Abstract
Introduction: The use of dipeptidyl peptidase-4 inhibitors in hospitalized patients is an area of active research. We aimed to compare the efficacy and the safety of the basal-bolus insulin regimen versus linagliptin-basal insulin in non-critically ill non-cardiac surgery patients in a real-world setting.
Methods: We enrolled patients with type 2 diabetes hospitalized in non-cardiac surgery departments with admission glycated haemoglobin level < 8%, admission blood glucose concentration < 240 mg/dL, and no at-home injectable treatments who were treated with basal-bolus (n = 347) or linagliptin-basal (n = 190) regimens between January 2016 and December 2017. To match patients on the two regimens, a propensity matching analysis was performed.
Results: After matching, 120 patients were included in each group. No differences were noted in mean blood glucose concentration after admission (p = .162), number of patients with a mean blood glucose 100–140 mg/dL (p = .163) and > 200 mg/dL (p = .199), and treatment failures (p = .395). Total daily insulin and number of daily insulin injections were lower in the linagliptin-basal group (both p < .001). Patients on linagliptin-basal insulin had fewer hypoglycaemic events (blood glucose < 70 mg/dL) (p < .001).
Conclusion: For type 2 diabetes surgery patients with mild to moderate hyperglycaemia without pre-hospitalization injectable therapies, linagliptin-basal insulin was an effective, safe alternative with fewer hypoglycaemic events in real-world practice.
Treatment with basal-bolus insulin regimens is the standard of care for non-critically ill hospitalized patients with type 2 diabetes.
A differentiated treatment protocol that takes into account glycaemic control and clinical factors should be implemented in the hospital setting.
Linagliptin-basal insulin is an effective, safe alternative with fewer hypoglycaemic events during the hospitalization of non-critically ill non-cardiac surgery patients with T2D in real-world practice.
Key messages
Introduction
Diabetes mellitus has been strongly associated with increased hospital mortality, greater incidence of complications, and longer hospital stays [Citation1–3]. Extensive data from several clinical trials in both critically and non-critically ill hospitalized patients with hyperglycaemia and diabetes mellitus have reported that improved glycaemic control reduces in-hospital deaths, length of stay, and infections [Citation1,Citation4–6].
Current clinical guidelines from professional societies recommend treatment with basal-bolus insulin regimens as the standard of care for non-critically ill hospitalized patients with type 2 diabetes (T2D) [Citation3,Citation7]. In the hospital setting, the use of a basal-bolus insulin regimen has resulted in improved glycaemic control and lower risk of complications compared to sliding scale insulin alone in both medicine and surgery departments [Citation5,Citation6]. However, this regimen requires multiple subcutaneous injections per day and has been associated with a significant risk of hypoglycaemia, which has been reported in up to 32% of non-critically ill patients with T2D in the hospital [Citation5,Citation6,Citation8,Citation9].
The potential side effects and contraindications of most non-insulin antihyperglycaemic agents have limited their use in routine in-hospital antihyperglycaemic management [Citation7,Citation10,Citation11]. However, dipeptidyl peptidase-4 inhibitors (DPP4i) are a class of antidiabetic agents with a well-established safety and efficacy profile. These agents entail a very low risk of hypoglycaemia due to their glucose-dependent mechanism of action. Moreover, DPP4is have a low risk of drug interactions and can be used in patients with cardiac or renal failure. Altogether, these advantages make DPP4i an attractive alternative for in-hospital hyperglycaemia treatment [Citation12].
In recent years, three randomized controlled trials of both non-critically ill medical and surgical inpatients with T2D have showed that a DPP4i (such as sitagliptin alone or in combination with basal insulin and saxagliptin alone) resulted in similar glycaemic control and safety compared to a basal-bolus insulin regimen [Citation13–Citation15]. The same results have been observed in our research group’s work. We conducted the first real-world study (Lina-Real-World Study) focused on the management of T2D inpatients in a medicine (non-surgery) department using linagliptin in combination with basal insulin [Citation16]. Recently, a new multicentre randomized clinical trial focused on patients with T2D undergoing non-cardiac surgery has showed daily linagliptin to be safe and effective alternative to multi-dose insulin therapy for patients with mild to moderate hyperglycaemia [Citation17]. In addition, linagliptin, when added to ongoing insulin treatment, has resulted in improved glycaemic control and neutral impact on major adverse cardiovascular events [Citation18]; and in the recent randomized clinical trial with linagliptin (CARMELINA) conducted in vulnerable patients with high cardiovascular and renal risk, patients in the linagliptin group were less likely to initiate insulin therapy or increase doses of pre-existing insulin therapy [Citation19].
In this study, we aimed to retrospectively compare the efficacy and safety of two in-hospital treatment regimens (basal-bolus insulin and linagliptin-basal insulin), according to our local antihyperglycaemic protocol, in surgery patients. We hypothesized that linagliptin in combination with basal insulin would result in similar glycaemic control and a lower number of complications compared to a basal-bolus insulin regimen in non-cardiac surgery department clinical practice.
Material and methods
Study design and hospital antihyperglycaemic protocol
We conducted an observational, multicentre, real-world study of patients with T2D hospitalized in non-cardiac surgery departments in two university hospitals (Hospital Regional Universitario de Málaga and Hospital Universitario Virgen de la Victoria) and two General Medical Clinics (HeliHospital (Marbella) and Hospital Cenyt (Estepona)) in Málaga, Spain, from January 2016 to December 2017.
Data on patients were collected from each medical centre via medical records from the electronic medical record system and a review of medical records. These data were manually reviewed by investigators; the same method was used in a previously published study [Citation16]. Hospitalized non-critically ill surgery patients with history of T2D who were aged ≥18 years and who were treated with a hospital antihyperglycaemic regimen according to our current clinical practice were included. Our local antihyperglycaemic regimen used for managing non-critically ill hospitalized patients with T2D has been published previously [Citation16]. In brief, the hospital antihyperglycaemic regimen implemented in our area includes 2 recommended protocols: the basal-bolus insulin regimen (standard of care) and the DPP4i (linagliptin)-basal insulin regimen (optional). This optional regimen can only be used for patients with mild to moderate hyperglycaemia – defined as an admission glycated haemoglobin (HbA1c) level <8% and admission blood glucose (BG) concentration <240 mg/dL – who are treated at home without injectable therapies. Patients who meet the following criteria are excluded from DPP4i-basal insulin treatment: history of acute diabetic complications, type 1 diabetes, hyperglycaemia without a known history of diabetes, concomitant hospital treatment with a systemic glucocorticoid, expected admission to an intensive care unit, heart surgery, clinically relevant liver disease or cirrhosis, acute renal function impairment, blood dyscrasias or any disorders causing haemolysis or unstable red blood cells, gastrointestinal obstruction, pregnancy, those expected to be without oral intake, history of pancreatitis episodes or active gallbladder disease, previous bariatric and other gastrointestinal surgeries that induce chronic malabsorption. Medical providers make the decision of starting with basal-bolus or linagliptin-basal regimen according to their own medical criteria. The basal-bolus regimen involves administration of once-daily basal insulin (insulin glargine (Lantus; Sanofi-Aventis, Gentilly, France) at 04:00 p.m. and rapid-acting insulin analogues before meals (insulin lispro (Humalog; Eli Lilly, Indianapolis, IN, United States of America) or insulin aspart (Novorapid; Novo Nordisk, Bagsvaerd, Denmark). The insulin dose is calculated according to admission BG concentrations, age and serum creatinine. The DPP4i-basal insulin regimen includes a single dose of linagliptin 5 mg (Trajenta; Boehringer Ingelheim, Ingelheim am Rhein, Germany) at 09:00 a.m. in combination with basal insulin (insulin glargine) at the same dose as the basal-bolus regimen. When there is treatment failure – defined as two consecutive measurements or a mean daily BG concentration >240 mg/dL – the linagliptin-basal insulin regimen is switched to basal-bolus regimen. In addition, the dose of insulin is modified during hospitalization when required (basal or fasting hyperglycaemia or hypoglycaemia), according to our protocols. The goal of therapy is to maintain fasting and pre-prandial glucose concentrations between 100 and 140 mg/dL. When supplemental rapid-acting insulin before meals and bedtime is required, the dose is calculated according to BG concentrations, total daily insulin units, and patient bodyweight. Fasting, pre-prandial, and bedtime capillary BG concentrations are measured using a point-of-care glucose meter. Additionally, BG concentration is measured any time a patient experiences symptoms of hypoglycaemia or when requested by the medical provider. Hypoglycaemia was defined as a measurable BG concentration <70 mg/dL. More in-depth information about our antihyperglycaemic regimen was reported in our previous published study (Lina-Real-World Study) [Citation16].
Some of the authors were also the treating physicians for these patients. Patients had to give written consent for the consultation of theirs medical records. This study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Research Ethics Committee of Málaga.
Study outcomes
The primary endpoint was to compare differences in glycaemic control during hospitalization, measured by mean daily BG concentrations, between treatment regimens. Secondary endpoints were differences between groups in regards to the total number of hypoglycaemic events, number of patients with 1 or ≥2 hypoglycaemic events, incidence rates for hypoglycaemia, the number of patients with hypoglycaemia (BG <70, <54 and <40 mg/dL), and hyperglycaemic events (BG >200 mg/dL), BG concentrations between 100 and 140 mg/dL, percentage of treatment failures; total daily dose of insulin (basal and prandial), number of daily insulin injections, length of hospital stay, hospital complications, and mortality.
Statistical analysis
Baseline characteristics were analysed using descriptive statistics. Continuous and categorical variables were shown as means ± standard deviation and as absolute value and percentage, respectively. The hypoglycaemia incidence rate per 100 patients-year was calculated. The differences between groups were determined using the two-sample Student’s t-test or the Mann–Whitney–Wilcoxon rank-sum test for continuous variables and Pearson’s Chi-squared test for categorical variables. Values were considered to be statistically significant when p < .05. Multiple comparisons across different days of therapy were adjusted conservatively using Tukey’s adjustment.
In order to match each patient who initiated basal-bolus regimen with a patient who initiated the DPP4i-basal regimen in a 1:1 manner, a propensity score with a calliper of 0.2 and a greedy matching algorithm were used. The probability of starting the DPP4i-basal regimen (as opposed to the basal-bolus regimen) was estimated using a logistic regression model that included variables that could have affected treatment assignment or outcomes as independent variables (age, gender, smoking and alcohol abuse status, history of hypertension, dyslipidaemia, chronic kidney disease, cerebrovascular disease, chronic obstructive pulmonary disease, liver disease, atrial fibrillation, coronary artery disease and heart failure, amount of time the patients has had T2D, admission BG and HbA1c, serum creatinine, transaminase levels, body mass index, principal admitting diagnosis, surgery procedure, and at-home treatment). In order to assess the adequacy of propensity matching, we used the standardized difference (SD) found in patient characteristics following matching. A significant imbalance in the group was considered to be present if a SD between baseline variables of higher than 10% was found.
Statistical analyses were performed using SPSS Statistics for Windows, version 15.0 and SAS for Windows, version 9.3 (SPSS, Chicago, IL, USA).
Results
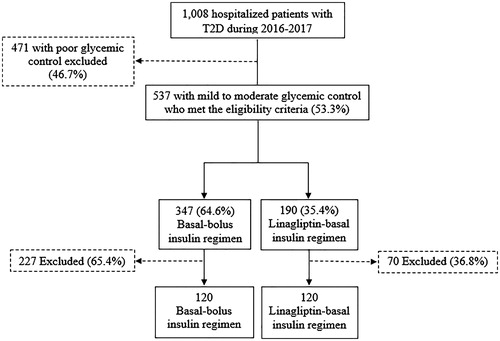
A flow chart for patient inclusion for both regimens is shown in . We identified a total of 1008 hospitalized surgery patients with T2D between January 2016 and December 2017. Of them, 537 patients (53.3%) had mild to moderate glycaemic control and met our protocol’s eligibility criteria for treatment with DPP4i-basal regimen. The basal-bolus insulin regimen was used in 347 patients (64.6%) and the DPP4i-basal insulin regimen in 190 (35.4%). After a matched-pair analysis, 120 patients were included in each treatment group.
Figure 1. Flow charts for patient inclusion for basal-bolus insulin versus Linagliptin-basal insulin regimens. T2D: type 2 diabetes.
The pre- and post-propensity matching baseline clinical characteristics of hospitalised surgery groups according to antihyperglycaemic regimen are shown in . After propensity matching, the groups were well-balanced and only negligible differences were observed (standardized difference ≤0.1). Non-significant differences were observed between groups in the post-propensity matching. Nevertheless, before propensity matching, a greater number of patients in the basal-bolus group were obese, were treated at home with a combination of oral antidiabetic agents, and had coronary artery disease. Patients treated with the linagliptin-basal regimen were more likely to have diet or monotherapy as home T2D treatment, had had diabetes for a longer time, and had higher admission blood glucose concentrations. In addition, these patients had more atrial fibrillation.
Table 1. Pre- and post-propensity matching baseline clinical characteristics of inpatient surgery groups according to antihyperglycaemic regimen.
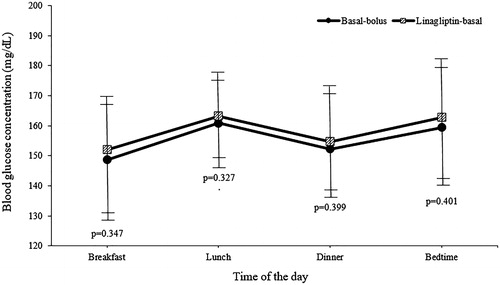
Both the linagliptin-basal and basal-bolus regimens resulted in similar improvements in mean daily BG concentrations after first day of therapy () and in mean BG concentrations before meals and at bedtime (). The length of hospital stay was similar in both treatment groups (), with a range between 4 and 10 d (98.3% of patients were hospitalized between 4 and 8 d).
Figure 2. Mean daily blood glucose concentrations. Values are shown as mean ± standard deviations. mg/dL: milligram/decilitre.
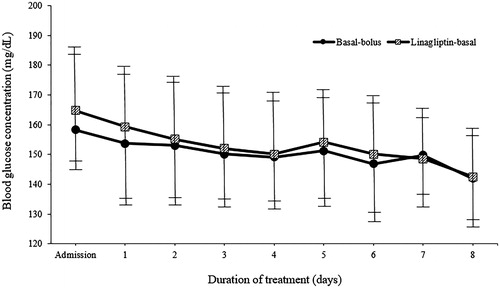
Figure 3. Mean blood glucose concentrations before meals and bedtime. Values are shown as mean ± standard deviations. mg/dL: milligram/decilitre.
After performing the post-matching between the groups (Basal-bolus versus Linagliptin-basal), no significant differences were noted in mean BG concentration after admission (149.5 ± 14.9 versus 151.8 ± 15.3 mg/dL, p = .162), the number of patients with mean BG reading between 100 and 140 mg/dL (20.8 versus 22.5%, p = .163) and higher than 200 mg/dL (9.2 versus 10.0%, p = .199), and the number and day of treatment failure (15.8 versus 16.7%, p = .395). Total daily insulin was significantly lower in the linagliptin-basal group compared with the basal-bolus group (22.8 ± 7.5 versus 32.5 ± 5.2 units per day, p < .001). There were no significant differences in the total basal (15.1 ± 2.6 versus 15.7 ± 2.6 units per day, p = .616) and supplemental rapid-acting insulin doses (5.3 ± 1.3 versus 5.6 ± 1.3 units per day, p = .238). In addition, patients treated with the linagliptin-basal insulin received significantly fewer insulin injections per day during hospitalization (2.6 ± 0.8 versus 4.0 ± 0.0, p < .001). In regards to hypoglycaemia, patients in the linagliptin-basal insulin group had fewer total number of hypoglycaemic events (12 versus 21, p < .001), patients with 1 or ≥2 hypoglycaemic events (7.5 versus 10.0%, p = .031; 2.1 versus 7.5%, p < .001; respectively), and any BG reading <70 mg/dL (9.2 versus 13.3%, p = .041). The incidence rate for hypoglycaemias was significantly higher in basal-bolus insulin than linagliptin-basal insulin patients (16.4 versus 8.1 events per 100 patients-year, p < .001). No differences were observed in severe hypoglycaemias (BG <54: 1.6 versus 3.3%, p = .190; and <40 mg/dL: 1.6 versus 3.3%, p = .183) or hospital complications (8.3 versus 9.2%, p = .202) when comparing the groups. On the other hand, before matching analysis, patients treated with basal-bolus insulin had lower mean daily BG concentrations after admission, received less total daily insulin, and had fewer injections. In addition, the total number of hypoglycaemic events, patients with 1 or ≥2 hypoglycaemic events, hypoglycaemia incidence rate, number of patients with any BG reading <70, <54 and <40 mg/dL, total hospital complications, infections, acute respiratory failure and a requirement of admission to an intensive care unit were lower in patients in the linagliptin-basal insulin group. These data are shown in .
Table 2. Pre- and post-propensity matching glycaemic control outcomes, treatment failures, insulin therapy, hypoglycaemic events and hospital complications of hospitalised surgery patients according to antihyperglycaemic regimen.
Discussion
This real-world study showed that the treatment with the linagliptin and basal insulin regimen resulted in similar glycaemic control with a significant reduction in hypoglycaemia events compared to the basal-bolus insulin regimen in non-critically ill non-cardiac surgery patients with T2D who had mild to moderate hyperglycaemia and who were treated at home without injectable therapies. In addition, patients treated with linagliptin-basal insulin received a lower daily total insulin dose and fewer injections during the hospitalization, thus simplifying management of T2D in surgery departments. No differences were observed in hyperglycaemic events, treatment failures, or complications.
To our knowledge, Lina-Surg Study is the first multicentre, real-world study focussed on the efficacy and safety of linagliptin in combination with basal insulin in regards to glycaemic control and complications in surgery patients with T2D.
Current clinical guidelines from professional societies recommend treatment with daily basal insulin and rapid-acting insulin before meals for non-critically ill hospitalized patients with T2D as the standard of care [Citation3,Citation7]. Although this multi-dose insulin regimen significantly improves glycaemic control and reduces the risk of complications compared to sliding scale insulin alone in the hospital setting [Citation5,Citation6], it is time- and labour-intensive, requiring several subcutaneous injections and capillary BG testing per day. It also entails significant risk of in-hospital hypoglycaemia [Citation5,Citation6,Citation8,Citation9].
Data from three randomized trials of non-critically ill medical and surgical patients with T2D have shown the DPP4is (such as sitagliptin, either alone or in combination with basal insulin, or saxagliptin alone) are effective and safe treatments for management of hyperglycaemia in the hospital setting [Citation13–15]. Although surgery patients were included in these randomized trials, the percentage varied widely (from 16% to 74.5%) and the absolute number was very limited for the purposes of obtaining definitive evidence on the use of DPP4i as a therapeutic alternative for non-critically ill patients with T2D hospitalized in surgery departments [Citation13–15]. However, results from a multicentre randomized clinical trial specifically focused on patients with T2D undergoing non-cardiac surgery, have recently been published [Citation17]. In this study, conducted by Vellanki et al. patients were randomized into groups which received either daily linagliptin or basal-bolus regimen, with once-daily glargine and rapid-acting insulin before meals. The treatment with linagliptin was inferior, resulting in higher mean daily BG concentrations compared to the basal-bolus group in the overall analysis. In regards to Vellanki et al.’s findings, they may be explained by the fact that patients may have had poorer glycaemic control than our patients, given that the inclusion criteria were admission BG levels of up to 400mg/dL; did not consider baseline HbA1c; and included treatment at home with any combination of oral agents except DPP4i, glucagon-like peptide-1 receptor agonists (GLP1-RA), or low-dose insulin therapy (≤0.5 units/kg/d) [Citation17]. Indeed, if those patients were stratified according to BG at the time of randomisation <200 mg/dL (63% of the cohort) or BG ≥200 mg/dL, those with a BG concentration <200 mg/dL who received daily linagliptin had similar glycaemic control to those on multi-dose insulin therapy. Additionally, in Vellanki et al.’s study, although there were no differences in length of hospital stay or the rate of perioperative complications between the groups, patients on linagliptin had fewer hypoglycaemic events with respect to those on the basal-bolus insulin regimen. In our real-word clinical practice study on surgical inpatients, which followed the same protocol used in our previous study focused on the management of medicine department inpatients with T2D [Citation16], we found similar results on glycaemic control, treatment simplification, and safety when using the combination of linagliptin with basal insulin.
In regards to hypoglycaemia, there were significantly fewer events (total number, patients with 1 or ≥2 events, hypoglycaemia incidence rate and BG reading <70 mg/dL) in the linagliptin-basal insulin regimen group than the basal-bolus regimen group in our study. No differences were observed in the number of severe hypoglycaemias episodes (BG <54 and <40 mg/dL). This finding was in accordance with the recently-published randomized trial by Vellanki et al. [Citation17]. However, in our real-world Lina-Surg Study, the percentage of patients who had an hypoglycaemic event (BG <70 mg/dL) in the linagliptin group was 9.2%, higher figure than in the recent clinical trial on surgery patients by Vellanki et al., which found a percentage of 1.6% [Citation17]. This difference could be explained as resulting from the use of insulin combined with DPP4i instead of DPP4i therapy alone. When the combination of DPP4i and basal insulin has been used in the previous studies [Citation13,Citation14], the percentage of hypoglycaemic events were similar to what was observed in our results.
Our patients’ glycaemic status before admission is a determining factor in deciding on an in-hospital antihyperglycaemic treatment [Citation17,Citation20]. Higher admission HbA1c levels have been related to poor clinical outcomes, poor glycaemic control, and poor response to insulin therapy in medicine and surgery department patients with T2D [Citation20,Citation21]. According to previous evidence [Citation13–17,Citation22] and our current study, it may be preferable to manage hospitalized non-critically ill T2D patients with mild to moderate glycaemic control and low dose of insulin at home as well as patients with clinical frailty at high risk of drug-induced hypoglycaemia with a combination of DPP4i and basal insulin. The use of a multi-dose insulin regimen could be more appropriate for hospitalized patients who have poor glycaemic status, receive a high-dose or multi-dose insulin regimen at home, or have clinically relevant conditions or severe complications that make it necessary to manage them with an intensive antihyperglycaemic regimen [Citation16,Citation21].
In the future, the development of differentiated treatment protocol that take into account glycaemic control and clinical factors – similar to what is routinely done for outpatient antidiabetic management [Citation23] – should be fully implemented in the hospital setting.
Other non-insulin antidiabetic agents as GLP1-RA have shown promise in the inpatient setting [Citation11,Citation24]. Recently, the first randomized controlled trial on the safety and efficacy of exenatide therapy for the inpatient management of general and surgery patients with T2D has been published [Citation25]. In this pilot study, exenatide resulted in similar glycaemic control and safety compared to a basal-bolus regimen. More evidence on the efficacy and safety of GLP1-RA will be necessary to routinely implement the use of GLP1-RA in our clinical practice.
Regarding the sodium-glucose transporter 2 (SGLT2) inhibitors, a recent review suggested that they should be avoided in routine in-hospital use until safety and effectiveness are established [Citation26].
Our findings on the efficacy and safety of linagliptin, which showed a reduction in the number of hypoglycaemia events, insulin doses, and need for injections, are important because they are real-world clinical practice data that support the feasibility of a simpler antihyperglycaemic hospital regimen involving a once-daily oral antidiabetic agent in combination with a once-daily injection of a basal insulin for surgery T2D patients with an admission BG <240 mg/dL and Hb1Ac <8%.
We would like to acknowledge some limitations of this study; any conclusions drawn should be assessed within this context. First of all, despite the propensity matching analysis performed and due to the fact that data were obtained retrospectively via medical records from the electronic medical record system, the possibility of unmeasured confounding factors cannot be ruled out. Second, though, there is an increasing body of evidence on the use of DPP4i alone or in combination with basal insulin in representable community-based sample [Citation13–17], this practice is not yet the standard of care. We implemented a protocol based on our current clinical practice for treating hyperglycaemia in non-critically ill patients with T2D in the surgical setting, but it is not fully developed. In fact, this protocol is only routinely used in a limited number of hospital and general clinics. Most physicians manage hospitalised patients with T2D using basal-bolus insulin without taking account patients’ glycaemic status or prehospitalization treatment. Third, our cohort had a lower number of events or complications, which could be explained in part by the fact that most surgical patients were admitted for elective non-cardiac surgery and were expected to be without oral intake for only a short time. Thus, the results of this study are not applicable to all patients undergoing surgery. Fourthly, we do not entirely know whether the benefits of adequate glycaemic control during hospitalization are due to improvement in BG concentration or due to the direct effect of the medication. Finally, in our local protocol, only linagliptin is used in in-hospital management of hyperglycaemia. Due to varying pharmacological characteristics [Citation27], our findings cannot be extrapolated to other DPP4is. We initially decided to use linagliptin because it does not require dose titration according to renal function, offering an easy alternative for glycaemic control to the basal-bolus insulin regimen used in most orthopaedic and general surgical patients with T2D.
In conclusion, linagliptin in combination with basal insulin was as effective and safe as basal-bolus insulin regimen and led to fewer hypoglycaemia events during the hospitalization of non-critically ill non-cardiac surgery patients with T2D. In addition, patients treated with linagliptin-basal insulin required lower daily total insulin doses and fewer injections. This antihyperglycaemic regimen is an adequate alternative to the intensive and laborious basal-bolus insulin regimen for patients with mild to moderate hyperglycaemia who are treated at home without injectable therapies. Further research on the management of hyperglycaemia with simpler regimens in the hospital setting could be helpful for full implementation of these regimens in real-world clinical practice.
Acknowledgements
The authors thank Claire Conrad for her help with the final English-language version.
Disclosure statement
Luis M. Pérez-Belmonte and Ricardo Gómez-Huelgas have received consulting fees and honoraria for membership of advisory boards from Boehringer Ingelheim, Eli Lilly, Novo Nordisk, Sanofi and Janssen. None of these financial contributions are related to the manuscript. No other potential conflicts of interest relevant to this article were reported.
Additional information
Funding
References
- Inzucchi SE. Clinical practice. Management of hyperglycemia in the hospital setting. N Engl J Med. 2006;355:1903–1911.
- Lara-Rojas CM, Pérez-Belmonte LM, López-Carmona MD, et al. National trends in diabetes mellitus hospitalization in Spain 1997–2010: analysis of over 5.4 millions of admissions. Eur J Intern Med. 2019;60:83–89.
- Umpierrez GE, Hellman R, Korytkowski MT, et al. Management of hyperglycemia in hospitalized patients in non-critical care setting: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2012;97:16–38.
- Van den Berghe G, Wilmer A, Hermans G, et al. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354:449–461.
- Umpierrez GE, Smiley D, Zisman A, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial). Diab Care. 2007;30:2181–2186.
- Umpierrez GE, Smiley D, Jacobs S, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery). Diab Care. 2011;34:256–261.
- American Diabetes Association. Diabetes care in the hospital: standards of medical care in diabetes – 2019. Diab Care. 2019;42:S173–S81.
- Boucai L, Southern WN, Zonszein J. Hypoglycemia-associated mortality is not drug-associated but linked to comorbidities. Am J Med. 2011;124:1028–1035.
- Umpierrez GE, Palacio A, Smiley D. Sliding scale insulin use: myth or insanity? Am J Med. 2007;120:563.
- Donner TW, Flammer KM. Diabetes management in the hospital. Med Clin North Am. 2008;92:407–425.
- Mendez CE, Umpierrez GE. Pharmacotherapy for hyperglycemia in noncritically ill hospitalized patients. Diab Spectr. 2014;27:180–188.
- Aschner P, Kipnes MS, Lunceford JK, et al. Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diab Care. 2006;29:2632–2637.
- Umpierrez GE, Gianchandani R, Smiley D, et al. Safety and efficacy of sitagliptin therapy for the inpatient management of general medicine and surgery patients with type 2 diabetes: a pilot, randomized, controlled study. Diab Care. 2013;36:3430–3435.
- Pasquel FJ, Gianchandani R, Rubin DJ, et al. Efficacy of sitagliptin for the hospital management of general medicine and surgery patients with type 2 diabetes (Sita-Hospital): a multicentre, prospective, open-label, non-inferiority randomized trial. Lancet Diab Endocrinol. 2017;5:125–133.
- Garg R, Schuman B, Hurwitz S, et al. Safety and efficacy of saxagliptin for glycemic control in non-critically ill hospitalized patients. BMJ Open Diab Res Care. 2017;5:e000394.
- Pérez-Belmonte LM, Gómez-Doblas JJ, Millán-Gómez M, et al. Use of linagliptin for the management of medicine department inpatients with type 2 diabetes in real-world clinical practice (Lina-Real-World Study). J Clin Med. 2018;7:271–286.
- Vellanki P, Rasouli N, Baldwin D, et al. Glycaemic efficacy and safety of linagliptin compared to basal-bolus insulin regimen in patients with type 2 diabetes undergoing non-cardiac surgery: a multicenter randomized clinical trial. Diab Obes Metab. 2019;21:837–843.
- Zinman B, Ahrén B, Neubacher D, et al. Efficacy and cardiovascular safety of linagliptin as an add-on to insulin in type 2 diabetes: a pooled comprehensive post hoc analysis. Can J Diab. 2016;40:50–57.
- Rosenstock J, Perkovic V, Johansen OE, et al. Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the CARMELINA randomized clinical trial. JAMA. 2019;321:69–79.
- Pasquel FJ, Gomez-Huelgas R, Anzola I, et al. Predictive value of admission hemoglobin A1c on inpatient glycemic control and response to insulin therapy in medicine and surgery patients with type 2 diabetes. Diab Care. 2015;38:202–203.
- Umpierrez GE, Hor T, Smiley D, et al. Comparison of impatient insulin regiments with determir plus aspart versus neutral protamine hagedorn plus regular in medical patients with type 2 diabetes. J Clin Endocrinol Metab. 2009;97:564–569.
- Nauck MA, Meier JJ. Sitagliptin plus basal insulin: simplifying in-hospital diabetes treatment? Lancet Diab Endocrinol. 2017;5:83–85.
- American Diabetes Association. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes – 2019. Diab Care. 2019;42:S90–S102.
- Umpierrez GE, Korytkowski M. Is incretin-based therapy ready for the care of hospitalized patients with type 2 diabetes? Insulin therapy has proven itself and is considered the mainstay of treatment. Diab Care. 2013;36:2112.
- Fayfman M, Galindo RJ, Rubin DJ, et al. A randomized controlled trial on the safety and efficacy of exenatide therapy for the inpatient management of general medicine and surgery patients with type 2 diabetes. Diab Care. 2019;42:450–456.
- Umpierrez G, Korytkowski M. Diabetic emergencies-ketoacidosis, hyperglycaemic hyperosmolar state and hypoglycaemia. Nat Rev Endocrinol. 2016;12:222–232.
- Deacon CF, Holst JJ. Linagliptin, a xanthine-based dipeptidyl peptidase-4 inhibitor with an unusual profile for the treatment of type 2 diabetes. Expert Opin Investig Drugs. 2010;19:133–140.
