Abstract
Background: Cardiovascular complications are strongly correlated with a higher risk of mortality during follow-up after noncardiac surgery. However, controversy remains regarding whether perioperative administration of hydroxymethylglutaryl-CoA reductase inhibitors (statins) has a beneficial effect on patient outcomes.
Objective: We performed a meta-analysis to validate the hypothesis that perioperative statins improve patient outcomes after noncardiac surgery.
Methods: Electronic databases (PubMed, Web of Science, EMBASE, and the Cochrane Library) were searched for randomized controlled trials (RCTs) published up to 10 November 2017. RCTs were eligible for inclusion if they compared perioperative statin treatment with control treatment in patients scheduled for noncardiac surgery and reported data pertaining to clinical outcomes.
Results: Twelve RCTs involving 4707 patients (2371 in the perioperative statin group and 2336 in the control group) were ultimately included in this meta-analysis. The incidences of postoperative myocardial infarction, composite of death/myocardial infarction/stroke and new cases of atrial fibrillation were all lower in patients treated with statins than in control group patients, as shown by the fixed-effects model (odds ratio (OR) = 0.460, 95% confidence interval (CI) = 0.324–0.653, p = 0 for myocardial infarction; OR = 0.617, 95% CI = 0.476–0.801, p = 0 for composite of death/myocardial infarction/stroke; OR = 0.406, 95% CI = 0.247–0.666, p = 0 for new atrial fibrillation). No significant differences in the incidences of stroke or transient ischemic attack, all-cause mortality and cardiovascular mortality were observed between the statin and control arms.
Conclusions: This meta-analysis supports the hypothesis that perioperative statins effectively reduce the incidences of postoperative myocardial infarction, composite of death/myocardial infarction/stroke and new cases of atrial fibrillation in patients undergoing noncardiac surgery.
Cardiovascular complications are strongly correlated with a higher risk of mortality during follow-up after noncardiac surgery.
We performed a meta-analysis to confirm the hypothesis that perioperative statins improve patient outcomes after noncardiac surgery.
Key Messages
Introduction
Three hundred million patients worldwide undergo major noncardiac surgery each year [Citation1]. Despite great advancements in perioperative treatment, more than 1 million patients die within 30 days after surgery [Citation2], and cardiovascular complications are strongly correlated with a higher risk of mortality during follow-up [Citation3,Citation4].
Several randomized controlled trials (RCTs) have recently assessed the effects of perioperative hydroxymethylglutaryl-CoA reductase inhibitors (statins) on patient outcomes after noncardiac surgery. However, all these studies were relatively small, which might have reduced the statistical power. Thus, we performed a meta-analysis to confirm the hypothesis that statins improve patient outcomes after noncardiac surgery.
Methods
Data sources and selection criteria
Electronic databases (PubMed, Web of Science, EMBASE and the Cochrane Library) were searched for RCTs published up to 10 November 2017. The following keywords were used: (“hydroxymethylglutaryl-CoA Reductase Inhibitors” OR = “HMG-CoA reductase inhibitors” OR = “statin” OR = “statins” OR = “lipid-lowering therapy” OR = “lipid-lowering treatment” OR = “lipid pharmacotherapy” OR = “atorvastatin” OR = “pravastatin” OR = “fluvastatin” OR = “simvastatin”) AND (“noncardiac surgery” OR = “non-cardiac vascular surgery” OR = “no-cardiac surgery” OR = “vascular surgery” OR = “vascular surgical procedures” OR = “peripheral arterial disease” OR = “peripheral vascular diseases” OR = “endarterectomy, carotid” OR = “carotid endarterectomy” OR = “aortic aneurysm, abdominal” OR = “aortic aneurysm repair” OR = “lower limb revascularization” OR = “lower extremity revascularization” OR = “lower limb bypass” OR = “lower extremity bypass” OR = “infrainguinal bypass”). Reference lists were also reviewed to identify relevant studies, regardless of the language in which the studies were published.
The following studies were included in the analysis: (1) RCTs; (2) studies including subjects undergoing noncardiac surgery; and (3) studies in which statins were administered to the treatment group and placebo to the control group. Non-RCTs, cohort studies, case reports, commentaries, and retrospective studies were excluded.
Study end-points and data extraction
The primary end-point was all-cause mortality, and the secondary end-points were cardiovascular mortality, myocardial infarction (MI), atrial fibrillation, stroke and MACE (composite of death, MI and stroke). Two reviewers independently extracted data for the following parameters from the identified studies: the first author, publication year, country, ages, clinical characteristics, type of procedure, type of statin therapy and number of patients. The risk of bias in the included RCTs was assessed with the method recommended by the Cochrane Collaboration [Citation5].
Statistical analysis
Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated for all outcomes and compared between the statin and control groups. Cochran’s test and I2 statistical means were used to assess the heterogeneity among trials [Citation6]. A random-effects model (the DerSimonian and Laird method) was used when p < .10. Otherwise, a fixed-effects model (Mantel–Haenszel method) was employed [Citation7,Citation8]. We used the Begg and Egger tests to determine whether publication bias was present with respect to all outcomes [Citation9]. All statistical analyses were conducted with Stata v.12.0 (Stata Corp., College Station, TX) and RevMan 5.2 (the Cochrane Collaboration) software. A p value <.05 was considered statistically significant.
Results
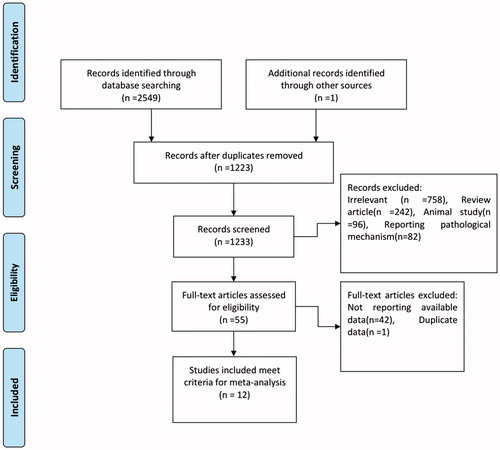
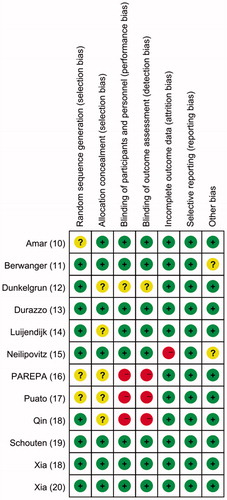
A flow chart describing the study selection process is shown in . Twelve RCTs involving 4707 patients (2371 in the perioperative statin group and 2336 in the control group) were ultimately included in this meta-analysis [Citation10–21]. The characteristics of the included studies are summarized in . Atorvastatin was the intervention drug in eight studies; its dose ranged from 10 mg to 80 mg [Citation1,Citation3,Citation12–16,Citation20]. Rosuvastatin was the intervention drug in the other studies; it was administered at doses of 80 mg [Citation5,Citation17], 20 mg [Citation21] and 10 mg [Citation10]. The results of the risk-of-bias assessment for the included studies are reported in .
Table 1. Baseline demographic and clinical characteristics.
Patients in the statin and control arms did not exhibit significant differences in all-cause mortality (OR = 0.729, 95% CI = 0.464–1.147, p = .172). Significant heterogeneity was not noted between the trials (I2 = 0%; p = .739), as shown in and , and we did not observe evidence of publication bias, as indicated by the Begg funnel plot (p = .462). Sensitivity analysis was performed using the leave-one-out approach. The pooled ORs were not significantly affected by the omission of individual trials from the analysis.
Figure 3. ORs for the associations between all-cause mortality and perioperative statin and placebo administration in patients undergoing noncardiac surgery. The sizes of the data markers are proportional to the weights of the individual studies.
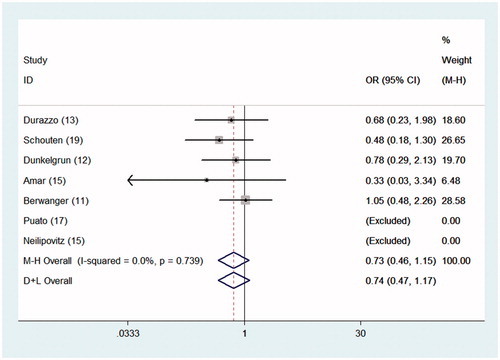
Table 2. Meta-analyses of the effects of perioperative statins on improving patient outcomes after noncardiac surgery.
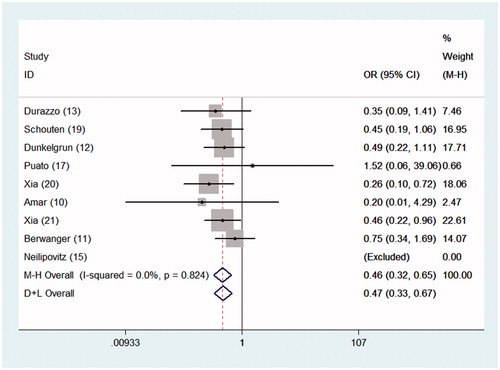
During the follow-up period, 48 of 1514 patients (3.2%) in the perioperative statin group developed MI; the incidence of MI in the statin group was significantly lower than that (6.9%) in the control group (OR = 0.460, 95% CI = 0.324–0.653, p = 0), as shown in . Incidences of postoperative MACE and new cases of atrial fibrillation were both lower in patients treated with statins than in control group patients, as shown by the fixed-effects model (OR = 0.617, 95% CI = 0.476–0.801, p = 0 for MACE; OR = 0.406, 95% CI = 0.247–0.666, p = 0 for new cases of atrial fibrillation). No significant differences in the incidences of stroke or TIA and cardiovascular mortality were observed between the statin and control arms (OR = 0.584, 95% CI = 0.226–1.505, p = .265 for stroke or TIA; OR = 0.749, 95% CI = 0.410–1.367, p = .346 for cardiovascular mortality).
Figure 4. ORs for the associations between MI and perioperative statin and placebo administration in patients undergoing noncardiac surgery. The sizes of the data markers are proportional to the weights of the individual studies.
Five of the 12 studies focused on noncardiovascular surgery, and another five studies assessed noncardiac vascular surgery. The other two studies investigated noncardiac surgery. We performed subgroup analyses based on the type of surgery. In the population that underwent noncardiovascular surgery, the incidences of MI, MACE and new cases of atrial fibrillation were all lower in patients treated with statins than in patients in the control group, as shown by the fixed-effects model (OR = 0.398, 95% CI = 0.248–0.638, p = 0 for MI; OR = 0.484, 95% CI = 0.340–0.688, p = 0 for MACE; OR = 0.370, 95% CI = 0.220–0.622, p = 0 for new cases of atrial fibrillation), as shown in . In the population that underwent noncardiac vascular surgery, a significant difference in the incidence of MI was observed between the statin and control arms (OR = 0.446, 95% CI = 0.220–0.901, p = .024). However, in the population that underwent noncardiac surgery, no significant differences in the incidences of MI, MACE and new cases of atrial fibrillation were observed between the statin and control arms.
Table 3. Subgroup analyses of the effects of perioperative statins on improving patient outcomes after noncardiac surgery.
Discussion
The present meta-analysis was designed to assess the effects of perioperative statins on patient outcomes after noncardiac surgery. Patients who received statin therapy had a significantly lower risk of developing postoperative MI, MACE and new cases of atrial fibrillation than did controls.
Statins (3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors) are used to treat hyperlipidaemia and exert pleiotropic effects [Citation22]. Statins have been shown to exert anti-arrhythmic effects by modulating systemic inflammatory marker expression [Citation23,Citation24], attenuate fibrosis after MI by alleviating the inflammatory response [Citation25], induce the vasodilation of coronary microvessels and exert direct antithrombotic effects [Citation26,Citation27]. Many studies have assessed the effects of perioperative statins on outcomes after cardiac surgery. Statin use may prevent postoperative atrial fibrillation, particularly in patients undergoing coronary artery bypass graft (CABG) surgery [Citation28–30]. Curtis et al. performed a retrospective study and showed that preoperative statin administration for 24 hours or less reduced the rate of 30-day all-cause mortality after CABG [Citation31].
More than 300 million noncardiac surgeries are performed annually. Perioperative myocardial injury (PMI) has been identified as an important complication of noncardiac surgery [Citation32]. Strategies for improving the treatments and outcomes of PMI may provide major medical benefits [Citation32]. A previous meta-analysis investigated whether perioperative statin therapy improves patient outcomes during and after noncardiac vascular surgery [Citation33]. Five eligible studies were included in the previous meta-analysis. However, this study did not obtain sufficient evidence to conclude that statin use improved patient outcomes after surgery. Another meta-analysis by Antoniou et al. was published in 2015 [Citation34]. This analysis aimed to investigate the role of perioperative statin therapy in noncardiac vascular and endovascular surgery. Four RCTs and 20 observational cohort or case–control studies were included in the analysis; the results suggested that statin therapy improves operative and interventional outcomes and lowers the risks of all-cause mortality, MI and MACE. As the conclusions of these studies were inconsistent, we collected nearly all relevant, published RCTs and conducted a meta-analysis to examine the effects of statin use after noncardiac surgery. In 2017, Bass et al. assessed the effect of atorvastatin on perioperative myocardial injury and inflammation after orthopaedic surgery. Twenty-two patients (11 in the perioperative statin group and 11 in the control group) were included, none of whom experienced MI, stroke, transient ischemic attack, atrial fibrillation or death. Nonetheless, the study was underpowered to reveal the effects of perioperative atorvastatin on patient outcomes after orthopaedic surgery [Citation35], and it was therefore not included in our analysis. Ultimately, our analysis included 12 studies, five of which focused on populations that underwent noncardiovascular surgery, five of which assessed populations that underwent noncardiac vascular surgery, and two of which investigated populations that underwent noncardiac surgery. Our analysis involved a greater number of participants than previous analyses; thus, its statistical power was increased. Based on our results, statin therapy has significant beneficial effects on patient outcomes after noncardiac surgery.
Our analysis had some limitations. First, although 12 studies were included in our meta-analysis, the total number of participants was still small. Second, various types of surgery, such as vascular, urological and dental surgery, were performed in all studies. We performed subgroup analyses based on the type of surgery, but we were unable to draw a precise conclusion about the impact of the type of surgery on postoperative outcomes because of the limited number of included studies. Another limitation was that varying doses of two different intervention drugs (atorvastatin and rosuvastatin) were employed in the studies included in our meta-analysis. Because the number of included participants was small, we could not perform subgroup analyses to assess the effects of different types and doses of statins on outcomes.
In conclusion, the current limited evidence supports the hypothesis that perioperative statins effectively reduce the rates of MI, MACE and new cases of atrial fibrillation in patients undergoing noncardiac surgery. Future studies should focus on the optimal statin type and dose and other clinical end-points, such as lengths of stay and infection complications.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Smilowitz NR, Gupta N, Guo Y, et al. Perioperative acute myocardial infarction associated with non-cardiac surgery. Eur Heart J. 2017;38:2409–2417.
- Gorka J, Polok K, Iwaniec T, et al. Altered preoperative coagulation and fibrinolysis are associated with myocardial injury after non-cardiac surgery. Br J Anaesth. 2017;118:713–719.
- Weiser TG, Regenbogen SE, Thompson KD, et al. An estimation of the global volume of surgery: a modelling strategy based on available data. Lancet. 2008;372:139–144.
- Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management. The Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur J Anaesthesiol. 2014;31:517–573.
- Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions version 5.1.0 [cited March 2011]. The cochrane collaboration. 2011. Available from: www.Cochrane-handbook.Org.
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560.
- Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188.
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101.
- Amar D, Park B, Zhang H, et al. Beneficial effects of perioperative statins for major pulmonary resection. J Thorac Cardiovasc Surg. 2015;149:1532–1538.
- Berwanger O, de Barros ESPG, Barbosa RR, et al. Atorvastatin for high-risk statin-naive patients undergoing noncardiac surgery: The Lowering the Risk of Operative Complications Using Atorvastatin Loading Dose (LOAD) randomized trial. Am Heart J. 2017;184:88–96.
- Dunkelgrun M, Boersma E, Schouten O, et al. Bisoprolol and fluvastatin for the reduction of perioperative cardiac mortality and myocardial infarction in intermediate-risk patients undergoing noncardiovascular surgery: a randomized controlled trial (DECREASE-IV). Ann Surg. 2009;249:921–926.
- Durazzo AE, Machado FS, Ikeoka DT, et al. Reduction in cardiovascular events after vascular surgery with atorvastatin: a randomized trial. J Vasc Surg. 2004;39:967–975.
- Luijendijk P, Bouma BJ, Vriend JW, et al. Beneficial effect of high dose statins on the vascular wall in patients with repaired aortic coarctation? Int J Cardiol. 2014;176:40–47.
- Neilipovitz DT, Bryson GL, Taljaard M. STAR VaS–short term atorvastatin regime for vasculopathic subjects: a randomized placebo-controlled trial evaluating perioperative atorvastatin therapy in noncardiac surgery. Can J Anesth/J Can Anesth. 2012;59:527–537. Anaesth.
- Parepa I-R, Suceveanu A-I, Mazilu L, et al. Preventing cardiac complications after non-cardiac non-vascular surgery by using perioperative statin therapy – a prospective study in Constanta county, Romania. Farmacia. 2017;65:120–124.
- Puato M, Faggin E, Rattazzi M, et al. Atorvastatin reduces macrophage accumulation in atherosclerotic plaques: a comparison of a nonstatin-based regimen in patients undergoing carotid endarterectomy. Stroke. 2010;41:1163–1168.
- Qin P. The effect of atorvastatin for heart disease on perioperative cardiovascular events in non-cardiac surgery [master’s thesis]. Hebei Medical University; 2015.
- Schouten O, Boersma E, Hoeks SE, et al. Fluvastatin and perioperative events in patients undergoing vascular surgery. N Engl J Med. 2009;361:980–989.
- Xia J, Qu Y, Shen H, et al. Patients with stable coronary artery disease receiving chronic statin treatment who are undergoing noncardiac emergency surgery benefit from acute atorvastatin reload. Cardiology. 2014;128:285–292.
- Xia J, Qu Y, Yin C, et al. Preoperative rosuvastatin protects patients with coronary artery disease undergoing noncardiac surgery. Cardiology. 2015;131:30–37.
- Sai C, Li J, Yingbin X, et al. Atorvastatin prevents postoperative atrial fibrillation in patients undergoing cardiac surgery. Hellenic J Cardiol. 2018 [Jan 4];[1–8]. doi: 10.1016/j.hjc.2017.12.012
- Saeed A, Amin VHM, Alireza M, et al. Evaluation of the effect of statins on post-surgical patients with acute kidney injury. Maedica (Buchar). 2017;12:95–100.
- An J, Shi F, Liu S, et al. Preoperative statins as modifiers of cardiac and inflammatory outcomes following coronary artery bypass graft surgery: a meta-analysis. Interact Cardiovasc Thorac Surg. 2017;25:958–965.
- Bao JW, Sun B, Ma PP, et al. Rosuvastatin inhibits inflammatory response and resists fibrosis after myocardial infarction. Eur Rev Med Pharmacol Sci. 2018;22:238–45.
- Sanguigni V, Pignatelli P, Lenti L, et al. Short-term treatment with atorvastatin reduces platelet CD40 ligand and thrombin generation in hypercholesterolemic patients. Circulation. 2005;111:412–419.
- Wassmann S, Faul A, Hennen B, et al. Rapid effect of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibition on coronary endothelial function. Circ Res. 2003;93:e98–103.
- Zhen-Han L, Rui S, Dan C, et al. Perioperative statin administration with decreased risk of postoperative atrial fibrillation, but not acute kidney injury or myocardial infarction: a meta-analysis. Sci Rep. 2017;7:10091.
- Calcagno S, Stio RE, Mancone M, et al. The statin therapy to prevent atrial fibrillation after cardiac surgery: Shakespearean dilemma. J Thorac Dis. 2016;8:2986–2990.
- Bockeria OL, Shvartz VA, Akhobekov AA, et al. Statin therapy in the primary prevention of early atrial fibrillation after coronary artery bypass grafting. Indian Heart J. 2016;68:792–797.
- Curtis M, Deng Y, Lee VV, et al. Effect of dose and timing of preoperative statins on mortality after coronary artery bypass surgery. Ann Thorac Surg. 2017;104:782–789.
- Puelacher C, Lurati Buse G, Seeberger D, et al. Perioperative myocardial injury after noncardiac surgery: incidence, mortality, and characterization. Circulation. 2018;137:1221–1232.
- Sanders RD, Nicholson A, Lewis SR, et al. Perioperative statin therapy for improving outcomes during and after noncardiac vascular surgery. Cochrane Database Syst Rev. 2013;(7):CD009971.
- Antoniou GA, Hajibandeh S, Vallabhaneni SR, et al. Meta-analysis of the effects of statins on perioperative outcomes in vascular and endovascular surgery. J Vasc Surg. 2015;61:519–532 e1.
- Bass AR, Szymonifka JD, Rondina MT, et al. Postoperative myocardial injury and inflammation is not blunted by a trial of atorvastatin in orthopedic surgery patients. Hss J. 2018;14:67–76.