Abstract
Introduction: Every fifth ischemic stroke is caused by thromboembolism originating from an atherosclerotic carotid artery plaque. While prevention is the most cost-effective stroke therapy, antiplatelet and cholesterol-lowering drugs have a ceiling effect in their efficacy. Therefore, discovery of novel pathophysiologic targets are needed to improve the primary and secondary prevention of stroke. This article provides a detailed study design and protocol of HeCES2, an observational prospective cohort study with the objective to investigate the pathophysiology of carotid atherosclerosis.
Materials and Methods: Recruitment and carotid endarterectomies of the study patients with carotid atherosclerosis were performed from October 2012 to September 2015. After brain and carotid artery imaging, endarterectomised carotid plaques (CPs) and blood samples were collected from 500 patients for detailed biochemical and molecular analyses.
Findings to date: We developed a morphological grading for macroscopic characteristics within CPs. The dominant macroscopic CP characteristics were: smoothness 62%, ulceration 61%, intraplaque hemorrhage 60%, atheromatous gruel 59%, luminal coral-type calcification 34%, abundant (44%) and moderate (39%) intramural calcification, and symptom-causing “hot spot” area 53%.
Future plans: By combining clinically oriented and basic biomedical research, this large-scale study attempts to untangle the pathophysiological perplexities of human carotid atherosclerosis.
This article is a rationale and design of the HeCES2 study that is an observational prospective cohort study with the objective to investigate the pathophysiology of carotid atherosclerosis.
The HeCES2 study strives to develop diagnostic algorithms including radiologic imaging to identify carotid atherosclerosis patients who warrant surgical treatment.
In addition, the study aims at finding out new tools for clinical risk stratification as well as novel molecular targets for drug development.
Key Messages
Introduction
Atherosclerotic cardiovascular diseases (ASCVD) constitute the largest cause of mortality in the graying Western world and the incidence is surging also in Eastern Europe, South America, Africa and Asia. [Citation1]. Prevalent risk factors such as obesity, hypertension, dyslipidemia, smoking, unhealthy dietary habits and sedentary lifestyle aggravate the atherosclerotic vascular process and increase cardiovascular events. The result has been a tremendous increase in the overall disease burden at the population level [Citation2]. Although the risk scores available today can identify individuals at high risk, large proportions of such individuals remain unidentified. Moreover, prevention of ASCVD by attacking the risk factors at an individual level has proven difficult.
By investigating the plaque dissected on carotid endarterectomy (CEA), investigators studying carotid artery atherosclerosis can exploit direct access to the cellular contents, which have or have not yet culminated in a clinically discernible symptom. They have alluded to an outstanding opportunity to use the carotid plaque material to establish a characteristic gene expression pattern or even a “fingerprint” that reflects either the stability or the progression of the atherosclerotic process throughout the entire cardiovascular system in order to predict systemic vascular outcomes [Citation3]. Supporting evidence arises from post-mortem observations in victims of myocardial infarction, revealing that, in addition to the culprit plaque, also other coronary plaques demonstrate significantly increased inflammation [Citation4]. In addition, it has been observed that when coronary disease presents with unstable clinical symptoms, CP morphology is also more unstable [Citation5]. Finally, when atherosclerosis has reached an advanced stage with clinical symptoms, such as in the case of large-vessel peripheral arterial disease of the lower limbs, or in the carotid arteries, the patients have a high risk of acute myocardial infarction, that is the development of a vulnerable coronary plaque which will cause an acute, often fatal, atherothrombotic event [Citation6,Citation7]. These findings support the idea that instability of the vascular wall is a systemic process and thus, a single atherosclerotic plaque could provide information on the inflammatory status of plaques elsewhere in the vascular system [Citation3].
Recent observations of the predictive value of discrete magnetic resonance imaging (MRI) characteristics of a carotid plaque (CP) have reinstated the concept of a vulnerable plaque in cerebrovascular disease and stroke. In a recent meta-analysis of CP MRI and stroke risk, the hazard ratios for intraplaque hemorrhage, lipid-rich necrotic core, and thinning/rupture of the fibrous cap as predictors of subsequent stroke/transient ischemic attack (TIA) were 4.59 (95% CI, 2.91–7.24), 3.00 (1.51–5.95), and 5.93 (2.65–13.20), respectively [Citation8]. The virtue of therapeutic removal of plaques of this nature to prevent strokes validates the vulnerable plaque paradigm inasmuch as it validates the CEA procedure itself. Still, imaging represents only one facet of ongoing search for valuable local or circulating biomarkers to improve stratification of stroke risk due to carotid atherosclerosis.
We have 20 years of research experience on multidisciplinary and longitudinal research in many aspects of carotid artery disease, based on previously explored different aspects of symptom-causing carotid artery disease in the Helsinki Carotid Endarterectomy Study (HeCES) cohort (see below). Our previous research identified a multitude of novel symptom-related molecular, hemodynamic and cognitive observations of a consecutive cohort of stenosing advanced carotid atherosclerosis, and building on these findings, it is the purpose of this report to outline in detail the methods used in the collection of CP specimens from 500 carotid patients in Helsinki to establish an extended biomarker database and enable more efficient discovery of novel drug targets.
Past progress in Helsinki Carotid Endarterectomy Study (HeCES)
The HeCES study started in 1997 in order to shed light into the still elusive reasons why some plaques advance even into complete occlusion without causing any clinical symptoms while others cause strokes. The original study included 92 consecutive patients with a high-grade carotid stenosis (>70%) who underwent CEA in the Helsinki University Hospital during 1997–2000.
Focusing on CP destabilization, our previous HeCES study has revealed associations with CP morphology and histology [Citation9–11], gene expression of iron/haeme metabolism (HO-1, CD163) [Citation12] and proteins related to intraplaque hemorrhage (PLIN2/adipophilin, CD163) [Citation13,Citation14]. We performed a microarray study which presented a comprehensive transcriptional profiling of stroke-associated CPs and demonstrated a significant transcriptome “fingerprint” difference between stroke-associated and silent CPs [Citation15]. Some important genes emerged from that study were, for example, FABP4 [15,16] and haptoglobin [Citation17]. In addition to the biochemical and morphological CP analyses, we have also studied radiological brain findings and cognition [Citation18–21], as well as future risk to thromboembolic complications in other vascular beds [Citation7], in patients with high-grade carotid stenosis.
Objectives
This observational study focuses on five objectives targeting the most pressing problems in the prevention of cardiovascular events in carotid artery disease.
To unravel the dominating pathomechanisms, which govern the destabilization of an atherosclerotic plaque and may have evolved over decades.
To develop a novel, clinically meaningful carotid artery-specific plaque classification score by combining data from detailed photographic and histologic morphologic examination of the culprit lesion with imaging findings and clinical data.
To find new tools for clinical risk stratification, such as circulating biochemical markers or genetic variants, in order to identify individuals with especially high or low risk of thromboembolism.
Based on clinically characterized patient material, to find novel molecular targets useful for drug development and to find out whether some of them are statin-sensitive.
To clarify which biomedical factors or genotypes represent long-term risk of restenosis, recurrent stroke or premature cardiac death based on 10-year follow-up.
Materials and methods
The HeCES 2 study is a cross-sectional and longitudinal prospective consecutive cohort study that was conducted at Helsinki University Hospital (HUH) in Finland in collaboration with the departments of neurology, vascular surgery and radiology. Recruitment of patients started during October 2012 and was continued consecutively until September 2015 to include altogether the predefined number of 500 symptomatic and asymptomatic carotid atherosclerosis patients scheduled for CEA. The CPs and blood samples were collected for detailed analyses and patients were interviewed and examined thoroughly. After this main recruitment period, the collection of bilaterally operated plaques as well as the recruitment for the brain and eye substudy (HECES-BEST, see Supplemental file p. 12) is still ongoing. The study was approved by the Ethics Committee of medicine of the Hospital District of Helsinki and Uusimaa, and all study patients gave written informed consent.
Patients
Patients were referred to CEA from the Hospital District of Helsinki and Uusimaa, and the decisions to perform CEA were based on the guidelines of European Stroke Organization [Citation22]. A computed tomography (CT) or MRI of the brain was performed on almost all study patients, but a small portion (n = 22) of asymptomatic patients did not have a brain scan. Most patients underwent a Doppler ultrasound examination, and all but one had either CT- or MR-angiography (CTA, MRA) of the carotid arteries. Carotid endarterectomies were performed according to the prevailing clinical standards. (More specifically, see Supplemental file p.2).
Assessment of the origin of clinical symptoms: plaque-derived or not
CEA patients occasionally had other potential aetiologies (e.g. atrial fibrillation or small vessel disease) for the clinical symptom in addition to the carotid atherosclerosis. Two neurologists (K.N., P.I.) independently evaluated if a patient had experienced a genuine ischaemic cerebrovascular symptom (defined as symptomatic patient), as well as whether the operated CP had caused the thromboembolic symptoms (defined as symptom-causing plaque). This was performed in each case by recording all possible major and minor aetiologies, and considering thoroughly the situation of symptom presentation, collateral status of cerebral circulation as well as location and extent of the putative cerebral ischemia. If the symptom was assumed to be caused by hemodynamic mechanism rather than thromboembolism, the symptom status was defined as ambiguous. In case of ambiguity regarding the symptom status of the plaque or between competing aetiologies, a panel of stroke neurologists (P.I., K.N., L.S. and P.J.L.) was consulted to reach a consensus. The diagnoses and plaque status were determined blinded to macroscopic evaluation of plaques. Subtypes of ischemic stroke were recorded according to the TOAST classification [Citation23], and the stroke risk after TIAs was classified by the ABCD2 prognostic score [Citation24].
Laboratory examinations
Blood samples where taken preoperatively for clinical purposes, as well as genotyping and biomarker assays. See more in the Supplement pp. 2–3.
Radiology
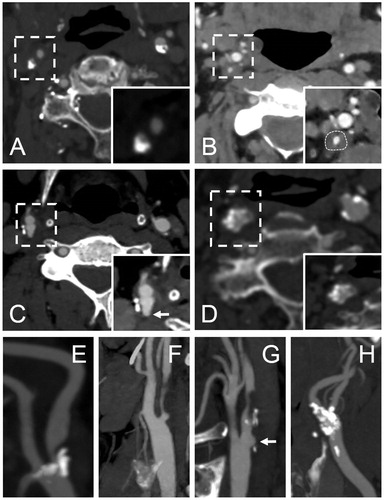
Before recruitment, nearly all patients underwent multi-detector CTA of the carotid arteries. If a patient had one or several contraindications to CTA, such as iodine allergy or severe kidney insufficiency, MRA of the carotid arteries was performed instead. One patient had contraindications for both CTA and MRA, and only had a preoperative carotid artery Doppler ultrasound. In addition, most of the patients underwent non-enhanced brain CT before recruitment. HeCES 2 imaging data was analysed more extensively than required for a standard clinico-radiological evaluation (), described in detail in the Supplemental file p. 3. The stenosis degree (%) of carotid arteries was calculated using the North American Symptomatic Carotid Endarterectomy Trial (NASCET)–criteria [Citation25]. The stenoses were classified as lipid-like soft, uniformly calcified (bulky calcification), and nodular-like calcified according to the dominant CTA plaque feature. Moreover, we recorded other known vulnerability markers, such as ulcerations and luminal thromboses. Over 1–2 mm deep ulcerations are visible in CTA axial images as contrast-filled “pouches” [Citation26].
Figure 1. Illustration of the used computed tomopraghy angiography (CTA) classification of the internal carotid artery (ICA) stenosis and an example of an ulcerative CP. Images A–D represent CTA axial plane source images showing the different classifications of the ICA stenosis. Images E – H represent the corresponding oblique sagittal maximum intensity projection reformat images. All the images visualize a right sided ICA stenosis. A and E = bulky calcification; B and F = soft plaque (delineated); C and G = ulcerative plaque (arrow marks the pouch of a plaque ulceration); D and H = calcified nodules.
Tissue processing and morphologic examination
Specific description and images of tissue processing, as well as preparation for RNA, protein and lipid analyses are provided in the Supplemental file pp. 3-6 (Supplementary Figures S1 and S2, Supplementary Table S1). Specimens were thoroughly photographed during tissue processing, specification is provided in the Supplemental file p. 7 (Supplementary Figure S3). In addition, morphologic evaluation included macroscopic and microscopic evaluation.
Macroscopic evaluation
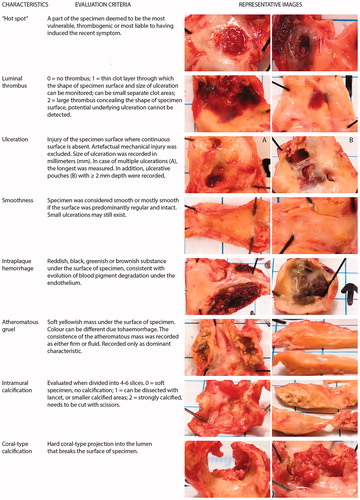
Based on the experience of our expert panel we felt that no uniform satisfactory morphological grading method covering each of the most important plaque characteristics exists. Therefore we developed some new morphological grades after numerous consensus meetings and a lead-in period (). During tissue processing, the carotid specimens were morphologically evaluated based on their visual and morphological characteristics, and the macroscopic observations were recorded in a specimen information form (Supplementary Figure S4, Supplementary file p. 8). The recorded parameters included six variables: luminal thrombus, ulceration, smoothness, intraplaque hemorrhage, atheromatous gruel, and calcification. Both intramural and luminal calcifications were recorded. In most instances, the luminal calcification was different from intramural calcification, and referred to as coral-type calcification due to a coral-type morphologic impression. In addition, the term “hot spot” was used for the area of the specimen judged to be the most vulnerable and thrombogenic area, or even considered to be the foremost likely area having triggered the recent thromboembolic symptom. As the name (“spot”) implicates, the “hot spot” was often a separate (small) area differing from the surroundings, where an ulceration or fresh thrombus could be seen. Details of the evaluation of the characteristics and images of specimens representing typical morphological features are shown in .
Histology
Gross histological evaluation was carried out from one representative longitudinal slice by means of two histological stainings, Hematoxylin-Eosin (HE) and Masson’s Trichrome (MT). Several parameters describing the general characteristics and measures of plaque vulnerability were registered from CPs for future comparison with detailed immunohistochemical analysis of the specimen, and with various patient characteristics including biochemical, radiological and genetic information. These parameters included: sample integrity, correlation of histology to macroscopic findings, plaque surface irregularity, overall plaque stability, intraplaque hemorrhage, fibrous cap thickness and rupture, plaque and cap inflammation, lipid core, thrombus, calcification, fibrous, cholesterol crystals, cellularity, neovessels and foam cells. Comprehensive information and details of the histological assessment are provided in the Supplemental file pp. 9–11 (Supplementary Tables S2 and S3).
HeCES-BEST
A specific substudy for consenting patients with hemodynamically significant stenosis (high-grade, exceeding 70% by NASCET criteria, projected n = 100) was launched during HeCES 2 study for a closer look into the effects of a potential perfusion deficit on the eye and brain parenchyma, as well as the endothelial function (Brain and Eye SubsTudy, HeCES-BEST). More details of this substudy are provided in the Supplemental file p. 12.
Follow-up
By the initiation of our study, the study patients have also consented to the follow-up of their health and medical data throughout the study. Information on recurrent cerebrovascular symptoms (TIA, stroke), cardiac events (myocardial infarctions, unstable angina, occurrence of atrial fibrillation) and other thromboembolic diseases will be retrieved from the National Care Register for Health Care up until 10 years from the recruitment. Deaths and the causes of death are collected from the Finnish public authority Statistics Finland. Study patients will be contacted no later than 10 years after their CEA, at which time they will be interviewed. A Doppler ultrasound examination will be performed in order to detect possible restenosis. Clinical records on general health, vascular events, medication and blood pressure will be revisited. Blood samples will be obtained as well.
Findings to date
During the main recruitment period altogether 500 patients were recruited. CEA patients operated in the Department of Vascular Surgery at the Helsinki University Hospital (HUH) were recruited in a consecutive fashion. During the recruitment period altogether 671 CEAs were performed to 631 patients, while non-recruitment due to exclusion criteria or planned study cessations during holidays was only 131 patients. Due to various reasons, sporadic study patients did not undergo CEA (n = 8) or the CP was lost (n = 7). A proportion of study patients (n = 35) underwent CEA to the contralateral asymptomatic side in a staged manner so that the operations were carried out at intervals of at least one month. Hence, the yield of CPs during the period October 2012 – September 2015 was n = 520 (Supplementary Figure S5, Supplemental file p. 13). Perioperative mortality was 0.4% (2 deaths within 1 month postoperatively) but these deaths were unrelated to the CEA. Nine patients out of 492 suffered from major postoperative stroke (Modified Rankin Scale ≥3) resulting in a complication rate of 1.8%.
Baseline characteristics and symptomatology
Clinical characteristics at the time of CEA are presented in the Supplemental file (Supplementary Table S4, p.14). There were more men (68%) than women among study patients, which reflects the general gender distribution of advanced carotid artery disease. The mean age of study patients was 69.8 years, ranging from 44.0 to 91.0 years.
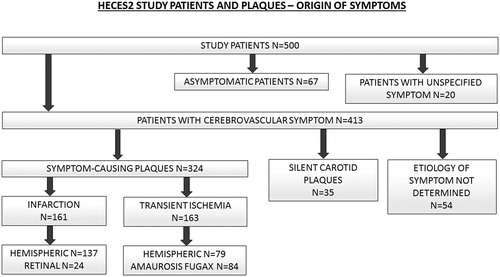
Based on the stringent consensus diagnoses by stroke neurologist panel, 413 (83%) of the 500 study patients had experienced a cerebrovascular symptom, 67 (13%) were asymptomatic and 20 (4%) had an unspecified symptom, that is, not possible to determine whether it represented a cerebrovascular or an alternative symptomatology such as epileptic, migrainous or musculoskeletal. More specific division of the symptom status of patients and CPs is presented in . All strokes were classified into subtypes according to the TOAST classification system [Citation23] (Supplementary Figure S6, Supplemental file p. 15).
Figure 3. HeCES2 study patients and carotid plaques – origin of symptoms. 413 (83%) of the 500 study patients had experienced a cerebrovascular symptom, 67 (13%) were asymptomatic and 20 (4%) had an unspecified symptom, that is not possible to determine whether it represented a cerebrovascular or an alternative symptomatology such as epileptic, migrainous or musculoskeletal. Among the 413 study patients with a cerebrovascular symptom, the operated CP was determined to be symptom-causing in 324 (79%) patients while in 35 (8%) patients it was deemed that the CP was innocent and the cerebrovascular symptom represented another aetiology or vascular territory. In another 54 (13%) patients, coinciding competing aetiologies of cerebrovascular symptoms prevented to declare whether the CP was symptom-causing or not. Among the symptom-causing CPs, 161 (50%) had caused a stroke and 163 (50%) a TIA, and further 137 (85%) of the strokes were hemispheric and 24 (15%) retinal artery occlusions, whereas 79 (48%) of the TIAs were hemispheric and 84 (52%) were amaurosis fugax.
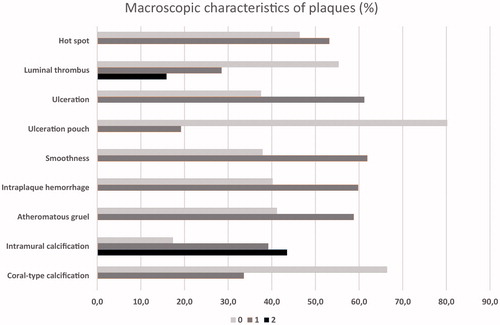
The results of the macroscopic grading are presented in . Typically, one plaque had several macroscopic changes which are defined in . The percentages of the macroscopic characteristics present were as follows: smoothness 62%, ulceration 61%, intraplaque hemorrhage 60%, atheromatous gruel 59%, “hot spot” 53%, luminal coral-type calcification 34%, abundant (44%) and moderate (39%) intramural calcification, luminal thrombus of moderate size (29%) and of abundant size (16%), and ulceration pouch 19%.
Figure 4. The proportion of macroscopic characteristics in carotid plaques. All characteristics were divided into two categories: 0 = not present or 1 = present, except intramural calcification and luminal thrombus, that had three different categories: 0 = not present, 1 = moderately present, 2 = abundantly present.
Discussion
Currently available screening and diagnostic methods are insufficient to identify the persons before a thromboembolic ischaemic stroke occurs. The concept of the vulnerable, unstable atherosclerotic plaque has promised new avenues of opportunity in the field of cardiovascular medicine, but the promises have yet to realize. While the rupture-prone plaques have a predilection segment in the carotid bifurcation, various types of atherosclerotic plaques with high likelihood of thrombotic complications and rapid progression should be considered as vulnerable plaques [Citation27]. Therefore, we wish to include among the vulnerable plaques not only the unstable rupture-prone but also the stable erosion-prone carotid plaques. Circulating blood mediators that promote thrombosis as well as prime inflammatory vasculopathy in the distal vascular bed also drive the progression of vulnerability to stroke. Despite longstanding studies and large-scale efforts, such as Athero-Express [Citation28] and the Oxford plaque study [Citation29], the clinical applicability of the vulnerable plaque concept in carotid atherosclerosis still remains obscure. However, due to its bilaterality, this concept can systematically be investigated only in the carotid artery system where strictly ipsilateral symptom generation followed by vascular imaging can justify symptom state-specific acquisition (i.e. CEA) of the respective plaque specimen for detailed pathological and molecular examinations. To this end, from all 500 patients, we have described the experimental protocol for collecting 520 CPs, from which 324 were symptom-causing, for careful macroscopic, microscopic and molecular investigations to follow.
Studies on the pathological substrate of coronary artery disease have demonstrated common association between acute myocardial infarction and rupture or erosion of a coronary atherosclerotic plaque [Citation30]. This observation has led to the paradigm of a “high-risk”, rupture-prone vulnerable plaque that could be identified and treated to prevent atherothrombotic events. Definitive proof of the vulnerable plaque being the very substrate of acute coronary events, remains, however, elusive because animal or human data supporting a cause-and-effect relationship are lacking [Citation31,Citation32]. Moreover, despite major advancements in imaging technology, clinical studies have not been able to demonstrate improved stroke risk prediction when compared to traditional approaches of risk prediction. In view of the existence of also clinically silent coronary plaque ruptures, investigators of coronary disease are turning from the vulnerable plaque paradigm to alternative, multifaceted hypotheses of the natural history of atherosclerotic plaque rupture [Citation33].
More specifically, a temporal shift from vulnerable plaques to more stable plaques both in coronary and carotid arteries has been recently observed [Citation34,Citation35]. Thus, plaques underlying thromboembolic cerebrovascular event nowadays reveal more fibrous and non-inflammatory characteristics compared with those some ten years ago [Citation36]. Thus, a paradigm shift from rupture of an unstable plaque to endothelial erosion of a stable plaque has taken place. The authors considered favourable changes in the major risk factors of ASCVD due to life-style changes and favourable effects of the widespread usage of statins as likely reasons for the shift from unstable to stable plaque as a cause of an atherothrombotic clinical event.
In addition to the accustomed methodology of morphologic plaque analysis, such as microscopic histopathologic examinations, the present study introduces a more unconventional and unique ex vivo gross morphological approach to complement the morphologic characterization of a CP. By combining plaque data from histologic examinations with macroscopic morphology as well as radiologic information, we aim at discovering new morphologic plaque features relative to plaque vulnerability and symptom generation. Vice versa, new degree of knowledge might be gained concerning the interpretation of carotid artery CTA by corroborating the radiologic characteristics with histologic and gross macroscopic findings ultimately leading to facilitated and accelerated decision making in clinical practice.
In the previous HeCES cohort of 92 patients, we were able to profile the gene expression pattern of symptom-causing CP, the molecular “fingerprint” of the culprit lesion [Citation15]. In that early cohort, 54% of the patients had not been receiving statins preoperatively, and thus about half of the patients were lacking the potential carotid plaque-stabilizing effects of this class of drugs [Citation37]. The present study includes a significant number of silent plaques, enabling us to replicate these findings and confirm the molecular “fingerprint” of the culprit lesion in the present era of widespread statin use.
To focus purely in carotid artery disease in triggering thromboembolic strokes, our previous HeCES cohort excluded patients with AF and other potential sources of cardiogenic emboli [Citation9–11,Citation18–20]. The present study, however, included 86 (17%) patients with AF. In the face of ever increasing AF epidemic, we are planning to try our putative biomarkers and imaging signs to extend their resolution in differentiating symptom-causing thromboembolic carotid plaques from silent ones even in this demanding subpopulation. In keeping with the consecutive nature of enrolment, this approach improves the versatility of the eventual results in relating them to a heterogeneous, real-life population of carotid atherosclerosis with common co-morbidities, with a diverse panel of antithrombotic medications and with other confounding conditions.
Even with modern imaging, the neurological delineation of a patient’s symptom is sometimes a challenging task. A cerebrovascular symptom is seldom crystal-clear, not even sudden right hemiparesis, with speech difficulty or diplopia with balance disturbance. When symptoms present with adjunct nonspecific features, penetrating clinical experience to accurately interpret patients’ symptoms is often required. At times, the arterial anatomy and collateral pathways evolved over time may diverge so that ischemia precipitated from carotid circulation presents as symptoms more typical for posterior cerebral circulation or vice versa. The decision explained previously did not ease our challenges as neurologists in dissecting the individual symptom-precipitating mechanisms - large artery thromboembolic, cardioembolic, hemodynamic or small vessel vascular occlusions - during almost everlasting, yet highly entertaining prerogative deliberations on most likely hypotheses. Infallible aetiopathogenetic classification can never be achieved, and for this reason, we arrived at classifying 54 (11%) patients to a group in which it could not be determined whether the plaque or a competing aetiology had precipitated the clinical symptom. Furthermore, we included 20 (4%) patients to a group with an ambiguous symptom, meaning that eventually it was not possible to determine whether the symptom was of cerebrovascular nature or due to some other neurological disturbance occurring ipsilaterally to the operated carotid artery.
For scientific purposes, the present study pays particular attention to the accuracy of the evaluation of the neurological symptoms of the patients, and, moreover, to the detailed macroscopic, microscopic, and molecular architecture of the endarterectomized CPs. In this study, it took two to four stroke neurologists to evaluate the symptom status with the aid of radiologic data as well as available medical records, while in daily clinical practice often hefty therapy decisions must be made with a smaller manpower. Erroneous initial interpretation of the symptom status waters down all otherwise sophisticated results and eloquent projections of individual disease course, and even endangers patients to flawed therapeutic regimens. It is for this purpose that the study aims to find novel biomarkers and imaging hallmarks, and thereby to provide clinicians some trustworthy tools to judge whether a given plaque is likely going to destroy the few remaining years of an elderly patient, or is prone to stay innocent through the projected remaining ‘good years’. Furthermore, benefit can be expected through identification of novel molecular targets for drug development.
Supplemental Material
Download MS Word (4.2 MB)Acknowledgments
Reeta Levo, Anita Mäkelä and Taru Puhakka are thanked for skilful technical assistance.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- WHO. The Top 10 Causes of Death. 2014; Available at: http://www.who.int/mediacentre/factsheets/fs310/en/
- GBD 2016 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017;39010100:1260-1344.
- Hellings WE, Peeters W, Moll FL, et al. From vulnerable plaque to vulnerable patient: the search for biomarkers of plaque destabilization. Trends Cardiovasc Med. 2007;17:162–171.
- Mauriello A, Sangiorgi G, Fratoni S, et al. Diffuse and active inflammation occurs in both vulnerable and stable plaques of the entire coronary tree: a histopathologic study of patients dying of acute myocardial infarction. J Am Coll Cardiol. 2005;45:1585–1593.
- Lombardo A, Biasucci LM, Lanza GA, et al. Inflammation as a possible link between coronary and carotid plaque instability. Circulation. 2004;109:3158–3163.
- Criqui MH, Langer RD, Fronek A, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326:381–386.
- Nuotio K, Soinne L, Hänninen H, et al. Life-threatening coronary disease is prevalent in patients with stenosing carotid artery disease. Int j Stroke. 2015;10:1217–1223.
- Gupta A, Baradaran H, Schweitzer AD, et al. Carotid plaque MRI and stroke risk: a systematic review and meta-analysis. Stroke. 2013;44:3071–3077.
- Nuotio K, Lindsberg PJ, Carpén O, et al. Adhesion molecule expression in symptomatic and asymptomatic carotid stenosis. Neurology. 2003;60:1890–1899.
- Nuotio K, Mäyränpää MI, Saksi J, et al. Endothelial apoptosis does not determine symptom status in carotid artery disease. Cerebrovasc Dis. 2007;23:27–34.
- Lehtonen-Smeds EM, Mäyränpää M, Lindsberg PJ, et al. Carotid plaque mast cells associate with atherogenic serum lipids, high grade carotid stenosis and symptomatic carotid artery disease. Results from the helsinki carotid endarterectomy study. Cerebrovasc Dis. 2005;19:291–301.
- Ijäs P, Nuotio K, Saksi J, et al. Microarray analysis reveals overexpression of CD163 and HO-1 in symptomatic carotid plaques. Arterioscler, Thrombo Vasc Biol. 2007;27:154–160.
- Nuotio K, Isoviita PM, Saksi J, et al. Adipophilin expression is increased in symptomatic carotid atherosclerosis: correlation with red blood cells and cholesterol crystals. Stroke. 2007;38:1791–1798.
- Isoviita PM, Nuotio K, Saksi J, et al. An imbalance between CD36 and ABCA1 protein expression favors lipid accumulation in stroke-prone ulcerated carotid plaques. Stroke. 2010;41:389–393.
- Saksi J, Ijäs P, Nuotio K, et al. Gene expression differences between stroke-associated and asymptomatic carotid plaques. J Mol Med. 2011;89:1015–1026.
- Saksi J, Ijäs P, Mäyränpää MI, et al. Low-Expression Variant of Fatty Acid-Binding Protein 4 Favors Reduced Manifestations of Atherosclerotic Disease and Increased Plaque Stability. Circulation Cardiovasc Gene. 2014;7:588–598.
- Ijäs P, Saksi J, Soinne L, et al. Haptoglobin 2 allele associates with unstable carotid plaque and major cardiovascular events. Atherosclerosis. 2013;230:228–234.
- Soinne L, Helenius J, Tatlisumak T, et al. Cerebral hemodynamics in asymptomatic and symptomatic patients with high-grade carotid stenosis undergoing carotid endarterectomy. Stroke. 2003;34:1655–1661.
- Soinne L, Helenius J, Saimanen E, et al. Brain diffusion changes in carotid occlusive disease treated with endarterectomy. Neurology. 2003;61:1061–1065.
- Soinne L, Saimanen E, Malmberg-Céder K, et al. Association of the fibrinolytic system and hemorheology with symptoms in patients with carotid occlusive disease. Cerebrovasc Dis. 2005;20:172–179.
- Soinne L, Helenius J, Tikkala I, et al. The effect of severe carotid occlusive disease and its surgical treatment on cognitive functions of the brain. Brain Cogn. 2009;69:353–359.
- European Stroke Organisation (ESO) Executive Committee. ESO Writing Committee. Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc Dis. 2008;25:457–507.
- Adams HPJ, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. Stroke. 1993;24:35–41.
- Johnston SC, Rothwell PM, Nguyen-Huynh MN, et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet. 2007;369:283–292.
- North American Symptomatic Carotid Endarterectomy Trial. Methods, patient characteristics, and progress. Stroke. 1991;22:711–720.
- Saba L, Caddeo G, Sanfilippo R, et al. Efficacy and sensitivity of axial scans and different reconstruction methods in the study of the ulcerated carotid plaque using multidetector-row CT angiography: comparison with surgical results. AJNR Am J Neuroradiol. 2007;28:1061–1723.
- Naghavi M, Libby P, Falk E, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part I. Circulation. 2003;108:1664–1672.
- Verhoeven BA, Velema E, Schoneveld AH, et al. Athero-express: differential atherosclerotic plaque expression of mRNA and protein in relation to cardiovascular events and patient characteristics. Rationale and design. Eur J Epidemiol. 2004;19:1127–1133.
- Redgrave JN, Lovett JK, Gallagher PJ, et al. Histological assessment of 526 symptomatic carotid plaques in relation to the nature and timing of ischemic symptoms: the Oxford plaque study. Circulation. 2006;113:2320–2328.
- Virmani R, Burke AP, Farb A, et al. Pathology of the vulnerable plaque. J Am Coll Cardiol. 2006;478: Suppl:13–18.
- Finn AV, Nakano M, Narula J, et al. Concept of vulnerable/unstable plaque. Arterioscler Thromb Vasc Biol. 2010;30:1282–1292.
- Stone GW, Maehara A, Lansky AJ, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364:226–235.
- Arbab-Zadeh A, Fuster V. The myth of the "vulnerable plaque": transitioning from a focus on individual lesions to atherosclerotic disease burden for coronary artery disease risk assessment. J Am Coll Cardiol. 2015;65:846–855.
- Libby P, Pasterkamp G. Requiem for the 'vulnerable plaque'. Eur Heart J. 2015;36:2984–2987.
- Pasterkamp G, den Ruijter HM, Libby P. Temporal shifts in clinical presentation and underlying mechanisms of atherosclerotic disease. Nat Rev Cardiol. 2017;14:21–29.
- van Lammeren GW, den Ruijter HM, Vrijenhoek JEP, et al. Time-dependent changes in atherosclerotic plaque composition in patients undergoing carotid surgery. Circulation. 2014;129:2269–2276.
- Artom N, Montecucco F, Dallegri F, et al. Carotid atherosclerotic plaque stenosis: the stabilizing role of statins. Eur J Clin Invest. 2014;44:1122–1134.