Abstract
Vitamin D (VD) has been shown to exert immunomodulatory activities, especially in promoting immune tolerance. For these properties VD has been proposed in the therapy of immunological conditions in which the loss of tolerance is the key pathogenetic aspect of the disease, such as allergies. Despite these properties available literature suggests VD is not useful in treating or preventing allergic diseases and whether low serum VD levels favor allergic sensitization and severity is debated. The level of VD is one of the many conditions that can influence allergic sensitization and therefore only a multivariate analysis on a numerically adequate cohort of patients, that considers all the factors that can favor allergy, would be able to assign the weight of each variable and determine the extent to which VD inhibits allergic sensitization and march. On the contrary, VD is able to potentiate the antigen-specific tolerogenic response induced by Allergen Immunotherapy (AIT) as demonstrated by the large majority of studies. In our experience, the association of VD and Sublingual AIT (LAIS, Lofarma, Italy) gave an excellent clinical and immune response in particular enhancing the differentiation of memory T regulatory cells. While waiting for a more extensive literature, VD/AIT combination should be always performed in treating allergies. In any case, the assessment of the level of VD should become a routine in allergic patients with an indication to AIT as, in case of VD deficiency or insufficiency, VD seems a particularly active adjuvant to the immune treatment.
KEY MESSAGES
Allergic patients treated with allergen immunotherapy benefit from the simultaneous administration of Vitamin D, which on the contrary does not offer benefits when used alone for the prevention or treatment of allergies. Vitamin serum levels should be always evaluated in patients treated with allergen immunotherapy because these patients have the maximum clinical and immunological benefit from simultaneous vitamin D supplementation
Introduction
Vitamin D (VD), used since its discovery as a rickets therapy, also has extra-skeletal effects, including immunomodulatory actions justifying its role in the treatment of immune-mediated diseases. VD acts within immune cells as a cytokine, being both a transcription- and a growth factor; as a cytokine, displays pleiotropy, synergy, redundancy, and interaction with surface receptors [Citation1,Citation2]. It induces the secretion and/or surface expression of inhibitory cytokines (IL-10 and TGF-β) promoting maturation of regulatory dendritic and regulatory T cells (Tregs) and up-regulating IL-10 induced FOXP3+ Tregs that play a pivotal role in autoimmunity and allergy control [Citation3,Citation4]. VD inhibits Th2 cell differentiation, B cells proliferation, differentiation, and IgE production [Citation4] and prevents ILC2 activation [Citation5]. The active form of VD, calcitriol, downregulates the production of the proinflammatory chemokine CXCL8 in primary sublingual epithelial cells when administered with mite allergen, favoring the effect of the specific desensitization [Citation6]. VD inhibits the expression of the Th1 (IFN-y, TNF-α), Th9 (IL-9), Th17 (IL-17) and Th22 (IL-22) cytokines in T cells [Citation7]. Macrophages, DCs and T and B cells are also able to produce VD locally, which can act on immune cells in an autocrine or paracrine manner by binding the VD receptor [Citation8].
The VD immunological properties are demonstrated in studies involving the host defense against bacteria, being VD able of antimicrobial effects in human macrophages [Citation9]. VD induces the clearance of Mycobacterium tuberculosis, and its antibacterial activity is mediated by autophagy through the upregulation of LL37 and the activation of Beclin-1 and ATG5 in monocytes and macrophages [Citation10,Citation11]. Moreover, VD replacement has been shown to boost antigen-specific immunity induced by vaccines in older adults with sub-optimal VD status [Citation12].
Although controversial findings exist, most epidemiological studies have indicated that multiple types of cancer risk, including colon, prostate, breast, and stomach cancers, correlate with serum VD status [Citation13]. Moreover, VD might enhance the activity of immune cells such as DC, NK cells, T cells, B cells as well as TILs in cancer and inflammation [Citation14].
Furthermore, the VD ability to modulate the immunotolerance is the substrate for considering VD useful in the treatment of immunologically based diseases such as autoimmunity and allergy. In fact, autoimmunity can be favored in conditions of VD deficiency, since the lack of the stimulus to the differentiation of IL-10-producing Treg cells favors the activation of Th17 and Th9 cells [Citation15]. Thus, the integration of VD might be useful in the treatment of autoimmune diseases. In experimental animals, VD-induced tolerogenic dendritic cells (DC) increased the population of CD4+CD25+Foxp3+ T cells, CD4+IL-10+ T cells, and CD19+CD5+CD1d+ B cells, resulting in attenuated experimental autoimmune encephalomyelitis [Citation16]. In a meta-analysis, complementary supplementation of VD greater than 50,000 IU/week significantly improved the effects of basic therapy on disease activity score and global pain score in rheumatoid arthritis [Citation17]. Data exist showing positive effects of VD administration in LES, Multiple Sclerosis, and some autoimmune endocrine diseases, even though the information is not sufficient to determine its thorough effectiveness [Citation18,Citation19].
Discussion
The ability of VD to promote maturation of FOXP3+ regulatory T cells, to induce the production of inhibitory cytokines (IL-10 and TGF-β), to inhibit Th2 cell differentiation and B cells proliferation, differentiation, and IgE production [Citation3,Citation20] simulates its effect in the treatment of allergies. When keeping in mind that the effectiveness of allergen immunotherapy (AIT) mostly depends on modification of immune regulation [Citation21], the natural activity of VD on Treg differentiation is highly attractive.
We recently published in Allergy [Citation22] a study showing that one-year mite monomeric allergoid sublingual-immunotherapy (LAIS, Lofarma, Milan Italy) was effective in increasing memory effector Tregs in mite sensitized asthmatic children, and this increase significantly correlated with the amelioration of clinical scores: VAS for symptoms and ARIA classification of rhinitis. We performed a post-hoc analysis, in a group taking VD3 during LAIS, as prescription of their pediatrician, independently from the study protocol with doses ranging from 400 to 1000 UI/die. No side effects were observed. Laboratory (Treg frequency and activation and total and mite specific IgE) and clinical data (VAS for symptoms, ARIA classification of rhinitis, and drug consumption) were compared between VD-supplemented versus non supplemented. No significant differences were observed in anthropometric parameters and medical history between the two cohorts. As expected, VD serum levels were significantly higher in the supplemented group after 12-months (). Both groups had a significant improvement of all clinical (VAS, ARIA, and antihistamine and nasal corticosteroid consumption) parameters after one-year LAIS (). Interestingly, the VD sub-cohort showed significantly better results. In particular, the improvement of ARIA and VAS and the reduction in antihistamine consumption were significantly greater as compared to the non-VD supplemented (p < 0.05). Moreover, non-VD supplemented showed an increase in total and mite specific IgE, whereas total and mite specific IgE decreased in VD sub-group, but only the differences in D farinae specific IgE were significant (p < 0.05) ().
Table 1. Time dependent changes of clinical and laboratory parameters of SLIT and SLIT + VD3 treated patients.
Significant differences between the two groups were also obtained when evaluating T regulatory cell changes. In fact, peripheral effector memory Tregs expressing HLA-DR were significantly higher in VD treated patients (p < 0.0001), with VD supplementation representing the main determinant factor for the increase of these cells in the whole LAIS treated initial group (). No patients reported local or systemic adverse reactions during the treatment, consistent with data from a pharmacovigilance study showing that the rate of adverse reactions of monomeric allergoid based AIT corresponds to 0.0004% of all administered doses, all local and mild (6), far below the commonly reported rates for native allergen sublingual AIT products, for which also anaphylactic reactions are reported (www.adrreports.eu. Accessed December 20, 2022).
Figure 1. Incremental variation of the expression of activation marker HLA-DR on the effector memory subset of Tregs (CD127neg/lowCD4posCD25posCD45RAnegCD39posHLA-DRpos) in the whole original cohort of AIT patients and the two sub-cohorts, either supplemented with VD3 or not.
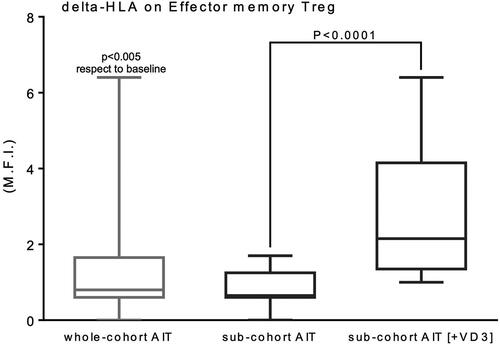
Our results are in line with the literature showing an adjuvant effect of VD for AIT both in animals and in humans.
Animal studies
All experimental data in animals showed that complementary VD supplementation potentiates the effect of AIT. In a mouse model of asthma, the combination of AIT/VD induced a more significant inhibition of bronchial hyperreactivity and serum OVA-specific IgE levels and increase in regulatory cytokines IL-10 and TGF-β than AIT alone and reduced the airway eosinophilia not ameliorated by AIT alone [Citation23]. The effect of the combined administration of VD with AIT on bronchial hyperreactivity and eosinophilia was confirmed by other authors [Citation24]. Consistently, VD supplementation was associated with an increase of Foxp3+ Treg cells, with respect to that induced by AIT alone, providing a mechanistic explanation for the significantly enhanced AIT efficacy [Citation25]. These effects seem to be dose dependent as the reduction of bronchial hyperreactivity and bronchial eosinophilia were particularly pronounced with the administration of high VD doses [Citation26]. Other authors demonstrated that the improved inhibitory effects of AIT on allergic airway inflammation (particularly eosinophilic inflammation) afforded by VD pretreatment was accompanied by reduction of IL-4 and IL-5 levels in BAL fluid and of serum sIgE, with an increase the regulatory cytokine IL-10 [Citation27]. Furthermore, it has been shown that OVA-specific IgE and IgG1 serum concentrations were increased in VD-deficient mice compared with control mice. Moreover, coadministration of VD with OVA-specific AIT reduced the Th2 cytokine expression in the lungs and the allergic airway inflammation and responsiveness upon OVA challenge more that AIT alone [Citation28]. In a mouse model of respiratory allergy, AIT with monomeric allergoid has been shown to be potentiated when in coadministration with VD. In fact, VD adjuvated allergoid vaccine induced the most prominent reduction of airway eosinophilia and Th2 cytokines with concomitant increase of Treg cells and IL-10 in the lung and Der p 2-specific IgG2a in the serum, as compared to AIT or VD alone [Citation29]. The effect of VD supplementation has been shown both in sublingual and in injection AIT, in an asthma mouse model, with a similar induction of blocking antibodies and suppression of airway inflammation, underscoring the relevance of proficient VD levels for a successful AIT [Citation30].
Human studies
Almost all human studies showed adjuvant effects of VD to AIT. Jerzynska [Citation31] showed that VD supplementation combined with sublingual AIT was significantly more efficacious than AIT alone on nasal symptoms, and combined symptom medication score in children with grass allergic rhinitis. Later, the same author [Citation32] reported that VD supplementation improved the clinical effects of AIT also in grass-allergic asthmatic children, with a significant reduction of asthma exacerbation and FENO. These effects were accompanied by a higher increase from baseline in the percentage of CD4 + CD25 + Foxp3+ cells in the VD group (p = 0.0058 compared to the AIT alone). Moreover, CD4 + CD25 + Foxp3+ cell induction had a significant correlation with FENO reduction in the VD supplemented group. Yu et al. [Citation33] showed that, comparing patients with allergic rhinitis treated with AIT or VD or AIT/VD or placebo, the combination of AIT/VD induced the greatest significant down regulation of miR-19a in B cells, with increased expression of IL10 with respect to the other treatments. These effects were parallel by an amelioration of clinical parameters (reduction of AR symptom scores and medication scores), serum specific IgE, SPT index, and serum levels of Th2 cytokines (IL-4 and IL-13) in AIT/VD treated patients. The authors concluded that administration with VD promotes the effect of SIT on suppression of allergic rhinitis via repressing the expression of miR-19a in peripheral B cells.
A recent 3-year AIT trial suggested an earlier onset of AIT-induced immunomodulation in patients supplemented with high-dose VD with respect to non-VD supplemented subjects, with increased specific IgG4/IgE ratio during the early phase of AIT and the increased VDR-inducible CD38+ B cells in the VD group. The clinical effect of the combination AIT/VD appeared relevant after the 2nd year of treatment, as the symptom medication scores were comparable after the first-year treatment among VD treated and non-treated [Citation20].
Only one study showed less encouraging results. Also in this case, however, some important favorable outcomes with a reduction of total asthma symptom score and Foxp3 expression were found in the VD supplemented patients [Citation34].
Unlike what has been demonstrated on the effectiveness of VD as an adjuvant for AIT, the usefulness of VD treatment alone in established allergies or VD supplementation in preventing the onset of allergic diseases is controversial. Results of various studies deny in most cases a relationship between VD basal level and the appearance of allergies. In a cross-sectional study involving only allergic patients [Citation35], the association between VD levels and allergies appeared weak. Overall, VD levels were lower in patients with asthma and rhinitis, but without statistical significance. Only patients with or without atopic dermatitis had significant different VD levels. VD levels were not related to seasonal allergies, whereas a significant, negative correlation was seen for house dust mite and dog dander. The authors concluded that studies involving larger samples would be required to better define the association between VD and allergies. Same considerations were made by other authors showing that the VD status in human allergic patients has a nonlinear relationship with immune parameters relevant for allergic disease (e. g. serum IgE levels) [Citation36]. Similarly, VD serum assessment seemed to be scarcely useful to pheno/endotyping allergic asthmatic children in clinical practice as the VD classes (normal, insufficient or deficient) were not able to discriminate clinical, functional and laboratory parameters in allergic asthma [Citation37]. Some authors also did not show association between maternal levels of VD and atopic disease outcomes (eczema, food allergy, asthma, allergic rhinitis) at 2 and 5 years in a disease-specific cohort in childhood with prospectively collected, validated atopic outcomes [Citation38].
Basal serum levels of VD were also studied in relation to the efficacy of AIT and no correlation was found between the efficacy of one-year injection AIT and serum VD levels on symptom and medication scores. A slight statistical difference was reported only in mite sIgE level between the VD deficiency and the sufficiency groups [Citation39].
On the contrary, two studies showed positive clinical outcomes. The first by Joudi et al. [Citation40] showed that both SNOT-22 and MiniRQLQ scores decreased significantly after AIT in rhinitis patients with different levels of VD, with significantly more pronounced effects when the level of VD was sufficient. The second study showed that a significant reduction in asthma symptoms score, and steroid-sparing effect of AIT were associated with high VD serum levels (higher than 30 ng/mL), with higher Foxp3 and TGF alfa induction [Citation41].
Other studies evaluated the possibility that VD supplementation may prevent the sensitization and onset of allergies and therefore may provide health benefits to humans by reducing a predisposition to allergic diseases when administered in sufficient doses [Citation42,Citation43].
An interventional study showed that VD supplementation during pregnancy and infancy was able to prevent aeroallergen sensitization and respiratory illness, with a reduction of the number of mite sensitized children at age 18 months [Citation44]. On the contrary, not statistically significant data were observed in other studies [Citation45–47]. Chawez et al. [Citation45] detected that the supplementation of 2800 IU/d of VD3 during the third trimester of pregnancy compared with 400 IU/d did not offer statistically significant reduction the risk of persistent wheeze in the offspring. Similarly, Litonjua et al. [Citation46] and Mirzakhani et al. [Citation47] indicated that the supplementation with VD 4400 IU/d in pregnant women at risk of having a child with asthma was followed by non-statistically significant reduction in the incidence of asthma and recurrent wheezing in their children at age 3 years with respect to women supplemented with VD 400 IU/d. Therefore, the potential impact of VD supplementation in preventing allergies remains uncertain.
The possibility of preventing and treating allergies by VD supplementation is a debated issue in many systematic reviews and meta-analysis of randomized and non-randomized studies. The conclusions were similar: VD level does not relate with either the prevalence of the current allergic rhinitis or its development [Citation48]; VD supplementation reduces the severity of atopic dermatitis and symptoms of allergic rhinitis and asthma exacerbation only in children with VD serum level lower than <10 ng/mL [Citation49]; the evidence does not support the notion that VD supplementation in pregnant women or in breastfeeding mothers prevents the development of allergic diseases in their children and that VD supplementation in infants prevents the development of allergic diseases [Citation50]; the is no evidence of an association between maternal antenatal or infant VD level or dietary intake and the development of food allergy or eczema in offspring, and an association between higher VD levels in cord blood and reduced risk of eczema in cohort studies [Citation51]; evidence indicates there is a nonlinear relationship between vitamin D and food allergy [Citation52].
Conclusions
Available literature suggests that VD is not useful in treating or preventing allergic diseases and there are some doubts that low serum VD levels is a crucial factor in favoring the onset of allergic diseases. Available studies are heterogeneous, and therefore longitudinal studies on VD effect on the prevention and treatment of allergy are needed to understand the impact of VD in early life and its potential role in stopping the atopic epidemic and the allergic march. The level of RV is one of the many conditions that can influence allergic sensitization and therefore a multivariate analysis on a numerically adequate cohort of patients, that takes into account all the factors that can favor allergy, will be able to assign the weight of each variable and determine to what extent VD play a role as an inhibitor of allergic sensitization and march. It is well known that VD effectiveness as immunomodulator has been demonstrated, in particular favoring immunoregulation. However, such activity is not directed towards a specific antigen. On the contrary, it appears that when supplemented during AIT, the activity of VD on B and Treg favors the AIT-induced antigen-specific tolerogenic response, and therefore VD becomes a potentiating factor. On the other hand, as shown, almost all literature agrees on the usefulness of VD as adjuvant to AIT and recent reviews listed VD among the possible adjuvant of AIT [Citation53–56]. In our experience, the association of LAIS and VD provided an excellent clinical and immune response, and we believe that this combination should be always performed, while waiting for a more extensive literature. In any case, the assessment of the level of VD should become a routine in allergic patients with an indication to AIT as, in case of VD deficiency or insufficiency, the VD supplementation seems a particularly active adjuvant to the immune treatment.
Contributions
Conceptualization: Claudia Petrarca, Mario Di Gioacchino; data curation: funding acquisition: Francesca Santilli; investigation: Claudia Petrarca, Rocco Mangifesta; clinical practice: Francesca Santilli, Mario Di Gioacchino; project administration Mario Di Gioacchino; supervision: Claudia Petrarca, Mario Di Gioacchino, Rocco Mangifesta; writing-original draft: Claudia Petrarca, Rocco Mangifesta; writing-review and editing: Mario Di Gioacchino, Francesca Santilli.
All authors have read and agreed to the published version of the manuscript.
Disclosure statement
Claudia Petrarca and Mario Di Gioacchino received research grants from Lofarma Allergeni (Milan Italy).
Data availability statement
The data that support the findings of this study are available upon reasonable request.
Additional information
Funding
References
- Umar M, Sastry KS, Chouchane AI. Role of vitamin D Beyond the skeletal function: a review of the molecular and clinical studies. Int J Mol Sci. 2018;19(6):1. doi: 10.3390/ijms19061618.
- Bivona G, Agnello L, Ciaccio M. The immunological implication of the new vitamin D metabolism. Cent Eur J Immunol. 2018;43(3):331–7. doi: 10.5114/ceji.2018.80053.
- Chambers ES, Suwannasaen D, Mann EH, et al. 1alpha,25-dihydroxyvitamin D3 in combination with transforming growth factor-beta increases the frequency of Foxp3(+) regulatory T cells through preferential expansion and usage of interleukin-2. Immunology. 2014;143(1):52–60. doi: 10.1111/imm.12289.
- Sassi F, Tamone C, D’Amelio P. Vitamin D: nutrient, hormone, and immunomodulator. Nutrients. 2018;10(11):1656. doi: 10.3390/nu10111656.
- Ruiter B, Patil SU, Shreffler WG. Vitamins A and D have antagonistic effects on expression of effector cytokines and gut-homing integrin in human innate lymphoid cells. Clin Exp Allergy. 2015;45(7):1214–1225. doi: 10.1111/cea.12568.
- Pelst MP, Höbart C, Wallaeys C, et al. Adjuvanting allergen extracts for sublingual immunotherapy: calcitriol downregulates CXCL8 production in primary sublingual epithelial cells. Front Immunol. 2020;11:1033. doi: 10.3389/fimmu.2020.01033.
- Pfeffer PE, Mann EH, Hornsby E, et al. Vitamin D influences asthmatic pathology through its action on diverse immunological pathways. Annals ATS. 2014;11(Supplement 5):S314–S321. doi: 10.1513/AnnalsATS.201405-204AW.
- Hufnagl K, Jensen-Jarolim E. Vitamin A and D in allergy: from experimental animal models and cellular studies to human disease. Allergo J Int. 2018;27(3):72–78. doi: 10.1007/s40629-018-0054-2.
- Fabri M, Stenger S, Shin DM, et al. Vitamin D is required for IFN-γ-mediated antimicrobial activity of human macrophages. Sci Transl Med. 2011;3(104):104ra2. doi: 10.1126/scitranslmed.3003045.
- Yuk JM, Shin DM, Lee HM, et al. Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin. Cell Host Microbe. 2009;6(3):231–243. doi: 10.1016/j.chom.2009.08.004.
- Campbell GR, Spector SA. Autophagy induction by vitamin D inhibits both Mycobacterium tuberculosis and human immunodeficiency virus type 1. Autophagy. 2012;8(10):1523–1525. doi: 10.4161/auto.21154.
- Chambers ES, Vukmanovic-Stejic M, Turner CT, et al. Vitamin D3 replacement enhances antigen-specific immunity in older adults. Immunother Adv. 2021;1:ltaa008. doi: 10.1093/immadv/ltaa008.
- Wu X, Hu W, Lu L, et al. Repurposing vitamin D for treatment of human malignancies via targeting tumor microenvironment. Acta Pharm Sin B. 2019;9(2):203–219. doi: 10.1016/j.apsb.2018.09.002.
- Min D, Lv XB, Wang X, et al. Downregulation of miR-302c and miR-520c by 1,25(OH)2D3 treatment enhances the susceptibility of tumour cells to natural killer cell-mediated cytotoxicity. Br J Cancer. 2013;109(3):723–730. doi: 10.1038/bjc.2013.337.
- Palmer MT, Lee YK, Maynard CL, et al. Lineage-specific effects of 1,25-dihydroxyvitamin D3 on the development of effector CD4 T cells. J Biol Chem. 2011;286(2):997–1004. doi: 10.1074/jbc.M110.163790.
- Xie Z, Chen J, Zheng C, et al. 1,25-dihydroxyvitamin D3-induced dendritic cells suppress experimental autoimmune encephalomyelitis by increasing proportions of the regulatory lymphocytes and reducing T helper type 1 and type 17 cells. Immunology. 2017;152(3):414–424. doi: 10.1111/imm.12776.
- Guan Y, Hao Y, Guan Y, et al. The effect of vitamin D supplementation on rheumatoid arthritis patients: a systematic review and Meta-Analysis. Front Med (Lausanne). 2020;7:596007. doi: 10.3389/fmed.2020.596007.
- Illescas-Montes R, Melguizo-Rodríguez L, Ruiz C, et al. Vitamin D and autoimmune diseases. Life Sci. 2019;233:116744. doi: 10.1016/j.lfs.2019.116744.
- Sîrbe C, Rednic S, Grama A, et al. An update on the effects of vitamin D on the immune system and autoimmune diseases. Int J Mol Sci. 2022;23(17):9784. doi: 10.3390/ijms23179784.
- Heine G, Francuzik W, Doelle-Bierke S, et al. Immunomodulation of high-dose vitamin D supplementation during allergen-specific immunotherapy. Allergy. 2021;76(3):930–933. doi: 10.1111/all.14541.
- Durham SR, Shamji MH. Allergen immunotherapy: past, present and future. Nat Rev Immunol. 2023;23(5):317–328. doi: 10.1038/s41577-022-00786-1.
- Petrarca C, Lanuti P, Petrosino MIMI, et al. Peripheral effector memory regulatory T cells are incremented and functionally enhanced in successful mite monomeric allergoid sublingual immunotherapy. Allergy. 2021;76(7):2208–2211. doi: 10.1111/all.14692.
- Taher YA, van Esch BC, Hofman GA, et al. 1alpha,25-dihydroxyvitamin D3 potentiates the beneficial effects of allergen immunotherapy in a mouse model of allergic asthma: role for IL-10 and TGF-beta. J Immunol. 2008;180(8):5211–5221. doi: 10.4049/jimmunol.180.8.5211.
- Grundström J, Neimert-Andersson T, Kemi C, et al. Covalent coupling of vitamin D3 to the major cat allergen fel d 1 improves the effects of allergen-specific immunotherapy in a mouse model for cat allergy. Int Arch Allergy Immunol. 2012;157(2):136–146. doi: 10.1159/000327546.
- Van Overtvelt L, Lombardi V, Razafindratsita A, et al. IL-10-inducing adjuvants enhance sublingual immunotherapy efficacy in a murine asthma model. Int Arch Allergy Immunol. 2008;145(2):152–162. doi: 10.1159/000108140.
- Hesse L, van Ieperen N, Petersen AH, et al. High dose vitamin D3 empowers effects of subcutaneous immunotherapy in a grass pollen-driven mouse model of asthma. Sci Rep. 2020;10(1):20876. doi: 10.1038/s41598-020-77947-6.
- Ma JX, Xia JB, Cheng XM, et al. 1,25-dihydroxyvitamin D3 pretreatment enhances the efficacy of allergen immunotherapy in a mouse allergic asthma model. Chin Med J (Engl). 2010;123:3591–3596.
- Heine G, Tabeling C, Hartmann B, et al. 25-hydroxvitamin D3 promotes the long-term effect of specific immunotherapy in a murine allergy model. J Immunol. 2014;193(3):1017–1023. doi: 10.4049/jimmunol.1301656.
- Petrarca C, Clemente E, Amato V, et al. Vitamin D3 improves the effects of low dose der p 2 allergoid treatment in der p 2 sensitized BALB/c mice. Clin Mol Allergy. 2016;14:7. doi: 10.1186/s12948-016-0044-1.
- Hesse L, Petersen AH, Oude Elberink JNG, et al. 1,25(OH)2VitD3 supplementation enhances suppression of grass pollen-induced allergic asthma by subcutaneous and sublingual immunotherapy in a mouse model. Sci Rep. 2020;10(1):8960. doi: 10.1038/s41598-020-65946-6.
- Jerzynska J, Stelmach W, Rychlik B, et al. The clinical effect of vitamin D supplementation combined with grass-specific sublingual immunotherapy in children with allergic rhinitis. Allergy Asthma Proc. 2016;37(2):105–114. doi: 10.2500/aap.2016.37.3921.
- Jerzyńska J, Stelmach W, Rychlik B, et al. Clinical and immunological effects of vitamin D supplementation during the pollen season in children with allergic rhinitis. Arch Med Sci. 2018;14(1):122–131. doi: 10.5114/aoms.2016.61978.
- Yu ZJ, Zeng L, Luo XQ, et al. Vitamin D3 inhibits micro RNA-17-92 to promote specific immunotherapy in allergic rhinitis. Sci Rep. 2017;7(1):546. doi: 10.1038/s41598-017-00431-1.
- Baris S, Kiykim A, Ozen O, et al. Vitamin D as an adjunct to subcutaneous allergen immunotherapy in asthmatic children sensitized to house dust mite. Allergy. 2014;69(2):246–253. doi: 10.1111/all.12278.
- Lombardi C, Passalacqua G. Italian vitamin D allergy group vitamin D levels and allergic diseases. An italian cross-sectional multicenter survey. Eur Ann Allergy Clin Immunol. 2017;49:75–79.
- Hypponen E, Berry DJ, Wjst M, et al. Serum 25-hydroxyvitamin D and IgE—a significant but nonlinear relationship. Allergy. 2009;64(4):613–620. doi: 10.1111/j.1398-9995.2008.01865.x.
- Licari A, Marseglia GL, Ciprandi G. Vitamin D3 in children with allergic asthma in clinical practice. Pediatr Pulmonol. 2019;54(3):225–227. doi: 10.1002/ppul.24229.
- Hennessy A, Hourihane JO, Malvisi L, et al. Antenatal vitamin D exposure and childhood eczema, food allergy, asthma and allergic rhinitis at 2 and 5 years of age in the atopic disease-specific Cork BASELINE birth cohort study. Allergy. 2018;73(11):2182–2191. doi: 10.1111/all.13590.
- Jia X, Zheng H, Yan X, et al. Effect of baseline serum vitamin D level on symptom and medication scores of subcutaneous immunotherapy in children with mite allergy. Front Pediatr. 2022;10:1018549. doi: 10.3389/fped.2022.1018549.
- Joudi M, Farid Hosseini R, Khoshkhui M, et al. Effects of serum vitamin D and efficacy of subcutaneous immunotherapy in adult patients With allergic rhinitis. Allergy Asthma Immunol Res. 2019;11(6):885–893. doi: 10.4168/aair.2019.11.6.885.
- Majak P, Jerzyńska J, Smejda K, et al. Correlation of vitamin D with Foxp3 induction and steroid-sparing effect of immunotherapy in asthmatic children. Ann Allergy Asthma Immunol. 2012;109(5):329–335. doi: 10.1016/j.anai.2012.08.002.
- Reinholz M, Ruzicka T, Schauber J. Vitamin D and its role in allergic disease. Clin Exp Allergy. 2012;42(6):817–826. doi: 10.1111/j.1365-2222.2011.03923.x.
- Yepes-Nunez JJ, Brozek JL, Fiocchi A, et al. Vitamin D supplementation in primary allergy prevention: systematic review of randomized and nonrandomized studies. Allergy. 2018;73(1):37–49. doi: 10.1111/all.13241.
- Grant CC, Crane J, Mitchell EA, et al. Vitamin D supplementation during pregnancy and infancy reduces aeroallergen sensitization: a randomized controlled trial. Allergy. 2016;71(9):1325–1334. doi: 10.1111/all.12909.
- Chawes BL, Bonnelykke K, Stokholm J, et al. Effect of vitamin D3 supplementation during pregnancy on risk of persistent wheeze in the offspring: a randomized clinical trial. JAMA. 2016;315(4):353–361. doi: 10.1001/jama.2015.18318.
- Litonjua AA, Carey VJ, Laranjo N, et al. Effect of prenatal supplementation with vitamin D on asthma or recurrent wheezing in offspring by age 3 years: the VDAART randomized clinical trial. JAMA. 2016;315(4):362–370. doi: 10.1001/jama.2015.18589.
- Mirzakhani H, Carey VJ, Zeiger R, et al. Impact of parental asthma, prenatal maternal asthma control, and vitamin D status on risk of asthma and recurrent wheeze in 3-year-old children. Clin Exp Allergy. 2019;49(4):419–429. doi: 10.1111/cea.13320.
- Kim YH, Kim KW, Kim MJ, et al. Vitamin D levels in allergic rhinitis: a systematic review and meta-analysis. Pediatr Allergy Immunol. 2016;27(6):580–590. doi: 10.1111/pai.12599.
- Li Q, Zhou Q, Zhang G, et al. Vitamin D supplementation and allergic diseases during childhood: a systematic review and Meta-Analysis. Nutrients. 2022;14(19):3947. doi: 10.3390/nu14193947.
- Luo C, Sun Y, Zeng Z, et al. Vitamin D supplementation in pregnant women or infants for preventing allergic diseases: a systematic review and meta-analysis of randomized controlled trials. Chin Med J (Engl). 2022;135(3):276–284. doi: 10.1097/CM9.0000000000001951.
- Zeng R, Li Y, Shen S, et al. Is antenatal or early-life vitamin D associated with eczema or food allergy in childhood? A systematic review. Clin Experimental Allergy. 2023;53(5):511–525. doi: 10.1111/cea.14281.
- Di T, Chen L. A narrative review of vitamin D and food allergy in infants and children. Transl Pediatr. 2021;10(10):2614–2620. doi: 10.21037/tp-21-396.
- Moingeon P, Lombardi V, Baron-Bodo V, et al. Enhancing allergen-presentation platforms for sublingual immunotherapy. J Allergy Clin Immunol Pract. 2017;5(1):23–31. doi: 10.1016/j.jaip.2016.07.020.
- Rajakulendran M, Tham EH, Soh JY, et al. Novel strategies in immunotherapy for allergic diseases. Asia Pac Allergy. 2018;8(2):e14. doi: 10.5415/apallergy.2018.8.e14.
- Nelson HS. Allergy immunotherapy: future directions for the 2020s. Allergy Asthma Proc. 2020;41(5):314–325. doi: 10.2500/aap.2020.41.200041.
- Sadeghi M, Keshavarz Shahbaz S, Dehnavi S, et al. Current possibilities and future perspectives for improving efficacy of allergen-specific sublingual immunotherapy. Int Immunopharmacol. 2021;101(Pt B):108350. doi: 10.1016/j.intimp.2021.108350.
