Abstract
Background: Massive transfusion in patients with upper gastrointestinal bleeding (UGIB) was not investigated. We developed a new scoring system to predict massive transfusion and to enhance care and early resource mobilization.
Methods: Massive transfusion was defined as transfusion with ≥10 units of red blood cells within the first 24 h. Data were extracted from a 10-year, six-hospital database. Logistic regression was applied to derive a risk score for massive transfusion using data from 2006 to 2010, in 24,736 patients (developmental cohort). The score was then validated using data from 2011 to 2015 in 27,449 patients (validation cohort). Area under the receiver operating characteristic (AUROC) curve was performed to assess prediction accuracy.
Results: Five characteristics were independently associated (p < .001) with massive transfusion: presence of band-form cells among white blood cells (band form >0), international normalized ratio (INR) >1.5, pulse >100 beats per minute or systolic blood pressure <100 mmHg (shock), haemoglobin <8.0 g/dL and endoscopic therapy. The new scoring system successfully discriminated well between UGIB patients requiring massive transfusion and those who did not in both cohorts (AUROC: 0.831, 95%CI: 0.827–0.836; AUROC: 0.822, 95% CI: 0.817–0.826, respectively).
Conclusions: The new scoring system predicts massive transfusion requirement in patients with UGIB well.
Massive transfusion is a life-saving management in massive upper gastrointestinal bleeding. How to identify patients requiring massive transfusion in upper gastrointestinal bleeding is poorly documented.
Approximately 3.9% of upper gastrointestinal bleeding patients require massive transfusion.
A new scoring system is developed to identify patients requiring massive transfusion with high accuracy.
Key messages
Introduction
Massive haemorrhage requiring massive transfusion (MT) occurs in a variety of clinical situations, such as surgery, trauma, gastrointestinal haemorrhage and remains a major cause of substantial morbidity and mortality [Citation1,Citation2]. MT accounts for 3%–5% of all civilian and 8%–10% of all military patients having suffered trauma [Citation3,Citation4]. The presence of coagulopathy is associated with poorer outcomes in patients with severe haemorrhage in both military and civilian studies [Citation5]. Blood component transfusion is one of the key elements in the management of patients with massive haemorrhage to correct potential coagulopathy. Abnormal coagulation parameters at the time of presentation are noticed in up to 25% of patients with trauma, which is associated with a 3-fold increase in mortality [Citation6,Citation7]. Several reports demonstrate that the rapid treatment of initial coagulation anomalies improves survival [Citation6,Citation8]. Clinical studies among patients with trauma highlight the importance of identification and treatment of coagulopathy in the early stages of presentation [Citation5]. Early initiation of a MT protocol has been associated with a reduction in an overall number of blood products transfused, organ failure and mortality in patients with trauma [Citation9–13]. Accordingly, early and accurate identification of the need for MT is essential for early activation of a MT protocol, and several appropriate scoring systems for MT protocol initiation have been developed [Citation14–16]. However, previous studies describing the scoring systems of MT have largely been limited to trauma setting, little is known in gastrointestinal bleeding patients with massive exsanguination requiring MT.
Our study aimed to develop a new scoring system in upper gastrointestinal bleeding (UGIB) patients to enhance the early determination of the need for MT.
Methods
Study data source
The electronic medical records obtained from the Chang Gung Research Database (CGRD) were used in the present study. The records consisted of unidentifiable data which were designed for research purposes; these data are stored in a secure server for data analysis. Presently, our study contains data from six different branches of the Chang Gung Memorial Hospital collected in CGRD. This includes one local hospital, three regional hospitals and two medical centres, distributed throughout central, northern and southern Taiwan. To record essential clinical information, including diagnoses, therapeutic management, prescriptions, laboratory examination records, invasive and non-invasive procedure information, patient demographics, patient vital signs, date of specialist consultation, and hospital admission and discharge date, a computerized system was developed. The CGRD anonymizes medical records, ensuring confidentiality. Each patient is allocated a unique reference number; this enables retrieval of data and further statistical analysis. The protocol of this study was approved by the Chang Gung Medical Foundation Institutional Review Board (IRB No: 201600990B0C101). The waiver of informed consent was applicable because of the anonymized data used in this study.
Study design
This study was a secondary analysis that used data obtained from the CGRD. The data collection and validation details are reported [Citation17]. Between January 2006 and December 2015, adult patients (>18 years) with UGIB admitted to the emergency department (ED) of the six different branches of Chang Gung Memorial hospital were reviewed. Using the International Classification of Diseases 9th revision (ICD-9) codes, UGIB was identified. To define the index diagnosis in this study, only the ICD-9 coding in the ED was used. The date of ED admission with UGIB was defined as the index date. Laboratory study results, such as routine chemistry and haematology, were obtained on the admission day. We excluded patients not receiving endoscopy within 24 h, having incomplete records, and those who were admitted for other conditions but developed UGIB during the hospital stay. Enrolled patients were divided into the developmental cohort and validation cohort. The developmental cohort included data obtained from 2006 to 2010, while the validation cohort included data obtained from 2011 to 2015.
Definition for massive transfusion
Massive transfusion was defined as transfusion with red blood cell (RBC) ≧10 unit within the initial 24 h following the presentation to the ED.
Shock definition
The definition of shock in this study was systolic blood pressure <100 mmHg or pulse >100 beats per minute at the ED triage.
Definition of endoscopic therapy
In the current study, endoscopic therapy was defined as any treatment to stop bleeding by endoscopy. Non-variceal bleedings were treated by argon plasma coagulation, endoscopic haemoclips or with hypertonic saline-epinephrine injection. Variceal bleedings were treated by endoscopic variceal ligation with overtube insertion or cyanoacrylate injection.
Covariates
To identify baseline medical conditions, we retrieved diagnosis records dated before the index date for all patients using ICD-9 codes from the CGRD (Supplementary Table 1). Each patient’s diagnosis was determined according to ICD-9 codes from the out-patient department or ICD-9 codes from discharge records of those who were admitted to the hospital. The severity of liver cirrhosis was classified by Child-Pugh classification system [Citation18].
Rockall score and modified Glasgow Blatchford score
Based on patient clinical features and endoscopic findings, the Rockall score, ranging from 0 to 11, was calculated for each patient [Citation19,Citation20]. Meanwhile, the modified Glasgow Blatchford score (mGBS), ranging from 0 to 16, was also calculated. The Glasgow Blatchford score (GBS) (range, 0–23) is a risk-scoring system for evaluating the need for clinical intervention in patients with UGIB [Citation21,Citation22]. Modified GBS (mGBS), which eliminated the subjective criteria of GBS (i.e. syncope, melena and the prior history of co-existent liver disease or heart failure) was comparable with GBS in predicting the needs for clinical interventions [Citation23,Citation24].
Definition of further bleeding
Further bleeding was defined as that requiring a repeat esophagogastroduodenoscopy (EGD) after initial endoscopic therapy, radiological intervention or surgery performed for haemostasis.
Statistical analysis
We presented categorical variables as percentages and continuous variables as medians with interquartile ranges (IQR). To construct a diagnostic scoring system, factors were entered as categorical variables. For patients’ age, an age of 65 was set as a cut-off. For all the laboratory variables, the cut-off points were determined based on a combination of the median of massive transfusion group and non-massive transfusion group, clinical experience and review of previous reports [Citation18,Citation21,Citation25,Citation26]. The methods of analyses used in the current study were univariate and multivariate logistic regression analyses. The initial model inclusion criterion was p < .05 with predictive factors entered using backward-stepwise selection and retained where p < .01. The assignment of score point was based on the corresponding regression coefficients. The regression coefficients were transformed by dividing the coefficients with the smallest coefficients in the model and then rounding to the nearest 0.5 to obtain item scores [Citation27].
The risk score of each patient was calculated by totalling the scores of each independent variable. To evaluate model calibration, we performed the Hosmer-Lemeshow goodness-of-fit test [Citation28]. The predictive accuracy of the risk score was expressed as area under the receiver operating characteristic (AUROC) curve [Citation29]. The curve represents the relationship between corresponding values of sensitivity and specificity with all possible values of probabilities as a cut-off point to predict for the presence of massive transfusion. All analyses were conducted using the MedCalc Statistical Software version 17.9.2 (MedCalc Software bvba, Ostend, Belgium; http://www.medcalc.org; 2017).
Risk score validation
The risk score derived from the derivation cohort was applied to the validation cohort. The AUROC curve was used to assess the ability of the model to discriminate between massive transfusion and non-massive transfusion UGIB patients. Using the sensitivity and specificity for each potential cut-off compared with the overall risk of massive transfusion, patients with UGIB were stratified into low, normal and high risk of massive transfusion.
Sensitivity analyses
Additional analyses were conducted in order to examine the robustness of our main results. To assess the potential effect of missing data, we performed multiple imputation using multivariate normal distribution with the SAS Enterprise Guide (version 5.1; SAS Institute, Cary, NC) [Citation30]. In addition, the Rockall score and mGBS were compared with the new score to evaluate the discrimination between massive transfusion and non-massive transfusion UGIB patients by AUROC in the validation cohort. The chi-squared test was used to compare the difference between AUC curves according to the method described by DeLong [Citation31].
Data availability
The raw datasets analyzed during this study and their corresponding processed datasets are available from the corresponding author on request.
Results
Study cohort
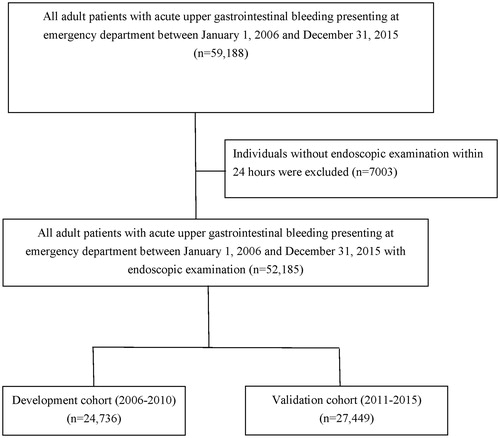
illustrates the process of patient selection. During the study period (2006–2015), a total of 59,188 adult UGIB patients presenting to the ED were identified. Of these, 7003 (11.8%) adult patients did not receive endoscopic examination within 24 h; thus, they were excluded. The entire study cohort consisted of 52,185 patients. From 2006 to 2010, 24,736 (47.4%) patients were enrolled as the developmental cohort, and from 2011 to 2015, 27,449 (52.6%) patients were enrolled as the validation cohort. Of the 52,185 patients, 5.7% (n = 2975) died during hospitalization and 16.4% (n = 8544) had further bleeding. Moreover, 3.9% (n = 2020) underwent massive transfusions, and, of which, 26.3% (n = 531) suffered hospital mortality. In the massive transfusion group, the median units of RBC transfused within 24 h were 15 (interquartile range; IQR: 12–23). The basic characteristics and comorbidities of the developmental and validation cohorts have been demonstrated in . The proportion of malignancy and Child-Pugh classification C of massive transfusion patients in the developmental cohort and in the validation cohort were similar (31.9% vs. 31.7%, 15.2% vs. 15.1%, respectively).
Table 1. Patient baseline characteristics in the developmental and validation cohorts.
summarizes the factors associated with massive transfusion in the univariate logistic analysis among patients in the developmental cohort. In the univariate analysis, male (odds ratio [OR], 1.2; 95% confidence interval [CI]: 1.1–1.4), chronic obstructive pulmonary disease (OR, 0.8; 95% CI: 0.7–0.9), severe renal disease (OR, 1.2; 95% CI: 1.1–1.4), malignancy (OR, 1.1; 95% CI: 1.0–1.3), and liver cirrhosis (OR, 1.6; 95% CI: 1.3–2.0) were associated with massive transfusion.
Table 2. Factors associated with massive transfusion in the univariate logistic analysis.
summarizes the factors associated with massive transfusion in multivariate logistic analysis among patients in the developmental cohort and the corresponding scoring system. In the final results of multivariate analysis, band form (band form cell in white blood cells) >0 (OR, 4.4; 95% CI: 4.0–4.8), international normalized ratio (INR) >1.5 (OR, 1.7; 95% CI: 1.6–2.0), shock (OR, 1.2; 95% CI: 1.1–1.3), haemoglobin (Hb) <8.0 g/dl (OR, 2.3; 95% CI: 2.1–2.5), and endoscopic therapy (OR, 5.1; 95% CI: 4.6–5.6) were associated with massive transfusion.
Table 3. Factors associated with massive transfusion in the final multivariate logistic analysis.
The score was constructed by dividing the regression coefficients of each independent factors with the smallest coefficient in the final logistic model then rounding (). The performance of the final model was very good (Hosmer-Lemeshow goodness of fit test, p = .99) [Citation27] and discriminated well between patients with massive transfusion and those with non-massive transfusion. AUROC for the developmental cohort was 0.831 (95% CI: 0.827–0.836) [Citation28].
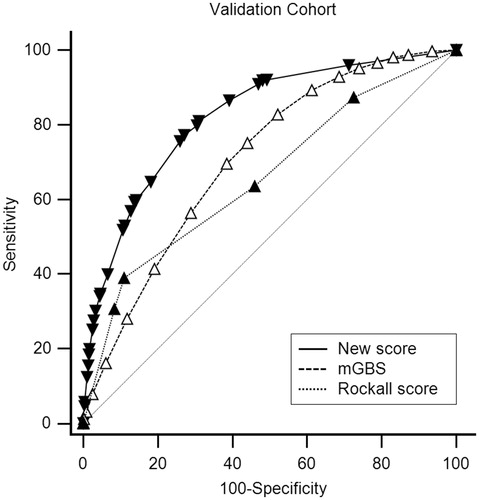
In the validation cohort of 27,449 patients, the model demonstrated a good reliability with AUROC of 0.822 (95% CI: 0.817–0.826).
summarizes the stratification of risks of massive transfusion by different new score cut-off values. The score cut-offs for the three different groups selected were based on the sensitivity and specificity of using different thresholds. The risks of massive transfusion were divided into three groups: low (1.1% risk of massive transfusion), normal (3.9% risk, similar to the overall population), and high (10.2% risk), with corresponding new score cut-off value of 0–5, 6.5–7.5 and >7.5, respectively ().
Table 4. Risk of massive transfusion in a new scoring system.
Sensitivity analyses
Multiple imputation revealed similar factors associated with massive transfusion (). In the validation cohort, the new score (AUROC: 0.822, 95% CI: 0.817–0.826) performed better than the Rockall score (AUROC: 0.693, 95% CI: 0.687–0.698, p < .001) and mGBS (AUROC: 0.708, 95% CI: 0.702–0.713, p < .001) for predicting the need for massive transfusion ().
Discussion
Our study demonstrated a new scoring system for massive transfusion in patients with UGIB, with high accuracy and simplicity.
In current literature, there is no available validated predictive scoring system to assess the need for MT and guide the determination of MT protocol activation in patients with non-trauma bleeding. Gastrointestinal bleeding is one of the major bleeding contexts requiring massive transfusion, ranging from 17.3% to 25% [Citation32,Citation33]. In UGIB, transfusions are definitely indicated in massive, exsanguinating haemorrhage and can be lifesaving; however, the most effective transfusion strategy is controversial because the majority of UGIB cases are minor bleeding without haemodynamic instability [Citation34,Citation35]. One recent (2013) randomized trial demonstrated that a restrictive transfusion strategy at lower transfusion thresholds had better outcomes in patients with UGIB [Citation25]. However, patients with massive exsanguinating bleeding were excluded in this study, thus an optimal transfusion strategy in UGIB patients with massive haemorrhage remains unclear.
The mortality rates for patients requiring MT are between 25 and 48% [Citation36,Citation37]. In our total cohort (n =52,185), about 4% of patients with UGIB required massive transfusion, which was with 26.3% (n = 531) mortality.
In the trauma setting, there is evidence that early transfusion of blood products with adequate amounts of plasma or platelet concentrates is beneficial to survival and reduction in blood product wastes [Citation9,Citation12,Citation13]. The discovery of acute traumatic coagulopathy (ATC) suggests the coagulopathy is established rapidly after injury and is best treated much earlier in the clinical course [Citation4,Citation6,Citation38]. In 2011, Australia’s National Blood Authority (NBA) published patient blood management guidelines for critical bleeding and MT, and recommended that institutions should develop an MT protocol that includes the dose, timing and ratio of blood component therapy for use in trauma patients with, or at risk of, critical bleeding requiring MT [Citation39].
Early identification of patients requiring MT is crucial in order to promptly activate an MT protocol; this early identification and activation of the protocol are essential to improve patient outcomes. In recent years, multiple scoring systems predicting the need for MT have been established and some have high accuracy in trauma patients [Citation14–16].
Convenience and rapid calculation are essential for a scoring system to be useful in predicting the need for MT, and accuracy is also important, since complications of transfusion can be severe [Citation40,Citation41]. Nevertheless, the components of these scoring systems are mainly based on the mechanisms of trauma, which is not fully applicable to patients with UGIB. The Rockall score and mGBS are both widely used scoring systems in predicting outcomes, such as mortality, further bleeding or the need for transfusion, interventions in patients with UGIB [Citation19–24]. In our study, the new scoring system has a significantly higher AUROC than the Rockall score and mGBS (0.822 vs. 0.693, p < .001, 0.822 vs. 0.708, p < .001, respectively). The result of this study shows that the accuracy of the new scoring system is high () and easy to use by stratifying the risk of MT into three categories: low, normal and high risk (). To our knowledge, our current study is the first report that demonstrates a new scoring system to evaluate the need for MT to facilitate the initiation of the MT protocol in UGIB patients.
There are several potential limitations in the present study. The first limitation is the retrospective observational design of our study; variables included in the score were limited to those that had been included in the database. Second, although the score has been validated in the current study, further clinical experience and external validation of this score will be required to assess its value in the management of individual patients.
In conclusion, this study presents a risk scoring system to predict MT in patients with UGIB that was developed and validated using a large clinical database. The main value of our results is to enhance clinical decision-making and activation of the MT protocol in patients most likely to benefit from them. Further research is recommended to investigate the risk scoring system’s effects on activation of MT protocol and patients’ clinical outcomes.
| Abbreviations | ||
| UGIB | = | upper gastrointestinal bleeding |
| RBC | = | red blood cell |
| ED | = | emergency department |
| AUROC | = | Area under the receiver operating characteristic curve |
| INR | = | international normalized ratio |
| MT | = | massive transfusion |
| CGRD | = | Chang Gung Research Database |
| ICD-9 codes | = | International Classification of Diseases, 9th revision codes |
| mGBS | = | modified Glasgow Blatchford score |
| GBS | = | Glasgow Blatchford score |
| EGD | = | esophagogastroduodenoscopy |
| IQR | = | interquartile ranges |
| ATC | = | acute traumatic coagulopathy |
| NBA | = | Australia's National Blood Authority |
Supplemental Material
Download MS Word (54 KB)Acknowledgements
This study had no financial support. We are grateful to the Chang Gung Research Database for the provision of the electronic health records for this study. YCC and MSH had complete access to the data analyzed in the study and take responsibility for the data integrity and the analysis accuracy of the data.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Malone DL, Hess JR, Fingerhut A. Massive transfusion practices around the globe and a suggestion for a common massive transfusion protocol. J Trauma. 2006;60:S91–S96.
- Lacroix J, Hebert PC, Fergusson DA, et al. Age of transfused blood in critically ill adults. N Engl J Med. 2015;372:1410–1418.
- Como JJ, Dutton RP, Scalea TM, et al. Blood transfusion rates in the care of acute trauma. Transfusion. 2004;44:809–813.
- Holcomb JB. Damage control resuscitation. J Trauma. 2007;62:S36–S37.
- Niles SE, McLaughlin DF, Perkins JG, et al. Increased mortality associated with the early coagulopathy of trauma in combat casualties. J Trauma. 2008;64:1459–1463; discussion 63–65.
- Brohi K, Singh J, Heron M, et al. Acute traumatic coagulopathy. J Trauma. 2003;54:1127–1130.
- Maegele M, Lefering R, Yucel N, et al. Early coagulopathy in multiple injury: an analysis from the German Trauma Registry on 8724 patients. Injury. 2007;38:298–304.
- Schreiber MA, Perkins J, Kiraly L, et al. Early predictors of massive transfusion in combat casualties. J Am Coll Surg. 2007;205:541–545.
- Holcomb JB, del Junco DJ, Fox EE, et al. The prospective, observational, multicenter, major trauma transfusion (PROMMTT) study: comparative effectiveness of a time-varying treatment with competing risks. JAMA Surg. 2013;148:127–136.
- Schuster KM, Davis KA, Lui FY, et al. The status of massive transfusion protocols in United States trauma centers: massive transfusion or massive confusion? Transfusion. 2010;50:1545–1551.
- Cotton BA, Gunter OL, Isbell J, et al. Damage control haematology: the impact of a trauma exsanguination protocol on survival and blood product utilization. J Trauma. 2008;64:1177–1182; discussion 82–83.
- O'Keeffe T, Refaai M, Tchorz K, et al. A massive transfusion protocol to decrease blood component use and costs. Arch Surg. 2008;143:686–690; discussion 90–91.
- Cotton BA, Au BK, Nunez TC, et al. Predefined massive transfusion protocols are associated with a reduction in organ failure and postinjury complications. J Trauma. 2009;66:41–48; discussion 8–9.
- Ogura T, Lefor AK, Masuda M, et al. Modified traumatic bleeding severity score: early determination of the need for massive transfusion. Am J Emerg Med. 2016;34:1097–1101.
- Nunez TC, Voskresensky IV, Dossett LA, et al. Early prediction of massive transfusion in trauma: simple as ABC (assessment of blood consumption)? J Trauma. 2009;66:346–352.
- Yucel N, Lefering R, Maegele M, et al. Trauma Associated Severe Hemorrhage (TASH)-Score: probability of mass transfusion as surrogate for life threatening haemorrhage after multiple trauma. J Trauma. 2006;60:1228–1236; discussion 36–37.
- Chen YC, Hsiao CT, Lin LC, et al. The association between red blood cell transfusion and outcomes in patients with upper gastrointestinal bleeding. Clin Transl Gastroenterol. 2018;9:138.
- Cholongitas E, Papatheodoridis GV, Vangeli M, et al. Systematic review: the model for end-stage liver disease–should it replace Child-Pugh's classification for assessing prognosis in cirrhosis? Aliment Pharmacol Ther. 2005;22:1079–1089.
- Rockall TA, Logan RF, Devlin HB, et al. Risk assessment after acute upper gastrointestinal haemorrhage. Gut. 1996;38:316–321.
- Vreeburg EM, Terwee CB, Snel P, et al. Validation of the Rockall risk scoring system in upper gastrointestinal bleeding. Gut. 1999;44:331–335.
- Blatchford O, Murray WR, Blatchford M. A risk score to predict need for treatment for upper-gastrointestinal haemorrhage. Lancet. 2000;356:1318–1321.
- Stanley AJ, Dalton HR, Blatchford O, et al. Multicentre comparison of the Glasgow Blatchford and Rockall Scores in the prediction of clinical end-points after upper gastrointestinal haemorrhage. Aliment Pharmacol Ther. 2011;34:470–475.
- Cheng DW, Lu YW, Teller T, et al. A modified Glasgow Blatchford Score improves risk stratification in upper gastrointestinal bleed: a prospective comparison of scoring systems. Aliment Pharmacol Ther. 2012;36:782–789.
- Quach DT, Dao NH, Dinh MC, et al. The performance of a modified Glasgow Blatchford Score in predicting clinical interventions in patients with acute nonvariceal upper gastrointestinal bleeding: a vietnamese prospective multicenter cohort study. Gut Liver. 2016;10:375–381.
- Saltzman JR, Tabak YP, Hyett BH, et al. A simple risk score accurately predicts in-hospital mortality, length of stay, and cost in acute upper GI bleeding. Gastrointest Endosc. 2011;74:1215–1224.
- Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368:11–21.
- Chen L, Magliano DJ, Balkau B, et al. AUSDRISK: an Australian Type 2 Diabetes Risk Assessment Tool based on demographic, lifestyle and simple anthropometric measures. Med J Aust. 2010;192:197–202.
- Lemeshow S, Hosmer DW, Jr. A review of goodness of fit statistics for use in the development of logistic regression models. Am J Epidemiol. 1982;115:92–106.
- Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148:839–843.
- Lee KJ, Carlin JB. Multiple imputation for missing data: fully conditional specification versus multivariate normal imputation. Am J Epidemiol. 2010;171:624–632.
- DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845.
- Chay J, Koh M, Tan HH, et al. A national common massive transfusion protocol (MTP) is a feasible and advantageous option for centralized blood services and hospitals. Vox Sang. 2016;110:36–50.
- Ruseckaite R, McQuilten ZK, Oldroyd JC, et al. Descriptive characteristics and in-hospital mortality of critically bleeding patients requiring massive transfusion: results from the Australian and New Zealand Massive Transfusion Registry. Vox Sang. 2017;112:240–248.
- Barkun AN, Bardou M, Kuipers EJ, et al. International consensus recommendations on the management of patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med. 2010;152:101–113.
- Barkun A, Bardou M, Marshall JK. Consensus recommendations for managing patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med. 2003;139:843–857.
- Sinha R, Roxby D, Bersten A. Experience with a massive transfusion protocol in the management of massive haemorrhage. Transfusion Med. 2013;23:108–113.
- Campos A, Munoz M, Garcia-Erce JA, et al. Incidence and mortality of massive transfusion in a university hospital: study of the period 2001-2005. Med Clin (Barc). 2007;129:366–371.
- Stanworth SJ, Morris TP, Gaarder C, et al. Reappraising the concept of massive transfusion in trauma. Crit Care. 2010;14:R239.
- National Blood Authority (NBA). Patient blood management guidelines: module 1—critical bleeding/massive transfusion. Canberra, Australia: NBA; 2011. [cited 26 October 2016]. [Available at: http://www.blood.gov.au/pbm-module-1]
- Carson JL, Grossman BJ, Kleinman S, et al. Red blood cell transfusion: a clinical practice guideline from the AABB*. Ann Intern Med. 2012;157:49–58.
- Kaufman RM, Djulbegovic B, Gernsheimer T, et al. Platelet transfusion: a clinical practice guideline from the AABB. Ann Intern Med. 2015;162:205–213.