Abstract
Objective
To explore the efficacy and safety of ultrasound-guided injection acupotomy as a minimally invasive intervention treatment of cervical spondylotic radiculopathy (CSR).
Methods
160 CSR subjects were recruited who met the inclusion criteria in our hospital from October 2019 to December 2021. The subjects were randomly divided into the experimental and control group, with 80 cases in each. The experimental group received ultrasound-guided injection acupotomy as a minimally invasive intervention therapy. The control group received ultrasound-guided selective nerve root block (SNRB). The Odom’s criteria clinical curative effect, visual analogue scale (VAS), neck disability index (NDI), and 36-Item Short Form Health Survey questionnaire (SF-36) were used to evaluate the outcome of subjects at several different points in time.
Results
At 30 min and 1 month after the end of treatment, there was no significant difference in any scores. However, after six months, the excellent and good rate was better in the experimental group compared to the control (RD = 0.175; 95% CI, 0.044–0.300, p = 0.009). The total effective rate was also better in the experimental group (RD = 0.126; 95% CI, 0.021–0.232, p = 0.018). In contrast, the VAS score (MD = −0.500; 95% CI, −1.000–0.000, p = 0.030) and NDI score (MD = −6.460; 95% CI, −11.067 to −1.852, p = 0.006) were lower in the experimental group compared to the control. The total SF-36 score was higher in the experimental group (MD = 7.568; 95% CI, 2.459–12.677, p = 0.004).
Conclusion
Ultrasound-guided injection acupotomy minimally invasive interventional treatment of CSR has no significant difference in short-term curative effect compared with ultrasound-guided SNRB, but the data indicators are significantly better than the latter at 6 months after the end of the course of treatment, showing better long-term efficacy.
Background
Cervical spondylotic radiculopathy (CSR) is a common disease in the department of pain, accounting for about 60–70% of all cervical spondylosis [Citation1]. It is mainly caused by the stimulation or compression of unilateral or bilateral spinal nerve roots. Symptoms include sensory, motor, and reflex disturbances consistent with the distribution of spinal nerve roots. The most common age for CSR is 50–60 years old [Citation2, Citation3]. About two per 1,000 middle-aged people suffer from CSR every year [Citation4]. An epidemiological survey of CSR in the USA found that about 83.2 per 100,000 people suffer from this condition [Citation5].
CSR has a high incidence rate, a long course of disease, and is prone to repeated attacks that seriously affect the work and life of people. CSR is mainly managed using conservative and surgical treatment. From the perspective of economy and safety risks, conservative treatment is usually the first choice for patients. At the early stage of CSR, it is widely recommended that conservative treatment is performed for six to 12 weeks, and that surgical treatment should be considered if symptoms do not improve significantly [Citation1, Citation6, Citation7]. According to statistics, only 10–25% of CSR patients ultimately need surgical treatment, with the rest achieving satisfactory results through conservative treatment [Citation6, Citation7].
Conservative treatments for CSR include transcutaneous electrical nerve stimulation, functional exercises, traction, manipulation, physical therapy, oral medication, and epidural steroid injections. Selective Nerve Root Block (SNRB), a type of epidural steroid injection, eliminates inflammation and edema of damaged nerve roots, and quickly relieves symptoms. SNRB has been used to treat CSR. SNRB is therapeutic and diagnostic, helping to identify affected nerve roots [Citation8]. In China, many physicians use acupotomy to treat CSR. Acupotomy helps to loosen the soft tissue around the nerve root, release the compression of the nerve root, and promote the absorption of inflammatory substances, providing better therapeutic effect [Citation9–11].
Several studies have investigated the potential of combining X-ray or ultrasound-based SNRB with acupotomy to treat lumbar disc herniation and entrapment of the inferior patellar branch of the saphenous nerve [Citation12, Citation13]. However, there is no report on whether this combination of treatments could be used to treat CSR. To treat CSR more effectively, we explored and developed a new treatment method integrating SNRB and acupotomy. Ultrasound-guided injection acupotomy was used as a minimally invasive intervention technology to treat CSR.
Materials and methods
Study design
This study is a single-center, prospective, randomized parallel-controlled, open-label trial, we followed the CONSORT guidelines in reporting this clinical trial. The research complied with the ethical principles and constraints of the Declaration of Helsinki [Citation14]. The research protocol was approved by the Ethics Committee of Zhangjiagang Second People’s Hospital (approval number: ZEY-2020004), and was registered in the China Clinical Trial Registration Center (http://www.chictr.org.cn) (registration number: ChiCTR1900026633). We divided the subjects into an experimental and control group. Subjects in the experimental group received ultrasound-guided injection acupotomy as a minimally invasive interventional therapy. Subjects in the control group received ultrasound-guided selective cervical nerve root block therapy. Every patient was randomly assigned to one of the two groups after written informed consent was obtained.
Sample size calculation
The primary efficacy parameter was the excellent and good rate. Based on our preliminary test, the excellent rate of the experimental and control groups was 87% and 68% at six months after the end of treatment. Based on a previous Pre-Experimen, a sample size of 73 subjects per group was calculated to provide 80% power to detect a clinically meaningful difference between the two groups at an alpha level of 0.05 (2-tailed test). Considering the risk of subjects dropping out, we added 10% to the original estimate. In the end, at least 80 subjects were enrolled in each group.
Eligibility criteria
From October 2019 to December 2021, we recruited adult subjects with neck, shoulder, back, and upper limb pain from the Department of Acupuncture and Department of Pain Medicine in Zhangjiagang Second People’s Hospital, China.
Because there are no unified diagnostic criteria for CSR, we developed diagnostic criteria based on the published literature [Citation15–18]. These criteria were: (1) Have suffered neck strain or a sprained or stiff neck; (2) Have radiation numbness, pain, or paresthesia in the neck and areas innervated by the root of the cervical nerve, which might be aggravated when the neck posture is improper; (3) Symptoms might be accompanied by the reduced muscle strength of the upper limbs, as well as inflexible finger movement, and hand muscle atrophy might occur in the elderly over the course of the disease; (4) There may be tenderness or muscle tension spasms in the neck during physical examination; (5) The neck extension test, brachial plexus traction test (Eaten test), intervertebral foramen extrusion test (Spurling test), head percussion test, and Hoffman sign might be positive; (6) X-ray and CT might show changes to the physiological curvature of the cervical spine, as well as vertebral joint instability, bone spur formation, and intervertebral foramen stenosis; and (7) MRI might show changes to cervical disc degeneration, nucleus pulposus, nerve root compression, and cervical spinal canal stenosis. Electromyography is useful for identifying responsible neural segments. CSR can be diagnosed if any one of (1)+(2)+(5)+other four items is met.
Inclusion criteria included: (1) subjects diagnosed with CSR; (2) the duration of CSR for each participant was less than 12 weeks; and (3) participants were between 20 to 80 years old. Patients voluntarily participated in this study, and signed the informed consent of the clinical trial. Exclusion criteria included: (1) Suffering from other neck, shoulder, back, and upper limb soft tissue injuries, upper limb peripheral neurovascular entrapment, cervical spinal cord lesions, and other diseases that could affect the research results; (2) Those who have received other treatments within one week of before the treatment, which could affect the curative effect; (3) Symptoms combined with severe heart, lung, liver, and kidney dysfunction, hematopoietic system diseases, mental disorders, and women who are pregnant; (4) Those who have been experienced open neck surgery; (5) Those who are allergic to the test drug; and (6) Those who were not willing to undergo minimally invasive interventional therapy after enrollment.
Randomization
A physician at our hospital who was not involved in the research used a random number generator on a computer to generate 1 to 160 random number sequences. The physician put these random number sequences in sealed opaque envelopes with continuous codes, in triplicate. The study director, intervention implementer, and statistician each keep a copy. After the subjects received the envelopes in the coded order, before entering their groups, three people simultaneously opened the envelopes face to face. Subjects were allocated to the intervention implementers, with those with odd and even numbers entering the experimental and control groups, respectively.
Treatment methods
In the experimental group, the posterior tubercle of the cervical vertebral transverse process and the cervical nerve root located between the anterior and posterior tubercle were targeted. In the control group, the cervical nerve root was targeted. One transverse process was selected for each treatment. Before treatment, the subjects in the two groups should first identify the cervical nerve roots to be blocked and the cervical transverse process to be released, (mainly C5, C6, C7 nerve roots and transverse processes) [Citation18–20], according to the symptoms, signs and relevant imaging examination, Ultrasound positioning was performed after confirmation.
During positioning, the subjects were asked to hold a lateral position, with the affected side on the top. A thin pillow was placed under the head. The cervical spine was slightly extended to expose the head and neck of the affected side fully. Routine ECG monitoring, neck iodophor disinfection, and aseptic towel were used. A Voluson P8 ultrasound produced by General Electric (USA) was used. It was set to a 5–12 MHz high-frequency linear array probe. Small organs were selected for the ultrasound mode. The appropriate depth was set, and the gain was adjusted depending on the size of the subject. The probe was coated with coupling agent. Disposable sterile gloves were worn, tightened, and sterilized for later use.
The same blocking solution was used on both groups of subjects A 10 ml disposable sterile syringe was used to draw 1.0 ml (5 + 2mg) Compound Betamethason Injection (Schering-Plough Labo N.V., Berg, Belgium), 1.5 ml (30 mg) of 2% lidocaine hydrochloride (Shanxi Jinxin Double Crane Pharmaceutical Co., Ltd., Shanxi, China), and 3.5 ml of 0.9% sodium chloride (Sinopharm Rongsheng Pharmaceutical Co., Ltd., Henan, China), totaling 6 ml blocker solution for standby.
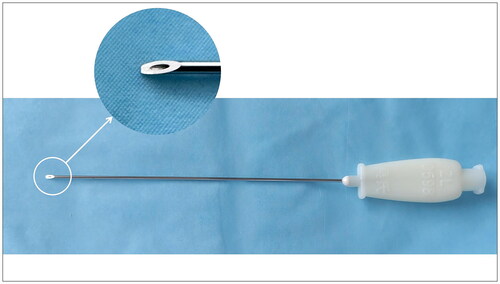
After confirming the posterior tubercle of the transverse process and the cervical nerve root that had to be released in the experimental group, the short-axis section of the probe was used, and the in-plane needle technique was applied. Hanqing brand disposable sterile injection acupotomy was used, with a specification of 0.9*85 mm (Henan Shangrui Medical Technology Co., Ltd., Henan, China). The incision line was parallel to the long axis of the neck. The best needle insertion was found using the ultrasound image angle and plane, puncturing from back to front. During puncturing, ultrasound was used to track the needle insertion process of the injection acupotomy in real time. The blade was inserted avoiding the blood vessels, and separated the muscle fascia layers until it touched the posterior nodule of the transverse process. After withdrawing bloodless and anergic cerebrospinal fluid, 2 ml blocking fluid was injected. The posterior tubercle of the transverse process was gently cut three times in a longitudinal direction. It was then peeled off three times horizontally. After confirming that the subject had incurred no adverse reactions, the injection acupotomy was passed obliquely through the posterior tubercle of the cervical spine, The advance of the needle was continued until it reached around the cervical nerve root. At this point, some subjects have numbness or pain radiating to the shoulder, back, or upper limbs. After withdrawing the bloodless and anergic cerebrospinal fluid again, the remaining 4 ml of blocking fluid was injected slowly. The skin of the incision was kept clean and dry for three days ().
Figure 1. Posterior tubercle of the sixth cervical transverse process and selective block C6 nerve root released by injection acupotomy. A. Posterior tubercle of the sixth cervical transverse process was released with injection acupotomy. B. Injection acupotomy selective block C6 nerve root. White arrow. Injection acupotomy; at. Transverse process anterior tubercle of C6; PT. Transverse process posterior tubercle of C6; TP. Transverse process of C6; C6. Nerve root C6; SCMM. Sternocleidomastoid muscle; ASM. Anterior scalene muscle; MSM. Middle scalene muscle.
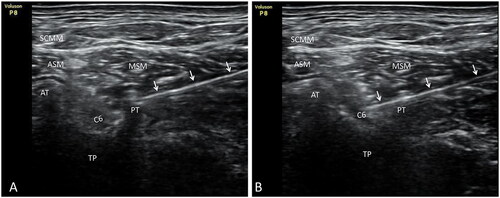
Figure 2. Posterior tubercle of the seventh cervical transverse process and selective block C7 nerve root released by injection acupotomy. A. Posterior tubercle of the seventh cervical transverse process was released with injection acupotomy. B. Injection acupotomy selective block C7 nerve root. White arrow. Injection acupotomy; PT. Transverse process posterior tubercle of C7; TP. Transverse process of C7; C6. Nerve root C6; C7. Nerve root C7; VA. Vertebral artery; SCMM. Sternocleidomastoid muscle; ASM. Anterior scalene muscle; MSM. Middle scalene muscle.
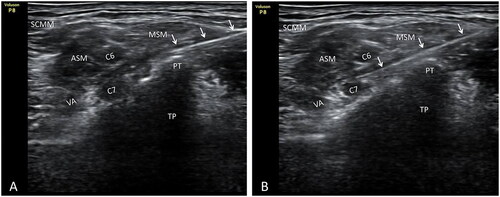
After confirming the cervical nerve root to be needed blocked in the control group, the short-axis section of the probe was also used. The in-plane needle technique was applied. A 22 G 0.7*80 mm ultrasound-guided special nerve block needle (Shenzhen Tuoren Biomedical Electronics Co., Ltd., Guangdong, China) was used to make the puncture. The needle tip was located near to the cervical nerve root, between the anterior and posterior nodules of the cervical spine, without deliberately looking for abnormal sensation. Before the routine injection, it needs to be gently withdrawn, and no blood or cerebrospinal fluid is flowing out, and 4 ml of blocking solution should be injected around the cervical nerve root. During the injection process, the needle tip should be clearly visible. After the treatment, the needle holes were covered with a band-aid.
Both groups of subjects received a slow injection of medicinal solution during treatment. After the treatment was completed, the subjects had to lie down for 30 min. If subjects’ symptoms did not improve significantly after the first treatment, a second treatment was given one week later.
Outcome measurements
The primary outcome was the excellent and good rate. The secondary outcome indicators included total effective rate, Visual Analogue Scale (VAS), Neck Disability Index (NDI) [Citation21], and The 36-Item Short Form Health Survey questionnaire (SF-36) total score [Citation22]. The SF-36 total score was calculated as an average of the scores of the eight dimensions. The clinical curative effect was evaluated using the Odom’s criteria clinical curative effect evaluation standard [Citation23]. This standard was divided into four grades: excellent, good, medium, and poor. The excellent and good rate = [(excellent + good) cases/total cases] × 100%. Total effective rate = [(excellent + good + moderate) number of cases/total number of cases] × 100%.
Adverse reactions to the safety indicators included vasovagal reactions during or after treatment, local anesthetic poisoning, intravascular injection, spinal nerve injury, spinal cord infarction, local hematoma or infection, and general spinal anesthesia [Citation24–26].
Follow-up protocol
Subjects were followed up in various ways, mainly based on outpatient follow-up, including telephone follow-up, family follow-up, and network follow-up as supplementary methods. Professionally trained physicians in statistics collected data before subjects were enrolled, 30 min after the first treatment, and then at one and six months after the end of the course of treatment. At each follow-up, it also was recorded whether the subjects had experienced any adverse reactions.
Statistical analysis
The data were statistically analyzed by a statistician in a blinded manner. The efficacy indicators of the two groups of subjects were analyzed using the Full Analysis Set (FAS). FAS refers to subjects who have received at least one complete treatment and have trial follow-up records. The safety analysis set (Safety-analysis-set, SS) was used to evaluate the safety of clinical trials. The safety analysis population included all subjects who received at least one treatment and had at least one subsequent safety-related follow-up visit. Statistical analysis was performed using SPSS 21.0 software. Missing values were dealt with by Multiple Imputation (MI). Categorical variables were expressed as the number of cases (%). The comparison of rates between groups was performed using the χ2 test or Fisher’s exact test. The Rate Difference (RD) and 95% Confidence Interval (95% CI) were calculated using the Wilson method. Continuous variables were first tested for normality using the S-W method (Shapiro-Wilk Test). Normally distributed variables were expressed as Mean ± Standard Deviation (Mean ± SD). Comparisons between groups were performed using independent sample t tests to calculate the Mean Difference (MD) and 95%CI. Variables with non-normal distributions were represented by median (first quartile, third quartile) [M (P25, P75)]. Comparison between groups was performed by Mann-Whitney U test and Hodges-Lehman estimation Calculate the Median Difference (MD), and 95% CI. Significance was set at p < 0.05.
Results
General characteristics of eligible subjects in the two groups
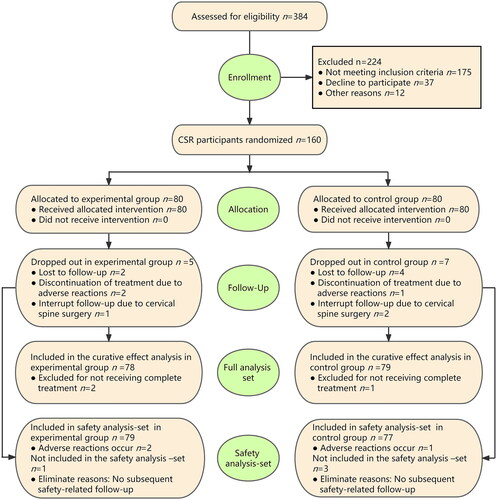
A total of 160 subjects participated in this study from October 2019 to December 2021. presents baseline data on the subjects in both groups, including demographic characteristics and clinical characteristics. Five and seven cases dropped out of the experimental and control groups over the entire program, respectively (drop-out rate: 6.3% and 8.7%, respectively). There was no statistically significant difference between the two groups (RD = 0.025; 95%CI, −0.063 − 0.115, p = 0.548). Of the cases that dropped out, two in the experimental group and one in the control group were excluded because of vasovagal reaction during treatment.
Table 1. Comparison of demographic and clinical characteristics of subjects in two groups.
Overall, 78 and 79 cases in the experimental and control groups were included in the efficacy analysis, respectively. One case in the experimental group and two cases in the control group were lost to follow-up at one month after the end of the course of treatment; one case in the experimental group and two cases in the control group were lost to follow-up at 6 months after the end of the course of treatment; one case in the control group interrupted the follow-up due to cervical spine surgery within one month of completing treatment; one case in the experimental group and one case in the control group interrupted the follow-up due to cervical spine surgery within 6 months after the end of the course of treatment ().
Results of the curative effect
One month after the end of the course of treatment, the excellent and good rate was 73.1% and 65.8% in the experimental and control groups, respectively. There was no significant difference between the two groups at this point (RD = 0.073; 95%CI, −0.071–0.212, p = 0.324). The total effective rate was 89.8% and 86.1% in the experimental and control groups, respectively, with no significant difference between the two groups (RD = 0.037; 95%CI, −0.069–0.142, p = 0.481). However, six months after the end of the course of treatment, the excellent and good rate was 85.9% versus 68.4% in the experimental and control groups, respectively, with the experimental group clearly outperforming the control group (RD = 0.175, 95%CI, 0.044–0.300, p = 0.009). The total effective rate was 93.6% and 81.0% for the experimental and control groups, respectively, with a clear statistically significant difference (RD = 0.126; 95%CI, 0.021–0.232, p = 0.018) ().
Table 2. Comparison of efficacy 1 Month and 6 months after the course of treatment of subjects in two groups (ODM).
VAS scores of the two groups
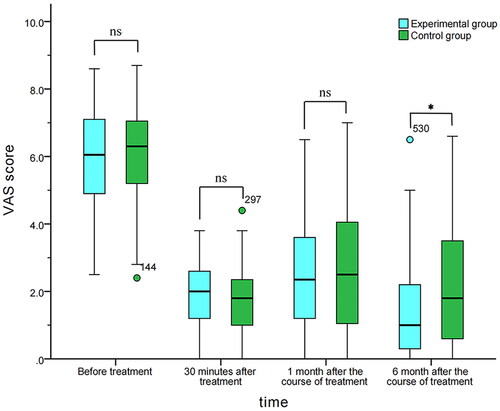
Before treatment, the VAS score of the test group was 6.1 (4.9, 7.1), and that of the control group was 6.3 (5.0, 7.1). There was no significant difference between the two groups (MD = -0.100; 95% CI, −0.600–0.300, p = 0.548). Thirty minutes after treatment, the VAS score was 2.0 (1.2, 2.6) and 1.8 (1.0, 2.4) for the experimental and control groups, respectively, with no significant difference (MD = 0.100; 95%CI, −0.200–0.500, p = 0.417). One month after completing treatment, the VAS score was 2.4 (1.2, 3.7) and 2.5 (1.0, 4.1) for the experimental and control groups respectively, with no significant difference (MD = -0.100; 95% CI, −0.700–0.400, p = 0.761). Six months after completing treatment, the VAS score was 1.0 (0.3, 2.2) and 1.8 (0.6, 3.7) for the experimental and control groups, with the difference being statistically significant (MD=-0.500; 95%CI, −1.000–0.000, p = 0.030) ().
NDI score of the two groups
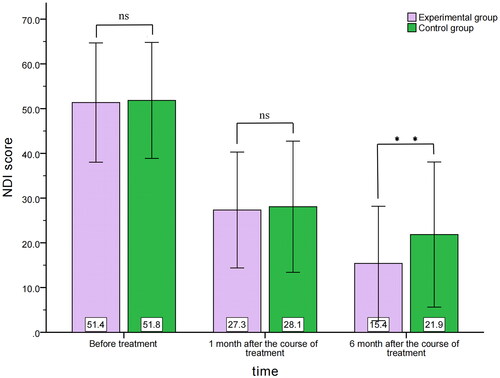
Before treatment, the NDI score was 51.4 ± 13.3 and 51.8 ± 13.0 in the experimental and control groups, respectively, with no significant difference (MD=-0.483; 95%CI, −4.629–3.663, p = 0.818). One month after completing treatment, the NDI score was 27.3 ± 12.9 and 28.1 ± 14.7, in the experimental and control groups, respectively, with no significant difference (MD=-0.732; 95%CI, −5.095–3.631, p = 0.741). Six months after completing treatment, the NDI score was 15.4 ± 12.8 and 21.9 ± 16.2 in the experimental and control groups respectively, with a statistically significant difference (MD=-6.460; 95%CI, −11.067– −1.852, p = 0.006) ().
SF-36 total score of the two groups
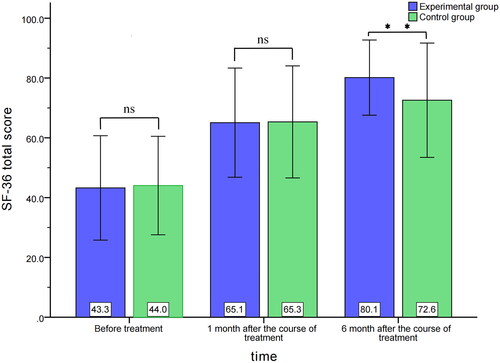
Before treatment, the total score of SF-36 was 43.3 ± 17.5 and 44.0 ± 16.5 in the control and experimental groups, respectively, with no significant difference (MD=-0.788; 95%CI, −6.139–4.563, p = 0.772). One month after completing treatment, the total score of SF-36 was 65.1 ± 18.3 and 65.3 ± 18.7 in the experimental and control groups, respectively, with no significant difference (MD=-0.250; 95%CI, −6.083–5.584, p = 0.933). At six months after completing treatment, the total score of SF-36 was 80.1 ± 12.6 and 72.6 ± 19.1 in the experimental and control groups, respectively, with a statistically significant difference (MD = 7.568; 95%CI, 2.459–12.677, p = 0.004) ().
Safety analysis
One and three cases in the experimental and control groups, respectively, were lost to follow-up within one month after completing treatment, and were excluded without any and subsequent safety-related follow-up. Overall, 79 and 77 cases in the experimental and control groups, respectively, were included in the safety analysis. Vasovagal reactions occurred in two subjects in the experimental group during the treatment (adverse reaction rate: 2.5%) and one subject in the control group (adverse reaction rate: 1.3%). There was no significant difference in the adverse reaction rate between the two groups (RD = 0.012; 95%CI, −0.048–0.076, p = 0.575). The two groups of subjects exhibited no other adverse reactions. All adverse reactions recovered after symptomatic treatment, with no adverse consequences to subjects.
Discussion
Injection acupotomy therapy is a minimally invasive treatment method of traditional Chinese medicine that integrates needle-knife loosening and local injection. It was invented by Professor Hanqing Wu, a famous Chinese researcher who is an expert on minimally invasive procedures. The procedure is based on the nine-acupuncture of ancient China [Citation27]. Injection acupotomy was given the Chinese utility model patent certificate in 2006 (patent number: ZL 2005 2 0031398.1). The equipment has a hollow pipe in the center of the acupotomy, through which the fluid can be injected into the soft tissue, the acupotomy can be released at the same time as the drug is injected (). This procedure improves anti-inflammatory and analgesic effects, reduces postoperative recurrence, and reduces the pain of patients. It has a clear curative effect on pain and dysfunction caused by soft tissue injury.
Ultrasound-guided puncture when treating CSR allows the knife edge or needle point to reach the target more quickly and accurately. This approach also avoids unforeseen damage to normal soft tissue, and is more efficient. Various studies have shown that there is no significant difference in the success rate, pain relief, and functional improvement between ultrasound-guided SNRB versus fluoroscopy-guided transforaminal injection when treating CSR. However, ultrasound-guided SNRB has the advantage of no radiation, no serious complications, and being more efficient [Citation8, Citation24, Citation28]. Some blood vessels are also present around some cervical nerve roots, with the Doppler mode of ultrasound being able to detect any located around the target. This procedure effectively avoids intravascular injection, and enhances the safety of the entire treatment process [Citation24, Citation28, Citation29]. In our study, three subjects in the two groups interrupted treatment after vasovagal reaction occurred (adverse reaction rate: 1.87%). No other serious adverse reactions were detected and the safety was satisfied. These findings support those of above-mentioned studies.
To avoid spinal cord or brainstem infarction caused by intravascular injection, non-granular steroids (such as dexamethasone injection) are recommended for use as the blocking solution [Citation8, Citation24, Citation30]. However, some studies confirmed that granular steroid injections are curative effect better than non-granular steroids, but that there is no significant difference in the incidence of adverse reactions [Citation31]. In 2012, study in Korea used triamcinolone acetonide for selective cervical nerve root block under fluoroscopy. This drug was considered to be more effective than non-granular Betamethasone Sodium Phosphate Injection, as it provided longer pain relief with no serious complications [Citation32]. In 2016, a study showed that the satisfaction of the same patients regarding the effectiveness of lumbar epidural injections was significantly higher for triamcinolone acetonide compared to dexamethasone, with the interval between re-injections also being longer [Citation33]. Ultrasound guidance allows blood vessels to be identified accurately, and the trajectory of the needle tip can be tracked in real time, avoiding intravascular injection. We also selected the granular steroid Compound Betamethason Injection during treatment, which guaranteed safety and improved efficacy. The improvement of the subjects’ symptoms was most obvious within one month after the end of the course of treatment. Compared with baseline, the VAS and NDI scores of the two groups significantly decreased between one months after completing treatment, whereas the total score of SF-36 significantly rose compared to baseline. This improvement continued until 6 months after the end of the course of treatment.
Acupotomy is a common therapy in China [Citation34, Citation35], with doctors attaining satisfactory curative effects with acupotomy for CSR. In 2020, a meta-analysis of acupotomy therapy for CSR showed that most subjects with CSR improved following acupotomy therapy in clinical practice [Citation9]. At present, ultrasound-guided acupotomy therapy is popular, because of its efficacy and safety [Citation36, Citation37]. Rao et al. [Citation13] suggested that ultrasound-guided acupotomy combined with peripheral nerve block represents a new method for treating saphenous nerve entrapment in the knee joint, with relatively long-lasting effects. Choi et al. [Citation38] demonstrated how ultrasound-guided acupotomy release combined with nerve block therapy has better curative effects than nerve block therapy alone when treating lumbosacral radiculopathy. Of importance, this procedure is less costly than other methods.
Using MRI, Chen et al. showed that, for many cases of cervical disc herniation, upper extremity symptoms were caused by nerve entrapment outside the cervical foramina [Citation39]. Dissection studies show that, after C5 and C6 nerve roots leave the intervertebral foramen, they are surrounded by the dense and tough starting points of the anterior and middle scalene muscles in the anterior and posterior tubercle of the transverse process of the cervical spine, with tendinous attachment points forming a ‘scissors style’ angle stuck at the beginning of the nerve [Citation40]. Therefore, tension and spasms of the scalene muscle caused by various factors result in the narrowing of the cervical intervertebral foramen and transverse process bone fiber canal. Consequently, compression of the cervical nerve root causes CSR. To address this issue, our group used injection acupotomy to loosen the soft tissue around the posterior tuberosity of the cervical vertebrae, while performing SNRB. This approach had better long-term efficacy compared to SNRB alone. Six months after the end of the course of treatment, we can also observe the continuous improvement of the symptoms of the two groups of subjects. It is generally believed that SNRB has only a short-term effect, so the long-term improvement of symptoms in the control group cannot be ruled out as the result of the subject’s self-healing. Compared with the control group, the improvement of symptoms in the experimental group was more significant. In addition to some self-healing factors, the improvement of nerve root entrapment after the release of the soft tissue attached to the posterior tuberosity of the cervical transverse process was also an important factor. In addition, the excellent rate and total effective rate of the experimental group were significantly better than those of the control group 6 months after the course of treatment.
This study has some limitations. First, only subjects with cervical spondylotic radiculopathy from a single center were analyzed. Consequently, the research sample had certain limitations that might have affected the extrapolation of the results. Second, the sample size was relatively small. Third, no long-term observation of the curative effect could be obtained beyond six months. Fourth, the study only included ultrasound-guided selective cervical nerve root block therapy in the control group for comparison. Ultrasound-guided acupotomy release of neck soft tissue was not conducted, which would have facilitated comparison of efficacy among the groups. Our research team plans to include more parallel observation groups to evaluate curative effect in future studies.
Conclusions
This study demonstrated that there was no significant difference in the short-term curative effects of ultrasound-guided injection acupotomy and ultrasound-guided SNRB when treating CSR. However, the long-term (six-months) curative effects of ultrasound-guided injection acupotomy therapy were significantly better compared to SNRB. Injection acupotomy technology is worthy of further research and promotion for use in practice.
Authors’ contributions
Ye Cao is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Ye Cao was supported by the research fund. Ye Cao and Jianfeng Pu devised and designed the study and treated the subjects. All subjects were recruited by Wenping Cao, Yeting Chen and Yunwu Fan. Yeting Chen performed the statistical analysis. Wenping Cao and Yunwu Fan carried out the literature search and data collection. Jianfeng Pu and Yeting Chen wrote this paper. The paper was revised and edited by Jianfeng Pu, Yeting Chen, and Ye Cao.
Acknowledgements
The authors thank the Suzhou Special Project of Diagnosis and Treatment Technology for Clinical Key Diseases and the Suzhou Innovative Project for Medical Health Science and Technology for the support provided to this study. The authors also thank Medjaden Bioscience Limited for editing and proofreading this paper and Huang Liqiang, Zhu Xiaojuan and Xu Jun for their contributions to this study.
Data sharing
The datasets analysed during the current study are available from the corresponding author on reasonable request.
Disclosure statement
The authors have declared that no competing interest exists.
Additional information
Funding
References
- Luyao H, Xiaoxiao Y, Tianxiao F, et al. Management of cervical spondylotic radiculopathy: a systematic review. Global Spine J 2022;12(8):1–13. doi:10.1177/21925682221075290.
- Salemi G, Savettieri G, Meneghini F, et al. Prevalence of cervical spondylotic radiculopathy: a door‐to‐door survey in a sicilian municipality. Acta Neurol Scand. 1996;93(2–3):184–188. doi:10.1111/j.1600-0404.1996.tb00196.x.
- Kolenkiewicz M, Włodarczyk A, Wojtkiewicz J. Diagnosis and incidence of spondylosis and cervical disc disorders in the University Clinical Hospital in Olsztyn, in years 2011-2015. Biomed Res Int. 2018;2018:5643839. doi:10.1155/2018/5643839.
- Son WS, Ahn M-W, Lee GW. JJoKSoSSWhen is the optimal time point for predicting the 1-year follow-up outcome of selective nerve root block for cervical radiculopathy? J Korean Soc Spine Surg. 2019;26(2):40–49. doi:10.4184/jkss.2019.26.2.40.
- Radhakrishnan K, Litchy WJ, O’Fallon WM, et al. Epidemiology of cervical radiculopathy. A population-based study from rochester, Minnesota, 1976 through 1990. Brain. 1994;117(2):325–335. doi:10.1093/brain/117.2.325.
- Dai W, Wang X, Xie R, et al. Acupotomy combined with massage for cervical spondylotic radiculopathy: a protocol for systematic review and meta-analysis. Medicine (Baltimore). 2020;99(32):e21587. doi:10.1097/MD.0000000000021587.
- Lin T, Wang Z, Chen G, et al. Predictive effect of cervical sagittal parameters on conservative treatment of Single-Segment cervical spondylotic radiculopathy. World Neurosurg. 2020;134:e1028–e36. doi:10.1016/j.wneu.2019.11.081.
- Yang D, Xu L, Hu Y, et al. Diagnosis and treatment of cervical spondylotic radiculopathy using selective nerve root block (SNRB): where are we now? Pain Ther. 2022;11(2):341–357. doi:10.1007/s40122-022-00357-1.
- Dai W, Xie R, Wang X, et al. Evidence for acupotomology in the management of cervical radiculopathy: a protocol for systematic review and meta-analysis. Medicine (Baltimore). 2020;99(36):e22007. doi:10.1097/MD.0000000000022007.
- Chen B, Zhang C, Zhang R-P, et al. Acupotomy versus acupuncture for cervical spondylotic radiculopathy: protocol of a systematic review and meta-analysis. BMJ Open. 2019;9(8):e029052. doi:10.1136/bmjopen-2019-029052.
- Li A-L, Wang X-W, Wang J-R, et al. [Clinical observation of acupotomy combined with warm needling for cervical spondylotic radiculopathy of qi and blood stagnation syndrome]. Zhen Ci Yan Jiu. 2022;47(10):914–916. doi:10.13702/j.1000-0607.20210968.
- Lu D, Ding W, Sheng H, et al. Clinical effect of needle knife injection under C-arm X-ray-imaging guidance for the treatment of lumbar disc herniation. Int J Clin Exp Med. 2018;11(9):10191–10198.
- Rao Y, Hou F, Huang H, et al. The combined treatment of entrapped infrapatellar branch of the saphenous nerve after ACL reconstruction: ultrasound-guided perineural injection and acupotomy. J Back Musculoskelet Rehabil. 2022;35(3):479–483. doi:10.3233/BMR-210110.
- Shrestha B, Dunn L. The declaration of helsinki on medical research involving human subjects: a review of seventh revision. J Nepal Health Res Counc. 2020;17(4):548–552. doi:10.33314/jnhrc.v17i4.1042.
- Childress MA, Becker BA. Nonoperative management of cervical radiculopathy. Am Fam Phys. 2016;93(9):746–754.
- Kim K-T, Kim Y-B. Cervical radiculopathy due to cervical degenerative diseases: anatomy, diagnosis and treatment. J Korean Neurosurg Soc. 2010;48(6):473–479. doi:10.3340/jkns.2010.48.6.473.
- Theodore N. Degenerative cervical spondylosis. N Engl J Med. 2020;383(2):159–168. doi:10.1056/NEJMra2003558.
- Kang K-C, Lee HS, Lee J-H. Cervical radiculopathy focus on characteristics and differential diagnosis. Asian Spine J. 2020;14(6):921–930. doi:10.31616/asj.2020.0647.
- Iyer S, Kim HJ. Cervical radiculopathy. Curr Rev Musculoskelet Med. 2016;9(3):272–280. doi:10.1007/s12178-016-9349-4.
- Rao RD, Currier BL, Albert TJ, et al. Degenerative cervical spondylosis: clinical syndromes, pathogenesis, and management. J Bone Joint Surg Am. 2007;89(6):1360–1378. doi:10.2106/00004623-200706000-00026.
- Joelson A, Fritzell P, Hägg O. Handling of missing items in the oswestry disability index and the neck disability index. A study from swespine, the national swedish spine register. Eur Spine J. 2022;31(12):3484–3491. doi:10.1007/s00586-022-07425-2.
- Lins L, Carvalho FM. SF-36 total score as a single measure of health-related quality of life: scoping review. SAGE Open Med. 2016;4:205031211667172. doi:10.1177/2050312116671725.
- Kim SH, Shin HC, Shin DA, et al. Early clinical experience with the mobi-C disc prosthesis. Yonsei Med J. 2007;48(3):457–464. doi:10.3349/ymj.2007.48.3.457.
- Jang JH, Lee WY, Kim JW, et al. Ultrasound-Guided selective nerve root block versus Fluoroscopy-Guided interlaminar epidural block versus Fluoroscopy-Guided transforaminal epidural block for the treatment of radicular pain in the lower cervical spine: a retrospective comparative study. Pain Res Manag. 2020;2020:9103421. doi:10.1155/2020/9103421.
- Huston CW, Slipman CW, Garvin C. Complications and side effects of cervical and lumbosacral selective nerve root injections. Arch Phys Med Rehabil. 2005;86(2):277–283. doi:10.1016/j.apmr.2004.02.018.
- Pobiel RS, Schellhas KP, Eklund JA, et al. Selective cervical nerve root blockade: prospective study of immediate and longer term complications. AJNR Am J Neuroradiol. 2009;30(3):507–511. doi:10.3174/ajnr.A1415.
- Pu J-F, Cao Y, Cao W-P, et al. [Clinical application study of acupotomy-injection technique with targeted three-point in the treatment of frozen shoulder]. Zhongguo Gu Shang. 2019;32(6):508–512. doi:10.3969/j.issn.1003-0034.2019.06.005.
- Cui X, Zhang D, Zhao Y, et al. An open-label non-inferiority randomized trail comparing the effectiveness and safety of ultrasound-guided selective cervical nerve root block and fluoroscopy-guided cervical transforaminal epidural block for cervical radiculopathy. Ann Med. 2022;54(1):2681–2691. doi:10.1080/07853890.2022.2124445.
- Murata S, Iwasaki H, Natsumi Y, et al. Vascular evaluation around the cervical nerve roots during Ultrasound-Guided cervical nerve root block. Spine Surg Relat Res. 2020;4(1):18–22. doi:10.22603/ssrr.2019-0006.
- Rathmell JP, Benzon HT, Dreyfuss P, et al. Safeguards to prevent neurologic complications after epidural steroid injections: consensus opinions from a multidisciplinary working group and national organizations. Anesthesiology. 2015;122(5):974–984. doi:10.1097/ALN.0000000000000614.
- Bensler S, Sutter R, Pfirrmann CWA, et al. Particulate versus non-particulate corticosteroids for transforaminal nerve root blocks: comparison of outcomes in 494 patients with lumbar radiculopathy. Eur Radiol. 2018;28(3):946–952. doi:10.1007/s00330-017-5045-z.
- Chung J-Y, Yim J-H, Seo H-Y, et al. The efficacy and persistence of selective nerve root block under fluoroscopic guidance for cervical radiculopathy. Asian Spine J. 2012;6(4):227–232. doi:10.4184/asj.2012.6.4.227.
- Kim JY, Lee JW, Lee GY, et al. Comparative effectiveness of lumbar epidural steroid injections using particulate vs. non-particulate steroid: an intra-individual comparative study. Skeletal Radiol. 2016;45(2):169–176. doi:10.1007/s00256-015-2277-3.
- Kwon C-Y, Yoon S-H, Lee B. Clinical effectiveness and safety of acupotomy: an overview of systematic reviews. Complem Ther Clin Pract. 2019;36:142–152. doi:10.1016/j.ctcp.2019.07.002.
- Liu F, Zhou F, Zhao M, et al. Acupotomy therapy for chronic nonspecific neck pain: a systematic review and Meta-Analysis. Evid Based Complem Alternat Med. 2017;2017:6197308. doi:10.1155/2017/6197308.
- Qiu Z, Jia Y, Shen Y, et al. Acupotomy by an ultrasound-guided technique: a protocol for a systematic review. Medicine (Baltimore). 2019;98(42):e17398. doi:10.1097/MD.0000000000017398.
- Zeng X, Ding Z-H, Fu L-Q, et al. [Progress of researches in acupotomy visualization under ultrasound]. Zhen Ci Yan Jiu. 2021;46(6):546–548. doi:10.13702/j.1000-0607.20210127.
- Choi S, Park S, Lim Y-S, et al. A comparative study of a nerve block therapy with and without a deeply inserted acupotomy applied to hyeopcheok points for lumbosacral radiculopathy: safety, effectiveness, cost-effectiveness (a randomized controlled, two-arm, parallel study, pilot study, assessor-blind). Medicine (Baltimore). 2022;101(9):e28983. doi:10.1097/MD.0000000000028983.
- Chen Y, Kumar N, Lim JWW, et al. High-resolution sonography detects extraforaminal nerve pathology in patients patients initially diagnosed with cervical disc disease: a case series. J Clin Ultrasound. 2013;41(1):46–54. doi:10.1002/jcu.20876.
- Wu J, Xu Y, Pu S, et al. US-Guided transforaminal cervical nerve root block: a novel lateral in-Plane approach. Pain Med. 2021;22(9):1940–1945. doi:10.1093/pm/pnab008.