Abstract
Background
Comorbidities are commonly seen in patients with coronavirus disease 2019 (COVID-19), but the clinical implication is not yet well-delineated. We aim to characterize the prevalence and clinical implications of comorbidities in patients with COVID-19.
Methods
This is a retrospective multi-centre study involving patients admitted between January 16th and March 10th 2020. The composite endpoint was defined as the presence of at least one of the following, intensive care unit (ICU) admission, or the need for mechanical ventilation, or death.
Results
A total of 472 consecutive cases admitted to 51 certified COVID-19 tertiary care hospitals were enrolled (median age was 43 [32–53.5] years and 53.0% were male). There were 101 (21.4%) patients presented with comorbidities, including hypertension (15.0%), diabetes mellitus (7.8%), coronary artery disease (2.6%), chronic obstructive pulmonary disease (1.3%) and cerebrovascular disease (1.9%). The composite endpoint occurred in 65 (13.8%) patients. Multivariate stepwise logistic regression analysis indicated that older age (odds ratio [OR] 1.39, 95% confidence interval (CI) 1.05–1.85, per 10-year increment), antecedent hypertension (OR 2.82, 95% CI 1.09–7.29), neutrophil counts (OR 1.33, 95% CI 1.14–1.56) and lactate dehydrogenase level (OR 1.01, 95% CI 1.00–1.01) were independently associated with the presence of composite endpoint. Hypertensive patients, compared with controls, had a greater chance of experiencing the composite endpoint (p < .001) and each individual endpoint, i.e. ICU admission (p < .001), mechanical ventilation (p < .001) and death (p = .012). In the stepwise regression analysis of anti-hypertensive medications, none of the therapy predicted the composite endpoint.
Conclusions
Hypertension is a common comorbidity in patients with COVID-19 and associated with adverse outcomes.
Hypertension was identified as the comorbidity associated with the prognosis of COVID-19 in this retrospective cohort.
Patients with hypertension could experience an increased risk of the composite endpoint.
Anti-hypertensive therapy did not affect patient outcomes.
KEY MESSAGES
Background
The coronavirus disease 2019 (COVID-19) continues to spread worldwide and poses great challenge to local health care systems. The service of intensive care unit (ICU) is also under pressure in this pandemic, with around 5% to 16% of patients requiring admission to ICU [Citation1,Citation2]. Although our knowledge of COVID-19 is accumulating, some clinical manifestations of interest remain to be delineated. The proportion of patients with comorbidities ranged from 23.7% to 46.4% in some preliminary cohorts [Citation3–5], which intuitively complicated patient management. As reported in previous outbreaks of coronavirus infection, the presence of comorbidities in patients with COVID-19 also seems to affect the need for intensive care within the disease course, and the prognosis including a higher mortality rate [Citation6]. Therefore, evaluating the prevalence and impact of these chronic conditions is essential to mitigate COVID-19 complications, disease progression and mortality. The presence of comorbidities may merely serve as a combination of confounders that contributes to adverse outcomes, while certain comorbidities could truly interplay with the underlying pulmonary disease. Current clinical cohorts to date, however, often take the presence of comorbidities as a whole, which might result in over- or under-estimation of the role of a specific accompanying disease on COVID-19. Thus, we sought to characterise the prevalence and clinical implications of pre-existing comorbidities in a large cohort of patients with laboratory-confirmed COVID-19.
Methods
Study population
This is a retrospective multi-centre study. A total of 472 consecutive patients with laboratory-confirmed COVID-19 admitted to 51 certified COVID-19 tertiary care hospitals (listed in the Supplementary Additional file 1) within Sichuan province, China between January 16th and March 10th 2020 were enrolled. Around two fifth of cases came from three centers (i.e. The Public Health Clinical Centre of Chengdu, Dazhou Central Hospital and Luzhou Infectious Disease Hospital). Laboratory-confirmed cases were defined by a positive result on high throughput sequencing or real-time reverse-transcriptase–polymerase chain-reaction (RT-PCR) assay of nasal and/or pharyngeal swabs. The study was approved by the institutional ethics committee (Study Number 2020-226) and all patients provided written informed consent.
Data collection
Medical records, the use of medications, the findings of laboratory investigations were collected for all patients and cleaned on 14th March 2020. Admission laboratory investigations included the complete blood count, biochemical analysis, liver and renal function, inflammatory markers (C-reactive protein, CRP; pro-calcitonin), and cardiac biomarkers (high-sensitivity troponin T, hs-TnT; creatine kinase, CK, and MB sub-fraction, CK-MB; N-terminal-pro B-type natriuretic peptide, NT-proBNP; lactate dehydrogenase, LDH).
Study outcomes
The composite endpoint was defined as any of the following, admission to an ICU, or the need for mechanical ventilation or death. COVID-19 severity classified by the Novel Coronavirus-Pneumonia diagnostic criteria and treatment regimens defined by the National Health Commission of China [Citation7] was considered as a secondary outcome. A severe case was defined as any of the following, respiratory rate ≥ 30/min, resting oxygen saturation ≤93%, or oxygenation index (=PaO2/FiO2) ≤ 300 mmHg. A critical case was defined as respiratory failure requiring mechanical ventilation, shock (defined as a state of cellular and tissue hypoxia due to either reduced oxygen delivery, increased oxygen consumption, inadequate oxygen utilization, or a combination of these processes) or the need for intensive care.
Statistical analysis
Continuous variables were expressed as medians (interquartile ranges, IQR). Categorical variables were expressed as frequencies (percentages). Continuous variables were compared using Student’s t test or Mann-Whitney U test, as appropriate, and categorical variables using Chi-squared test or Fisher’s exact test, as appropriate. Logistic regression analyses were carried out to determine the odds ratio (OR) and 95% confidence intervals (CI) for covariates with the composite endpoint being the outcome. The multivariate stepwise logistic regression model on composite endpoints was performed with a p-value for entry (p < .20) and removal (p < .10) in the model. When exploring the effect of anti-hypertensive medications on the outcomes, we performed the stepwise logistic regression models by forcing the medications to be kept in the model. The variables entered in step 1 were the baseline characteristics. All statistical analyses were performed using Stata/MP 16.0 and a two-side p-value <.05 was considered as statistically significant.
Results
The median age was 43 years (IQR, 32–53.5 years) and 250 cases (53.0%) were male in this cohort (). The hospitalization duration was 15 days (IQR, 11–20 days). A total of 101 (21.4%) patients were presented with comorbidities, including hypertension (n = 71, 15.0%), diabetes mellitus (n = 37, 7.8%), coronary artery disease (n = 12, 2.6%), chronic obstructive pulmonary disease (n = 6, 1.3%) and cerebrovascular disease (n = 9, 1.9%). A total of 91 (19.3%) patients were classified as severe/critical case and 7 patients were intubated. The composite endpoint occurred in 65 (13.8%) patients. Patients with composite endpoints were older (p < .001); were more frequently to have comorbidities (hypertension: p < .001, diabetes mellitus: p < .001, coronary artery disease: p < .001, cerebrovascular disease: p < .001, chronic obstructive pulmonary disease: p = .01); had lower oxygenation index (p < .001), lymphocyte counts (p < .001), uric acid level (p = .025), but higher neutrophil counts (p < .001), aspartate aminotransferase (AST) (p < .001), CRP (p < .001) and LDH (p < .001) levels compared with patients without composite endpoints ().
Table 1. Baseline characteristics of enrolled patients.
Table 2. Predictors of the composite endpoint by multivariate stepwise logistic regression.
Multivariate stepwise logistic regression analysis indicated that an older age (OR 1.39, 95% CI 1.05–1.85), antecedent hypertension (OR 2.82, 95% CI 1.09–7.29), neutrophil counts (OR 1.33, 95% CI 1.14–1.56) and LDH level (OR 1.01, 95%CI 1.00–1.01) were independently associated with the presence of the composite endpoint.
Being the only comorbidity that remained significant for the presence of the composite endpoint in the multivariate analysis, subgroups stratified by the history of hypertension were compared. Hypertensive patients were older (p < .001); presented more frequently with other comorbidities (diabetes mellitus: p < .001, coronary artery disease: p < .001, cerebrovascular disease: p < .001); were more likely to be treated with angiotensin-converting enzyme inhibitor (ACEi)/angiotensin II receptor blocker (ARB), β-blockers, calcium channel blocker (CCB) (p < .001) and statins (p = .006) (). They also had lower values of serum albumin (p < .001), but higher values of AST (p < .001), total triglyceride (p = .003) and LDH (p = .002). Hypertensive patients, compared with controls, had a greater chance of experiencing the composite endpoint (31.0% vs. 10.7%, p < .001) and each individual endpoint, including ICU admission (21.7 vs. 5.7%, p < .001), mechanical ventilation (22.5% vs. 6.7%, p < .001) and death (2.8% vs. 0.3%, p = .012) ().
Figure 1. Clinical outcomes in COVID-19 patients with or without antecedent hypertension. (*p < 0.05; ***p < 0.001).
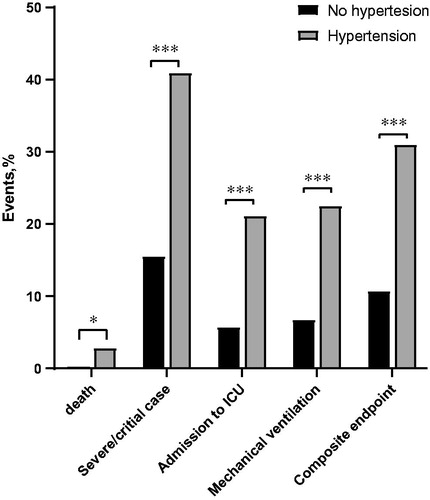
Table 3. Baseline characteristics and outcomes in patients with or without antecedent hypertension.
In the stepwise regression analysis for anti-hypertensive medications, none of the therapy was statistically significant for the presence of the composite endpoint (). Around 40% of patients with antecedent hypertension did not receive any anti-hypertensive medications. However, the occurrence of adverse events did not differ between patients treated with and without anti-hypertensive medications ().
Table 4. Multivariate stepwise logistic regression for each anti-hypertensive medication.
Table 5. Outcomes in patients with antecedent hypertension grouped by the usage of anti-hypertensive medications.
Discussion
Our study provides the few structured information concerning the role of comorbidities in the setting of COVID-19. We demonstrated that (1) Hypertension was identified as the comorbidity associated with the prognosis of COVID-19 in this retrospective cohort. Pre-existing hypertension was associated with a nearly 3-fold risk of the composite endpoint (i.e. admission to ICU, the need for mechanical ventilation and death) after adjusting for baseline characteristics, other comorbidities and the results of laboratory tests. (2) Anti-hypertensive therapy did not affect patient outcomes.
It is believed that patients with pre-existing medical conditions are particularly vulnerable to severe diseases. During previous outbreaks of coronavirus infections or pandemic influenzas, the reported risks of developing severe complications or progressing to a severe case are constantly higher in patients with underlying chronic diseases [Citation8,Citation9]. The notion of comorbidity is relatively large as it involves a range of diseases of different pathologies, thus it is worthwhile to weigh the impact separately in order to identify the disease type that truly contributes to adverse events. Patients with underlying chronic diseases are also likely to have a different clinical profile, making it difficult to determine whether the detrimental effects of comorbidities came from their susceptibility to acute damage or pathological interactions between comorbidities and the pulmonary illness.
In this analysis, we identified that hypertension was associated with adverse outcomes in COVID-19 after adjusting for baselines, other common comorbidities and laboratory indicators. The finding is consistent with previous reports which linked severe COVID-19 disease in patients with conditions associated with the therapy blocking renin-angiotensin-aldosterone system (RAAS), such as hypertension and diabetes mellitus [Citation3]. It is speculated that the dysregulation of RAAS may play a central role in the pathophysiology of COVID-19 related acute lung injury [Citation10]. Some other hypotheses also seem to support the observed harmful effect of hypertension. Adaptive immune disorders might be one reason why people with hypertension are at a higher risk for coronavirus and disease progression [Citation11]. While coronavirus also damages the heart directly [Citation12], in patients with hypertension whose heart function has already been weakened by the effects of high blood pressure, the double-hit by the virus infection is likely to cause more severe injury. More recently, higher risks of death and ICU admission in COVID-19 patients with hypertension were demonstrated by meta-analyses [Citation13,Citation14], further suggesting the detrimental role of hypertension in this pandemic. This characteristic could potentially contribute to a “phenotype” triage to determine patients of a higher risk and eventually benefit patient management. A validation of such findings should be confirmed in other ethnic groups as Chinese cohorts currently remain the main source of evidence.
Besides the above discussed theories, the possibility of anti-hypertensive therapy contributing to the detrimental role of hypertension in COVID-19 has gained popularity. Ever since angiotensin-converting enzyme 2 (ACE2) being identified as a functional receptor for coronaviruses [Citation15], there are ongoing concerns that patients with hypertension may face worse outcomes given many anti-hypertensive medications inhibit ACE which may induce increases in ACE2 activity [Citation16]. However, increased ACE2 expression does not guarantee automatic activation of downstream processes essential for viral entry, thus it remains questionable whether the increase of ACE 2 level leads to increase susceptibility to COVID-19 infection [Citation17]. In the same time, ACE2 also has a protective function as it degrades angiotensin II, which triggers a variety of adverse reactions such as enhanced inflammation and endothelial dysfunction [Citation18]. It should be noted that there are no current reliable data to justify the alteration of ACEi or ARB treatment in patients with COVID-19. In our analysis, although it was not designed to assess the effect of medications for comorbidities on COVID-19, anti-hypertensive therapy did not seem to influence the outcomes. Similarly, large retrospective studies aiming to evaluate the association of the use of ACEi or ARB with COVID-19 also revealed that ACEi or ARB did not affect the risk of COVID-19 [Citation19,Citation20]. The withdrawal of RAAS blockers in patients who commonly used these medications can otherwise lead to the risk of complications from their pre-existing comorbidities. Both the American College of Cardiology and the European Society of Cardiology have published statements advising against the discontinuation of ACEi/ARB treatment during this pandemic [Citation21].
Our study has certain limitations inherent to the retrospective design. The use of RT-PCR to group patients may exclude some infected patients due to the false negative issue in this test. Some patients in this cohort were admitted at an early stage of COVID-19, thus the complete documentation of exposure history and laboratory testing was not available for every patient. Obesity as a major comorbidity with a high prognostic value was not included in the multivariate analysis due to a significant deficiency of values for either weight or height of the patients. The lack of these parameters in retrospectively collected data may be resulted from a shortage of medical staff during patient management and the fact that weight and height did not affect clinical decisions much. The history of hypertension was obtained from the medical records and we were unable to further stratify the patients with either stable or fluctuating blood pressure during treatment. Events that might be worsened or improved during in-hospital period such as the severity of COVID-19 and the timing of intubation were not recorded as serial changes in our dataset.
Conclusions
Hypertension is a common comorbidity in patients with COVID-19 and associated with increased risk of the need for ICU admission, mechanical ventilation and death, but anti-hypertensive therapy did not affect patient outcomes. Preventative strategies and a close monitoring of blood pressure may be of use in the management of COVID-19 cases with hypertension.
Supplemental Material
Download MS Word (14.6 KB)Disclosure statements
No potential conflict of interest was reported by the author(s).
Availability of data and materials
The data are available from the corresponding author on reasonable request.
References
- Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239.
- Grasselli G, Pesenti A, Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA. 2020;323(16):1545.
- Guan W-J, Ni Z-Y, Hu Y, et al. China medical treatment expert group for Covid-19, clinical characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708–1720.
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506.
- Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 Novel Coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069.
- Novel Coronavirus Pneumonia Emergency Response Epidemiology Team, [The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41:145–151.
- National Health Commission of the People’s Republic of China, Novel Coronavirus-Pneumonia diagnostic and treatment regimens, n.d.; [cited 2020 July 27]. Available from: http://www.nhc.gov.cn/yzygj/s7653p/new_list.shtml.
- Badawi A, Ryoo SG. Prevalence of comorbidities in the Middle East respiratory syndrome coronavirus (MERS-CoV): a systematic review and meta-analysis. Int J Infect Dis. 2016;49:129–133.
- Mertz D, Kim TH, Johnstone J, et al. Populations at risk for severe or complicated influenza illness: systematic review and meta-analysis. BMJ. 2013;347:f5061
- Sanchis-Gomar F, Lavie CJ, Perez-Quilis C, et al. Angiotensin-converting enzyme 2 and antihypertensives (angiotensin receptor blockers and angiotensin-converting enzyme inhibitors) in Coronavirus Disease 2019. Mayo Clin Proc. 2020;95(6):1222–1230.
- Rai A, Narisawa M, Li P, et al. Adaptive immune disorders in hypertension and heart failure: focusing on T-cell subset activation and clinical implications. J Hypertens. 2020. [cited 2020 July 28]. DOI:10.1097/HJH.0000000000002456
- Xiong T-Y, Redwood S, Prendergast B, et al. Coronaviruses and the cardiovascular system: acute and long-term implications. Eur. Heart J. 2020;41(19):1798–1800.
- Zuin M, Rigatelli G, Zuliani G, et al. Arterial hypertension and risk of death in patients with COVID-19 infection: systematic review and meta-analysis. J Infect. 2020;81(1):e84–e86.
- Roncon L, Zuin M, Zuliani G, et al. Patients with arterial hypertension and COVID-19 are at higher risk of ICU admission. Br. J Anaesth. 2020;125(2):e254–e255.
- Turner AJ, Hiscox JA, Hooper NM. ACE2: from vasopeptidase to SARS virus receptor. Trends Pharmacol Sci. 2004;25(6):291–294.
- Ferrario CM, Jessup J, Chappell MC, et al. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111(20):2605–2610.
- Bourgonje AR, Abdulle AE, Timens W, et al. Angiotensin‐converting enzyme 2 (ACE2), SARS‐CoV‐2 and the pathophysiology of coronavirus disease 2019 (COVID‐19). J Pathol. 2020;251(3):228–248.
- Verdecchia P, Cavallini C, Spanevello A, et al. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur J Intern Med. 2020;76:14–20.
- Mehta N, Kalra A, Nowacki AS, et al. Association of use of angiotensin-converting enzyme inhibitors and angiotensin ii receptor blockers with testing positive for Coronavirus Disease 2019 (COVID-19). JAMA Cardiology. 2020.
- Mancia G, Rea F, Ludergnani M, et al. Renin–angiotensin–aldosterone system blockers and the risk of Covid-19. N Engl J Med. 2020;382(25):2431–2440.
- Busse LW, Chow JH, McCurdy MT, et al. COVID-19 and the RAAS—a potential role for angiotensin II? Crit Care. 2020;24(1):136.
