Abstract
Aim
To evaluate diagnostic performance of metagenomic next-generation sequencing (mNGS) for Pneumocystis jirovecii pneumonia (PCP), in comparison with polymerase chain reaction (PCR), Gomori methenamine silver (GMS) staining and serum 1,3-β-d-Glucan (BG) assay.
Methods
52 PCP patients and 103 patients with non-pneumocystic jirovecii pneumonia (non-PCP) were enrolled, and comparative analysis was conducted of different diagnostic tests. Clinical features and co-pathogen characteristics were reviewed.
Results
The diagnostic sensitivity (92.3%) and specificity (87.4%) of mNGS did not show significant differences compared with that of PCR while mNGS had the advantage over PCR in the detection of co-pathogens. Despite its excellent specificity, the sensitivity of GMS staining (9.3%) was inferior to that of mNGS (p < .001). The combination of mNGS with serum BG statistically outperformed mNGS or serum BG alone in the areas under the receiver operating characteristic curves (AUCs, p = .0013 and p = .0015, respectively). Notably, all the blood samples showing positive mNGS for Pneumocystis jirovecii came from PCP patients. The leading co-pathogens among patients with PCP were cytomegalovirus, Epstein-Barr virus and Torque teno virus.
Conclusions
mNGS shows superiority over several common clinical methods in the diagnosis of suspected PCP. Serum BG in conjunction with mNGS further improved the diagnostic efficacy of mNGS.
Introduction
Pneumocystis jirovecii pneumonia (PCP), an opportunistic pulmonary infection caused by Pneumocystis jirovecii (P. jirovecii), has become a threat not only to HIV-infected population, but also to large numbers of HIV-negative immunosuppressed patients due to increasing use of corticosteroids, chemotherapy and other immunosuppressants [Citation1]. While the mortality of HIV-positive PCP has decreased to less than 20%, PCP in HIV-negative patients is still of great concern with high mortality rates of 30–60% [Citation2]. Early diagnosis and timely treatment are important in improving prognosis. However, the clinical manifestations of PCP are sometimes atypical, but rapidly progressive, which may lead to delayed initiation of anti-PCP therapy and unfavorable outcomes. Therefore, diagnostic tools with high sensitivity and specificity have always been pursued by clinicians.
P. jirovecii is extremely difficult to culture [Citation3]. The most commonly used Gomori methenamine silver (GMS) staining relies on identifying cyst forms of P. jirovecii, and its diagnostic efficacy can be affected by the burden of the fungus, the quality of samples and the skill of technicians reviewing slides. Thus, false negatives could be an issue, and GMS staining may underestimate the infection rate [Citation4]. In contrast, polymerase chain reaction (PCR) for P. jirovecii has been shown to be more sensitive in the detection of PCP among patients with or without HIV [Citation5,Citation6]. A negative PCR is highly suggestive of the absence of P. jirovecii, whereas a positive PCR cannot distinguish colonization from active infection. Besides, serum 1,3-β-d-Glucan (BG) is widely used in the diagnosis of PCP, but it is more of an indicator for fungal infections [Citation7,Citation8]. A positive BG is not specific to PCP. However, as a non-invasive approach, serum BG could be an adjunct testing, potentially integrated with other tests to make a definitive diagnosis [Citation9].
In recent years, metagenomic next-generation sequencing (mNGS) has been increasingly applied to clinical microbiology [Citation10–12]. With high-throughput capacity and rapid turnaround time, mNGS is able to detect nucleic acids of pathogens in a comprehensive and unbiased manner. It shows significant advantages for the high sensitivity and the ability to diagnose mixed infections [Citation13]. Nevertheless, the diagnostic performance of mNGS for PCP has not been fully evaluated.
Although mNGS has been reported to be efficient in diagnosing PCP in non-HIV-infected and immunocompromised patients [Citation14,Citation15], those studies did not analyze the data from blood and respiratory samples separately, which may provide mixed information and affect the result interpretation. Moreover, both mNGS and PCR detect nucleic acids, but few have ever compared the diagnostic efficacy of mNGS and PCR for PCP.
Therefore, this study aimed to assess the diagnostic performance of mNGS for PCP with lower tract respiratory specimens and figure out the potential superior strategy in the diagnosis of PCP by comparing different methods. Concomitant microbes were also analyzed to provide a pathogen profile in PCP and non-PCP patients.
Methods
Study design and PCP diagnosis
This was a retrospective study conducted in Peking Union Medical College Hospital, a tertiary care hospital in Beijing, China, between October 2020 and September 2022. We investigated patients diagnosed with pneumonia whose samples from the lower respiratory tract were collected for mNGS. A total of 52 PCP patients and 103 non-PCP patients infected by other pathogens were enrolled in this study. All patients included had been admitted to our department and got definite etiologic diagnoses.
The clinical diagnosis of PCP was made by two experienced pulmonologists based on clinical manifestations (such as fever, dyspnea or progressive hypoxemia), radiological findings (such as typical bilateral ground glass opacity on chest computed tomography), and microbiological tests [Citation16]. A microbiological test was considered positive when P. jirovecii cyst was detected in microscopic examination with GMS staining or P. jirovecii DNA (PCP-DNA) was revealed positive by mNGS or PCR assay of a sputum or bronchoalveolar lavage fluid (BALF) specimen. All sputum specimens were quality-assessed with cytological screening [Citation17]. Patients with positive PCP-DNA specimens who did not have typical clinical or radiological features for PCP were excluded from our analysis.
The inclusion criteria of non-PCP: (1) Clinical characteristics satisfied diagnostic criteria of pneumonia. (2) Lower respiratory tract specimens were collected for mNGS. Cases that had been diagnosed with PCP were excluded.
Metagenomic next-generation sequencing analysis
Bronchoalveolar lavage was performed in accordance with previous practice guideline for qualified BALF specimens [Citation18]. mNGS was performed on DNA extracted from lower respiratory tract specimens in all patients while RNA sequencing was also performed for cases suspected of viral pneumonia. The procedure of mNGS consists of nucleic acid extraction, library construction, sequencing, and bioinformatic analysis.
DNA was extracted from all samples using a QIAamp® UCP Pathogen DNA Kit (Qiagen) following the manufacturer’s instructions. Human DNA was removed using Benzonase (Qiagen) and Tween20 (Sigma) [Citation19]. Total RNA was extracted with a QIAamp® Viral RNA Kit (Qiagen) and ribosomal RNA was removed by a Ribo-Zero rRNA Removal Kit (Illumina). cDNA was generated using reverse transcriptase and dNTPs (Thermo Fisher). Libraries were constructed for the DNA and cDNA samples using a Nextera XT DNA Library Prep Kit (Illumina, San Diego, CA) [Citation20]. Library quality was assessed by a Qubit dsDNA HS Assay kit followed by a High Sensitivity DNA kit (Agilent) on an Agilent 2100 Bioanalyzer. Library pools were then loaded onto an Illumina Nextseq CN500 sequencer for 75 cycles of single-end sequencing to generate approximately 20 million reads for each library. For negative controls, we also prepared peripheral blood mononuclear cell (PBMC) samples with 105 cells/mL from healthy donors in parallel with each batch, using the same protocol, and sterile deionized water was extracted alongside the specimens to serve as non-template controls [Citation20,Citation21].
Trimmomatic was used to remove low-quality reads, adapter contamination, and duplicate reads, as well as those shorter than 50 bp [Citation22]. Low complexity reads were removed by Kcomplexity with default parameters. Human sequence data were identified and excluded by mapping to a human reference genome (hg38) using Burrows-Wheeler Aligner software [Citation23]. We designed a set of criteria similar to the National Center for Biotechnology Information (NCBI) criteria for selecting representative assembly for microorganisms (bacteria, viruses, fungi, protozoa, and other multicellular eukaryotic pathogens) from the NCBI Nucleotide and Genome databases (https://www.ncbi.nlm.nih.gov/assembly/help/anomnotrefseq/). Pathogen lists was selected according to three references: (1) Johns Hopkins ABX Guide (https://www.hopkinsguides.com/hopkins/index/Johns_Hopkins_ABX_Guide/Pathogens), (2) Manual of Clinical Microbiology (12th Edition), and (3) clinical case reports or research articles published in current peer-reviewed journals [Citation24]. The final database consisted of about 13,000 genomes. Microbial reads were aligned to the database with SNAP v1.0beta.18 (https://arxiv.org/abs/1111.5572). Virus-positive detection results (DNA or RNA viruses) were defined as the coverage of three or more non-overlapping regions on the genome. For the identification of bacteria, fungi and parasites, a positive detection was reported for a given species or genus if the reads per million (RPM) ratio, or RPM-r was ≥5, where the RPM-r was defined as the RPMsample/RPMNC (i.e. the RPM corresponding to a given species or genus in the clinical sample divided by the RPM in the NC/negative control) [Citation20]. In addition, to minimize cross-species misalignments among closely related microorganisms, we penalized (reduced) the RPM of microorganisms sharing a genus or family designation, if the species or genus appeared in non-template controls. A penalty of 5% was used for species [Citation25]. Hence, non-specific reads would be filtered and only the reads mapped to unique species could be classified to the species level.
Oral commensals were not considered as clinically significant microbes (CSMs) regardless of the relative abundance unless proven otherwise or strongly suggested by clinical manifestation. For bacteria (except mycobacteria), fungi (except molds) and viruses, a certain microbe was considered as CSM when its relative abundance was >30% at the species level with literature-based evidence of pulmonary pathogenicity. Molds were considered to be CSMs when the stringently mapped read number (SMRN) at the species level was >10 and literature supported their possible pathogenicity in the lungs. Mycobacteria were defined as positive in mNGS as long as the SMRN at the species level was >3, given the low possibility for contamination and the difficulty of DNA extraction [Citation10].
PCR, GMS staining and serum BG assay
P. jirovecii DNA can be detected by real-time PCR. The respiratory sample was mixed with 4% NaOH solution, from which 1 mL was placed in an experimental tube and centrifuged at 12,000 rpm/min for 5 min. Then, the supernatant was discarded. The remaining solution was mixed with normal saline and centrifuged at the same speed for 1 min, and again the supernatant was discarded. Next, 50 μL of magnetic beads (Da An Gene Co., Ltd. of Sun Yat-sen University) were added and incubated for 5 min at 100 °C after oscillating centrifugation and used for PCR amplification immediately. Real-time PCR was performed using PCR detection kits (PCR fluorescence probe method, Beijing Zhuo Cheng Hui Sheng Biotechnology Co., Ltd., Beijing, China). P. jirovecii-specific primer and fluorescent probe were designed targeting the mitochondrial larger subunit (mtLSU) rRNA region, and the human albumin gene was used for internal control. The PCR reaction mixture contained 35 μL of nucleic acid amplification reaction solution, 5 μL of the primer-probe mixture, and 10 μL of extracted DNA. The reaction process included 1 cycle of pre-incubation at 50 °C for 2 min, denaturation at 95 °C for 5 min, followed by 40 PCR cycles constituting denaturation at 95 °C for 15 s, annealing/extension and signal acquisition at 60 °C for 45 s, followed by a 1 min instrument hold at 12 °C. The results were analyzed with ABI 7500 fluorescence quantitative PCR software (Thermo Fisher Scientific, Massachusetts, USA). A cycle threshold (Ct) value of ≤37 was considered positive for P. jirovecii by PCR [Citation26].
The GMS staining was performed as follows: Formalin-fixed samples on glass slides were incubated with periodic acid for 10 min, stained by silver solution for 1.5 h in a water bath at 56 °C, and then stained with 0.25% gold chloride solution, 3% sodium thiosulfate and brilliant green. The results were read with immunofluorescence microscopy. Serum BG levels were measured by kinetic chromogenic method (fungal 1,3-β-d-Glucan detection kit produced by CHARLES RIVER Company, Zhanjiang, China) [Citation27].
Statistical analysis
Statistical analyses were performed using Stata 14.0 SE or GraphPad Prism 6.0. Continuous variables were presented as median with interquartile range (IQR) and analyzed by Mann-Whitney U test. Categorical variables were described as absolute numbers and frequencies and analyzed with the Chi-square test or with Fisher’s exact test, as appropriate. Sensitivity, specificity, positive predict value (PPV) and negative predict value (NPV) were calculated using the clinical diagnosis of PCP as the reference, and 95% confidence intervals (95% CIs) were obtained using Wilson’s method. For diagnostic performance evaluation, the non-parametric approach was taken in comparison of the areas under the receiver operating characteristic (ROC) curves (AUCs); logistic regression was used in combination of serum BG with mNGS. p-value of < .05 was considered statistically significant.
Results
Diagnostic performance
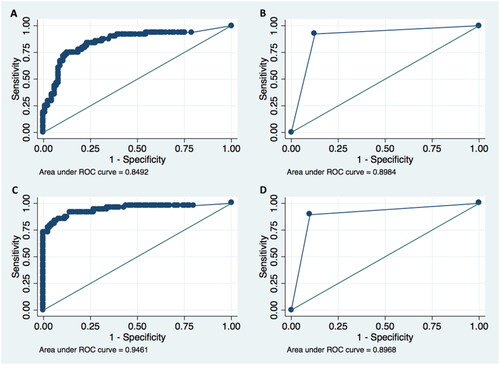
To evaluate the diagnostic efficacy of mNGS, 52 PCP and 103 non-PCP patients were included, and mNGS of BALF or qualified sputum was performed in all patients. The diagnostic performance of mNGS was compared with that of PCR, GMS staining and serum BG (). The mNGS and PCR were comparable in terms of diagnostic sensitivity (92.3% vs. 89.4%, p = .739), specificity (87.4% vs. 90.0%, p = .739), PPV (78.7% vs. 91.3%, p = .767) and NPV (95.7% vs. 87.8%, p = .773). The sensitivity of mNGS (92.3%) was superior to that of GMS staining (9.3%, p < .001) and serum BG (72.9%, p = .003). Also, mNGS (NPV, 95.7%) showed significantly higher NPV when compared to GMS staining (68.8%, p < .001) and serum BG (85.2%, p = .003). However, as the specificity of GMS staining reached 100%, GMS staining outperformed mNGS (87.4%, p < .001) in the diagnostic specificity.
Table 1. Comparison of diagnostic performance among mNGS, PCR, GMS staining and serum BG.
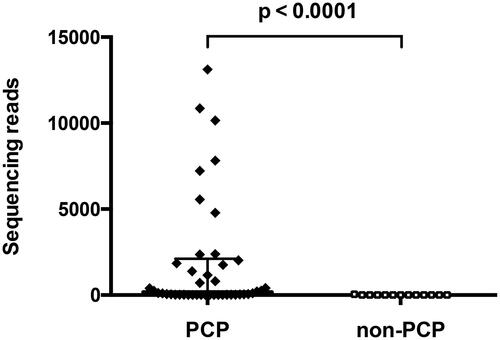
For patients with P. jirovecii dectected by mNGS, 13/103 (12.6%) were false positive in the non-PCP group without clinical diagnostic confirmation. The median reads of P. jirovecii DNA in the PCP group were 168 (IQR, 20-2020), which was remarkably higher than that of non-PCP patients with P. jirovecii DNA detected by mNGS (median, 3; IQR, 2–8; ).
Figure 1. Sequencing reads among PCP and non-PCP patients with P. jirovecii detected by mNGS. PCP group: n = 48, median 168; non-PCP group: n = 13, median 3. Error bar, median with IQR.
Besides, from the 155 patients with pneumonia, 51 blood samples were also collected for mNGS, of which 9 were mNGS positive for P. jirovecii. All these 9 blood samples came from PCP patients. The median level of serum BG from the 9 patients was 1116.9 pg/ml (IQR, 317.0–1608.4), which was higher than that of the overall PCP patients (median, 210.9; IQR, 82.8–589.8).
ROC curve analysis
In this study, ROC curves were graphed for mNGS, serum BG, a combination of mNGS and BG (mNGS + BG), and PCR, as is shown in . The AUC of mNGS (0.898) showed no statistical difference when compared with that of PCR (0.897, p = .970). The AUC for serum BG was 0.849, and the cut-off value of 91.3 pg/ml was calculated, with sensitivity of 75.0% and specificity of 87.2%. Furthermore, when combining mNGS with serum BG, it showed significant differences in AUC (0.946) compared to mNGS (p = .0013) or serum BG (p = .0015) alone, suggesting better diagnostic performance in combination of mNGS and serum BG. In addition, when we combined PCR with serum BG, the AUC (0.944) did not show significant difference in comparison with that of mNGS and serum BG combination (p = .883).
Pathogen profile
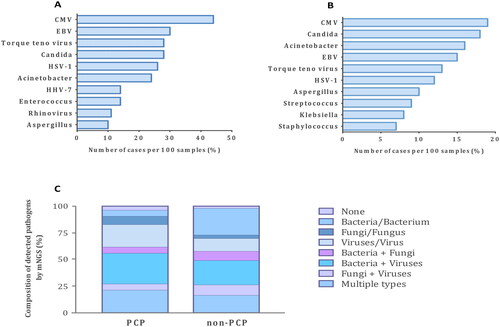
Compared with non-PCP, the pathogen spectrum of PCP patients had its own characteristics (). Virus co-infection was commonly seen among patients with PCP. The leading co-pathogens were cytomegalovirus (CMV, 44%) and Epstein-Barr virus (EBV, 30%), followed by Torque teno virus (28%) and Candida (28%). The most common bacteria detected by mNGS were Acinetobacter and Enterococcus in patients with PCP.
Figure 3. Distribution of pathogens detected by mNGS of lower respiratory tract specimens from PCP and non-PCP patients. (A) Top 10 co-pathogens identified from PCP patients. (B) Top 10 co-pathogens identified from patients with non-PCP pneumonia. (C) Composition of detected pathogens based on microbial types. P. jirovecii was not taken into calculation when cases were attributed to certain categories. CMV: cytomegalovirus; EBV: Epstein-Barr virus; HSV: herpes simplex virus; HHV: human herpes virus.
Clinical characteristics
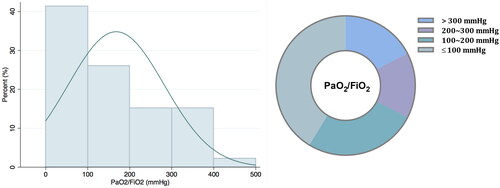
Among patients with PCP, most (78.8%) were immunocompromised with rheumatic autoimmune diseases (48.1%) being the leading underlying condition (). Forty-two patients (80.8%) were receiving corticosteroids at the diagnosis of PCP, and the median dose was 40 mg (IQR, 30–60). Other immunosuppressive agents were used in 67.3% of patients with PCP; nevertheless, PCP prophylaxis with trimethoprim/sulfamethoxazole (TMP-SMX) was seldom used (3.8%). 31 out of 50 patients with PCP (62.0%) suffered from CMV viremia in the clinical course. Dyspnea was more common among patients with PCP (82.7%) compared with those with non-PCP pneumonia (43.7%). For patients with PCP, the median oxygenation index (PaO2/FiO2) was 119 mmHg (IQR, 75–256), and 82.6% were suffering from hypoxemia with PaO2/FiO2 ≤ 300 mmHg at admission (). Twenty patients with PCP (38.5%) received high-flow nasal cannula and 2 (3.8%) were treated with non-invasive ventilator, whereas 32 (61.5%) required invasive mechanical ventilation. Nine patients (17.3%) got supported by extracorporeal membrane oxygenation (ECMO) among those with PCP. Overall, the 30-d mortality of patients with PCP was 36.5% ().
Table 2. Clinical characteristics of PCP and non-PCP patients.
Table 3. Managements and outcomes of PCP patients.
Discussion
From the 1980s, PCP, as an opportunistic yet severe pulmonary infection, has emerged as a concern for HIV and non-HIV immunocompromised patients [Citation28]. Since then, different testing methods have been utilized in detecting P. jirovecii, and thus assisting clinicians in diagnosis. With the rapid development of mNGS in recent years, it has become a promising diagnostic tool for suspected pneumonia, which has led to the question ‘Will mNGS potentially supplant traditional methods in diagnosing pneumonia?’ [Citation10,Citation29,Citation30]. To address this in PCP, by investigating patients with PCP and non-PCP pneumonia, we evaluated the diagnostic efficacy of mNGS and compared it with that of PCR, GMS staining and serum BG.
Before the clinical introduction of mNGS, the diagnosis of PCP was based on positive findings in PCR or GMS staining of lower respiratory tract specimens [Citation31,Citation32]. Both mNGS and PCR are able to detect the DNA of P. jirovecii, yet few have ever conducted a comparative analysis of the two in PCP. In the current study with PCP, the diagnostic performance of mNGS did not show significant differences as compared to that of PCR in regard to sensitivity and specificity. However, the result of mNGS could provide extra information on co-infected pathogens; and the relative abundance and specific reads offered may help with result interpretation in many cases. It has been reported that the P. jirovecii sequencing reads may fluctuate as the infection state changes, suggesting the potential application of mNGS in monitoring therapeutic efficacy [Citation33]. In this respect, mNGS is superior to PCR if cost is not taken into account. Nevertheless, as the specificity and PPV of mNGS were below 90%, similar to PCR, mNGS could not distinguish P. jirovecii colonization from infection, and thus clinical manifestation and other laboratory indicators should be integrated to make a proper diagnosis.
Previous studies have shown that the sensitivity and NPV of mNGS reach 100% in the diagnosis of PCP, which seems a little higher in comparison with the present study [Citation14,Citation15]. Though mNGS is expected to excel in the detection capability, still we would put a question mark in its ability to detect P. jirovecii with a ‘100% guarantee’ given the variability of clinical conditions and the complex process of sample acquisition and processing.
Direct microscopic visualization of P. jirovecii using GMS staining has been considered as a gold standard method historically in the diagnosis of PCP. Consistent with previous research on PCP, we have demonstrated that GMS staining has an excellent performance in specificity and PPV (100%), suggesting that if the cyst of P. jirovecii is detected with GMS staining, it will overwhelmingly support the diagnosis of PCP [Citation15,Citation34]. However, the sensitivity of GMS staining was quite low (9.3%), making it far from a satisfactory method in diagnosing PCP. In fact, other research has raised the concern that using BALF GMS staining as a sole method for PCP may lead to missed or delayed diagnoses in HIV-negative immunocompromised population due to its poor sensitivity for PCP [Citation35].
1,3-β-d-Glucan is a component of the P. jirovecii’s cyst wall, and therefore, serum BG is considered as a useful indicator for PCP diagnosis [Citation27,Citation36]. Yet it is not a specific marker as it can show positivity in other fungal infections and can also be affected by intravenous infusion of immunoglobin, albumin and others. Thus, serum BG assay could serve as an adjunct testing for PCP. A study among oncology patients has suggested that elevated serum BG level in conjunction with positive PCR results is associated with clinically significant PCP [Citation37]. In this study, similarly, we combined serum BG with mNGS, and it further improved the diagnostic efficacy of mNGS.
It is worth noting that the DNA of P. jirovecii was detected by mNGS in 9 blood samples from PCP patients, but not in blood samples from non-PCP patients, which implied outstanding specificity of 100% for blood sample mNGS in diagnosing PCP. Normally, P. jirovecii almost exclusively inhabits the lungs and should be undetectable in the blood. So, the rationale here is that, if P. jirovecii is detected in blood, we would expect that it has grown to a severe pathologic state where P. jirovecii may circulate into peripheral blood from its primary site. This could be supported in the current study by the higher levels of serum BG among the 9 patients (with positive blood sample mNGS for P. jirovecii). Still, the pathophysiological mechanism needs to be elucidated in the future. On the other hand, this finding also offers evidence to perform blood sample mNGS alternatively for the diagnosis of PCP when respiratory samples are not available, which is also supported by some other studies [Citation38,Citation39].
Furthermore, using mNGS, we explored the pathogen profile of PCP and non-PCP patients. Notably, viruses topped at the list of co-pathogens detected, especially for PCP patients. It was alarming that CMV was detected by mNGS from nearly half of PCP patients, and 62% of them were suffering from CMV viremia. Hence physicians should be alert to CMV re-activation and co-infection when treating patients with PCP. Interestingly, bacteria co-infection seemed not as prevalent as expected among PCP patients. This may be explained by the fact that empirical antibiotic treatment is usually initiated at a very early stage, even prior to hospital admission. Nevertheless, co-infection should be a concern for patients with PCP as most of them are in an immunocompromised state. As for patients with non-PCP pneumonia, besides viruses, Candida and Acinetobacter also ranked among the top co-pathogens. The explanation might be that most patients were critically ill in ICU (some were even immunocompromised) and there may be quite an amount of microbial contamination and colonization in patients and the environment, as was supported by previous studies, which would be detected by mNGS with excellent sensitivity [Citation40–42]. Therefore, despite the high incidence of pathogen detection by mNGS in respiratory specimens, it does not necessarily indicate true infection of a certain pathogen. The result of mNGS should be interpreted with great caution.
Among PCP patients, the majority suffered from hypoxemia, of which some even met the criteria for acute respiratory distress syndrome (ARDS). While mechanical ventilation was an important tool in managing these patients, 38.5% of PCP patients were treated with a high-flow nasal cannula in this study. Oxygen therapy and respiratory support is generally necessary for patients with PCP, but there are always some worries about ventilator-associated pneumonia for these patients mostly with lymphocytopenia and compromised immunity. Thus, a high-flow nasal cannula could be an option, especially for those with mild ARDS.
Our study has several limitations. It was a retrospective study and data were collected from a single center. As our hospital receives thousands of immunocompromised patients every year, the pathogen spectrum of pneumonia may differ from other centers. Besides, clinical mNGS testing is expensive with a single run costing $500–1000, whereas it may help shorten the time for a definitive diagnosis, and thereby reduce the length of hospital stay and other medical costs. The overall medical economic efficiency has not been evaluated in this study. Furthermore, it is expected that monitoring sequencing reads of mNGS may indicate therapeutic efficacy and guide treatment, which has not been addressed with our study. Finally, we have not explored the application of mNGS in drug resistance. The drug-resistant genes can be tested with mNGS; whether it is in line with conventional antimicrobial sensitivity testing is of interest to investigate in the future.
Conclusions
Taken together, as compared with PCR and GMS staining, mNGS would be a preferable method in diagnosing PCP, especially for those with mixed infection; the performance of mNGS can get boosted in combination with serum BG, indicating a potential strategy of choice in the diagnosis of PCP. In addition, blood sample mNGS can be performed alternatively for the diagnosis of PCP when respiratory samples are unavailable, yet further studies are needed to address its sensitivity and specificity. Co-infection, especially with viruses, should be of concern for patients with PCP in clinical practice.
Author contributions
Conception and design: Jing Yang
Acquisition of data: Xiaoning Wang
Analysis and interpretation of data: Yang Liu
Writing, review and revision of the manuscript: Yang Liu, Jun Xu and Huadong Zhu
Technical support: Qiwen Yang
Study supervision: Yang Jing
All the authors read and approved the final version of the manuscript.
Ethical approval
The study was approved by the Medical Ethics Committee of Peking Union Medical College Hospital, and written informed consent was waived due to the anonymized retrospective nature of the analysis. All methods were performed in accordance with relevant guidelines and regulations.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request if permitted by Peking Union Medical College Hospital.
Additional information
Funding
References
- Roux A, Gonzalez F, Roux M, et al. Update on pulmonary Pneumocystis jirovecii infection in non-HIV patients. Med Mal Infect. 2014;44(5):1–10. doi: 10.1016/j.medmal.2014.01.007.
- Rego de Figueiredo I, Vieira Alves R, Drummond Borges D, et al. Pneumocystosis pneumonia: a comparison study between HIV and non-HIV immunocompromised patients. Pulmonology. 2019;25(5):271–274. doi: 10.1016/j.pulmoe.2019.04.003.
- Thomas CFJr, Limper AH. Pneumocystis pneumonia. N Engl J Med. 2004;350(24):2487–2498. doi: 10.1056/NEJMra032588.
- Bateman M, Oladele R, Kolls JK. Diagnosing Pneumocystis jirovecii pneumonia: a review of current methods and novel approaches. Med Mycol. 2020;58(8):1015–1028. doi: 10.1093/mmy/myaa024.
- Azoulay E, Bergeron A, Chevret S, et al. Polymerase chain reaction for diagnosing pneumocystis pneumonia in non-HIV immunocompromised patients with pulmonary infiltrates. Chest. 2009;135(3):655–661. doi: 10.1378/chest.08-1309.
- Fillaux J, Malvy S, Alvarez M, et al. Accuracy of a routine real-time PCR assay for the diagnosis of Pneumocystis jirovecii pneumonia. J Microbiol Methods. 2008;75(2):258–261. doi: 10.1016/j.mimet.2008.06.009.
- Karageorgopoulos DE, Qu JM, Korbila IP, et al. Accuracy of beta-D-glucan for the diagnosis of Pneumocystis jirovecii pneumonia: a meta-analysis. Clin Microbiol Infect. 2013;19(1):39–49. doi: 10.1111/j.1469-0691.2011.03760.x.
- Koo S, Baden LR, Marty FM. Post-diagnostic kinetics of the (1 –> 3)-beta-D-glucan assay in invasive aspergillosis, invasive candidiasis and Pneumocystis jirovecii pneumonia. Clin Microbiol Infect. 2012;18(5):E122–E127. doi: 10.1111/j.1469-0691.2012.03777.x.
- Li WJ, Guo YL, Liu TJ, et al. Diagnosis of pneumocystis pneumonia using serum (1-3)-beta-D-Glucan: a bivariate meta-analysis and systematic review. J Thorac Dis. 2015;7(12):2214–2225.
- Peng JM, Du B, Qin HY, et al. Metagenomic next-generation sequencing for the diagnosis of suspected pneumonia in immunocompromised patients. J Infect. 2021;82(4):22–27. doi: 10.1016/j.jinf.2021.01.029.
- Shi Y, Peng JM, Qin HY, et al. Metagenomic next-generation sequencing: a promising tool for diagnosis and treatment of suspected pneumonia in rheumatic patients with acute respiratory failure: retrospective cohort study. Front Cell Infect Microbiol. 2022;12:941930. doi: 10.3389/fcimb.2022.941930.
- Wilson MR, Sample HA, Zorn KC, et al. Clinical metagenomic sequencing for diagnosis of meningitis and encephalitis. N Engl J Med. 2019;380(24):2327–2340. doi: 10.1056/NEJMoa1803396.
- Chiu CY, Miller SA. Clinical metagenomics. Nat Rev Genet. 2019;20(6):341–355. doi: 10.1038/s41576-019-0113-7.
- Wang D, Fang S, Hu X, et al. Metagenomic next-generation sequencing is highly efficient in diagnosing Pneumocystis Jirovecii pneumonia in the immunocompromised patients. Front Microbiol. 2022;13:913405. doi: 10.3389/fmicb.2022.913405.
- Jiang J, Bai L, Yang W, et al. Metagenomic next-generation sequencing for the diagnosis of Pneumocystis jirovecii pneumonia in non-HIV-infected patients: a retrospective study. Infect Dis Ther. 2021;10(3):1733–1745. doi: 10.1007/s40121-021-00482-y.
- Lagrou K, Chen S, Masur H, et al. Pneumocystis jirovecii disease: basis for the revised EORTC/MSGERC invasive fungal disease definitions in individuals without human immunodeficiency virus. Clin Infect Dis. 2021;72(Suppl 2):S114–S120. doi: 10.1093/cid/ciaa1805.
- Bartlett JG, Breiman RF, Mandell LA, et al. Community-acquired pneumonia in adults: guidelines for management. The infectious diseases Society of America. Clin Infect Dis. 1998;26(4):811–838. doi: 10.1086/513953.
- Meyer KC, Raghu G, Baughman RP, et al. An official American Thoracic Society clinical practice guideline: the clinical utility of bronchoalveolar lavage cellular analysis in interstitial lung disease. Am J Respir Crit Care Med. 2012;185(9):1004–1014. doi: 10.1164/rccm.201202-0320ST.
- Amar Y, Lagkouvardos I, Silva RL, et al. Pre-digest of unprotected DNA by benzonase improves the representation of living skin bacteria and efficiently depletes host DNA. Microbiome. 2021;9(1):123. doi: 10.1186/s40168-021-01067-0.
- Miller S, Naccache SN, Samayoa E, et al. Laboratory validation of a clinical metagenomic sequencing assay for pathogen detection in cerebrospinal fluid. Genome Res. 2019;29(5):831–842. doi: 10.1101/gr.238170.118.
- Li H, Gao H, Meng H, et al. Detection of pulmonary infectious pathogens from lung biopsy tissues by metagenomic next-generation sequencing. Front Cell Infect Microbiol. 2018;8:205. doi: 10.3389/fcimb.2018.00205.
- Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for illumina sequence data. Bioinformatics. 2014;30(15):2114–2120. doi: 10.1093/bioinformatics/btu170.
- Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25(14):1754–1760. doi: 10.1093/bioinformatics/btp324.
- Fiorini N, Lipman DJ, Lu Z. Towards PubMed 2.0. ELife. 2017;6:e28801. doi: 10.7554/eLife.28801.
- Gu W, Deng X, Lee M, et al. Rapid pathogen detection by metagenomic next-generation sequencing of infected body fluids. Nat Med. 2021;27(1):115–124. doi: 10.1038/s41591-020-1105-z.
- Liu W, Li M, Xu Y, et al. Evaluation of the performance of a multiplex real-time PCR assay for the identification of Aspergillus, Cryptococcus neoformans, and Pneumocystis jirovecii simultaneously from sputum in multicenter. Infect Drug Resist. 2022;15:6009–6017. doi: 10.2147/IDR.S379043.
- Sun R, Lv D, Xiao M, et al. Diagnostic accuracy of the 1,3-beta-D-glucan test and lactate dehydrogenase for pneumocystis pneumonia in non-HIV patients. Sci Rep. 2021;11(1):9226. doi: 10.1038/s41598-021-88729-z.
- Maini R, Henderson KL, Sheridan EA, et al. Increasing Pneumocystis pneumonia, England, UK, 2000–2010. Emerg Infect Dis. 2013;19(3):386–392. doi: 10.3201/eid1903.121151.
- Zheng Y, Qiu X, Wang T, et al. The diagnostic value of metagenomic NEXT-GENERATION sequencing in lower respiratory tract infection. Front Cell Infect Microbiol. 2021;11:694756. doi: 10.3389/fcimb.2021.694756.
- Mitchell SL, Simner PJ. Next-Generation sequencing in clinical microbiology: are we there yet? Clin Lab Med. 2019;39(3):405–418. doi: 10.1016/j.cll.2019.05.003.
- Alanio A, Hauser PM, Lagrou K, et al. ECIL guidelines for the diagnosis of Pneumocystis jirovecii pneumonia in patients with haematological malignancies and stem cell transplant recipients. J Antimicrob Chemother. 2016;71(9):2386–2396. doi: 10.1093/jac/dkw156.
- Liu Y, Zheng K, Liu Y, et al. Pneumocystis jirovecii pneumonia in patients with nephrotic syndrome: application of lymphocyte subset analysis in predicting clinical outcomes. Can J Infect Dis Med Microbiol. 2020;2020:4631297. doi: 10.1155/2020/4631297.
- Zhang F, Chen J, Huang H, et al. Application of metagenomic next-generation sequencing in the diagnosis and treatment guidance of Pneumocystis jirovecii pneumonia in renal transplant recipients. Eur J Clin Microbiol Infect Dis. 2021;40(9):1933–1942. doi: 10.1007/s10096-021-04254-x.
- Homayouni MM, Rostami A, Gholizadeh H, et al. Comparison of three cost effective staining methods for detection of Pneumocystis jirovecii. J Parasit Dis. 2017;41(1):298–301. doi: 10.1007/s12639-016-0776-3.
- Azar MM, Slotkin R, Abi-Raad R, et al. Gomori methenamine silver stain on bronchoalveolar lavage fluid is poorly sensitive for diagnosis of Pneumocystis jiroveci pneumonia in HIV-Negative immunocompromised patients and may lead to missed or delayed diagnoses. Arch Pathol Lab Med. 2020;144:1003–1010.
- Wood BR, Komarow L, Zolopa AR, et al. Test performance of blood beta-glucan for Pneumocystis jirovecii pneumonia in patients with AIDS and respiratory symptoms. AIDS. 2013;27(6):967–972. doi: 10.1097/QAD.0b013e32835cb646.
- Morjaria S, Frame J, Franco-Garcia A, et al. Clinical performance of (1,3) Beta-D glucan for the diagnosis of Pneumocystis pneumonia (PCP) in cancer patients tested with PCP polymerase chain reaction. Clin Infect Dis. 2019;69(8):1303–1309. doi: 10.1093/cid/ciy1072.
- Li J, Li J, Yu Y, et al. Pneumocystis pneumonia and rheumatic disease: diagnostic potential of circulating microbial cell-free DNA sequencing. Rheumatol Adv Pract. 2022;6(1):rkab105.
- Zhang Y, Ai JW, Cui P, et al. A cluster of cases of pneumocystis pneumonia identified by shotgun metagenomics approach. J Infect. 2019;78(2):158–169. doi: 10.1016/j.jinf.2018.08.013.
- Meena DS, Kumar D. Candida pneumonia: an innocent bystander or a silent killer? Med Princ Pract. 2022;31(1):98–102. doi: 10.1159/000520111.
- Raro OHF, Gallo SW, Ferreira CAS, et al. Carbapenem-resistant Acinetobacter baumannii contamination in an intensive care unit. Rev Soc Bras Med Trop. 2017;50(2):167–172. doi: 10.1590/0037-8682-0329-2016.
- Collingwood A, Blostein F, Seekatz AM, et al. Epidemiological and microbiome associations between Klebsiella pneumoniae and vancomycin-resistant enterococcus colonization in intensive care unit patients. Open Forum Infect Dis. 2020;7(1):ofaa012. doi: 10.1093/ofid/ofaa012.