Abstract
The chemokine CCL5 plays a potential role in the occurrence and development of colorectal cancer (CRC). Previous studies have shown that CCL5 directly acts on tumor cells to change tumor metastatic rates. In addition, CCL5 recruits immune cells and immunosuppressive cells into the tumor microenvironment (TME) and reshapes the TME to adapt to tumor growth or increase antitumor immune efficacy, depending on the type of secretory cells releasing CCL5, the cellular function of CCL5 recruitment, and the underlying mechanisms. However, at present, research on the role played by CCL5 in the occurrence and development of CRC is still limited, and whether CCL5 promotes the occurrence and development of CRC and its role remain controversial. This paper discusses the cells recruited by CCL5 in patients with CRC and the specific mechanism of this recruitment, as well as recent clinical studies of CCL5 in patients with CRC.
CCL5 plays dual roles in colorectal cancer progression.
CCL5 remodels the tumor microenvironment to adapt to colorectal cancer tumor growth by recruiting immunosuppressive cells or by direct action.
CCL5 inhibits colorectal cancer tumor growth by recruiting immune cells or by direct action.
Key Messages
1. Introduction
Colorectal cancer (CRC) is the third most common solid tumor in the world and one of the leading causes of cancer-related death [Citation1]. The development of immunotherapy, including immune checkpoint inhibitors, chimeric antigen receptor T cells and tumor vaccines, has led to great progress in cancer treatment by unleashing the beneficial killing ability of T-cells in a variety of cancers [Citation2]. CRC has shown considerable resistance to multiple immunotherapies, especially blocking immune checkpoints, which have exhibited therapeutic effects on many other types of cancer [Citation3]. Cytotoxic CD8+ T cells constitute a main group of effector immune cells in antitumor immunity. The amount of intratumoral infiltration of CD8+ T cells is regarded as a positive prognostic indicator for many human cancer types [Citation4]. For example, cancers with a high tumor mutation burden and genomic instability, such as microsatellite unstable CRC, high CD8+ T-cell infiltration has been significantly associated with a good prognosis [Citation5]. Although there are a large number of circulating active CD8+ T cells in tumor patients, the lack of CD8+ T cells in the central tumor area has become a major obstacle to immunotherapy for solid tumors, especially in patients with CRC [Citation6]. The enhancement of CD8+ T cell-mediated antitumor immunity and the transport of CD8+ T cells to the tumor sites are essential for effective cancer treatment, abd chemokines regulate T-cell aggregation in solid tumors [Citation7].
Increasing evidence shows that chemokines play important roles in tumor growth and metastasis. Different members of the chemokine family promote or inhibit tumor growth by promoting or inhibiting tumor angiogenesis [Citation8]. With molecular weights ranging from 7 to 15 kDa, chemokines are the main proteins secreted to the extracellular space. To date, 50 chemokines have been identified [Citation9]. Although their main role is recruitment and activation of the immune response, their important role in the process of tumor cell invasion, metastasis and immune response escape has been increasingly recognized [Citation10]. Most tumors promote their own growth by recruiting stromal cells to shape the local chemokine network [Citation11]. Among the known human chemokines, CCL4, CCL5, CXCL9 and CXCL10 are closely related to CD8+ T-cell infiltration [Citation12]. Among these proteins, CCL5 affects tumor progression in an autocrine or a paracrine manner, such as by directly affecting cancer cell proliferation, migration and survival through its autocrine function or by indirectly recruiting inflammatory cells into the tumor microenvironment (TME)through paracrine function, thus shaping the TME for its own survival [Citation13]. In this study, we explored the role played by the chemokine CCL5in the occurrence and development of CRC. Herein, we explain the relevant regulatory mechanism of CCL5, to provide a reference for clinical research.
2. CCL5 and its receptors
The chemokine (cysteine-cysteine motif) ligand 5 (CCL5/RANTES) is a secretory small molecule protein that is expressed in the blood and TME. It was discovered in 1988 by Schall TJ et al. [Citation14] using a cDNA library enriched with T-cell-specific sequences [Citation14]. The gene product of CCL5 is 10 kDa (91 amino acids), and the signal peptide is approximately 8 kDa after cleavage. Of the 68 residues, 4 are cysteine residues and harbor no N-linked glycosylation sites [Citation14]. CCL5 is a member of the CC chemokine family because it has two pairs of adjacent cysteine residues near the amino terminus and is expressed and secreted by macrophages, T cells, tubular epithelial cells, synovial fibroblasts and certain types of cancer cells [Citation15]. As a chemokine, it mediates the involvement of a variety of cells in the inflammatory response, including the transport and homing of T cells, monocytes and NK cells [Citation16,Citation17]. The regulation of CCL5 expression is complex. IFN, IL-1 and TNF-α induce the activation of CCL5 through transcription factors, including NF-kB, interferon regulatory factor 1, interferon regulatory factor 3, interferon regulatory factor 7, STAT1 or STAT3 [Citation18–20] ().
Figure 1. Receptors related of the chemokine CCL5. In addition to the cognate receptor CCR5, specific receptors include CCR1, CCR3 and CCR4. Noncanonical receptors include ACKR1, ACKR2, CCRL2 and CD44.
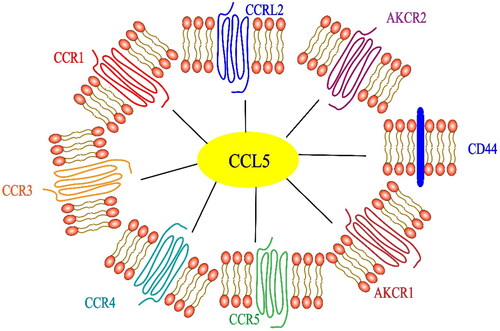
CCL5 induces a cell-type specific signaling response by binding to specific G protein-coupled receptors CCR1, CCR3, CCR4 and CCR5 on the surface of target cells [Citation21,Citation22]. CD44 is the coreceptor of CCL5 [Citation23]. The atypical receptor (nonsignal receptors such as ACKR1, ACKR2 and CCRL2) also interact with CCL5 [Citation24]. Among these receptors, CCR1 and CCR3 reside exclusively on the surface of immune cells [Citation25], but CCR5 expression has been found in all CRC samples [Citation26]. CCR5 is the main signal receptor of CCL5 and a potential drug target for most immune diseases [Citation27]. CCL5 has been proven to be the main coreceptor of human immunodeficiency virus-1 (HIV-1), which is very important for the pathogenesis of HIV-1 [Citation28]. There have been a large number of studies devoted to the role of CCR5 in HIV-1 infection. Specifically, HIV-1 binds to CD4+ T cells and enters CD4+ T cells with CD4 being the main receptor, while CCR5 is also a necessary receptor. The small-molecule inhibitor maraviroc and humanized monoclonal antibody leronlimab are CCR5 antagonists that inhibit the entry of the HIV-1 virus into CD4+ T cells [Citation29]. At present, the most promising method to block CCR5 is by administering the drug maraviroc, which is an allosteric inverse agonist of CCR5 [Citation30,Citation31]. However, recent studies have been more focused on cancer than on HIV-1 treatment. CCR5 is overexpressed in many types of cancers. Experiments in vivo and in vitro have shown that acquired resistance to trastuzumab in cancer patients was inhibited by maraviroc blocking CCL5/CCR5 binding [Citation31]. Maraviroc inhibits the binding of CCL5 and CCR5 by reducing the proliferation and metastasis of cancer cells [Citation32]. Although its potential role in cancer progression has been reported in some studies of various tumor types, including CRC, the CCL5/CCR5 axis has not been extensively studied in cancer treatment, and the role of CCL5 in CRC is still controversial. We believe that the main reason for the lack of clarity is because CCL5 is expressed in different cells and its specific receptors include CCR1, CCR3, CCR4 and CCR5 (see for details of its receptor-related functions). Different cells can be recruited to participate in the inflammatory response; therefore, the effect of CCL5 on the immune environment is different in physiological and pathological processes, even in different cancer models and tissue types, as explained in detail in .
Table 1. Function of the CCL5 receptor.
Table 2. Expression of CCL5 in human cells and its role.
2.1. The relationship between immunosuppressive cells recruited by CCL5 and tumorigenesis and progression
CCL5 can recruit a variety of cell types to respond to inflammation, including monocytes, macrophages, mast cells, eosinophils, basophils and dendritic cells. It is also involved in the migration of leukocytes to inflammatory tissues and the proliferation and activation of natural killer (NK) cells [Citation71,Citation72]. These cells and cancer cells constitute the TME, and most immunosuppressive cells undergo metabolic reprogramming in the TME. The metabolic changes in these cells lead to immunosuppression of CD8+ T cells and limit the antitumor immune response in advanced cancer. Here, we discuss the role and efficacy of some immunosuppressive cells such as mast cells (MCs) and tumor-associated macrophages in tumorigenesis and development ().
Figure 2. CCL5 promotes tumor progression by recruiting immunosuppressive cells. Mast cells recruited by CCL5 promote tumor neovascularization by secreting the cytokine TNF- α and activating the PI3K/AKT/GSK3 β/AM pathway; mast cells inhibit the activity of CD8+ T cells by secreting the cytokines IL-10 and TGF-β, and play immunosuppressive roles; mast cells and tumor-associated macrophages can directly promote tumor metastasis by secreting MMP-9. Tumor-associated macrophages are recruited by CCL5 to promote tumor neovascularization via the secretion VEGF; and TAMs directly inhibit the activity of CD8+ T cells.
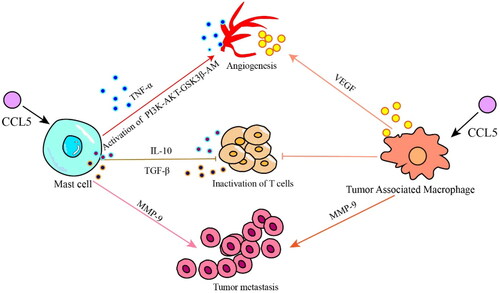
CCL5 is a chemoattractant for MCs. MCs are an immunosuppressive cells that inhibit the function of NK and other immune cells. MCs play a central role in the activation of gastrointestinal cells and endothelial cells, promoting tumor angiogenesis and progression. It has been reported that MCs play a role in promoting angiogenesis switching during tumor growth [Citation73,Citation74], and MCs and their secreted cytokines play important roles in inflammation-mediated tumorigenesis by regulating proinflammatory cytokines and inducible inflammatory enzymes [Citation75]. The main mechanism involves MCs secretion of TNF- α, which is the factor that induces tumor angiogenesis, and MCs activate the PI3K/AKT/GSK3β/AM signaling pathway to promote tumor microangiogenesis [Citation76]; MCs secrete excessive immunosuppressive cytokines such as IL-10 and TGF- β to inhibit the infiltration and function of CD8+ T cells, while CD8+ T cells are the key cell subsets that inhibit tumor growth and progression [Citation59,Citation77]; MCs also promote metastasis and escape of tumor cells from immune responses by upregulating matrix metalloproteinases (such as MMP9) [Citation78]. Studies by Tanaka et al. [Citation75] showed that mice lacking MCs were less susceptible to inflammation-related CRC [Citation75].
CCL5 binds to the receptor CCR5 and plays a role similar to that of oncogenes that is, it promotes tumor growth, induces extracellular matrix remodeling, recruits immune cells and polarizes tumor-associated macrophages (TAMs) [Citation64,Citation79]. CCL5 directly induce TAMs to secrete MMP9 and promote tumor cell metastasis [Citation80]. In the initial stage of cancer development, TAMs in the TME show typical activation or an M1-like phenotype and robust antitumor activity and secrete proinflammatory cytokines such as IL-1β, IL-6, IL-12 and IL-23 [Citation81]. However, in the later stage of cancer development, when growth factors and anti-inflammatory cytokines such as IL-4, IL-10 and TGF-β are enriched in the TME, TAMs polarize to anti-inflammatory M2-like phenotype, which helps to maintain an immunosuppressive TME [Citation82]. M2 TAMs constitute heterogeneous cell type that promotes malignant tumors through the production of angiogenic growth factors, extracellular matrix remodeling and immunosuppression [Citation83]. The epigenetic modification of cancer cells promotes the transport of TAMs to the TME, resulting in high PD-1 protein expression on CD8+ T cells and tumor progression [Citation84]. The expression of PD-1 on CD8+ T cells suppresses the activation of CD8+ T cells mediated by T-cell receptors [Citation85]. Moreover, blocking PD-1/PD-L1 in vivo increases the phagocytosis by TAMs and reduces tumor growth, indicating that PD-1/PD-L1 therapy may exert a direct effect on TAMs [Citation86]. The deletion of TAMs in the TME or inhibition of CSF1/CSF1R, the main survival ligand/receptor pair secreted by TAMs, significantly enhances the recruitment of CD8+ T cells and induces tumor regression [Citation87] ().
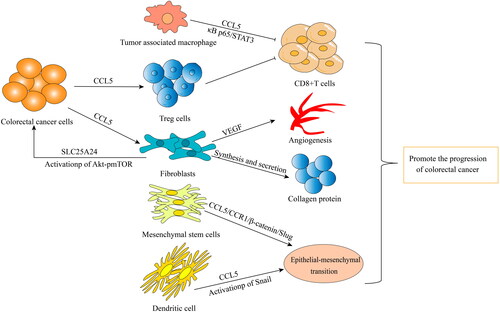
Figure 3. All kinds of cells in tumor microenvironment promote tumor progression in different ways: TAMs inhibit the activity of CD8+ T cells through CCL5/Kappa p65/STAT3 signaling pathway. Colorectal cancer cells recruit Treg cells to inhibit CD8+ T cell activity by secreting CCL5, or act on Flibroblasts by secreting CCL5. Flibroblasts promote tumor angiogenesis by secreting VEGF, synthesizing and secreting collagen protein, and activating SLC25A24 and Akt/pmTOR signaling pathways. Mesenchymal stem cells and dendritic cells promote stromal epithelial transformation by secreting CCL5.
3. Regulation of protective antitumor immunity mediated by CCL5
Some of the results show that the expression of CCL5 in CRC tissue has an antitumor effect, and its mechanism is realized by recruiting immune cells to the TME. Among these cells, type-1 dendritic cells (cDC1s) and CD8+ T cells play important roles in antitumor immunity. Their abundance in tumor and their activation triggered by therapy may enhance antitumor immunity and increase the response of cancer patients to immunotherapy [Citation88]. In fact, the increase in the rate of cDC1s infiltration into tumors and the protective effect it confers depend on the expression of CCL5 and CCR5. In addition, the expression of CCL5 and CCR5 is related to an increase in cDC1s in the TME and the prolonged overall survival time of cancer patients [Citation89]. The specific mechanisms of cDC1s action in the TME include cDC1s absorption of dead tumor cells and the transport of tumor antigens to the lymph nodes that drain the tumor [Citation90]. In addition to this transport, intratumoral cDC1s attract CD8+ T cells restimulating and attracting tumor-specific CD8+ T cells to play an antitumor immune role [Citation91]. Other studies have shown that cDC1-derived IL-12 is important for the antitumor activity of NK cells [Citation92]. In addition, infiltrating CD8+ T cells or other types of cells that produce CCL5 may maintain cDC1 recruitment [Citation88]. This possibility suggests that the activation of CCL5/CCR5 signaling may enhance the antitumor immune effect of CRC cancer patients. CCL5 inhibits tumor growth by promoting the infiltration of antitumor immune cells cDC1s into the TME, producing a more effective antitumor immune response. In addittion, some studies have suggested that in CRC tissue, the increase in CCL5 level is positively correlated with the number and activity of CD8+ cytotoxic T cells. It has been suggested that the expression of CCL5 in CRC tissue has exerts an antitumor effect [Citation93]. Therefore, some people think that cancer immunotherapy should be combined with a strategy to increas the expression of CCL5 in tumors, thereby enhancing the infiltration of various immune cells into the TME, to increase the therapeutic effect [Citation94].
4. The role of CCL5 in the tumor microenvironment
Tumorigenesis is a multistage process that is usually initiated by activating oncogenes or suppressing mutations in tumor suppressor genes. However, tumor cells often need additional factors in the TME to maintain their survival, growth and angiogenic functions [Citation95]. The TME is an important component of the invasive and metastatic potential of CRC cells [Citation96]. The tumor matrix in the TME includes the extracellular matrix and a variety of host cells, including immune cells, vascular cells and mesenchymal cells [Citation97]. Stromal cells, inflammatory cells and cancer cells communicate directly through cell contact and indirectly through paracrine signaling [Citation98]. These signals include chemokines, with CCL5 involved in many mechanisms of cancer progression, including cell proliferation, migration, invasion, angiogenesis, metastasis and colonization and the regulation of the extracellular matrix and immune escape mechanism of cancer, as signaling [Citation99,Citation100]. The expression level of CCL5 is related to the growth and metastasis of many kinds of cancers [Citation101–105], including CRC, and CCL5 not only plays an important role in the progression of CRC but can also be used to evaluate the curative effect of treatment and for diagnostics [Citation106,Citation107]. It may even predict severe hand and foot skin reactions in mCRC patients treated with repagenil [Citation108].
In addition to acting directly on CRC cells as a tumor-promoting factor, CCL5 appears to be a regulator of inflammatory cell infiltration into tumor tissue [Citation100]. Compared with that in control mice, the infiltration of CD8+ T cells into primary CRC tissues in CCL5−/− mice was significantly increased. CCL5 deficiency upregulated the expression of PD-1 and PD-L1, and reduced drug resistance to anti-PD-1 antibody therapy. Knocking out CCL5 can lead to metabolic disorders in TAMs in the TME of CRC patients, which promotes the migration of CD8+ T cells in the TME [Citation6]. TAMs are important to the TME and play roles in the occurrence and development of tumors by promoting the immune escape of tumor cells. CCL5 secreted by TAMs inhibits T cells and promotes tumor cell immune escape by stabilizing PD-L1 in vitro and in vivo. Mechanistically, CCL5 leads to the formation of the nuclear factor κρρa p65/STAT3 complex, which binds to the COP9 signal 5 (CSN5) promoter to upregulates CSN5 gene expression. Then, CSN5 regulates the deubiquitination and stability of PD-L1. High expression of CSN5 in CRC patients is associated with significantly shorter survival time [Citation57]. The role played human mesenchymal stem cells in promoting CRC progression in the TME is due to the activation of epithelial-mesenchymal transition (EMT) mediated by the CCL5/CCR1/β-catenin/Slug pathway, indicating that CCL5 is an important factor in the regulation of CRC development through its mediation of stromal cell and cancer cell interaction [Citation61]. In addition, chemokines secreted by mesenchymal cells may be important factors in the interaction between the TME mesenchymal cells and cancer cells. Specifically, the CCL3/4/5-CCR5 axis promotes tumor progression through the interactions between mesenchymal cells and CRC cells [Citation97]. Jung-Yu Kan et al. [Citation109] found that tumor-associated dendritic cells regulated the EMT and promoted the progression of CRC through CCL5-mediated Snail (a basic helix-loop-helix transcription factor) upregulation and downregulation in the protein expression of E-cadherin and other connective and adhesion proteins [Citation109]. Moreover, CCL5 from tumor buds recruitment fibroblasts by acting on CCR5 receptors on fibroblasts. CCL5 derived from tumor buds also positively regulated the expression of solute carrier family 25 member 24 (SLC25A24) in fibroblasts, which may have activated the p Akt/pmTOR signal transduction pathway. In addition, CCL5 increased the number of fibroblasts in the TME, promoting tumor angiogenesis by enhancing VEGFA expression and fibroblasts transdifferentiation into vascular endothelial cells. Finally, the results showed that CCL5 promoted the synthesis of collagen in fibroblasts, promoting the progress of CRC [Citation110]. Interestingly, the expression of CCL5 not only promoted the migration of Treg cells to tumors but also enhanced the ability of Treg cells to kill CD8+T cells [Citation50]. In addition, CCL5 is an important factor in CRC cell immune escape by increasing the accumulation of bone marrow-derived suppressor cells during development CRC [Citation111].
Increased expression of CCL5 has been found in the tumor tissues of CRC patients who drank alcohol. Further studies found that CCL5 enhanced autophagy in tumor cells and increased the migration ability of CRC cells by activating the AMPK signaling pathway, promoting the progression of CRC [Citation112]. In addition, the expression of CCL5 in tumor tissues of patients with CRC may promote tumor invasion and lymph node metastasis [Citation113]. In vitro, it was found that CCL5 promoted the migration of CRC cells and the proliferation of tumor cells in a dose-dependent manner [Citation100]. In a mouse experiment, abnormal intestinal flora in NLRP6 inflammatory factor deficient mice induced colonic inflammation by inducing an increase in the level of chemokine CCL5, leading to the eventually development of CRC [Citation114].
5. Promising clinical results indicate the use of CCL5 in colorectal cancer immunotherapy
CCL5 itself or the cells it recruits are closely related to the progression of CRC. Some studies have used CCL5 as a treatment index to evaluate CRC patients or cells recruited by CCL5 as carriers for targeted treatment in CRC patients. For example, CCL5 is involved in angiogenesis and is associated with the increased expression of vascular endothelial growth factor in cancer cells and vascular endothelial cells [Citation115]. Therefore, in the NCT04397601 study performed by Ottaiano et al. CCL5 was regarded as an indicator to evaluate the efficacy of bevacizumab and aflibercept in the treatment of mCRC. In addition, an ongoing NCT04713891 study to evaluate the safety and antitumor activity of oral KF-0210 as a single drug in advanced solid tumor participants was also based on CCL5 as an evaluation indicator. In addition, genetic variations in CCL5 and CCR5 single nucleotide polymorphisms may predict the efficacy of first-line chemotherapy based on cetuximab in mCRC patients, as well as the efficacy of bevacizumab in mCRC patients [Citation26,Citation99]. Further study on how the CCL5/CCR5 signaling axis acts on host immune cells and cancer cells to create an antitumor and anticancer microenvironment may provide useful insights into options for future drug development [Citation92]. Gene therapy has been widely studied in recent years, especially for the treatment of metastatic diseases. Mesenchymal cells in the TME are excellent gene carriers, in part because they are relatively easy to culture and expand in vitro. Importantly, these cells also show a significant natural tendency to migrate towards solid tumors [Citation116]. Kerstin Knoop et al. used the sodium iodide transporter-encoding gene in mesenchymal stem cell-mediated tumor matrix-targeted radioiodine therapy for metastatic colon cancer [Citation117]. Similarly, TAMs in the TME are excellent gene delivery vectors, and TAMs constitute the main cell type secreting CCL5. Considering the emerging TAM-targeting nanomaterials in recent years [Citation118], TAM-targeting nanomaterials loaded with CCL5-neutralizing antibodies may be a promising strategy to inhibit the activity of TAMs/CCL5.
6. Conclusion
Generally, recent research results on the role played by CCL5 in the immune regulatory network in patients with CRC have been controversial. Some studies suggested that the secretion of CCL5 directly affects the proliferation, migration and survival of CRC cancer cells or indirectly recruit inflammatory cells into the TME, shaping the TME of CRC patients and playing a variety of roles in their own survival. In contrast, other studies have shown that the expression of CCL5 in CRC tissue exerts an antitumor effect, and its mechanism of antitumor immunity involves recruiting immune cells, mainly cDC1s and CD8+ T cells, to the TME. We believe that the main reason is because CCL5 binds many receptors, including CCR1, CCR3, CCR4 and CCR5.In addition, the recruitment rates of CCL5 secreted by different cells and the underlying mechanisms of action differ. After binding to a specific receptor, CCL5 triggers signaling through different downstream cascades. Although research on the role of CCL5 in the immune regulatory network in CRC patients has led to significant increases in understanding, determining the effects of changes to CCL5 actions in CRC patients, will provide further theoretical and practical directions for targeted immunotherapy strategies.
In the future, relevant studies on whether CCL5 is involved in the occurrence and development of colorectal cancer and tumor immunosuppression can be initiated with cells known to secrete CCL5 and by determining other kind of cells that secrete CCL5 and can be continued by studying the activation of downstream signaling pathways after the CCL5 interacts with CCR5. The relationships among pathway activation and tumor microenvironment changes, tumor angiogenesis, tumor cell invasion and metastasis, lymph node metastasis, immune cell recruitment and immunosuppressive cell recruitment are related to targeted pathways and related cell activation, providing ideas for clinical immunotherapy.
Author contributions
X-L Z, X-F Z, H-Y L: conceived and designed the paper. H-Y L: Conceptualization and financial support. X-L Z drafted the manuscript. X-F Z: contributed equally with first author to this work. X-L Z and X-F Z extracted the data. Y-F, Y-J W, M-L L and X-Y L: interpreted the data and manuscript revision. X-F Z and Y-J W made serious changes to the revision opinions and provided figures for the revised draft. Y-T provided revision advice and guidance for the final draft. Y-T revised final draft critically for important intellectual content. All authors contributed to the article and approved the submitted version.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article.
Additional information
Funding
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):1–11.
- Zhong Z, Sanchez-Lopez E, Karin M. Autophagy, inflammation, and immunity: a troika governing cancer and its treatment. Cell. 2016;166(2):288–298.
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454.
- Fridman WH, Pages F, Sautes-Fridman C, et al. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12(4):298–306.
- Ogino S, Galon J, Fuchs CS, et al. Cancer immunology–analysis of host and tumor factors for personalized medicine. Nat Rev Clin Oncol. 2011;8(12):711–719.
- Zhang S, Zhong M, Wang C, et al. CCL5-deficiency enhances intratumoral infiltration of CD8(+) T cells in colorectal cancer. Cell Death Dis. 2018;9(7):766.
- Dangaj D, Bruand M, Grimm AJ, et al. Cooperation between constitutive and inducible chemokines enables T cell engraftment and immune attack in solid tumors. Cancer Cell. 2019;35(6):885.e810–900.e810.
- Lv D, Zhang Y, Kim HJ, et al. CCL5 as a potential immunotherapeutic target in triple-negative breast cancer. Cell Mol Immunol. 2013;10(4):303–310.
- Palomino DC, Marti LC. Chemokines and immunity. Einstein. 2015;13(3):469–473.
- Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4(7):540–550.
- Viola A, Sarukhan A, Bronte V, et al. The pros and cons of chemokines in tumor immunology. Trends Immunol. 2012;33(10):496–504.
- Romero JM, Grunwald B, Jang GH, et al. A four-chemokine signature is associated with a T-cell-Inflamed phenotype in primary and metastatic pancreatic cancer. Clin Cancer Res. 2020;26(8):1997–2010.
- Broekman ML, Maas SLN, Abels ER, et al. Multidimensional communication in the microenvirons of glioblastoma. Nat Rev Neurol. 2018;14(8):482–495.
- Schall TJ, Jongstra J, Dyer BJ, et al. A human T cell-specific molecule is a member of a new gene family. J Immunol. 1988;141(3):1018–1025.
- Soria G, Ben-Baruch A. The inflammatory chemokines CCL2 and CCL5 in breast cancer. Cancer Lett. 2008;267(2):271–285.
- Hinrichs AC, Blokland SLM, Lopes AP, et al. Transcriptome analysis of CCR9+ T helper cells from primary Sjogren’s syndrome patients identifies CCL5 as a novel effector molecule. Front Immunol. 2021;12:702733.
- Hinrichs AC, Blokland SLM, Kruize AA, et al. CCL5 release by CCR9+ CD8 T cells: a potential contributor to immunopathology of primary Sjogren’s syndrome. Front Immunol. 2022;13:887972.
- Mladinich MC, Conde JN, Schutt WR, et al. Blockade of autocrine CCL5 responses inhibits Zika virus persistence and spread in human brain microvascular endothelial cells. mBio. 2021;12(4):e0196221.
- Liu J, Guan X, Ma X. Interferon regulatory factor 1 is an essential and direct transcriptional activator for interferon {gamma}-induced RANTES/CCl5 expression in macrophages. J Biol Chem. 2005;280(26):24347–24355.
- Ma J, Shayiti F, Ma J, et al. Tumor-associated macrophage-derived CCL5 promotes chemotherapy resistance and metastasis in prostatic cancer. Cell Biol Int. 2021;45(10):2054–2062.
- Nagarsheth N, Wicha MS, Zou W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat Rev Immunol. 2017;17(9):559–572.
- Seki E, De Minicis S, Gwak GY, et al. CCR1 and CCR5 promote hepatic fibrosis in mice. J Clin Invest. 2009;119(7):1858–1870.
- Roscic-Mrkic B, Fischer M, Leemann C, et al. RANTES (CCL5) uses the proteoglycan CD44 as an auxiliary receptor to mediate cellular activation signals and HIV-1 enhancement. Blood. 2003;102(4):1169–1177.
- Bronte V, Bria E. Interfering with CCL5/CCR5 at the tumor-stroma interface. Cancer Cell. 2016;29(4):437–439.
- Halama N, Zoernig I, Berthel A, et al. Tumoral immune cell exploitation in colorectal cancer metastases can be targeted effectively by anti-CCR5 therapy in cancer patients. Cancer Cell. 2016;29(4):587–601.
- Suenaga M, Stintzing S, Cao S, et al. Role of CCL5 and CCR5 gene polymorphisms in epidermal growth factor receptor signalling blockade in metastatic colorectal cancer: analysis of the FIRE-3 trial. Eur J Cancer. 2019;107:100–114.
- Gerard C, Rollins BJ. Chemokines and disease. Nat Immunol. 2001;2(2):108–115.
- Berger EA, Murphy PM, Farber JM. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu Rev Immunol. 1999;17:657–700.
- Griffith JW, Sokol CL, Luster AD. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu Rev Immunol. 2014;32:659–702.
- Rosenbaum DM, Rasmussen SG, Kobilka BK. The structure and function of G-protein-coupled receptors. Nature. 2009;459(7245):356–363.
- Zazo S, Gonzalez-Alonso P, Martin-Aparicio E, et al. Autocrine CCL5 effect mediates trastuzumab resistance by ERK pathway activation in HER2-positive breast cancer. Mol Cancer Ther. 2020;19(8):1696–1707.
- Velasco-Velazquez M, Jiao X, De La Fuente M, et al. CCR5 antagonist blocks metastasis of basal breast cancer cells. Cancer Res. 2012;72(15):3839–3850.
- Weber C, Weber KS, Klier C, et al. Specialized roles of the chemokine receptors CCR1 and CCR5 in the recruitment of monocytes and T(H)1-like/CD45RO(+) T cells. Blood. 2001;97(4):1144–1146.
- Zernecke A, Shagdarsuren E, Weber C. Chemokines in atherosclerosis: an update. Arterioscler Thromb Vasc Biol. 2008;28(11):1897–1908.
- Furuichi K, Gao JL, Horuk R, et al. Chemokine receptor CCR1 regulates inflammatory cell infiltration after renal ischemia-reperfusion injury. J Immunol. 2008;181(12):8670–8676.
- Bertrand CP, Ponath PD. CCR3 blockade as a new therapy for asthma. Expert Opin Investig Drugs. 2000;9(1):43–52.
- Pease JE. Targeting chemokine receptors in allergic disease. Biochem J. 2011;434(1):11–24.
- Willems LI, Ijzerman AP. Small molecule antagonists for chemokine CCR3 receptors. Med Res Rev. 2010;30(5):778–817.
- Pease JE, Horuk R. Recent progress in the development of antagonists to the chemokine receptors CCR3 and CCR4. Expert Opin Drug Discov. 2014;9(5):467–483.
- Yamaguchi M, Takagi K, Narita K, et al. Stromal CCL5 promotes breast cancer progression by interacting with CCR3 in tumor cells. Int J Mol Sci. 2021;22:1981.
- Iellem A, Mariani M, Lang R, et al. Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4(+)CD25(+) regulatory T cells. J Exp Med. 2001;194(6):847–853.
- Purandare AV, Somerville JE. Antagonists of CCR4 as immunomodulatory agents. Curr Top Med Chem. 2006;6(13):1335–1344.
- Ishida T, Ueda R. CCR4 as a novel molecular target for immunotherapy of cancer. Cancer Sci. 2006;97(11):1139–1146.
- Scheu S, Ali S, Ruland C, et al. The C-C chemokines CCL17 and CCL22 and their receptor CCR4 in CNS autoimmunity. Int J Mol Sci. 2017;18:2306.
- Matsuo K, Nagakubo D, Komori Y, et al. CCR4 is critically involved in skin allergic inflammation of BALB/c mice. J Invest Dermatol. 2018;138(8):1764–1773.
- Jiao X, Velasco-Velazquez MA, Wang M, et al. CCR5 governs DNA damage repair and breast cancer stem cell expansion. Cancer Res. 2018;78(7):1657–1671.
- Do HTT, Lee CH, Cho J. Chemokines and their receptors: multifaceted roles in cancer progression and potential value as cancer prognostic markers. Cancers. 2020;12(2):287.
- Novak M, Koprivnikar Krajnc M, Hrastar B, et al. CCR5-Mediated signaling is involved in invasion of glioblastoma cells in its microenvironment. Int J Mol Sci. 2020;21:4199.
- Aldinucci D, Borghese C, Casagrande N. Formation of the immunosuppressive microenvironment of classic hodgkin lymphoma and therapeutic approaches to counter it. Int J Mol Sci. 2019;20:2416.
- Chang LY, Lin YC, Mahalingam J, et al. Tumor-derived chemokine CCL5 enhances TGF-beta-mediated killing of CD8(+) T cells in Colon cancer by T-regulatory cells. Cancer Res. 2012;72(5):1092–1102.
- Ban Y, Mai J, Li X, et al. Targeting autocrine CCL5-CCR5 axis reprograms immunosuppressive myeloid cells and reinvigorates antitumor immunity. Cancer Res. 2017;77(11):2857–2868.
- Wang SW, Liu SC, Sun HL, et al. CCL5/CCR5 axis induces vascular endothelial growth factor-mediated tumor angiogenesis in human osteosarcoma microenvironment. Carcinogenesis. 2015;36(1):104–114.
- Yang X, Hou J, Han Z, et al. One cell, multiple roles: contribution of mesenchymal stem cells to tumor development in tumor microenvironment. Cell Biosci. 2013;3(1):5.
- Wang X, Lang M, Zhao T, et al. Cancer-FOXP3 directly activated CCL5 to recruit FOXP3(+) treg cells in pancreatic ductal adenocarcinoma. Oncogene. 2017;36(21):3048–3058.
- Wang B, Qin Y, Wu Q, et al. mTOR signaling pathway regulates the release of proinflammatory molecule CCL5 implicated in the pathogenesis of autism spectrum disorder. Front Immunol. 2022;13:818518.
- Yang T, Deng Z, Xu L, et al. Macrophages-aPKCi-CCL5 feedback loop modulates the progression and chemoresistance in cholangiocarcinoma. J Exp Clin Cancer Res. 2022;41(1):23.
- Liu C, Yao Z, Wang J, et al. Correction: macrophage-derived CCL5 facilitates immune escape of colorectal cancer cells via the p65/STAT3-CSN5-PD-L1 pathway. Cell Death Differ. 2020;27(7):2293.
- Chen D, Bao X, Zhang R, et al. Depiction of the genomic and genetic landscape identifies CCL5 as a protective factor in colorectal neuroendocrine carcinoma. Br J Cancer. 2021;125(7):994–1002.
- Liu T, Xia Q, Zhang H, et al. CCL5-dependent mast cell infiltration into the tumor microenvironment in clear cell renal cell carcinoma patients. Aging. 2020;12(21):21809–21836.
- Marin Oyarzun CP, Glembotsky AC, Goette NP, et al. Platelet Toll-Like receptors mediate thromboinflammatory responses in patients with essential thrombocythemia. Front Immunol. 2020;11:705.
- Chen K, Liu Q, Tsang LL, et al. Human MSCs promotes colorectal cancer epithelial-mesenchymal transition and progression via CCL5/beta-catenin/slug pathway. Cell Death Dis. 2017;8(5):e2819.
- Keophiphath M, Rouault C, Divoux A, et al. CCL5 promotes macrophage recruitment and survival in human adipose tissue. Arterioscler Thromb Vasc Biol. 2010;30(1):39–45.
- Eriksson EE. Mechanisms of leukocyte recruitment to atherosclerotic lesions: future prospects. Curr Opin Lipidol. 2004;15(5):553–558.
- Long H, Xie R, Xiang T, et al. Autocrine CCL5 signaling promotes invasion and migration of CD133+ ovarian cancer stem-like cells via NF-kappaB-mediated MMP-9 upregulation. Stem Cells. 2012;30(10):2309–2319.
- Moriyama M, Hayashida JN, Toyoshima T, et al. Cytokine/chemokine profiles contribute to understanding the pathogenesis and diagnosis of primary Sjogren’s syndrome. Clin Exp Immunol. 2012;169(1):17–26.
- Manfroi B, De Grandis M, Moreaux J, et al. The microenvironment of DLBCL is characterized by noncanonical macrophages recruited by tumor-derived CCL5. Blood Adv. 2021;5(21):4338–4351.
- Pham K, Huynh D, Le L, et al. E-cigarette promotes breast carcinoma progression and lung metastasis: macrophage-tumor cells crosstalk and the role of CCL5 and VCAM-1. Cancer Lett. 2020;491:132–145.
- Wang X, Li X, Wei X, et al. PD-L1 is a direct target of cancer-FOXP3 in pancreatic ductal adenocarcinoma (PDAC), and combined immunotherapy with antibodies against PD-L1 and CCL5 is effective in the treatment of PDAC. Signal Transduct Target Ther. 2020;5:38.
- Jia J, Zhang H, He L, et al. Cutaneous neurofibroma cells with active Yap promotes proliferation of macrophages resulting in increased accumulation of macrophages by modulating CCL5 and TGFbeta1. Oncol Rep. 2020;43(4):1319–1330.
- Huang R, Wang S, Wang N, et al. CCL5 derived from tumor-associated macrophages promotes prostate cancer stem cells and metastasis via activating beta-catenin/STAT3 signaling. Cell Death Dis. 2020;11(4):234.
- Aldinucci D, Casagrande N. Inhibition of the CCL5/CCR5 axis against the progression of gastric cancer. Int J Mol Sci. 2018;19:1477.
- Marcuzzi E, Angioni R, Molon B, et al. Chemokines and chemokine receptors: orchestrating tumor metastasization. Int J Mol Sci. 2018;20:96.
- Marech I, Ammendola M, Gadaleta C, et al. Possible biological and translational significance of mast cells density in colorectal cancer. World J Gastroenterol. 2014;20:8910–8920.
- Coussens LM, Raymond WW, Bergers G, et al. Inflammatory mast cells up-regulate angiogenesis during squamous epithelial carcinogenesis. Genes Dev. 1999;13(11):1382–1397.
- Tanaka T, Ishikawa H. Mast cells and inflammation-associated colorectal carcinogenesis. Semin Immunopathol. 2013;35(2):245–254.
- Chen Y, Li C, Xie H, et al. Infiltrating mast cells promote renal cell carcinoma angiogenesis by modulating PI3K–>AKT–>GSK3beta–>AM signaling. Oncogene. 2017;36(20):2879–2888.
- Rigoni A, Colombo MP, Pucillo C. The role of mast cells in molding the tumor microenvironment. Cancer Microenviron. 2015;8(3):167–176.
- Krstic J, Santibanez JF. Transforming growth factor-beta and matrix metalloproteinases: functional interactions in tumor stroma-infiltrating myeloid cells. ScientificWorldJournal. 2014;2014:521754.
- Cao P, Ma B, Sun D, et al. Hsa_circ_0003410 promotes hepatocellular carcinoma progression by increasing the ratio of M2/M1 macrophages through the miR-139-3p/CCL5 axis. Cancer Sci. 2022;113(2):634–647.
- Robinson SC, Scott KA, Balkwill FR. Chemokine stimulation of monocyte matrix metalloproteinase-9 requires endogenous TNF-alpha. Eur J Immunol. 2002;32(2):404–412.
- Derlindati E, Dei Cas A, Montanini B, et al. Transcriptomic analysis of human polarized macrophages: more than one role of alternative activation? PLOS One. 2015;10(3):e0119751.
- Rolny C, Mazzone M, Tugues S, et al. HRG inhibits tumor growth and metastasis by inducing macrophage polarization and vessel normalization through downregulation of PlGF. Cancer Cell. 2011;19(1):31–44.
- DeNardo DG, Ruffell B. Macrophages as regulators of tumour immunity and immunotherapy. Nat Rev Immunol. 2019;19(6):369–382.
- Wang D, Li X, Li J, et al. APOBEC3B interaction with PRC2 modulates microenvironment to promote HCC progression. Gut. 2019;68(10):1846–1857.
- Sheppard KA, Fitz LJ, Lee JM, et al. PD-1 inhibits T-cell receptor induced phosphorylation of the ZAP70/CD3zeta signalosome and downstream signaling to PKCtheta. FEBS Lett. 2004;574(1-3):37–41.
- Gordon SR, Maute RL, Dulken BW, et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature. 2017;545(7655):495–499.
- Etzerodt A, Tsalkitzi K, Maniecki M, et al. Specific targeting of CD163(+) TAMs mobilizes inflammatory monocytes and promotes T cell-mediated tumor regression. J Exp Med. 2019;216(10):2394–2411.
- Bottcher JP, Bonavita E, Chakravarty P, et al. NK cells stimulate recruitment of cDC1 into the tumor microenvironment promoting cancer immune control. Cell. 2018;172(5):1022.e14–1037.e14.
- Cueto FJ, Del Fresno C, Brandi P, et al. DNGR-1 limits Flt3L-mediated antitumor immunity by restraining tumor-infiltrating type I conventional dendritic cells. J Immunother Cancer. 2021;9(5):e002054.
- Salmon H, Idoyaga J, Rahman A, et al. Expansion and activation of CD103(+) dendritic cell progenitors at the tumor site enhances tumor responses to therapeutic PD-L1 and BRAF inhibition. Immunity. 2016;44(4):924–938.
- Broz ML, Binnewies M, Boldajipour B, et al. Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell. 2014;26(5):638–652.
- Mittal D, Vijayan D, Putz EM, et al. Interleukin-12 from CD103(+) Batf3-Dependent dendritic cells required for NK-Cell suppression of metastasis. Cancer Immunol Res. 2017;5(12):1098–1108.
- Zumwalt TJ, Arnold M, Goel A, et al. Active secretion of CXCL10 and CCL5 from colorectal cancer microenvironments associates with GranzymeB + CD8+ T-cell infiltration. Oncotarget. 2015;6(5):2981–2991.
- Lapteva N, Aldrich M, Weksberg D, et al. Targeting the intratumoral dendritic cells by the oncolytic adenoviral vaccine expressing RANTES elicits potent antitumor immunity. J Immunother. 2009;32(2):145–156.
- Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9(4):239–252.
- Mueller MM, Fusenig NE. Friends or foes - bipolar effects of the tumour stroma in cancer. Nat Rev Cancer. 2004;4(11):839–849.
- Nishikawa G, Kawada K, Nakagawa J, et al. Bone marrow-derived mesenchymal stem cells promote colorectal cancer progression via CCR5. Cell Death Dis. 2019;10(4):264.
- Tlsty TD, Coussens LM. Tumor stroma and regulation of cancer development. Annu Rev Pathol. 2006;1:119–150.
- Seo W, Shimizu K, Kojo S, et al. Runx-mediated regulation of CCL5 via antagonizing two enhancers influences immune cell function and anti-tumor immunity. Nat Commun. 2020;11(1):1562.
- Cambien B, Richard-Fiardo P, Karimdjee BF, et al. CCL5 neutralization restricts cancer growth and potentiates the targeting of PDGFRbeta in colorectal carcinoma. PLOS One. 2011;6(12):e28842.
- Sugasawa H, Ichikura T, Kinoshita M, et al. Gastric cancer cells exploit CD4+ cell-derived CCL5 for their growth and prevention of CD8+ cell-involved tumor elimination. Int J Cancer. 2008;122(11):2535–2541.
- Raghavakaimal A, Cristofanilli M, Tang CM, et al. CCR5 activation and endocytosis in circulating tumor-derived cells isolated from the blood of breast cancer patients provide information about clinical outcome. Breast Cancer Res. 2022;24(1):35.
- Xu H, Zhao J, Li J, et al. Cancer associated fibroblast-derived CCL5 promotes hepatocellular carcinoma metastasis through activating HIF1alpha/ZEB1 axis. Cell Death Dis. 2022;13(5):478.
- Yang T, Chen M, Yang X, et al. Down-regulation of KLF5 in cancer-associated fibroblasts inhibit gastric cancer cells progression by CCL5/CCR5 axis. Cancer Biol Ther. 2017;18(10):806–815.
- Melese ES, Franks E, Cederberg RA, et al. CCL5 production in lung cancer cells leads to an altered immune microenvironment and promotes tumor development. Oncoimmunology. 2022;11(1):2010905.
- Suenaga M, Cao S, Zhang W, et al. Genetic variants in CCL5 and CCR5 genes and serum VEGF-A levels predict efficacy of bevacizumab in metastatic colorectal cancer patients. Int J Cancer. 2019;144(10):2567–2577.
- Ucuncu M, Serilmez M, Sari M, et al. The diagnostic significance of PDGF, EphA7, CCR5, and CCL5 levels in colorectal cancer. Biomolecules. 2019;9(9):464.
- Suenaga M, Schirripa M, Cao S, et al. Gene polymorphisms in the CCL5/CCR5 pathway as a genetic biomarker for outcome and Hand-Foot skin reaction in metastatic colorectal cancer patients treated with regorafenib. Clin Colorectal Cancer. 2018;17(2):e395–e414.
- Kan JY, Wu DC, Yu FJ, et al. Chemokine (C-C motif) ligand 5 is involved in Tumor-Associated dendritic Cell-Mediated Colon cancer progression through Non-Coding RNA MALAT-1. J Cell Physiol. 2015;230(8):1883–1894.
- Gao LF, Zhong Y, Long T, et al. Tumor bud-derived CCL5 recruits fibroblasts and promotes colorectal cancer progression via CCR5-SLC25A24 signaling. J Exp Clin Cancer Res. 2022;41(1):81.
- Zhang Y, Lv D, Kim HJ, et al. A novel role of hematopoietic CCL5 in promoting triple-negative mammary tumor progression by regulating generation of myeloid-derived suppressor cells. Cell Res. 2013;23(3):394–408.
- Zhao H, Chen D, Cao R, et al. Alcohol consumption promotes colorectal carcinoma metastasis via a CCL5-induced and AMPK-pathway-mediated activation of autophagy. Sci Rep. 2018;8(1):8640.
- Chen M, Yang X, Yang M, et al. Identification of a novel biomarker-CCL5 using antibody microarray for colorectal cancer. Pathol Res Pract. 2019;215(5):1033–1037.
- Hu B, Elinav E, Huber S, et al. Microbiota-induced activation of epithelial IL-6 signaling links inflammasome-driven inflammation with transmissible cancer. Proc Natl Acad Sci U S A. 2013;110(24):9862–9867.
- Sax MJ, Gasch C, Athota VR, et al. Cancer cell CCL5 mediates bone marrow independent angiogenesis in breast cancer. Oncotarget. 2016;7(51):85437–85449.
- Dwyer RM, Kerin MJ. Mesenchymal stem cells and cancer: tumor-specific delivery vehicles or therapeutic targets? Hum Gene Ther. 2010;21(11):1506–1512.
- Knoop K, Schwenk N, Schmohl K, et al. Mesenchymal stem cell-mediated, tumor stroma-targeted radioiodine therapy of metastatic Colon cancer using the sodium iodide symporter as theranostic gene. J Nucl Med. 2015;56(4):600–606.
- Ovais M, Guo M, Chen C. Tailoring nanomaterials for targeting Tumor-Associated macrophages. Adv Mater. 2019;31(19):e1808303.
