Abstract
Introduction
Risks of low-dose aspirin-associated upper and lower gastrointestinal bleeds (UGIB/LGIB) may vary by severity and presence of cardiovascular disease (CVD). No study has quantified these risks for UGIB and LGIB in the same real-world study population.
Patients and methods
Using UK primary care data, 199,049 new users of low-dose aspirin (75–300 mg/day) and 1:1 matched non-users were followed to identify incident UGIB (N = 1843)/LGIB (N = 2763) cases. Nested case-control analyses compared current low-dose aspirin vs. non-use on UGIB/LGIB risk.
Results
Adjusted incidence rate ratios (ORs; 95% CIs) were 1.62 (1.42–1.86) for non-fatal UGIB, 1.63 (1.47–1.81) for non-fatal LGIB, 0.77 (0.51–1.16) for fatal UGIB, 1.29 (0.50–3.36) for fatal LGIB. For hospitalizations, adjusted ORs (95% CIs) were 1.55 (1.32–1.81) for UGIB and 1.89 (1.58–2.27) for LGIB; for referred only cases, they were 1.52 (1.26–1.84) for UGIB and 1.54 (1.37–1.73) for LGIB. In primary CVD prevention, adjusted ORs (95% CI) were 1.62 (1.38–1.90) for UGIB and 1.60 (1.42–1.81) for LGIB; in secondary CVD prevention, they were 1.16 (0.89–1.50) for UGIB and 1.67 (1.34–2.09) for LGIB.
Conclusion
Low-dose aspirin was associated with increased risks of non-fatal but not fatal UGIB/LGIB.
Low-dose aspirin is associated with an increased risks of non-fatal UGIB/LGIB but not fatal UGIB/LGIB.
Key message
Introduction
Long-term use of low-dose aspirin remains the mainstay recommended therapy for the secondary prevention of ischaemic vascular events [Citation1–3]. Evidence also support a protective effect of low-dose aspirin against colorectal cancer (CRC) [Citation4,Citation5] has led to it being advocated for use in certain groups of patients without established ischaemic vascular disease but who are considered at high enough risk to warrant preventative therapy [Citation6–8], with the benefits regarded as outweighing the risks of bleeding.
The recent publication of findings of three large randomized controlled trials (RCTs) of 100 mg daily aspirin in patients without pre-existing cardiovascular disease – ASCEND (A Study of Cardiovascular Events in Diabetes) [Citation9], ASPREE (Aspirin in Reducing Events in the Elderly) [Citation10], and ARRIVE (Aspirin to Reduce Risk of Initial Vascular Events) among individuals at average cardiovascular risk based on specific risk factors [Citation11] – has seen increasing discussion in the benefits and risks of aspirin for primary CVD prevention [Citation12]. These clinical trials each saw an increased bleeding risk (mostly gastrointestinal [GI] bleeding) with aspirin vs. placebo, in line with previous findings [Citation1,Citation13]. The differing trial designs and composition of study participants, however, underscores the need for robust data addressing bleeding outcomes with preventative aspirin at the larger general population level – from well-designed population-based studies that include individuals with GI comorbidities in routine care settings, to support low-dose aspirin clinical decision-making. In addition to assessing bleeding risks according to the target population (primary or secondary CVD prevention), a full evaluation of low-dose aspirin-associated GI bleeding would cover the severity of bleeds (case-fatality, need for hospitalization) and the bleed location (upper or lower GI tract) – data on low-dose aspirin-associated risks of lower GI bleeding are scarce. A recent meta-analysis of data from aspirin clinical trials (where doses ranged from 75 mg to 1900 mg daily) found no significant increase in the risk of fatal GI bleeds in subjects randomized to aspirin compared with placebo [Citation14].
Using data from a validated UK primary care database – The Health Improvement Network (THIN) – we aimed to quantify the association between new use of low-dose aspirin and risk of upper and lower gastrointestinal bleeding (UGIB/LGIB), including by level of healthcare assistance received (hospitalized or referred only), case-fatality, specific GI tract location, characteristics of aspirin (dose and duration) and according to primary/secondary CVD prevention population. To minimize confounding arising from differences between aspirin users and non-users that are difficult to control, a matched observational cohort study of new users of low-dose aspirin was carried out, with a subsequent nested case-control analysis to evaluate aspirin as a time-dependent variable.
Patients and methods
The study protocol was approved by an independent scientific review committee for THIN (reference number 14-088A1). This study was part of a wider observational research programme evaluating the benefits and risks of low-dose aspirin in the general UK population; the design of this present study is depicted in . Details of the data source (THIN) and identification of the source population and study cohorts have been described previously [Citation15]. Briefly, the source population included 1,840,253 individuals in THIN aged 40–84 years between 01 January 2000 and December 2012 who met the study eligibility criteria (see Supplementary Methods). We identified 199,079 individuals with a first prescription for low-dose aspirin (start date) matched each 1:1 to a non-user of low-dose aspirin on their start date by age, sex, time since study entry, and number of primary care practitioner (PCP) visits in the previous year (as a proxy measure for general health status). Two separate follow-ups of the cohorts were undertaken to identify incident cases of UGIB and LGIB. Details of the follow-up process and identification, classification and multi-step validation of UGIB and LGIB cases have been described previously in an analysis restricted to the low-dose aspirin cohort [Citation16]. The same methods were applied in this present study for individuals in both the low-dose aspirin and non-user comparator cohort, and for all identified incident UGIB/LGIB cases (Supplementary Figure). The index date was the date of the recorded UGIB/LGIB diagnosis. Positive predictive values in THIN of 95% for UGIB diagnoses and 82% for LGIB diagnoses have been previously reported after applying our validation processes [Citation17–20].
Figure 1. Flowchart depicting the cohort study with nested case-control analysis study design. EMR: electronic medical record; GIB: gastrointestinal bleed; HES: hospital episode statistics; LGIB: lower gastrointestinal bleed; PCP: primary care practitioner; UGIB: upper gastrointestinal bleed; THIN: The Health Improvement Network.
Control selection
The sampling of 5000 UGIB controls and 10,000 LGIB controls was performed among the two study cohorts. Controls were frequency matched to UGIB/LGIB cases by the calendar year, sex and age. Matching was a two-step process: firstly, we identified all eligible individuals within risk set strata defined by the index year of cases. Secondly, within each specific year stratum, we further frequency matched (random sample) according to the distribution of sex and age of cases in that stratum year. Controls met the same eligibility criteria as cases, and their index date was a computer-generated random date within their individual observation period.
Low-dose aspirin use and covariates
We have previously described the classification of exposure to low-dose aspirin and other medications [Citation15]. In this analysis, current use was defined as use 0–30 days before the index date, and the comparison group was “non-use,” which was defined as either never use of low-dose aspirin or distant use (use of low-dose aspirin more than 365 days before the index date). Although low-dose aspirin is available over-the-counter (OTC) in the UK, we have previously shown that misclassification of low-dose aspirin in THIN owing to unrecorded use of OTC low-dose aspirin, is minimal [Citation21]. In addition to patient demographics, lifestyle factors (smoking status, alcohol consumption, body mass index), medications and morbidities were identified any time before the index date; healthcare use (PCP encounters, secondary care referrals and hospitalizations) was captured in the year before the index date using the most recent status/value applicable. Individuals with missing values for variables were coded in a separate category “unknown.”
Statistical analysis
Separate nested case-control analyses were performed for UGIB and LGIB using STATA version 12.0. Analyses were undertaken for UGIB/LGIB cases, with sub-group analyses by level of healthcare assistance (hospitalized or referred only), case-fatality (death from any cause within the first 30 days), specific location of the bleed (duodenal ulcer, gastric ulcer or mucosal erosion for UGIB; diverticular disease or polyps for LGIB), duration of low-dose aspirin and specific dose. Unconditional logistic regression was used to estimate odds ratios with 95% confidence intervals (CIs) to quantify the association between low-dose aspirin (and other explanatory variables), with UGIB/LGIB, after adjustment for potential confounders. By using incidence density sampling to select controls, the OR is an unbiased estimator of the incidence rate ratio (RR) [Citation22]. For all analyses, current low-dose aspirin use (≤30 days before the index date) was compared with non-use as the reference group.
Results
After a median follow-up of 5.4 years (for both the UGIB and LGIB follow-ups), 1843 incident UGIB cases and 2763 incident LGIB cases were identified and confirmed after applying all our validation processes. Sixty per cent of UGIBs and 28% of LGIBs were hospitalized; the majority of other cases were referred only (eight UGIBs and three LGIBs died at home before any referral or hospitalization). Case-fatality rates were 6.9% (128/1843) for UGIB and 0.9% (25/2763) for LGIB (case-fatality rates among hospitalized cases only were 8.1% [90/1106] for UGIB and 2.2% [17/771] for LGIB). Among UGIB cases, 66.2% had the reason for the bleed recorded; of these, 45.2% were due to mucosal erosion, 30.3% were due to a gastric ulcer and 24.5% were due to a duodenal ulcer. For LGIB, 53.7% of cases had the reason for the bleed recorded and, among these the reason was a diverticular disease in 80.1% and polyps in the remaining cases.
Comorbidities and other patient characteristics, and risk of UGIB/LGIB
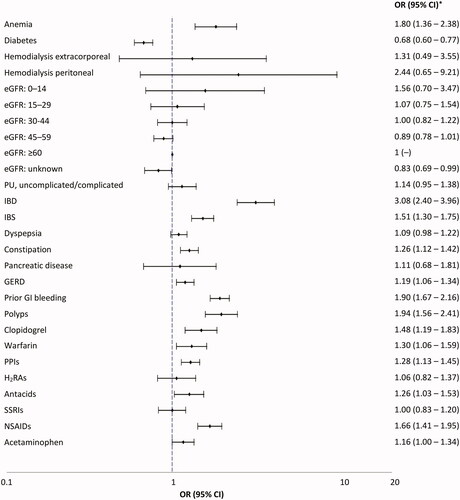
Associations between patient comorbidities and medication use and the risk of UGIB and LGIB are shown in and , respectively. Haemodialysis extracorporeal was the strongest risk factor for UGIB; adjusted OR: 6.04 (95% CI: 1.84–19.82). Antecedents of peptic ulcer carried an adjusted OR of 2.36 (95% CI: 1.90–2.93). The main risk factor for LGIB was inflammatory bowel disease conferring a three-fold increased risk; adjusted OR: 3.08 (95% CI: 2.40–3.96). Patients taking clopidogrel, warfarin and non-steroidal-anti-inflammatory drugs had an increased risk of both UGIB and LGIB. Among patients with an international normalized ratio ≥3, a ∼ five-fold increased risk of UGIB (adjusted OR: 5.67, 95% CI: 2.82–11.39) and ∼3-fold risk of LGIB (adjusted OR: 2.84, 95% CI: 1.64–4.94) was seen. There were too few patients with a prescription for other oral anticoagulants to provide any meaningful results; only 10 UGIB cases and 9 controls, and 11 LGIB cases and 12 controls were current or recent users of these medications. The adjusted ORs among patients currently taking proton pump inhibitors (PPIs) were 1.21, 95% CI: 1.04–1.41 for UGIB and 1.28, 95% CI: 1.13–1.45 for LGIB, using no previous PPI use as the reference group. When distant PPI use (PPI use discontinued >1 year before the index date) was used as the reference group (as a more suitable reference group to minimize confounding by indication), short-term current PPI use (≤3 months) was associated with an increased risk of UGIB (OR: 2.22, 95% CI: 1.68–2.94) and to a lesser extent of LGIB (OR: 1.38, 95% CI: 1.09–1.74). Use of PPI for ≥3 months reduced the risk of UGIB by 21% (OR: 0.79, 95% CI: 0.65–0.96) but had no effect on LGIB risk (OR: 1.07, 95% CI: 0.93–1.24). No major associations were seen between patient demographics, lifestyle factors, healthcare use or polypharmacy and the risk of UGIB/LGIB (data not shown).
Figure 2. Odds ratio (95% CI) for the associations between morbidities and medications and the risk of UGIB (nested case-control analysis). CI: confidence interval; eGFR: estimated glomerular filtration rate; GERD: gastro-oesophageal reflux disease; H2RA: histamine H2-receptor antagonist; IBD: inflammatory bowel disease; IBS: irritable bowel syndrome; lower gastrointestinal bleed; OR: odds ratio; NSAID: non-steroidal anti-inflammatory drugs; PPI: proton pump inhibitors; PU: peptic ulcer; SSRI: selective serotonin reuptake inhibitors; relative risk; UGIB: upper gastrointestinal bleed. *Adjusted by age, sex, calendar year, number of PCP visits in the year before the index date, smoking, alcohol consumption, prior UGIB, prior LGIB, prior GIB unspecified, pancreatic disease, uncomplicated peptic ulcer, polypharmacy, use of NSAIDs, PPIs, clopidogrel and warfarin. Peptic ulcer complicated were events that presented with haematemesis and/or perforation, unlike peptic ulcer uncomplicated events.
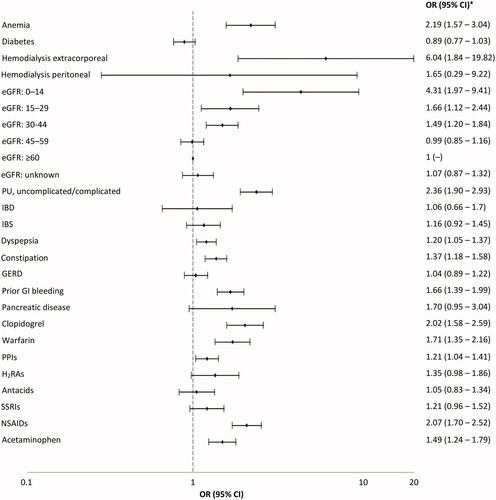
Figure 3. Odds ratio (95% CI) for the associations between morbidities and medications and the risk of LGIB (nested case-control analysis). CI: confidence interval; eGFR: estimated glomerular filtration rate; GERD: gastro-oesophageal reflux disease; H2RA: histamine H2-receptor antagonist; IBD: inflammatory bowel disease; IBS: irritable bowel syndrome; lower gastrointestinal bleed; OR: odds ratio; NSAID: non-steroidal anti-inflammatory drugs; PPI: proton pump inhibitors; PU: peptic ulcer; SSRI: selective serotonin reuptake inhibitors; relative risk; UGIB: upper gastrointestinal bleed. *Adjusted by age, sex, calendar year, number of PCP visits in the year before the index date, smoking, alcohol consumption, BMI, history of polyps, history of LGIB, history of unspecified GIB, PU diseases (complicated and uncomplicated), GERD, IBD, IBS, polypharmacy, use of NSAIDs, PPIs, clopidogrel and warfarin and low-dose aspirin. Peptic ulcer complicated were events that presented with haematemesis and/or perforation, unlike peptic ulcer uncomplicated events.
Low-dose aspirin and risk of UGIB/LGIB
Recency, duration and dose of low-dose aspirin
Adjusted ORs (95% CI) for the association between UGIB/LGIB and low-dose aspirin by recency, duration and dose are shown in and , respectively. Compared with non-use of low-dose aspirin, current low-dose aspirin use was associated with an increased risk of both UGIB (adjusted OR: 1.53, 95% CI: 1.34–1.75) and LGIB risk (adjusted OR: 1.63, 95% CI: 1.47–1.81). The risk of LGIB was constant over the duration of low-dose aspirin therapy, whereas UGIB risk was slightly higher in the first 3 months following the start of therapy (adjusted ORs: 1.89, 95% CI: 1.51–2.37 for <3 months and 1.47, 95% CI: 1.29–1.69 for ≥3 months). No dose-response relationship was seen between current use of low-dose aspirin and LGIB for the aspirin doses evaluated (75 mg–300 mg per day); however, the results were suggestive of a dose-response relationship for UGIB; adjusted ORs were 1.50, 95% CI: 1.31–1.71 for 75 mg/day and 2.02, 95% CI: 1.47–2.78 for >75 mg/day.
Table 1. ORs (95% CI) for the association between recency, dose and duration of low-dose aspirin and risk of UGIB.
Table 2. RRs (95% CI) for the association between recency, dose and duration of low-dose aspirin and risk of LGIB.
Primary or secondary CVD prevention population
As shown in , for UGIB, the ORs for current low-dose aspirin use were 1.16 (95% CI: 0.89–1.50) among individuals with ischaemic vascular antecedents and 1.62 (95% CI: 1.38–1.90) among those without ischaemic vascular antecedents; while the latter estimate is higher, the CIs overlap suggesting no significant difference between the two estimates. For LGIB, there was no evidence for a significant difference in low-dose aspirin associated risks between these two patient populations; 1.67 (95% CI: 1.34–2.09) and 1.60 (95% CI: 1.42–1.81) for those with and without ischaemic vascular antecedents, respectively.
Figure 4. Odds ratio (95% CI) for the association between current use of low-dose aspirin (current use ≤30 days vs. non-use [no use in the 365 days before the index date]) and risk of UGIB/LGIB stratified by primary/secondary CVD prevention population. CI: confidence interval; CVD: cardiovascular; LGIB: lower gastrointestinal bleed; OR: odds ratio; UGIB: upper gastrointestinal.
![Figure 4. Odds ratio (95% CI) for the association between current use of low-dose aspirin (current use ≤30 days vs. non-use [no use in the 365 days before the index date]) and risk of UGIB/LGIB stratified by primary/secondary CVD prevention population. CI: confidence interval; CVD: cardiovascular; LGIB: lower gastrointestinal bleed; OR: odds ratio; UGIB: upper gastrointestinal.](/cms/asset/9bc8ff2b-c6f4-4448-be9e-4a2ea1cc94cf/iann_a_1591635_f0004_b.jpg)
Dual antiplatelet therapy
For both UGIB and LGIB, the use of dual antiplatelet therapy (DAT) carried a greater risk than the sum of each antiplatelet used in monotherapy. An approximate four-fold increased the risk of UGIB (adjusted OR: 3.69, 95% CI: 2.59–5.26) and LGIB (adjusted OR: 3.59, 95% CI: 2.65–4.85) was seen among current users of DAT with low-dose aspirin and clopidogrel compared with non-users of either medication. Greater risks of UGIB and LGIB were also seen when low-dose aspirin was used concomitantly with warfarin; adjusted ORs: 3.22, 95% CI: 1.93–5.39 for UGIB and 3.11, 95% CI: 2.02–4.79, respectively.
Case-fatality and level of healthcare assistance
Associations between current use of low-dose aspirin and the risk of UGIB/LGIB by case-fatality and level of healthcare assistance are shown in . Compared with non-use, there was no evidence of an association between current use of low-dose aspirin and risk of fatal UGIB or fatal LGIB, but increased risks of non-fatal UGIB and LGIB were seen; adjusted ORs: 1.62, 95% CI: 1.42–1.86 for non-fatal UGIB and 1.63, 95% CI: 1.47–1.81 for non-fatal LGIB. Similar ORs were seen for hospitalized and referred-only cases of UGIB and only a small difference in risk was seen between hospitalized and referred cases of LGIB.
Figure 5. Odds ratio (95% CI) for the association between current use of low-dose aspirin (vs. non-use [no use in the 365 days before the index date]) and risk of (A) UGIB, and (B) LGIB, by case-fatality and level of healthcare assistance. CI: confidence interval; LGIB: lower gastrointestinal bleed; OR: odds ratio; UGIB: upper gastrointestinal bleed.
![Figure 5. Odds ratio (95% CI) for the association between current use of low-dose aspirin (vs. non-use [no use in the 365 days before the index date]) and risk of (A) UGIB, and (B) LGIB, by case-fatality and level of healthcare assistance. CI: confidence interval; LGIB: lower gastrointestinal bleed; OR: odds ratio; UGIB: upper gastrointestinal bleed.](/cms/asset/9190c505-c133-464b-8349-14c2e43db273/iann_a_1591635_f0005_b.jpg)
Bleed location
For UGIB, the increased risk was slightly higher for duodenal ulcers (adjusted RR: 2.37, 95% CI: 1.77–3.17) than for gastric ulcers (adjusted OR: 2.00, 95% CI: 1.54–2.60) or mucosal erosions (adjusted OR: 1.35, 95% CI: 1.09–1.66). For LGIB, the increased risk was higher for polyps (adjusted OR: 1.87, 95% CI: 1.41–2.48) than for diverticular diseases (adjusted OR: 1.76, 95% CI: 1.53–2.04).
Discussion
To our knowledge, this is the first large population-based study to provide estimates of low-dose aspirin-associated UGIB and LGIB risk in a routine primary care setting by various characteristics of the bleed – fatality, hospitalization and bleed location – and according to primary and secondary CVD prevention populations. In the latter population, we found low-dose aspirin to be associated with a 60% increased risk of LGIB but only a minor non-significant increase in UGIB risk. The Antithrombotic Trialists’ Collaboration’s (ATC) meta-analyses of secondary prevention RCT data (mostly involving low-dose aspirin) clearly showed aspirin to be associated with a 60% increased risk of major extracranial bleeds (mostly gastrointestinal) when used for secondary CVD prevention [Citation23]; however, separate risk estimates by upper/lower bleed location were not reported, which hinders comparisons. In our primary CVD prevention population, low-dose aspirin was associated with a 60% increased risk of both UGIB and LGIB. This estimate is very close to the 58% increased risk of major GI bleeding reported from the US Preventive Task Force’s (USPTF) meta-analysis of data from both primary prevention RCTs and cohort studies comparing placebo with no treatment to prevent CVD or cancer in adults [Citation13]. This increase in bleeding risk was not, however, deemed by the USPTF to outweigh the benefits of low-dose aspirin in reducing the risk of CVD and CRC in a large subgroup of middle-aged adults, and they currently recommend low-dose aspirin initiation for the primary prevention of CVD and CRC in adults aged 50–59 years who have a 10% or greater CVD risk, who are not at increased risk for bleeding and have a life expectancy of at least 10 years [Citation8]. Our estimate of UGIB risk in primary CVD prevention is also in line with that reported by the ATC meta-analysis of primary CVD prevention RCT data (54% increased risk of GI bleeding) [Citation1]. Our estimate of aspirin-associated UGIB in primary CVD prevention is also broadly comparable with those from ASCEND in diabetics (29% increased risk of major bleeding [mostly GI]) [Citation9] and ASPREE in the healthy elderly (87% increased risk of UGIB [Citation10]. In ARRIVE, a two-fold increased risk of GI bleeding (mostly minor events) was reported among study participants, all of whom were recruited based on being at moderate CVD risk but who were deemed by study investigators as more representative of a low-risk population due to the low event rates seen [Citation11]. The differences in GI bleeding definitions in these trials, along with the differences in trial design and study population highlight the value of our estimates of GI bleeding among patients in a routine clinical care setting, which cover GI bleeds of all severity in both the upper and lower GI tract.
In our overall study population, low-dose aspirin was associated with a 60% increased risk of both non-fatal UGIB and non-fatal LGIB, but no significantly increased risk of fatal cases of UGIB and LGIB. Analyses by the level of healthcare assistance showed that the increased risk of UGIB and LGIB with low-dose aspirin were similar for hospitalized (more serious cases) and referred only (less serious) cases of both. Aspirin-associated risks of hospitalized or emergency-room attended LGIB have been reported in other studies. Using a nationwide health insurance research database, Chen et al. [Citation24] reported a relative risk of 2.75 among 1-year low-dose aspirin users vs. non-users in the Taiwanese general population. A hospital-based case–control study in Spain [Citation19] found a 40% increased risk of LGIB associated with low-dose aspirin use, based on 415 cases. Among observational cohorts of US health professionals free from GI antecedents, a two-fold increased risk of diverticular bleeding risk in men [Citation25] and a 21% increase in LGIB risk in women [Citation26] have been reported.
The risk of LGIB in our study appeared to be constant over the duration of low-dose aspirin use, as found by others [Citation25,Citation26], whereas, for UGIB, the risk was slightly higher in the first 3 months following start of therapy and decreased thereafter, a pattern previously seen for UGIB in a similar UK primary care database to THIN [Citation27] and in the hospital-based case-control study in Spain [Citation28]. A similar pattern for low-dose aspirin has also been described previously for peptic ulcer bleeding in a case-control study in the UK using both hospital and community controls [Citation28]. Our results were also suggestive of a dose-response effect of low-dose aspirin on UGIB risk within the 75 mg–300 mg/day range. Point estimates from previous studies from the UK [Citation29], US [Citation25,Citation26], and Spain [Citation28] also suggest a possible increased UGIB risk with higher aspirin dose, while no apparent association has been found in other studies [Citation27,Citation30–32].
A main strength of this study is the population-based sample of matched low-dose aspirin users and non-users at the start of follow-up, which helped reduce confounding from imbalances between aspirin first-time users and non-users in factors relating to GI bleeding risk that may not otherwise have been sufficiently controlled. The study sample included low-dose aspirin new users and non-users from the UK general population, covering the broad spectrum of preventative aspirin users – whether using the drug for primary or secondary CVD prevention purposes. This included individuals with multiple morbidities, including GI antecedents, and those taking other medications related to GI bleeding risk, which is important, because in routine practice these represent the range of patients for whom decisions whether to prescribe preventative low-dose aspirin are made.
Other strengths include the large sample size enabling the calculation of precise risk estimates, the inclusion of only new users of low-dose aspirin thereby minimizing survivor bias [Citation33], and the validation of recorded UGIB/LGIB diagnoses through a review of both THIN medical records (including free text) and linked hospitalized records. By analyzing actual use of low-dose aspirin and all other drugs in the time period relative to the index date we accounted for potential changes in exposure over the follow-up period and reduced potential bias arising from misclassification of low-dose aspirin exposure. Any misclassification of low-dose aspirin exposure that may have still been present, for example, because we cannot be sure whether individuals always took their medication, would have biased the observed associations towards the null. Confounding due to differences between low-dose aspirin users and non-users at start of the study that are difficult to control for was minimized by the 1:1 matching by PCP visits in the year before start of follow-up as a proxy measure for general health status, and other confounders were adjusted for in the analysis. However, we acknowledge that residual confounding, including from unknown confounders, cannot be ruled out. Another limitation of our study is that we did not have access to all clinical information regarding the GI bleeding event (e.g. aetiology, results of endoscopy reports), because these data are not systematically recorded in THIN and as such were not necessarily recorded for all cases. Therefore, there could be some ascertainment bias in that some true cases may not have been “confirmed” based on the available information, yet this is likely to have been non-differential between low-dose aspirin users and non-users leading to risk estimates biased towards the null.
In a similarly designed study in THIN, we have previously investigated the relationship between the use of low-dose aspirin and risk of intracranial bleeding [Citation15]. The risk estimates of UGIB and LGIB in this current study, which cover the whole spectrum of GI bleeds, will further build upon the existing safety profile of preventative low-dose aspirin and aid key clinical decision regarding its use in routine clinical practice. The impact of these bleeding events in terms of their relationship to longer-term care needs and healthcare costs would be a valuable line of investigation for future research, and would further help guide prescribing decisions.
Supplemental Material
Download Zip (383.2 KB)Acknowledgements
We thank EpiMed Communications Ltd (Oxford, UK) for medical writing assistance funded by Bayer AG.
Disclosure statement
LCS and LAGR work for CEIFE, which has received research funding from Bayer AG. LAGR has received honoraria for serving on advisory boards for Bayer AG. AL has previously received a research grant from Bayer AG and has served as an advisory board member for Bayer AG and Bayer HealthCare. MS-G is a full-time employee of Bayer AG.
Data availability
The data supporting the results of this study are helped by the corresponding author.
Additional information
Funding
References
- Baigent C, Blackwell L, Collins R. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849–1860.
- Roffi M, Patrono C, Collet JP. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: task force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:267–315.
- Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC Guideline for the Management of Patients with Non-ST-Elevation Acute Coronary Syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64:e139–e228.
- Rothwell PM, Wilson M, Elwin CE, et al. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet. 2010;376:1741–1750.
- Garcia Rodriguez LA, Soriano-Gabarro M, Bromley S, et al. New use of low-dose aspirin and risk of colorectal cancer by stage at diagnosis: a nested case-control study in UK general practice. BMC Cancer. 2017;17:637.
- Vandvik PO, Lincoff AM, Gore JM, et al. Primary and secondary prevention of cardiovascular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e637S–e668S.
- Halvorsen S, Andreotti F, ten Berg JM, et al. Aspirin therapy in primary cardiovascular disease prevention: a position paper of the European Society of Cardiology working group on thrombosis. J Am Coll Cardiol. 2014;64:319–327.
- Bibbins-Domingo K. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: U.S. preventive services task force recommendation statement. Ann Intern Med. 2016;164:836–845.
- Bowman L, Mafham M, Wallendszus K, et al. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018;379:1529–1539.
- McNeil JJ, Wolfe R, Woods RL, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. 2018;379:1509–1518.
- Gaziano JM, Brotons C, Coppolecchia R, et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, double-blind, placebo-controlled trial. Lancet. 2018;392:1036–1046.
- Pignone M, DeWalt DA. More evidence to help guide decision making about aspirin for primary prevention. Ann Intern Med. 2018;169:804–805.
- Whitlock EP, Burda BU, Williams SB, et al. Bleeding risks with aspirin use for primary prevention in adults: a systematic review for the U.S. preventive services task force. Ann Intern Med. 2016;164:826–835.
- Elwood PC, Morgan G, Galante J, et al. Systematic review and meta-analysis of randomised trials to ascertain fatal gastrointestinal bleeding events attributable to preventive low-dose aspirin: no evidence of increased risk. PLoS One. 2016;11:e0166166.
- Cea Soriano L, Gaist D, Soriano-Gabarró M, et al. Low-dose aspirin and risk of intracranial bleeds: an observational study in UK general practice. Neurology. 2017;89:2280–2287.
- Cea Soriano L, Lanas A, Soriano-Gabarró M, et al. Incidence of upper and lower gastrointestinal bleeding in new users of low-dose aspirin. Clin Gastroenterol Hepatol. 2019;17:887–895.e6.
- García Rodríguez LA, Barreales Tolosa L. Risk of upper gastrointestinal complications among users of traditional NSAIDs and COXIBs in the general population. Gastroenterology. 2007;132:498–506.
- Margulis AV, García Rodríguez LA, Hernández-Díaz S. Positive predictive value of computerized medical records for uncomplicated and complicated upper gastrointestinal ulcer. Pharmacoepidem Drug Safe. 2009;18:900–909.
- González-Pérez A, Sáez ME, Johansson S, et al. Risk of bleeding after hospitalization for a serious coronary event: a retrospective cohort study with nested case-control analyses. BMC Cardiovasc Disord. 2016;16:164.
- Rodríguez L, Johansson S, Soriano LC. Use of clopidogrel and proton pump inhibitors after a serious acute coronary event: risk of coronary events and peptic ulcer bleeding. Thromb Haemost. 2013;110:1014–1024.
- Cea Soriano L, Soriano-Gabarró M, García Rodríguez LA. Validation of low-dose aspirin prescription data in The Health Improvement Network: how much misclassification due to over-the-counter use? Pharmacoepidemiol Drug Saf. 2016;25:392–398.
- Pearce N. What does the odds ratio estimate in a case-control study? Int J Epidemiol. 1993;22:1189–1192.
- Antithrombotic Trialists' Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71–86.
- Chen W-C, Lin K-H, Huang Y-T, et al. The risk of lower gastrointestinal bleeding in low-dose aspirin users. Aliment Pharmacol Ther. 2017;45:1542–1550.
- Huang ES, Strate LL, Ho WW, et al. A prospective study of aspirin use and the risk of gastrointestinal bleeding in men. PLoS One. 2010;5:e15721.
- Huang ES, Strate LL, Ho WW, et al. Long-term use of aspirin and the risk of gastrointestinal bleeding. Am J Med. 2011;124:426–433.
- de Abajo FJ, Garcia Rodriguez LA. Risk of upper gastrointestinal bleeding and perforation associated with low-dose aspirin as plain and enteric-coated formulations. BMC Clin Pharmacol. 2001;1:1.
- Lanas A, Garcia-Rodriguez LA, Arroyo MT, et al. Risk of upper gastrointestinal ulcer bleeding associated with selective cyclo-oxygenase-2 inhibitors, traditional non-aspirin non-steroidal anti-inflammatory drugs, aspirin and combinations. Gut. 2006;55:1731–1738.
- Cea Soriano L, Rodriguez LA. Risk of upper gastrointestinal bleeding in a cohort of new users of low-dose ASA for secondary prevention of cardiovascular outcomes. Front Pharmacol. 2010;1:126.
- Sorensen HT, Mellemkjaer L, Blot WJ, et al. Risk of upper gastrointestinal bleeding associated with use of low-dose aspirin. Am J Gastroenterology. 2000;95:2218–2224.
- Ibanez L, Vidal X, Vendrell L, et al. Upper gastrointestinal bleeding associated with antiplatelet drugs. Aliment Pharmacol Ther. 2006;23:235–242.
- Garcia Rodriguez LA, Lin KJ, Hernandez-Diaz S, et al. Risk of upper gastrointestinal bleeding with low-dose acetylsalicylic acid alone and in combination with clopidogrel and other medications. Circulation. 2011;123:1108–1115.
- Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol. 2003;158:915–920.
