Abstract
Aims
To quantify the incidence and prevalence of heart failure (HF) in persons with type 2 diabetes (T2DM) and to examine the 1-year survival after the diagnosis of HF.
Materials and methods
All cases of HF (n = 295,990) and T2DM in Finland were identified from national electronic health care registers for the period 1996–2012. The annual incidence and prevalence rates of HF and 1-year survival after the first diagnosis of HF were calculated for persons with T2DM and without diabetes using Poisson regression for the event rates.
Results
The age-adjusted rate ratio for incident HF among men with T2DM in the age group 35–74 years declined from 3.73 (95% CI, 3.46–4.02) in 1996 to 2.17 (2.04–2.31) in 2012 and among women from 3.90 (3.61–4.22) to 2.36 (2.16–2.58). The multivariate-adjusted hazard ratio of 1-year death after the diagnosis of HF declined from 1.15 (1.11–1.21) to 1.07 (1.05–1.10) from the first to the second half of the study period.
Conclusions
Individuals aged <75 years with T2DM had a considerably higher incidence of HF than individuals without diabetes. The prognosis of HF was worse in individuals with T2DM than in individuals without diabetes. However, the gap between the groups had narrowed over time.
The incidence of heart failure is 2–3 times higher among patients under 75 years of age with type 2 diabetes than among individuals without diabetes.
The prognosis of heart failure patients is worse among patients with type 2 diabetes than it is among patients without diabetes although it is improving.
Key messages
Introduction
The incidence of heart failure (HF) is 350–1000 a year per 100,000 persons [Citation1–3]. The prevalence of HF is 1000–2000 per 100,000 persons, the numbers increasing with age, being over 10,000 per 100,000 persons in the general population of western countries among people over 70 years of age [Citation1]. HF is hazardous to the quality of life, has high mortality and bears a major burden to health care [Citation4–6]. This has led to extensive trials to improve the prognosis of patients [Citation7]. Reports show varying results with no improvement or only a little improvement both in the prevention of HF [Citation8] and prognosis of HF patients [Citation8,Citation9]. Two recent Scandinavian register studies have shown increasing trends in the incidence HF among young people [Citation3,Citation10].
Diabetes mellitus has strong adverse effects on human body causing complications, often with a fatal outcome. HF is among the most serious. The risk for developing HF is about 2-fold among persons with diabetes compared to persons without diabetes [Citation11]. It is estimated that 12% of HF can be explained by diabetes [Citation12]. The main risk factors that can lead to HF are hypertension and coronary disease (CHD) which both are common among patients with diabetes. The hazards in coexistence of T2DM and HF have recently been pointed out [Citation13].
Information on the changes in incidence, prevalence and survival of HF among persons with diabetes is scarce. Recent clinical trials suggest, however, that new classes of medications are likely to be effective in the prevention and treatment of HF in individuals with diabetes [Citation14]. Full picture of the burden of HF in patients with diabetes is needed for evidence-based assessment of the potential benefits and limitations of these new medications. Our aim in this study was to examine the trends in the occurrence and survival of HF in patients with type 2 diabetes (T2DM) and to compare them with the corresponding trends in individuals without diabetes using electronic health care registers that cover the whole population in Finland.
Materials and methods
Country-wide health care registers and determination of diabetes
We executed a register study using data from the drug reimbursement registers of the Social Insurance Institution of Finland, National Hospital Discharge Register (NHDR) and Causes of Death Register of Statistics Finland (CDR). Information from these registers can be linked together at the individual level using the personal ID code, which is unique for each permanent resident of Finland.
The incidence of diabetes was determined by the use of hypoglycaemic medication (reimbursement registers) or diabetes diagnosis in the NHDR or in the CDR. The drug reimbursement register covers all medications prescribed by a doctor and the NHDR covers all hospitalizations in the country, including ambulatory visits to specialized care. Identification of individuals with diabetes made it possible to construct a diabetes register, which includes all people with diagnosed and treated diabetes in the country. Our register-based data missed, however, the major part of patients with diabetes treated in ambulatory care without hypoglycaemic medication (about 10%).
A proxy variable for the type of diabetes was determined as follows: type 1 diabetes (T1DM) included all those who were under 30 years of age and treated with insulin only or in combination with metformin, all persons aged 30–40 years when insulin only was started and persons with diabetes under 30 years of age in chronic institutional care if there was no information about hypoglycaemic medication used or hospital discharge diagnoses were not unambiguous. All other persons with diabetes were considered to have T2DM. Patients with gestational diabetes or T1DM were excluded from the study. The incidence and prevalence figures of T2DM for the year 2012 were extrapolated from the figures of previous 5 years.
Heart failure, prevalence, incidence and survival
HF was diagnosed in two ways. First, the diagnostic code (any of the ICD-10-codes I11.0, I13.0, I13.2, I50, or ICD-9-codes 4029B, 4148, 428 or ICD-8-codes 42700, 42710, 428, 78240) in any position in the NHDR including the outpatient visits (also in the emergency units) to the hospitals or CDR, and second, the right to specially reimbursed medicines for HF, or at least three purchases of furosemide and no other obvious reason for furosemide use such as chronic kidney disease. The validity of this approach to HF diagnoses has been shown earlier [Citation15]. The validation study showed that the register-based HF diagnosis had the specificity of 99.7%, positive predictive value 85.9%, negative predictive value 97.9% and sensitivity 48.5%. The ICD-codes listed above were also used for analysing causes of death. The first mentioning of diabetes or HF in any of the registers was considered as the date of onset of the disease.
We compared the annual incidence and prevalence rates of HF separately for men and women in three age groups, 35–64, 65–74 and 75–94 years for a 17-year study period in 1996–2012. Finally, we examined the 1-year survival in HF.
Statistical methods
Age-specific annual denominators for the incidence and prevalence rates among people with T2DM were obtained from our diabetes register. The denominators for populations without diabetes were obtained from the Finnish National Population Information System by subtracting the numbers of individuals with diabetes from the total population count.
Age-adjusted rate ratios for the incidence of HF among T2DM patients compared to population without diabetes were computed by gender. Age-adjustment was done also within the age groups to allow secular comparisons. The average ages in HF incidence were compared in the two populations. The trend estimates were computed using Poisson regression for the logarithm of the age-stratified and within-strata age-adjusted event rate and the study year as the explanatory variable. The comparison of 1-year survival of individuals with T2DM and without diabetes after the first diagnosis of HF was performed using multivariate Cox proportional hazards regression for the first and the second half of the study period. Proportional hazard assumptions were evaluated graphically through plotting the Schoenfeld residuals and no strong evidence against proportionality was obtained.
As covariates in survival analyses we used age, sex, valvular diseases (ICD-10-codes I05-08, I34-37, I39.0-39.4 and ICD-9-codes 394*, 395*, 396*, 4240*, 4241*, 4243*), history of myocardial infarction, CHD and cardiomyopathy (ICD-10-codes I21-23, I25, I42-43 and ICD-9-codes 410*, 411*, 413*, 414*,425*), and hypertension (ICD-10-codes I10-15 and ICD-9-codes 401-405) or special reimbursement for medicines to lower blood pressure.
Ethics
Our study was approved by the ethics committee of the National Institute for Health and Welfare.
Results
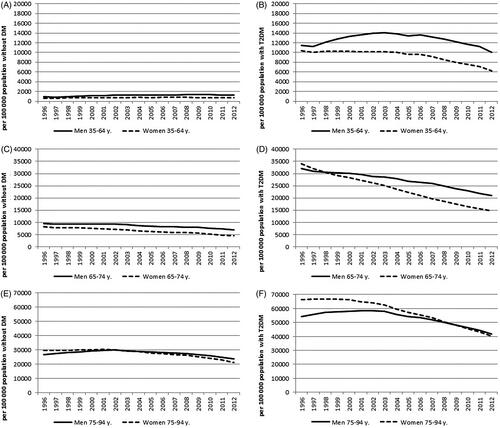
We identified 295,990 cases of incident HF during the study period, of them, 70,045 (24%) had T2DM before HF. The rest (76%) of HF cases occurred in individuals without diabetes. In the last study year 2012, 36% of all HF cases were observed in patients with T2DM and 64% in individuals without diabetes (Appendix 1). For comparison, it should be mentioned that 43,905 (27%) of the HF patients had a history of hypertension and 22,946 (14%) a history of myocardial infarction, CHD or cardiomyopathy. In 2012, the incidence of HF was 1200 and prevalence 17,500 per 100,000 persons among patients with T2DM. A decline was observed in the incidence and prevalence of HF in both genders and all three age groups among persons with T2DM ( and , Appendix 1). The incidence rates were lower among women than men with T2DM throughout the study period.
Figure 1. The incidence of heart failure during 1996–2012 in the population without diabetes (DM) in the age groups 35–64 years (A), 65–74 years (C) and 75–94 years (E) and among persons with type 2 diabetes (T2DM) in the age groups 35–64 years (B), 65–74 years (D) and 75–94 years (F).
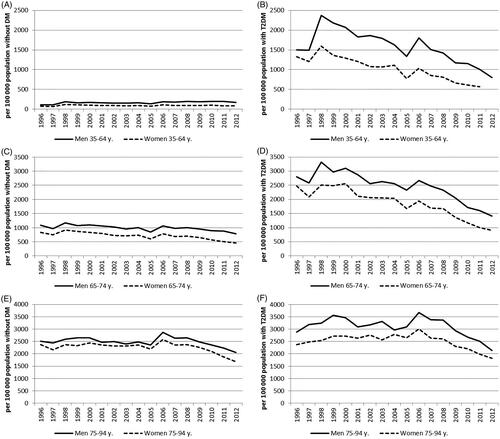
Figure 2. The prevalence of heart failure during 1996–2012 in the population without diabetes (DM) in the age groups 35–64 years (A), 65–74 years (C) and 75–94 years (E) and among persons with type 2 diabetes (T2DM) in the age groups 35–64 years (B), 65–74 years (D) and 75–94 years (F).
The age-adjusted incidence rate ratios of HF comparing persons with T2DM with persons without diabetes were significantly elevated in both genders and all three age groups. Especially elevated rate ratios were seen in the younger age groups. The rate ratios declined significantly during our observation period in the younger age groups of men and women, but not in the older age group. The rate ratio for men in the age group 35–74 declined from 3.73 (95% CI, 3.46–4.02) in 1996 to 2.17 (2.04–2.31) in 2012 and for women from 3.90 (3.61–4.22) to 2.36 (2.16–2.58) (). The rate ratio for the three last study years (2010–2012) was 3.18 (2.91–3.47) for men and 3.67 (3.20–4.22) for women in the age group 35–64 years. The corresponding figures for the age group 65–74 were 1.81 (1.66–1.97) and 1.59 (1.43–1.78).
Table 1. The age adjusted rate ratios (with 95% confidence intervals) of heart failure incidence from the years 1996–1998 to the years 2010–2012Table Footnote* among persons with type 2 diabetes compared to persons without diabetes in the population of Finland.
We observed an increase in the duration from the diagnosis of T2DM to the first diagnosis of HF. The mean HF-free duration rose from the first 3 years of the study period (1996–1998) to the last 3 years (2010–2012) in the total T2DM population from 8.3 to 8.9 years, for women from 8.0 to 9.2 (p<.0001, adjusted for age) and for men from 8.5 to 8.8 (p=.032, adjusted for age).
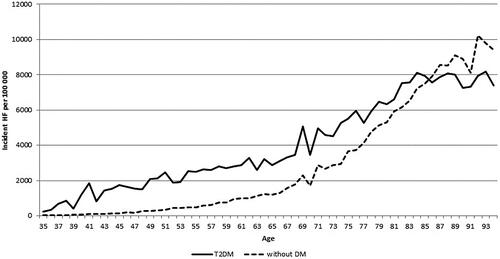
The incidence of HF was clearly higher among patients with T2DM until the age of 83 where the incidence among patients with T2DM levelled off but continued to grow among persons without diabetes (). The incidence of HF was approximately 4000 per year at the age of 70 years and rose to 8000 per 100,000 persons at the age of 83 years among persons with T2DM. Among persons without diabetes, the corresponding incidence figures were 2000 and 7000.
Figure 3. The incidence of heart failure (HF) by age among persons with type 2 diabetes (T2DM) and persons without diabetes (DM) during 1996–2012 in Finland.
The trends in the incidence and prevalence of HF showed a significant decline among patients with T2DM in both age groups (35–74 and 75–94) and both genders, but not among men without DM in the younger age group which had a significant increase instead ().
Table 2. The trends with 95% confidence intervals (CI) in the age adjusted incidence and prevalence of heart failure (HF) among men and women with type 2 diabetes (T2DM) and without diabetes (DM) during the period 1996–2012 in Finland.
In the 1-year follow-up after the diagnosis of HF, the presence of T2DM before HF was associated with significantly increased risk of death compared to HF patients without diabetes (). The adjusted hazard ratio declined from 1.15 (95% CI, 1.11–1.21) during the first half of the study period to 1.07 (1.05–1.10) during the second half. No other covariates, except the age, influenced the hazard ratios.
Table 3. The 1-year survival with heart failure of persons with type 2 diabetes compared to persons without diabetes, hazard ratios (HR) with 95% confidence intervals (CI) during the periods 1996–2003 and 2004–2012 in Finland.
Discussion
The main finding of our country-wide study was a very high incidence and prevalence of HF among persons with T2DM, especially those aged under 75 years compared to persons of similar age without diabetes. The age-adjusted rate ratios declined by increasing age and also over time. However, the risk for HF was still more than 3–4 times higher for persons with T2DM than for persons without diabetes towards the end of the study period in the youngest age group (35–64 years of age).
We showed a significant decline in the incidence and prevalence of HF among persons with T2DM. Among the youngest age-group of men with T2DM, the decline in the prevalence started only in the middle of the study period. The declining trends were weaker among women without diabetes than among women with T2DM. Men aged 35–64 years without diabetes had a slight increase in the incidence and prevalence of HF. Another interesting finding was that during the study period, the time from the diagnosis of T2DM to incident HF increased although the diagnostic tests for HF have become more sensitive. The increase in the average age of the study population did not explain this but the earlier diagnosis of T2DM can play a role.
The yearly decline in the incidence of HF among men and women with T2DM was highly significant. Also, in the prevalence of HF, the decline was faster in both age groups and both genders among persons with T2DM than among persons without diabetes. There was a 1–2% yearly decline among men and 3–4% yearly decline among women with T2DM. The rapid increase in the number of patients with T2DM together with the earlier diagnosis of T2DM interferes with the interpretation of results and may have caused overestimation of the positive development among patients with T2DM.
The stronger positive trends among individuals with T2DM compared to individuals without diabetes were also seen in the trends of survival after the diagnosis of incident HF. The adjusted hazard ratio for 1-year survival fell clearly from the first half of the observation period to the second half. However, the risk for fatal outcome remained higher among persons with T2DM.
Our findings suggest that the prevention of HF has been successful among patients with T2DM. The treatment of hypertension and hyperglycaemia, prevention of CHD by lowering of cholesterol and smoking cessation prevent HF. The benefit of treating hypertension has been shown in the UKPDS study [Citation16]. A reduction in HF hospitalizations was shown in a statin treatment study of CHD patients with diabetes [Citation17]. Higher glycated haemoglobin (HbA1c) levels increased the risk for HF in a cohort study [Citation18], but in controlled trials lowering of blood glucose did not reduce the risk of HF [Citation19]. The risk of hospitalization for HF was consistently higher among patients with diabetes than among controls irrespective of their risk factor levels [Citation20].
It is tempting to assume that the use of angiotensin converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) plays a role in the declining rates of HF, especially in patients with T2DM because they are widely used for treatment of hypertension and prevention of kidney disease. ACEI is also the dominating treatment of HF with reduced ejection fraction where the evidence of decreased mortality in randomized trials is shown [Citation21]. The use of ACEIs and ARBs in patients with T2DM can have a cardioprotective effect but also it may hide the early symptoms of HF.
It has been shown earlier that the treatment of risk factors among patients with T2DM has improved in Finland [Citation22]. That can give one explanation to the declining incidence of HF. Nevertheless, there is still space for improvement because differences in the incidence and prevalence of HF are big, especially among younger patients with T2DM compared to persons without diabetes.
HF is common among patients with diabetes [Citation11], but usually, only hypertension and CHD are considered as major risk factors for HF [Citation12,Citation23]. Our register study showed that T2DM should be considered as one of the major risk factors for HF, because 36% of incident HF patients had T2DM, 27% had hypertension and 14% had a history of myocardial infarction, CHD or cardiomyopathy. The burden of T2DM in causing HF has been shown also by other researchers [Citation20,Citation24,Citation25]. Although T2DM often co-occurs with other risk factors of HF, a notable proportion of HF patients do not have a history of other risk factors than T2DM. Consequently, the role of T2DM in the development of HF has probably been underestimated. We checked also the incidence of T2DM in the HF population after the incidence of HF and found 16,437 new cases of T2DM within 3 years after the diagnosis of HF. This is about the same incidence as in the general population, so it does not affect our conclusions.
It has been argued that HF is an increasing problem due to the ageing of population and better treatment possibilities of patients with myocardial infarction [Citation26]. There are studies which confirm this argument [Citation27] but also studies examining whether the HF epidemic could be prevented [Citation28]. Our study suggests that the unfavourable development could at least be slowed down.
There is evidence that the age- and sex-adjusted incidence of HF has declined in the general population [Citation2,Citation28]. Our study focused on patients with T2DM, but we also counted the results for the population without diabetes. In our study, the trends in the population without diabetes were not as good as they were in the T2DM population. We think that it is important to distinguish the trends by gender in populations with diabetes because diabetes is shown to be a major cardiovascular risk factor especially for women.
The big burden of HF comes from the increase in prevalence and repeated hospital visits [Citation24]. The frequency of hospitalizations among patients with HF has been higher and increasing more among women than among men. Hospitalizations may give an explanation as to why HF is considered more of a female problem. Our figures showed that the HF incidence was higher among men than women both in persons with T2DM and those without diabetes. However, when comparing gender differences in HF prevalence, it was higher only among men under the age of 75 with T2DM. There was no gender difference in the prevalence among people without diabetes or patients with T2DM aged 75 or more.
In our study, the extra burden caused by T2DM seemed to be heavy on the 1-year survival after the diagnosis of incident HF. Studies from Denmark and Sweden have earlier shown the adverse impact of diabetes on HF survival [Citation29,Citation30]. A benefit from sodium glucose cotransporter 2 inhibitors and possibly from some glucagon-like-peptide-1 receptor agonists [Citation14] is expected in treatment of HF patients with T2DM in the future.
There are strengths but also some limitations in our study. One of the strengths is that it covers a long period. The second strength is that it covers the whole population of the country with comprehensive hospitalization, causes of death and medication registers. Limitations are that the registers are not very sensitive for HF diagnoses although the specificity has been shown to be good [Citation15] and not comprehensive on ambulatory visits in specialised care and do not cover primary care visits. Patients with T2DM without hypoglycaemic medication and without hospitalizations were not included in our diabetes register. Furthermore, the age and treatment-based distinction between T1DM and T2DM is a proxy and minor random misclassification may have occurred. Other limitations were that we did not have information on the type of HF (maintenance of ejection fraction) and the lack of detailed risk factor data (like blood pressure and HbA1c) did not enable specific analyses on the aetiology of HF. Furthermore, the diagnostic practices of HF, such as the availability of cardiac ultrasound and measurements of natriuretic peptides, have changed over time, which may have improved the sensitivity and specificity of HF diagnoses. The likelihood of getting a HF diagnosis may also have been better among patients with T2DM because they are hospitalized more often due to diabetic complications.
In conclusion, individuals under 65 years of age with T2DM have 3–4-fold excess HF morbidity and those under 75 years of age have 2–3-fold excess compared to their counterparts without diabetes. Also, the prognosis of HF is worse in persons with T2DM than in individuals without diabetes. Our data suggest, however, that the gap between individuals with T2DM and individuals without diabetes has narrowed over time, probably due to improved treatment of hyperglycaemia and cardiovascular risk factors in T2DM.
Supplemental Material
Download MS Word (15.7 KB)Disclosure statement
KW has participated in a conference trip sponsored by Novo Nordisk and received honorariums for lectures from Novo Nordisk, Bristol-Myers-Squipp, AstraZeneca and Boehringer-Ingelheim and for an advisory board meeting from Sanofi. VS has participated in a conference trip sponsored by Novo Nordisk and received honorarium from the same source for participating in an advisory board meeting. He also has ongoing research collaboration with Bayer Ltd.
References
- Mosterd A, Hoes A. Clinical epidemiology of heart failure. Heart. 2007;93:1137–1146.
- Conrad N, Judge A, Tran J, et al. Temporal trends and patterns in heart failure incidence: a population-based study of 4 million individuals. Lancet. 2018;391:572–580.
- Christiansen MN, Kober L, Weeke P, et al. Age-specific trends in incidence, mortality, and comorbidities of heart failure in Denmark, 1995 to 2012. Circulation. 2017;135:1214–1223.
- Hobbs FD, Kenkre JE, Roalfe AK, et al. Impact of heart failure and left ventricular systolic dysfunction on quality of life: a cross-sectional study comparing common chronic cardiac and medical disorders and a representative adult population. Eur Heart J. 2002;23:1867–1876.
- Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics-2017 update: a report from the American heart association. Circulation. 2017;135:e146–e603.
- Cook C, Cole G, Asaria P, et al. The annual global economic burden of heart failure. Int J Cardiol. 2014;171:368–376.
- Fonarow GC, Albert NM, Curtis AB, et al. Improving evidence-based care for heart failure in outpatient cardiology practices: primary results of the Registry to Improve the Use of Evidence-Based Heart Failure Therapies in the Outpatient Setting (IMPROVE HF). Circulation. 2010;122:585–596.
- Levy D, Kenchaiah S, Larson MG, et al. Long-term trends in the incidence of and survival with heart failure. N Engl J Med. 2002;347:1397–1402.
- Nakano A, Johnsen SP, Frederiksen BL, et al. Trends in quality of care among patients with incident heart failure in Denmark 2003–2010: a nationwide cohort study. BMC Health Serv Res. 2013; 13:391–399.
- Barasa A, Schaufelberger M, Lappas G, et al. Heart failure in young adults: 20-year trends in hospitalization, aetiology, and case fatality in Sweden. Eur Heart J. 2014;35:25–32.
- He J, Ogden LG, Bazzano LA, et al. Risk factors for congestive heart failure in US men and women: NHANES. Arch Intern Med. 2001;161:996–1002.
- Echouffo-Tcheugui JB, Xu H, DeVore AD, at al. Temporal trends and factors associated with diabetes mellitus among patients hospitalized with heart failure: findings from get with the guidelines-heart failure registry. Am Heart J. 2016;182:9–20.
- Seferović PM, Petrie MC, Filippatos GS, et al. Type 2 diabetes mellitus and heart failure: a position statement from the heart failure association of the European society of cardiology. Eur J Heart Fail. 2018;20:853–872.
- Bassi N, Fonarow GC. Prevention of heart failure in patients with diabetes: role of diabetes medications. Curr Cardiol Rep. 2018; 20:112.
- Mähönen M, Jula A, Harald K, et al. The validity of heart failure diagnoses obtained from administrative registers. Eur J Prev Cardiolog. 2013;20:254–259.
- UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703–713.
- Shepherd J, Barter P, Carmena R, et al. Effect of lowering LDL cholesterol substantially below currently recommended levels in patients with coronary heart disease and diabetes: the Treating to New Targets (TNT) study. Diabetes Care. 2006;29:1220–1226.
- Iribarren C, Karter AJ, Go AS, et al. Glycemic control and heart failure among adult patients with diabetes. Circulation. 2001;103:2668–2673.
- Castagno D, Baird-Gunning J, Jhund PS, et al. Intensive glycemic control has no impact on the risk of heart failure in type 2 diabetic patients: evidence from a 37,229 patient meta-analysis. Am Heart J. 2011;162:938–948.
- Rawshani A, Rawshani A, Franzén S, et al. Risk factors, mortality, and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2018;379:633–644.
- Flather MD, Yusuf S, Køber L, et al. Long-term ACE-inhibitor therapy in patients with heart failure or left-ventricular dysfunction: a systematic overview of data from individual patients. ACE-inhibitor myocardial infarction collaborative group. Lancet. 2000;355:1575–1581.
- Winell K, Soveri P, Heikkinen K, et al. [Systematic quality work improves cardiovascular prevention]. Suom Lääkäril. 2011;66:1835–1839. Finnish.
- Ponikowski P, Voors AA, Anker SD, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:67–119.
- Boonman-de Winter LJ, Rutten FH, Cramer MJ, et al. High prevalence of previously unknown heart failure and left ventricular dysfunction in patients with type 2 diabetes. Diabetologia. 2012;55:2154–2162.
- Shah AD, Langenberg C, Rapsomaniki E, et al. Type 2 diabetes and incidence of cardiovascular diseases: a cohort study in 1.9 million people. Lancet Diabetes Endocrinol. 2015;3:105–113.
- Heidenreich PA, Albert NM, Allen LA, et al. Forecasting the impact of heart failure in the United States: a policy statement from the American heart association. Circ Heart Fail. 2013;6:606–619.
- Roger V, Weston S, Redfield M, et al. Trends in heart failure incidence and survival in a community‐based population. JAMA. 2004;292:344–350.
- Gerber Y, Weston SA, Redfield MM, et al. A contemporary appraisal of the heart failure epidemic in Olmsted county, Minnesota, 2000 to 2010. JAMA Intern Med. 2015;175:996–1004.
- Kristensen SL, Køber L, Jhund PS, et al. International geographic variation in event rates in trials of heart failure with preserved and reduced ejection fraction. Circulation. 2015;131:43–53.
- Johansson I, Dahlström U, Ener M, et al. Type 2 diabetes and heart failure: characteristics and prognosis in preserved, mid-range and reduced ventricular function. Diabetes Vasc Dis Res. 2018;15:494–503.
