Abstract
Background: The Klotho protects the cardiovascular system by protecting against cell apoptosis, inhibiting the production of reactive oxygen species, and modulating inflammation. We aimed to investigate relationship of plasma Klotho concentrations with functional outcome at 3 months after acute cerebral infarction.
Methods: We prospectively enrolled 262 first-ever acute cerebral infarction patients from whom a blood sample was acquired within 24 h of admission. An enzyme-linked immunosorbent assay was used for evaluating plasma Klotho concentration. Functional outcome on admission and three months was evaluated.
Results: Of the 262 patients, 152 (58.0%) were men. The mean age of these patients was 64.7 years. The mean ± standard deviation of plasma Klotho concentrations was 312.7 ± 153.3 pg/mL. As opposed to patients with good outcome, plasma Klotho levels were lower in the poor outcome group (207.8 ± 96.2 vs. 342.5 ± 153.5 pg/mL, p = .001). In multivariate analysis, increased plasma Klotho concentrations were independently associated with good functional outcome (Odds ratio: 2.42, 95% confidence interval: 1.45–4.04, p < .001).
Conclusions: Increased plasma Klotho concentrations were associated with good functional outcome in patients with acute ischemic stroke. We attribute these associations to the pleiotropic effects of Klotho in stroke and vascular diseases.
Increased plasma Klotho concentrations were associated with good functional outcome in patients with acute ischemic stroke.
Key message
1. Introduction
Klotho, identified as a gene that suppresses ageing, is predominantly present in the renal tubular epithelium, parathyroid gland, and the choroid plexus of the brain [Citation1]. The importance of Klotho has been emphasized following studies involving the Klotho knock-out mouse model, which reported that decreased Klotho was associated with human aging-like phenotypes shortened life expectancy and that overexpression of Klotho was associated with an extended life span [Citation2,Citation3]. Klotho exist in form of membrane or circulatory Klotho in humans. In the kidney, membrane Klotho works as a co-receptor for fibroblast growth factor (FGF)-23, and leads to phosphate secretion into the urine [Citation4]. Circulatory Klotho is involved in regulation of endothelium derived nitric oxide production, and calcium homeostasis in the kidney [Citation5,Citation6].
The incidence of stroke has caused a major global burden due to its high rate of mortality and residual disability [Citation7]. Therefore, it is important for patients, as well as for socio-economic reasons to regulate and identify factors associated with the prognosis of stroke. As mentioned above, because Klotho is involved in molecular mechanisms related to vascular pathophysiology, concentrations of Klotho might be associated with occurrence and outcome of vascular disease, including stroke. Previously, some studies reported that increased plasma Klotho concentrations were an independently associated risk for cardiovascular disease, variation of the Klotho gene was associated with chance of early onset coronary disease [Citation2,Citation8]. However, previous biomarkers related to the stroke outcome have been reported [Citation9,Citation10], studies on the association Klotho with prognosis of stroke have been limited to date.
In our study, we hypothesized that increased plasma Klotho concentrations would be independently associated with good functional outcome at three months in patients with acute cerebral infarction.
2. Materials and methods
2.1. Subjects
Between June 2014 and May 2016, we prospectively enrolled 262 patients with first-ever acute ischemic stroke who were admitted to the hospital within seven days of symptom onset, and whose subtypes of stroke were classified as large artery atherosclerosis, cardioembolism, or lacunar infarction. All patients were evaluated by protocol of our hospital, which includes 12-lead electrocardiography, chest x-ray, routine blood tests [white blood cell count and creatinine levels at admission; and levels of vitamin D (25(OH)D), fasting glucose, HbA1c, triglyceride, total cholesterol, low density lipoprotein, hemoglobin, total calcium, phosphate, albumin, alkaline phosphatase, uric acid and C-reactive protein after 12 hours fasting period], imaging studies (CT and/or MRI, CT angiography, MR angiography or digital subtraction angiography) [Citation11,Citation12]. Patients were not enrolled if they did not consent to participate in the study and did not consent to blood collection for plasma Klotho analysis and when they had a previous history of cancer or autoimmune disease or bone fractures in the last two months. The definition of risk factors was reported in the Supplementary Methods in a previous study [Citation11]. Neurological status was calculated using the National Institutes of Health Stroke Scale (NIHSS) as soon as patient got admitted [Citation13]. Cerebral atherosclerosis was defined as the presence of one or more vessels with over 50% stenosis or occlusion in extracranial or intracranial cerebral arteries [Citation14]. High-grade white matter hyperintensities (HWMHs) were regarded as a Fazekas score of ≥2 in periventricular white matter and/or deep white matter [Citation15]. The Kappa value was 0.92 for the presence of high grade white matter hyperintensities. Subtypes of stroke were grouped by the Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification system [Citation16]. Briefly, large artery atherosclerosis was diagnosed if the patient had significant (≥50%) stenosis or occlusion of the large artery supplying the vascular territory of lesion. Cardioembolism was identified if the patient had at least one definite cardioembolic source. Lacunar infarction was defined as a single, small ( < 15 mm), penetrating artery infarction of deep area but no significant stenosis ( < 50%) of the artery supplying the vascular territory of the infarction site and no possibility of identification of the cardioembolic source [Citation14,Citation17,Citation18]. Acute ischemic lesions were considered as hyperintense lesions on diffusion weighted images (DWI) and relevant hypointense lesions on apparent diffusion coefficient map images. Cerebral infarction volume was checked in all enrolled patients by using semi-automated computerized software (Xelis, Infinitt, Korea) [Citation17]. Three month modified Rankin scale (mRS) outcome was assessed by a well-trained research nurse [Citation19]. This study was approved by our Institutional Review Board (ECT 2014-04-023), and we received informed consent from all participants and their closest relatives.
2.2. Measurement of plasma Klotho concentrations
For estimation of plasma Klotho concentrations, blood samples in EDTA tubes were immediately collected at admission. Plasma was separated by centrifugation (1900 g, 15 min) at 4 °C [Citation17]. The acquired plasma was stored at −80 °C until analysis. An enzyme-linked immunosorbent assay (Immuno-Biological Laboratories, Gunma, Japan) was used for the evaluation of plasma Klotho concentration [Citation20]. Plasma Klotho concentrations were measured by researchers who were blinded to the clinical data (Y.C. and Y.M.J). Intra-assay and inter-assay coefficients of variability were 3.1 and 6.9%, respectively.
2.3. Statistical analysis
All statistical analysis was conducted by using SPSS software for Windows version 21.0 (SPSS, Chicago, IL, USA) or R package for Windows version 3.0.2 (R Foundation for Statistical Computing, Vienna, Austria). Categorical variables were analysed using Chi-square or Fisher’s exact test, and continuous variables were analysed using independent t-test, Mann–Whitney test or one-way ANOVA test.
To evaluate correlations of plasma Klotho concentrations with stroke severity (NIHSS), cerebral infarction volume on DWI, and other blood laboratory findings, Spearman correlation analyses were performed. Multivariate analyses were conducted to investigate associated factors for good functional outcome. Intracranial and extracranial cerebral atherosclerosis were dichotomized as cerebral atherosclerosis due to over-fitting in logistic regression. For logistic regression, plasma Klotho and FGF-23 were transformed by dividing 100 unit. Patients were grouped by good (mRS < 3) or poor (mRS ≥3) outcome for logistic regression. Age, sex, and other variables showing p < .1 during univariate analysis were entered into multivariate analyses. Subgroup analysis for the association of plasma Klotho concentrations with functional outcome was performed according to stroke subtype, NIHSS, and cerebral infarction volume. Owing to multicollinearity, the variables of NIHSS and cerebral infarction volume were separately entered in multivariate analysis and subgroup analysis. For investigating the prognostic ability of plasma Klotho levels in predicting good functional outcomes, receiver operating characteristic curve analysis was performed. The area under the curve (AUC) was calculated and the optimal cut-off value of plasma Klotho levels was defined at the level with the highest Youden index (sensitivity + specificity–1). To measure improvement in prediction performance by addition of plasma Klotho levels, change of AUC was computed. All p values were calculated using the two-tailed test. The p values below .05 indicate statistical significance.
3. Results
Demographic data of study patients are shown in . Of the 262 patients, 152 (58.0%) were men. The mean age was 64.7 years. The mean ± standard deviation of plasma Klotho concentrations was 312.7 ± 153.3 pg/mL. The study population was categorized into quartiles according to the plasma Klotho concentrations (149.7 ± 29.1, 242.9 ± 23.2, 337.1 ± 26.7 and 522.1 ± 128.8 pg/mL). The NIHSS, cerebral infarction volume, cerebral atherosclerosis, FGF-23 and white blood cell count were significantly different according to quartiles of the plasma Klotho concentrations (). Comparing patients had good outcome with those who had poor outcome, as opposed to patients with good outcome, plasma Klotho levels were lower in patients with poor outcome (207.8 ± 96.2 vs. 342.5 ± 153.5 pg/mL, p = .021). As opposed to patients with good outcome, patients with poor outcome were older (69.1 ± 13.0 vs. 63.5 ± 11.8 years), and more frequently had cerebral atherosclerosis (58.6% vs. 38.7%, p = .007), cardioembolic (31.0% vs. 14.2%) and large artery atherosclerosis (46.6% vs. 39.7%) subtypes (p = .001), and less frequently had taken pre-stroke statin medications (12.1% vs. 22.5%, p = .080). Patients with poor outcome also had higher NIHSS scores (median 8, interquartile range [Citation6–13] vs. 3 [1–5]), FGF-23 (759.1 ± 986.9 vs. 230.5 ± 229.7 pg/mL), white blood cell counts (8.5 ± 2.8 vs. 7.0 ± 2.2, count × 103) and C-reactive protein (2.2 ± 5.1 vs. 0.6 ± 1.4 mg/L) ().
Table 1. Characteristics of the study subjects according to quartile of plasma Klotho concentrations.
Table 2. Comparison of clinical and brain image findings according to clinical outcome at three months after index stroke.
The plasma Klotho concentrations were significantly and negatively associated with NIHSS (Rho = −0.253, p < .001), cerebral infarction volume (Rho = −0.223, p < .001), FGF-23 (Rho = −0.325, p = .001), creatinine (Rho = −0.130, p = .035) and C-reactive protein (Rho = −0.181, p = .003) (). However, there were no significant differences of Klotho concentration according to stroke subtype (p = .766) (Supplementary Table 1).
Table 3. Correlation of plasma Klotho with clinical and blood laboratory findings.
3.2. Association between plasma Klotho levels and functional outcome
In total enrolled patients, good functional outcome (mRS < 3) was noted in 204 (77.2%) patients. After adjusting for age, sex and other variables (statin intake, NIHSS, cerebral atherosclerosis, stroke subtype, FGF-23, white blood cell count and C-reactive protein) with p < .1 during univariate analysis, decreased plasma Klotho concentrations were independently associated with good functional outcome (odds ratio: 2.42; 95% confidence interval: 1.45–4.04; p < .001) (, Supplementary Table 2).
Table 4. Multivariable analysis for good clinical outcome at three months after index stroke with following dependent variables.
In subgroup analysis, the positive association of Klotho concentration with good functional outcome was consistently noted regardless of stroke subtype (p for interaction = .670) and quartiles cerebral infarction volume (p for interaction = .481). In contrast, the positive association of Klotho concentration with good functional outcome was significant in patients with relatively high (≥3 quartile) NIHSS (p for interaction = .017) (Supplementary Table 3).
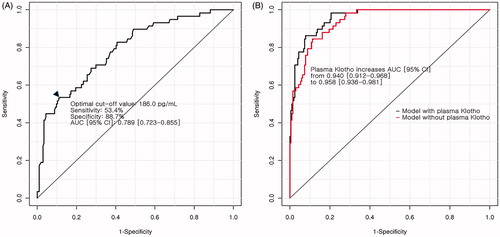
In receiver operating characteristic curve analysis, the AUC of plasma Klotho concentrations for predicting good functional outcome was 0.789 (95% confidence interval: 0.723–0.855). The optimal cut-off value of plasma Klotho concentrations were 186.0 pg/mL. Comparing the AUC of multivariate analysis with and without plasma Klotho concentrations, the AUC was significantly increased from 0.940 (95% confidence interval: 0.912–0.968) to 0.958 (95% confidence interval: 0.936–0.981; p = .022) ().
Figure 1. Results of receiver operating characteristic analysis for plasma alpha-Klotho concentration. (A) Receiver-operating characteristic curve using plasma alpha-Klotho concentrations alone for investigating poor functional outcome. Black arrowhead indicates the optimal cut-off point of plasma alpha-Klotho concentrations by Youden method. (B) Change in the AUC after the addition of plasma alpha-Klotho concentrations to the multivariate model for poor functional outcome.
4. Discussion
Our study demonstrated that increased plasma Klotho concentrations were significantly associated with good functional outcome at 3 months in patients with acute stroke.
Studies analysing the relationship between Klotho and cardiovascular disease, including stroke in human, are rare and most of them are genetic studies. These genetic studies showed that genetic variant of Klotho was correlated with risk for early-onset ischemic stroke [Citation21], and was a predictor of stroke [Citation22]. With regard to Klotho levels, previous clinical research showed that low circulating Klotho levels correlated with increased brachial-ankle pulse wave velocity, representing arterial stiffness in patients with renal failure [Citation23]. Moreover, low Klotho concentrations were also associated with vascular cognitive impairment [Citation24], and were related to the presence and severity of coronary artery diseases in patients where coronary angiography or cardiac surgery was performed [Citation25]. On the other hand, some studies did not show a relationship between Klotho and cardiovascular outcome. For example, plasma Klotho was not related to atherosclerotic events/death or decompensated heart failure/death in patients with chronic renal failure [Citation26], cardiovascular disease in dialysis patients [Citation27], mean platelet volume and platelet distribution width that may underlie thromboembolic disorders as opposed to FGF-23 [Citation28]. Although there are discrepancies, our study found new information suggesting that increased plasma Klotho concentrations were associated with good functional outcome, particularly in patients with acute ischemic stroke.
In our study, Klotho concentration was associated with not only functional outcome but also initial stroke severity (NIHSS and cerebral infarction volume), particularly in patients with severe stroke (high NIHSS). In other words, the reason for the positive relationship between Klotho concentrations and good functional outcome may be that the Klotho concentrations are related to the initial stroke severity. However, even if the NIHSS was adjusted in multivariate analysis, Klotho concentration was significantly associated with a functional outcome. Therefore, it can be assumed that Klotho concentration is independently related to functional recovery or outcome in addition to Klotho’s relationship with initial stroke severity.
Although our study did not suggest an exact mechanism for the relationship of plasma Klotho with poor functional outcome and stroke severity, some hypotheses may explain this association. First, endothelial dysfunction contributes to pathogenesis of stroke, leading to aggravated stroke severity, progression of the stroke and poor outcome [Citation29–31]. In a previous animal study, in-vivo gene delivery of Klotho protected against endothelial dysfunction [Citation32]. The Klotho is cleaved and released into the blood (circulatory form) where it may work as a vasculo-protective hormone, increasing nitric oxide availability in the endothelium [Citation33]. Therefore, decreased plasma Klotho concentrations may decrease protection against endothelial dysfunction, and hence may be associated with poor functional outcome. Second, Klotho is concerned in the vascular protection through inhibition of angiotensin II-induced reactive oxygen species, which is involved in cell apoptosis and inflammation [Citation34,Citation35]. In stroke, reactive oxygen species is closely correlated with stroke severity, stroke progression and poor outcome [Citation36,Citation37]. Down-regulation of inflammation and the vascular protective effect of Klotho may support our results. Third, arterial stiffness is closely correlated with functional outcome of acute cerebral infarction and initial stroke severity [Citation38,Citation39]. Klotho is involved in preservation of arterial wall integrity and pathogenesis of arterial stiffness or vascular calcification [Citation23,Citation40]. Therefore, decreased Klotho, an independent factor for arterial stiffness, may lead to poor outcome or severe stroke in acute ischemic stroke.
There are some limitations to this study. We did not estimate blood samples from the normal population. However, the main goals of our study were to compare plasma Klotho concentrations between good and poor functional outcome patients at 3 months after index stroke. Second, all of the blood samples were acquired from patients at admission. Therefore, we could not examine serial changes in Klotho during the course of a stroke. Third, the median NIHSS of the South Korea ischemic stroke was four points [Citation41], which was almost similar to our dataset but slightly higher. This is presumably due to our study exclusion of the stroke undetermined stroke subtype such as two or more causes identified subtype, which is known to have relatively poor prognosis [Citation42].
In conclusion, increased plasma Klotho concentrations were independently associated with good functional outcome in patients with acute ischemic stroke. We attribute these associations to the pleiotropic effects of Klotho in stroke and vascular diseases.
Supplemental Material
Download Zip (27 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Drueke TB, Massy ZA. Circulating Klotho levels: clinical relevance and relationship with tissue Klotho expression. Kidney Int. 2013;83:13–15.
- Semba RD, Cappola AR, Sun K, et al. Plasma Klotho and cardiovascular disease in adults. J Am Geriatr Soc. 2011;59:1596–1601.
- Kurosu H, Yamamoto M, Clark JD, et al. Suppression of aging in mice by the hormone Klotho. Science. 2005;309:1829–1833.
- Urakawa I, Yamazaki Y, Shimada T, et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444:770–774.
- Chang Q, Hoefs S, van der Kemp AW, et al. The beta-glucuronidase Klotho hydrolyzes and activates the TRPV5 channel. Science. 2005;310:490–493.
- Imura A, Tsuji Y, Murata M, et al. alpha-Klotho as a regulator of calcium homeostasis. Science. 2007;316:1615–1618.
- Kim JY, Kang K, Kang J, et al. Executive summary of stroke statistics in Korea 2018: a report from the epidemiology research council of the Korean stroke society. J Stroke. 2018;21:42–59.
- Arking DE, Becker DM, Yanek LR, et al. Klotho allele status and the risk of early-onset occult coronary artery disease. Am J Hum Genet. 2003;72:1154–1161.
- Xu T, Zuo P, Wang Y, et al. Serum omentin-1 is a novel biomarker for predicting the functional outcome of acute ischemic stroke patients. Clin Chem Lab Med. 2018;56:350–355.
- Furtner M, Ploner T, Hammerer-Lercher A, et al. The high-sensitivity cardiac troponin T assay is superior to its previous assay generation for prediction of 90-day clinical outcome in ischemic stroke. Clin Chem Lab Med. 2012;50:2027–2029.
- Song TJ, Kim YD, Yoo J, et al. Association between aortic atheroma and cerebral small vessel disease in patients with ischemic stroke. J Stroke. 2016;18:312–320.
- Chang Y, Kim J, Kim MH, et al. Interarm blood pressure difference is associated with early neurological deterioration, poor short-term functional outcome, and mortality in noncardioembolic stroke patients. J Clin Neurol. 2018;14:555–565.
- Oh MS, Yu KH, Lee JH, et al. Validity and reliability of a Korean version of the national institutes of health stroke scale. J Clin Neurol. 2012;8:177–183.
- Chang Y, Choi GS, Lim SM, et al. Interarm systolic and diastolic blood pressure difference is diversely associated with cerebral atherosclerosis in noncardioembolic stroke patients. Am J Hypertens. 2018;31:35–42.
- Song TJ, Chang Y, Kim AR, et al. High dietary glycemic load was associated with the presence and burden of cerebral small vessel diseases in acute ischemic stroke patients. Nutr Res. 2018;51:93–101.
- Adams HP, Jr., Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41.
- Song TJ, Kim J, Yang SH, et al. Association of plasma osteoprotegerin levels with stroke severity and functional outcome in acute ischaemic stroke patients. Biomarkers. 2012;17:738–744.
- Song TJ, Cho HJ, Chang Y, et al. Low plasma proportion of omega 3-polyunsaturated fatty acids predicts poor outcome in acute non-cardiogenic ischemic stroke patients. J Stroke. 2015;17:168–176.
- Song TJ, Chang Y, Chun MY, et al. High dietary glycemic load is associated with poor functional outcome in patients with acute cerebral infarction. J Clin Neurol. 2018;14:165–173.
- Yamazaki Y, Imura A, Urakawa I, et al. Establishment of sandwich ELISA for soluble alpha-Klotho measurement: age-dependent change of soluble alpha-Klotho levels in healthy subjects. Biochem Biophys Res Commun. 2010;398:513–518.
- Majumdar V, Nagaraja D, Christopher R. Association of the functional KL-VS variant of Klotho gene with early-onset ischemic stroke. Biochem Biophys Res Commun. 2010;403:412–416.
- Arking DE, Atzmon G, Arking A, et al. Association between a functional variant of the Klotho gene and high-density lipoprotein cholesterol, blood pressure, stroke, and longevity. Circ Res. 2005;96:412–418.
- Kitagawa M, Sugiyama H, Morinaga H, et al. A decreased level of serum soluble Klotho is an independent biomarker associated with arterial stiffness in patients with chronic kidney disease. PLoS One. 2013;8:e56695.
- Brombo G, Bonetti F, Ortolani B, et al. Lower plasma Klotho concentrations are associated with vascular dementia but not late-onset Alzheimer’s disease. Gerontology. 2018;64:414–421.
- Navarro-Gonzalez JF, Donate-Correa J, Muros de Fuentes M, et al. Reduced Klotho is associated with the presence and severity of coronary artery disease. Heart. 2014;100:34–40.
- Seiler S, Rogacev KS, Roth HJ, et al. Associations of FGF-23 and sKlotho with cardiovascular outcomes among patients with CKD stages 2–4. Clin J Am Soc Nephrol. 2014;9:1049–1058.
- Buiten MS, de Bie MK, Bouma-de Krijger A, et al. Soluble Klotho is not independently associated with cardiovascular disease in a population of dialysis patients. BMC Nephrol. 2014;15:197.
- Takeda Y, Fujita S, Ikemoto T, et al. The relationship of fibroblast growth factors 21 and 23 and alpha-Klotho with platelet activity measured by platelet volume indices. Clin Chem Lab Med. 2015;53:1569–1574.
- Cosentino F, Rubattu S, Savoia C, et al. Endothelial dysfunction and stroke. J Cardiovasc Pharmacol. 2001;38(2):S75–S78.
- Roquer J, Segura T, Serena J, et al. Endothelial dysfunction, vascular disease and stroke: the ARTICO study. Cerebrovasc Dis. 2009;27:25–37.
- Simak J, Gelderman MP, Yu H, et al. Circulating endothelial microparticles in acute ischemic stroke: a link to severity, lesion volume and outcome. J Thromb Haemost. 2006;4:1296–1302.
- Saito Y, Nakamura T, Ohyama Y, et al. In vivo Klotho gene delivery protects against endothelial dysfunction in multiple risk factor syndrome. Biochem Biophys Res Commun. 2000;276:767–772.
- Saito Y, Yamagishi T, Nakamura T, et al. Klotho protein protects against endothelial dysfunction. Biochem Biophys Res Commun. 1998;248:324–329.
- Rakugi H, Matsukawa N, Ishikawa K, et al. Anti-oxidative effect of Klotho on endothelial cells through cAMP activation. Endocrine. 2007;31:82–87.
- Maekawa Y, Ishikawa K, Yasuda O, et al. Klotho suppresses TNF-alpha-induced expression of adhesion molecules in the endothelium and attenuates NF-kappaB activation. Endocrine. 2009;35:341–346.
- Rodrigo R, Fernandez-Gajardo R, Gutierrez R, et al. Oxidative stress and pathophysiology of ischemic stroke: novel therapeutic opportunities. CNS Neurol Disord Drug Targets. 2013;12:698–714.
- Weston RM, Lin B, Dusting GJ, et al. Targeting oxidative stress injury after ischemic stroke in conscious rats: limited benefits with apocynin highlight the need to incorporate long term recovery. Stroke Res Treat. 2013;2013:648061.
- Lee YB, Park JH, Kim E, et al. Arterial stiffness and functional outcome in acute ischemic stroke. J Cerebrovasc Endovasc Neurosurg. 2014;16:11–19.
- Ormerod E, Ali K, Cameron J, et al. The association between arterial stiffness, initial stroke severity, and 3-week outcomes in patients with ischemic stroke. J Stroke Cerebrovasc Dis. 2017;26:2541–2546.
- Vervloet MG, Adema AY, Larsson TE, et al. The role of Klotho on vascular calcification and endothelial function in chronic kidney disease. Semin Nephrol. 2014;34:578–585.
- Kim BJ, Park JM, Kang K, et al. Case characteristics, hyperacute treatment, and outcome information from the clinical research center for stroke-fifth division registry in South Korea. J Stroke. 2015;17:38–53.
- Nam HS, Kim HC, Kim YD, et al. Long-term mortality in patients with stroke of undetermined etiology. Stroke. 2012;43:2948–2956.
