts all-cause mortality in women with suspected myocardial ischemia: a report from the Women's Ischemia Syndrome Evaluation (WISE). Am Heart J. 2009;157:548–555.
- Arsenault BJ, Rana JS, Stroes ES, et al. Beyond low-density lipoprotein cholesterol: respective contributions of non-high-density lipoprotein cholesterol levels, triglycerides, and the total cholesterol/high-density lipoprotein cholesterol ratio to coronary heart disease risk in apparently healthy men and women. J Am Coll Cardiol. 2009;55:35–41.
- Ridker PM, Rifai N, Cook NR, et al. Non-HDL cholesterol, apolipoproteins A-I and B100, standard lipid measures, lipid ratios, and CRP as risk factors for cardiovascular disease in women. JAMA. 2005;294:326–333.
- Ingelsson E, Schaefer EJ, Contois JH, et al. Clinical utility of different lipid measures for prediction of coronary heart disease in men and women. JAMA. 2007;298:776–785.
- Elshazly MB, Quispe R, Michos ED, et al. Patient-level discordance in population percentiles of the total cholesterol to high-density lipoprotein cholesterol ratio in comparison with low-density lipoprotein cholesterol and non-high-density lipoprotein cholesterol: the very large database of lipids study (VLDL-2B). Circulation. 2015;132:667–676.
- Mathews SC, Mallidi J, Kulkarni K, et al. Achieving secondary prevention low-density lipoprotein particle concentration goals using lipoprotein cholesterol-based data. PLoS One. 2012;7:e33692.
- Salazar MR, Carbajal HA, Espeche WG, et al. Relation among the plasma triglyceride/high-density lipoprotein cholesterol concentration ratio, insulin resistance, and associated cardio-metabolic risk factors in men and women. Am J Cardiol. 2012;109:1749–1753.
- Bhalodkar NC, Blum S, Enas EA. Accuracy of the ratio of triglycerides to high-density lipoprotein cholesterol for predicting low-density lipoprotein cholesterol particle sizes, phenotype B, and particle concentrations among Asian Indians. Am J Cardiol. 2006;97:1007–1009.
- Katakami N, Kaneto H, Osonoi T, et al. Usefulness of lipoprotein ratios in assessing carotid atherosclerosis in Japanese type 2 diabetic patients. Atherosclerosis. 2011;214:442–447.
- Tsuruya K, Yoshida H, Nagata M, et al. Association of the triglycerides to high-density lipoprotein cholesterol ratio with the risk of chronic kidney disease: analysis in a large Japanese population. Atherosclerosis. 2014;233:260–267.
- Tsuruya K, Yoshida H, Nagata M, et al. Impact of the triglycerides to high-density lipoprotein cholesterol ratio on the incidence and progression of CKD: a longitudinal study in a large Japanese population. Am J Kidney Dis. 2015;66:972–983.
- Zhang L, Yuan Z, Chen W, et al. Serum lipid profiles, lipid ratios and chronic kidney disease in a Chinese population. IJERPH. 2014;11:7622–7635.
- Kim JY, Kang HT, Lee HR, et al. Comparison of lipid-related ratios for prediction of chronic kidney disease stage 3 or more in Korean adults. J Korean Med Sci. 2012;27:1524–1529.
- Li Z, Bai Y, Guo X, et al. Alcohol consumption and cardiovascular diseases in rural China. Int J Cardiol. 2016;215:257–262.
- Sun GZ, Guo L, Wang XZ, et al. Prevalence of atrial fibrillation and its risk factors in rural China: a cross-sectional study. Int J Cardiol. 2015;182:13–17.
- Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612.
- Stevens PE, Levin A. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158:825–830.
- de Boer IH, Astor BC, Kramer H, et al. Lipoprotein abnormalities associated with mild impairment of kidney function in the multi-ethnic study of atherosclerosis. Clin J Am Soc Nephrol. 2008;3:125–132.
- Sandhu S, Wiebe N, Fried LF, et al. Statins for improving renal outcomes: a meta-analysis. J Am Soc Nephrol. 2006;17:2006–2016.
- Rahman M, Baimbridge C, Davis BR, et al. Progression of kidney disease in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin versus usual care: a report from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Am J Kidney Dis. 2008;52:412–424.
- Baigent C, Landray MJ, Reith C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377:2181–2192.
- Kang HT, Shim JY, Lee YJ, et al. Association between the ratio of triglycerides to high-density lipoprotein cholesterol and chronic kidney disease in Korean adults: the 2005 Korean National Health and Nutrition Examination Survey. Kidney & Kidney Blood Press Res. 2011;34:173–179.
- Cerasola G, Mule G, Nardi E, et al. Clinical correlates of renal dysfunction in hypertensive patients without cardiovascular complications: the REDHY study. J Hum Hypertens. 2010;24:44–50.
- Harper CR, Jacobson TA. Managing dyslipidemia in chronic kidney disease. J Am Coll Cardiol. 2008;51:2375–2384.
- Vaziri ND, Deng G, Liang K. Hepatic HDL receptor, SR-B1 and Apo A-I expression in chronic renal failure. Nephrol Dialy Transplant. 1999;14:1462–1466.
- Glass CK, Witztum JL. Atherosclerosis. the road ahead. Cell. 2001;104:503–516.
- Dogra G, Irish A, Chan D, et al. Insulin resistance, inflammation, and blood pressure determine vascular dysfunction in CKD. Am J Kidney Dis. 2006;48:926–934.
