Abstract
Background
The emergence of genetically-modified human proteins and glucagon-like peptide-1 (GLP-1) receptor agonists have presented a promising strategy for effectively managing diabetes. Due to the scarcity of clinical trials focusing on the safety and efficacy of semaglutide as an adjunctive treatment for patients with type 2 diabetes who had inadequate glycemic control with metformin, we conducted a systematic review and meta-analysis. This was necessary to fill the gap and provide a comprehensive assessment of semaglutide compared to sitagliptin, a commonly prescribed DPP-4 inhibitor, in this patient population.
Methods
A comprehensive and systematic search was carried out on reputable databases including PubMed, the Cochrane Library, and Elsevier’s ScienceDirect to identify relevant studies that compared the efficacy of once-weekly Semaglutide with once-daily Sitagliptin in individuals diagnosed with type 2 diabetes mellitus. The analysis of the gathered data was performed utilizing the random-effects model, which considers both within-study and between-study variations.
Results
The meta-analysis incorporated three randomized controlled trials (RCTs), encompassing 2401 participants, with a balanced distribution across the treatment groups. The primary focus of the study revolved around evaluating changes in HbA1C, blood pressure, pulse rate, body weight, waist circumference, and BMI. The findings revealed that once-weekly Semaglutide showed substantially improved HbA1C (WMD: −0.98; 95% CI: −1.28, −0.69, p-value: < 0.0001; I2: 100%), systolic (WMD: −3.73; 95% CI: −5.42, −2.04, p-value: <0.0001; I2: 100%) and diastolic blood pressures (WMD: −0.66; 95% CI: −1.02, −0.29, p-value: 0.0005; I2: 100%), and body weight (WMD: −3.17; 95% CI: −3.84, −2.49, p-value: <0.00001; I2: 100%) compared to once-daily Sitagliptin. However, there was an observed increase in pulse rate (WMD: 3.33; 95% CI: 1.61, 5.06, p-value: <0.00001; I2: 100%) associated with Semaglutide treatment. Regarding secondary outcomes, there was an elevated risk of total adverse events and premature treatment discontinuation with Semaglutide. The risk of serious, severe, moderate, and mild adverse events did not significantly differ between the two treatments.
Conclusions
In conclusion, the administration of once-weekly Semaglutide exhibited a substantial reduction in HbA1c, average systolic blood pressure (SBP), mean diastolic blood pressure (DBP), body weight, waist circumference, body mass index (BMI), and a rise in pulse rate, as opposed to the once-daily administration of Sitagliptin.
Introduction
Diabetes mellitus, a spectrum of metabolic disorders, has emerged as a global challenge affecting people worldwide. Interestingly, it is often referred to as a ‘lifestyle disorder,’ indicating its strong connection to our modern way of living [Citation1]. It is a multifaceted and gradual disease; despite the numerous treatment options accessible, a large number of patients with type 2 diabetes fail to reach the suggested blood glucose levels (HbA1c <7·0% [53·0 mmol/mol]; HbA1c ≤6·5% [48·0 mmol/mol]) [Citation2]. While treating diabetes can be challenging, it is not without hope. Clinical practice recommendations are promptly revised based on growing knowledge regarding many facets of diabetes management. The landscape of treatment approaches is evolving, particularly as a result of some groundbreaking clinical trials on more recent anti-diabetic drugs [Citation3]. According to the latest data from the World Health Organization (WHO), the prevalence of diabetes among adults aged 18 years and older was 8.5% in 2014. In 2019, diabetes accounted for 1.5 million deaths directly, with 48% occurring in individuals under 70. Furthermore, diabetes was responsible for an additional 460,000 deaths related to kidney disease. Raised blood glucose levels also contributed to approximately 20% of cardiovascular deaths. These statistics underscore the significant impact of diabetes on global health and highlight the need for effective prevention, management, and increased awareness surrounding this condition [Citation4]. The American Diabetes Association (ADA) has placed significant emphasis on tailoring diabetes management approaches to each patient’s unique circumstances, leveraging technology where appropriate, and prioritizing the prevention of complications [Citation5]. Individualized treatment strategies, such as medication, are required when diet and exercise alone are unable to maintain long-term glycaemic control in persons with type 2 diabetes (T2D) [Citation5]. Despite the availability of many different treatments, maintaining glycemic control in clinical settings without experiencing negative outcomes like hypoglycemic episodes is still exceedingly difficult [Citation6]. Conventional synthetic drugs, combined with the principles of naturopathy, offer a promising approach to managing this condition. This holistic combination can empower individuals to take charge of their health and make positive lifestyle choices, ultimately contributing to the effective management of diabetes. By embracing both modern medicine and natural therapies, individuals with diabetes have the opportunity to navigate their condition and lead fulfilling lives [Citation1]. illustrates the pathophysiology of diabetes and the comprehensive approach employed to maintain healthy blood sugar levels, prevent complications, and promote overall well-being in patients with diabetes.
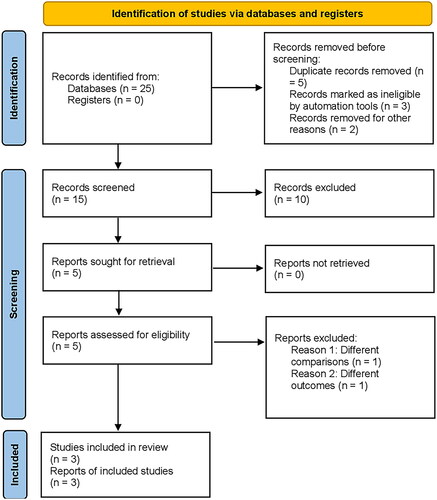
Figure 1. Prisma flow chart; flowchart illustrating the selection process of articles included in the meta-analysis, from an initial 25 articles identified, to the final inclusion of 3 randomized controlled trials (RCTs) after eliminating duplicates, assessing titles and abstracts, and considering only comparative studies for the analysis.
The development of genetically-engineered human proteins and glucagon-like peptide-1 (GLP-1) receptor agonists have emerged as a promising approach for effectively managing diabetes. The ability to reduce HbA1c levels and body weight while avoiding the risk of hypoglycemia has made this treatment approach particularly valuable for addressing the needs of obese individuals with type 2 diabetes. Nevertheless, the degradation of these proteins by Dipeptidyl Peptidase-4 (DPP-4) and Neutral Endopeptidase represents a significant challenge, as it limits their effectiveness by reducing their half-life to only 1-2 min [Citation7]. Sitagliptin, a DPP-4 inhibitor, is an oral antihyperglycemic agent used for treating T2D in adults in many countries. Sitagliptin inhibits DPP-4, leading to stabilization of the short-lived incretin peptides GLP-1 and GIP. The use of DPP-4 inhibitors in managing hyperglycemia in adults with T2D varies based on local practice guidelines [Citation8]. Both the GLP-1 receptor agonists and DPP-4 inhibitors have the potential to serve as second-line treatments in situations where initial treatments (primarily metformin) fail to achieve glycemic control [Citation9]. The efficacy and safety of semaglutide compared to various comparators, including sitagliptin, have been assessed in six global phase III clinical trials called the ‘Semaglutide Unabated Sustainability in Treatment of Type 2 Diabetes’ (SUSTAIN) program. These trials also evaluated cardiovascular outcomes [Citation10].
We conducted a systematic review and meta-analysis because there is a limited number of clinical trials with a restricted scope that assessed the safety and efficacy of semaglutide compared to sitagliptin, a frequently prescribed DPP-4 inhibitor, as adjunctive therapy for patients with type 2 diabetes who had suboptimal glycemic control with metformin. To our understanding, this is the initial meta-analysis to compare semaglutide 0.5 mg and 1.0 mg with the standard 100 mg dose of sitagliptin to establish the superiority of both options as second-line therapy.
Methods
In terms of methodology, this meta-analysis adheres to the established guidelines outlined by the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) [Citation11].
Data sources and search strategy
PubMed, the Cochrane Library and Elsevier’s ScienceDirect databases were systematically searched for clinical studies until April 2023 without language limitations. To gather relevant literature, a combination of specific keywords and medical subject headings (MeSH) such as ‘semaglutide,’ ‘sitagliptin,’ ‘once weekly,’ ‘once daily,’ ‘randomized controlled trial,’ and ‘safety and efficacy’ was utilized. Boolean operators (AND, OR) were employed in the search terms to ensure the inclusion of relevant studies. Supplementary Table S1 provides detailed information about the search methodology. The PICO (population, intervention, comparison, outcome) methodology was adapted with certain modifications. The study population comprised patients with type 2 diabetes. Three researchers (MK, SK, and KM) independently assessed the titles and abstracts of potentially eligible studies to ensure accuracy.
Inclusion and exclusion criteria
Inclusion criteria
The study selection criteria for this research involved several essential factors. Firstly, double-blind, randomized controlled trials (RCTs) were considered, emphasizing the use of rigorous scientific methodology to minimize bias. Secondly, a comparison was made between the effects of semaglutide once weekly and sitagliptin once daily, enabling a comprehensive evaluation of their respective impacts. The study’s target population comprised individuals aged 18 and above, with a specific focus on adult patients. Moreover, the inclusion criteria required that the studies involved human participants diagnosed with type 2 diabetes, ensuring direct relevance to the research question. Another crucial aspect was that the selected studies had to report safety and efficacy outcomes, providing valuable insights into the treatments’ effectiveness and any potential associated risks. Lastly, the articles considered for inclusion were explicitly limited to those published in English, ensuring that they were accessible and understandable to the intended readership.
Exclusion criteria
The study implemented the following criteria for exclusion. Firstly, nonclinical studies were excluded from consideration. Secondly, studies without controls were also excluded. Additionally, observational studies were not considered, including cohort, case-control, cross-sectional case reports, case series studies, editorials, review articles, and conference abstracts. Furthermore, studies with a sample size smaller than 20 were excluded. Finally, any studies with equivocal results were also excluded from the analysis.
Data extraction
The information from each research study was systematically gathered and recorded using a standardized data collection template. This comprehensive data included details such as the author’s name, publication year, country of origin, study population, participants’ demographic information, medications taken before enrollment in the trial, and clinical outcomes.
The primary outcomes assessed in the studies encompassed several factors, namely the alteration in glycated hemoglobin (HbA1c) levels, changes in both systolic (SBP) and diastolic (DBP) blood pressures, fluctuations in pulse rate, shifts in body weight, variations in waist circumference, and modifications in body mass index (BMI). These outcomes were measured in terms of percentage, millimeters of mercury (mmHg), beats per minute, kilograms (kg), centimeters (cm), and kilograms per square meter (kg/m2), respectively.
In addition to the primary outcomes, the studies also examined various secondary outcomes, which included the total occurrence of adverse events (AEs) and the severity levels of these events categorized as serious, severe, moderate, or mild. Furthermore, the number of patients who prematurely discontinued the treatment was also recorded and analyzed.
Quality assessment of the included studies
The quality assessment of all randomized controlled trials (RCTs) included in the study was conducted by employing the Cochrane risk of bias tool [Citation12].
Data analysis
The statistical analysis for this meta-analysis was performed using Review Manager version 5.4.1, a tool developed by the Nordic Cochrane Center in collaboration with the Cochrane Collaboration in Denmark in 2014. Only comparative studies were considered in the analysis. The outcomes were presented using forest plots, illustrating the combined effect sizes of relative risks (RRs) for dichotomous outcomes and weighted mean differences (WMDs) for continuous outcomes. A random-effects model with generic-inverse variance was employed to ensure the accuracy of the results.
To assess the potential presence of publication bias, funnel plots were generated for each primary outcome. Higgin’s I2 test was employed to evaluate the level of heterogeneity, classified as low, moderate, or high. In instances where significant heterogeneity (> 75%) [Citation13] was observed, a sensitivity analysis was conducted by systematically excluding individual studies to investigate their impact on the overall findings.
For all analyses, a p-value less than 0.05 was considered statistically significant. The authors thoroughly examined the data to guarantee the precision and dependability of their findings.
Given that the data in this study were collected from previous clinical trials where participants had already provided informed consent, ethical approval from a research ethics committee was not required for this investigation.
Results
Eligible studies
The preliminary output of the literature review consisted of 25 articles, which were subsequently refined by eliminating duplicates and assessing the titles and abstracts. From this process, three randomized controlled trials (RCTs) were identified [Citation10,Citation14,Citation15]. Only comparative studies were considered for inclusion in the meta-analysis, and a detailed depiction of the search methodology is presented in using the PRISMA diagram. All three RCTs were multicenter comparative studies, with two of them being double-blind and one being open-label [Citation10]. The mean duration of follow-up was 43 weeks, and the collection of articles included in this study ranged from the years 2017 to 2021.
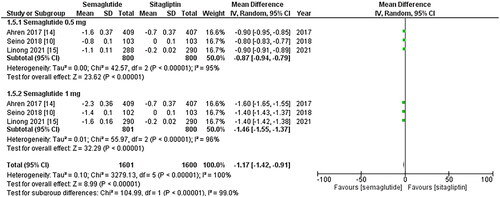
Figure 2. Forest plot of change in HbA1C (Glycated hemoglobin); the figure provides a comparison of the effects of once-weekly Semaglutide treatment and once-daily Sitagliptin on HbA1c values. The analysis demonstrates that once-weekly Semaglutide treatment leads to a significant decrease in HbA1c values compared to once-daily Sitagliptin. Notably, the 1 mg dose of Sitagliptin exhibits a more substantial reduction in HbA1c values; WMD- weighted mean difference; CI- Confidence interval; M-H- Mantel Hansel.
Baseline characteristics of the included patients
This meta-analysis comprised a total of 2401 participants, with 800 (33.3%) subjects assigned to the Semaglutide 0.5 mg group, 801 (33.4%) to the Semaglutide 1 mg group, and 800 (33.3%) to the Sitagliptin 100 mg group. The gender distribution of the participants showed that 1199 (49.9%) were male and 1096 (45.6%) were female. The average age of the participants was 55.4 ± 10.4 years. The mean HbA1C was 8.1 ± 0.9%, and the mean duration of diabetes was 7 ± 5.2 years. Most of the participants were overweight, with an average BMI of 28.6 ± 4. Prior to enrollment in the respective trials, 1690 out of 2401 (70.4%) participants were taking biguanides, while others were on medications such as sulphonylureas, thiazolidinediones, DPP-4 inhibitors, and alpha-glucosidase inhibitors. A detailed summary of the baseline characteristics of the participants can be found in and .
Table 1. Baseline characteristics of the included participants.
Table 2. Baseline Demographics of the included participants.
Quality assessment and publication bias
Through the implementation of the Cochrane methodology for evaluating RCTs (Supplemental Figure 1), trials of medium to high quality were identified. Moreover, based on the funnel plots, it was determined that the results were not affected by publication bias (Supplemental Figure 2).
Primary outcomes
The primary outcomes comprised of change in HbA1C, change in mean systolic and diastolic blood pressures, change in pulse rate, change in weight, change in BMI, and change in waist circumference.
Change in HbA1C
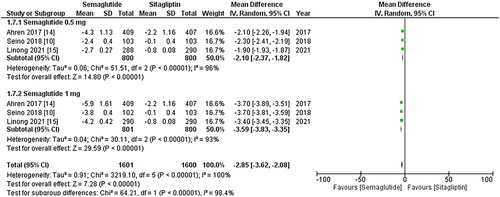
All three studies reported change in HbA1c levels, and upon pooled analysis, it was found that once-weekly Semaglutide treatment resulted in a significant decrease in HbA1c values after treatment (WMD: −0.98; 95% CI: −1.28, −0.69, p-value: < 0.0001; I2: 100%) compared to once daily Sitagliptin, particularly the 1 mg dose (WMD: −1.13; 95% CI: −1.54, −0.72, p-value: <0.00001; I2: 100%) as shown in . A leave-one-out sensitivity analysis was conducted to investigate the high heterogeneity observed among the studies. It was determined that the heterogeneity was not due to any specific study.
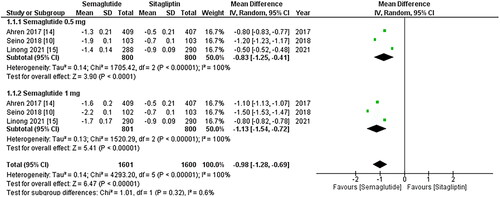
Figure 3. Forest plot of change in mean SBP (systolic blood pressure); the Figure demonstrates a pooled analysis comparing once-weekly Semaglutide and once-daily Sitagliptin, revealing a significant reduction in mean SBP with Semaglutide treatment compared to Sitagliptin. The decrease is more noticeable when the 1 mg dose of Sitagliptin is taken into account; WMD- weighted mean difference; CI- Confidence interval; M-H- Mantel Hansel.
Changes in SBP and DBP
The results from all three studies included the SBP and DBP values, and after conducting a pooled analysis, it was found that treatment with once-weekly Semaglutide resulted in a significant reduction in the mean SBP (WMD: −3.73; 95% CI: −5.42, −2.04, p-value: <0.0001; I2: 100%) compared to once-daily Sitagliptin, which was more noticeable with the 1 mg dose (WMD: −4.94; 95% CI: −6.19, −3.69, p-value: <0.00001; I2: 100%) as shown in . Similarly, the pooled analysis for DBP showed a significant decrease in mean DBP (WMD: −0.66; 95% CI: −1.02, −0.29, p-value: 0.0005; I2: 100%) with the use of once-weekly Semaglutide compared to once-daily Sitagliptin. This reduction was more pronounced with the 1 mg dose (WMD: −1.10; 95% CI: −1.99, −0.20, p-value: <0.00001; I2: 99%) as shown in . A leave-one-out sensitivity analysis was performed to address the high heterogeneity in the results of these two outcomes, which determined that any specific study did not cause heterogeneity.
Figure 4. Forest plot of change in mean DBP (Diastolic blood pressure); the figure demonstrates a significant decrease in mean DBP with once-weekly Semaglutide compared to once-daily Sitagliptin, with greater reduction observed for the 1 mg dose; WMD- weighted mean difference; CI- Confidence interval; M-H- Mantel Hansel.
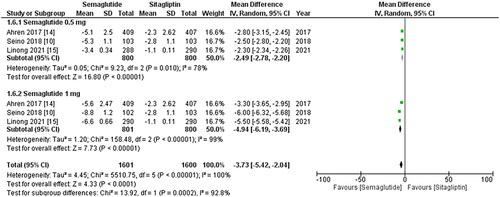
Figure 5. Forest plot of change in pulse rate; the figure demonstrates a significant increase in pulse rate associated with once-weekly Semaglutide treatment compared to once-daily Sitagliptin. Furthermore, the increase in pulse rate was more pronounced in patients taking the 1 mg once-weekly dose of Semaglutide; WMD- weighted mean difference; CI- Confidence interval; M-H- Mantel Hansel.
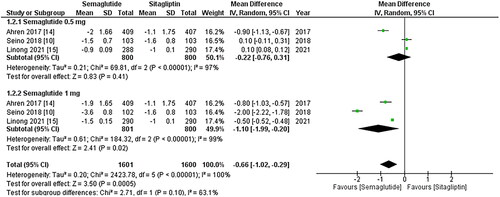
Change in pulse rate
All three studies included pulse rate values, and the pooled analysis revealed a significant increase in pulse rate associated with once-weekly Semaglutide treatment (WMD: 2.97; 95% CI: 2.35, 3.59, p-value: <0.00001; I2: 100%) compared to once-daily Sitagliptin. The increase was more pronounced in patients taking the 1 mg once-weekly dose of Semaglutide (WMD: 3.33; 95% CI: 1.61, 5.06, p-value: <0.00001; I2: 100%), as depicted in . A leave-one-out sensitivity analysis was conducted to account for the high in-study heterogeneity, and the results indicated that no particular study contributed to the observed heterogeneity.
Figure 6. Forest plot of change in body weight; the figure shows a significant decrease in body weight after treatment with once-weekly Semaglutide compared to once-daily Sitagliptin. Additionally, the 1 mg dose of Semaglutide exhibited a more remarkable reduction in body weight than the 0.5 mg dose; WMD- weighted mean difference; CI- Confidence interval; M-H- Mantel Hansel.
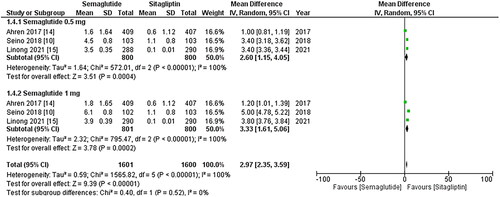
Change in body weight, waist circumference, and BMI
The outcomes mentioned above were reported by all three studies, and the pooled analysis revealed that once-weekly Semaglutide treatment led to a significant decrease in body weight after treatment (WMD: −3.17; 95% CI: −3.84, −2.49, p-value: <0.00001; I2: 100%) compared to once-daily Sitagliptin. However, the 1 mg dose of Semaglutide resulted in a more remarkable reduction in body weight than the 0.5 mg dose (WMD: −3.96; 95% CI: −4.14, −3.77, p-value: <0.00001; I2: 93%), as illustrated in . Furthermore, treatment with once-weekly Semaglutide was linked to a significant reduction in waist circumference (WMD: −2.85; 95% CI: −3.62, −2.08, p-value: <0.00001; I2: 100%), with the 1 mg dose of Semaglutide showing more noticeable effects (WMD: −3.59; 95% CI: −3.83, −3.35, p-value: <0.00001; I2: 93%), as depicted in . Likewise, the once-weekly Semaglutide regimen was associated with a significant decrease in BMI after treatment (WMD: −1.17; 95% CI: −1.42, −0.91, p-value: <0.00001; I2: 100%) compared to the once-daily Sitagliptin regimen. However, the 1 mg dose of Semaglutide resulted in a more notable reduction in BMI than the 0.5 mg dose (WMD: −1.46; 95% CI: −1.55, −1.37, p-value: <0.00001; I2: 96%), as shown in . A sensitivity analysis was conducted to account for the high heterogeneity in the outcomes, indicating that no study caused heterogeneity.
Figure 7. Forest plot of change in waist circumference; the analysis showed waist circumference reduction with once-weekly Semaglutide, Highlighting enhanced effects at 1 mg dose; WMD- weighted mean difference; CI- Confidence interval; M-H- Mantel Hansel.
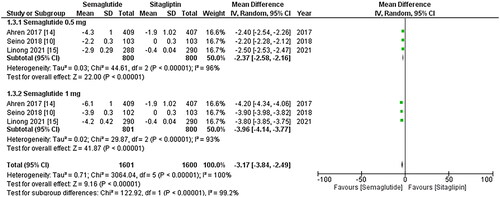
Figure 8. Forest plot of change in Body mass index (BMI); the figure demonstrates a significant decrease in BMI after treatment with the once-weekly Semaglutide regimen compared to the once-daily Sitagliptin regimen. Notably, the 1 mg dose of Semaglutide resulted in a more notable reduction in BMI than the 0.5 mg dose; WMD- weighted mean difference; CI- Confidence interval; M-H- Mantel Hansel.
Secondary outcomes
In this study, various adverse effects were examined as secondary outcomes, including different levels of adverse events such as total adverse events, serious adverse events, severe adverse events, moderate adverse events, mild adverse events, and premature treatment discontinuation. For a detailed breakdown of these secondary outcomes, please refer to . After analyzing the combined data, it was found that using a once-weekly Semaglutide treatment was associated with a significantly higher risk of total adverse events and premature treatment discontinuation. The risk of serious, severe, moderate, and mild adverse events also increased in the group receiving once-weekly Semaglutide, but the results were not statistically significant. Furthermore, when analyzing specific subgroups, it was observed that the risk of premature treatment discontinuation and severe adverse events was more pronounced with the 1 mg dose of Semaglutide, whereas the total, serious, moderate, and mild adverse events were more noticeable in patients who received the 0.5 mg dose of Semaglutide.
Table 3. Secondary outcomes.
Discussion
The continuous increase in the global occurrence of type 2 diabetes has emerged as a significant concern within the healthcare sector, primarily due to the subsequent rise in patient morbidity and mortality resulting from cardiovascular, renal, and neurological complications. Furthermore, the escalating financial and resource expenditures required to handle these complications have added to the gravity of the issue [Citation16]. Two broad categories of incretin therapies have been formulated with the intention of leveraging the antihyperglycemic properties of GLP-1 [Citation17]. Both agents, known as glucagon-like peptide (GLP)-1 receptor agonists and dipeptidyl peptidase-4 (DPP-4) inhibitors, contribute to the increased activation of GLP-1 receptors. GLP-1 receptor agonists deliver significant concentrations of the respective substances in the bloodstream, leading to targeted interaction with GLP-1 receptors. The question remains debatable whether combining GLP-1 receptor agonists and DPP-4 inhibitors would result in a more pronounced reduction in glucose levels compared to using either category of medication independently [Citation18]. A previous analysis conducted by Scheen et al. in 2012 indicated that GLP-1 receptor agonists exhibited greater potency in reducing glucose levels, promoting weight loss, and lowering systolic blood pressure compared to DPP-4 inhibitors [Citation19]. Conversely, DPP-4 inhibitors were found to be more convenient to administer, cost-effective, and better tolerated regarding gastrointestinal effects. However, it is worth noting that the NICE guidelines and analysis did not specifically focus on obese patients. In a separate study in 2017, Li et al. in examined the impact of sitagliptin on obese patients undergoing insulin treatment [Citation20]. They discovered that sitagliptin led to a reduction in body mass index (BMI) and a decrease in hypoglycemic episodes. Similarly, a cohort study by Kodera et al. in 2017 demonstrated the efficacy of sitagliptin in managing glucose metabolic disorders in obese Japanese patients with T2DM [Citation21]. These findings prompt the question of how effective Semaglutide and Sitagliptin are in managing T2DM in patients who are using metformin [Citation22]. Therefore, we conducted a comprehensive review and meta-analysis to assess the effectiveness and hypoglycemic risk of standard doses of Semaglutide (0.5 mg and 1 mg) with 100 mg sitagliptin as an add-on to metformin in patients with T2DM.
This meta-analysis of 3 randomized controlled trials (RCT) with 2401 participants compares the efficacy and safety profile of recently introduced standard doses of Semaglutide (0.5 mg and 1 mg) with 100 mg sitagliptin as an add-on to metformin in patients with T2DM. Our research aimed to address various outcome measures, including alterations in HbA1C levels, modifications in SBP and DBP, shifts in pulse rate, changes in body weight, waist circumference, and BMI. Moreover, our investigation also assessed several secondary outcome measures, encompassing overall adverse events, significant adverse events, severe adverse events, moderate adverse events, mild adverse events, and premature cessation of treatment. The investigation we conducted appraised the effect of Semaglutide therapy on HbA1c values and found a substantial reduction in contrast to Sitagliptin administered once per day. Nonetheless, previous research conducted by BUSE et al. in 2020 indicated a minor rise in HbA1c initially, followed by a decline until it stabilized. This finding challenges the efficacy of Semaglutide. In addition, during the extension, patients who continued with Sitagliptin maintained a constant HbA1c level without any additional decrease achieved after the primary phase [Citation23]. The administration of Semaglutide on a weekly basis led to a notable decrease in the average SBP and DBP. Lavernia et al. in 2020 also reported similar findings, demonstrating that individuals receiving oral semaglutide experienced mean reductions of 2–5 mmHg in systolic blood pressure and 1–2 mmHg in diastolic blood pressure upon completion of the study [Citation24].
In the study conducted by Hong et al.in 2018, the effects of semaglutide on blood pressure control were examined. The results indicated a significant reduction in systolic blood pressure (SBP) when compared to other treatments (weighted mean difference [WMD]: −0.29 mmHg, 95% confidence interval [CI]: −0.65 to 0.07, p = 0.113) [Citation25]. However, no significant difference was observed in diastolic blood pressure (DBP) (WMD: −0.29 mmHg, 95% CI: −0.65 to 0.07, p = 0.113). On the other hand, semaglutide exhibited a notable decrease in SBP (WMD: −2.55 mmHg, 95% CI: −3.22 to −1.88, p < 0.001) compared to other therapies for blood pressure management. Interestingly, the use of semaglutide resulted in a higher pulse rate compared to alternative treatments (WMD: 2.21 bpm, 95% CI: 1.54 to 2.88, p < 0.001), although there was moderate heterogeneity observed among the studies [Citation25]. Following the treatment with semaglutide, a significant decline in body weight, waist circumference, and BMI was observed compared to the administration of sitagliptin on a daily basis. The superior efficacy of oral semaglutide in reducing HbA1c levels and body weight, in contrast to sitagliptin, aligns with findings from other comparative trials that have demonstrated the superiority of GLP-1 receptor agonists (GLP-1RAs) over DPP-4 inhibitors in terms of glycemic control and weight reduction. These outcomes achieved with oral semaglutide hold clinical importance since enhanced glycemic control is associated with improved outcomes in diabetes management, and some patients may have a preference for oral medications to attain such enhanced glycemic control [Citation26]. Moreover, significant weight loss, which is clinically important, contributes to improved glycemic control and mitigates cardiovascular risk factors. In a study conducted by Wilding et al. in 2021, the response to Semaglutide in obese patients without diabetes was examined [Citation27]. The study found that when Semaglutide was used as a supplementary treatment alongside lifestyle intervention, adults classified as obese (or overweight with weight-related coexisting conditions) experienced a substantial average weight loss of 14.9% from their initial weight. This weight loss was significantly more significant than that observed in the placebo group combined with lifestyle intervention, exceeding it by an additional 12.4 percentage points. The observed mean weight loss of 14.9% with Semaglutide also exceeded the weight loss range of 4.0 to 10.9% reported with approved antiobesity medications compared to baseline [Citation27]. These findings highlight the positive response to Semaglutide and suggest its potential efficacy in promoting weight loss in obese individuals without diabetes when used in conjunction with lifestyle intervention. A study conducted by Rubino et al. [Citation28] in 2021 found that continuing semaglutide treatment beyond the initial randomized period resulted in sustained and significant weight loss. The weight loss achieved during the run-in period continued and reached a plateau at around week 60 to week 68, resulting in an estimated reduction of 17.4% over the entire trial. In contrast, participants who switched to placebo at week 20 gradually regained weight. The study’s findings highlight the importance of maintaining semaglutide treatment for longer, as it showed more significant benefits compared to switching to placebo. This emphasizes the chronic nature of obesity and the need for remedies that can sustain and maximize weight loss. Furthermore, the sustained weight loss with semaglutide was accompanied by improvements in waist circumference, lipid profiles, and glucose metabolism, which are essential cardiometabolic risk factors. Similar sustained weight loss has been associated with improvements in obesity-related complications, particularly type 2 diabetes, with treatment guidelines recommending a sustained weight loss of 5% to 15% for individuals with these conditions [Citation28]. In a phase 3 trial conducted by Miles et al. [Citation29] in 2018, the efficacy and safety of semaglutide were compared with placebo and other pharmacologic therapy for diabetes (PTD) in multicenter SUSTAIN trials. Semaglutide demonstrated lower hemoglobin A1c (HbA1c) levels by approximately −1.5% and weight reductions of approximately −4.5 kg, comparable to dulaglutide’s effects on HbA1c lowering. Additionally, semaglutide showed significant cardiovascular outcomes, including a reduced risk of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke, with a hazard ratio of 0.74 and a 95% confidence interval of 0.58–0.95. These findings highlight the efficacy of semaglutide in improving glycemic control, promoting weight loss, and reducing cardiovascular risks in patients with diabetes [Citation29].
To ensure the accurate interpretation and applicability of our meta-analysis findings, it is crucial to consider and evaluate the presence of substantial heterogeneity thoroughly. Various methodologies, such as leave-one-out analysis, were utilized to investigate and elucidate the sources of heterogeneity while conducting sensitivity analyses to ascertain the robustness of the results. Potential factors contributing to heterogeneity may encompass dissimilarities in study design, including variances in participant characteristics, outcome measurements, and durations of follow-up. Methodological disparities and variations in clinical and demographic attributes could also contribute to the observed heterogeneity. Even when the included studies share similarities in design and characteristics, statistical heterogeneity can arise due to random fluctuations in the results. Statistical tests, such as the I-squared (I2) statistic or Cochran’s Q test, are commonly employed to assess the presence of statistical heterogeneity.
It is important to note some limitations in our study. One limitation that can affect the validity of a study is the need for adequate sample size and a limited number of trials conducted. When the sample size is small, and the number of attempts is insufficient, it can lead to reduced statistical power and compromise the reliability of the findings. Secondly, Possible variations in ethnic backgrounds, as well as differences in the initial characteristics of the participants, may have played a role in the clinical heterogeneity observed. Furthermore, a limited number of studies provided information on various treatments besides metformin that patients were utilizing. Consequently, incorporating additional therapies like Diet and exercise therapy, sulphonylurea, α-glucosidase inhibitor, and thiazolidinediones, which might influence the outcomes, was not adequately reported in most studies.
Conclusion
In summary, the administration of once-weekly Semaglutide led to a significant reduction in HbA1c, average systolic blood pressure (SBP), mean diastolic blood pressure (DBP), body weight, waist circumference, and BMI, as well as an increase in pulse rate, compared to the once-daily administration of Sitagliptin. Moreover, Semaglutide demonstrated a favorable safety profile similar to other GLP-1 receptor agonists. Based on these findings, we conclude that once-weekly Semaglutide shows great promise as an adjunctive treatment to metformin when monotherapy fails to achieve adequate glycemic control in individuals with type 2 diabetes.
Authors’ contribution
1. Tirath patel: Conceived and designed the study, formulated the research question, and developed the methodology.
2. FNU Nageeta: Conceived and designed the study, formulated the research question, and developed the methodology.
3. Rohab Sohail: Conceived and designed the study, formulated the research question, and developed the methodology.
4. Tooba Shaukat Butt: Conceived and designed the study, formulated the research question, and developed the methodology.
5. Shyamala Ganesan: Conceived and designed the study, formulated the research question, and developed the methodology.
6. FNU Madhurita: Assisted in the study design and methodology development, conducted the literature search, and contributed to data extraction and quality assessment of the included studies
7. Mohammad Ahmed: Provided expertise on diabetes management and treatment, contributed to the study design, and assisted in the interpretation of the results.
8. Mahrukh Zafar: Assisted in the study design, contributed to the literature search, data extraction, and quality assessment of the included studies.
9. Wirda Zafar: Assisted in the study design, contributed to the literature search, data extraction, and quality assessment of the included studies.
10. Mohammad Uzair Zaman: Contributed to the study design, participated in the statistical analysis and interpretation of the results.
11. Giustino Varassi: Contributed to the study design, participated in the statistical analysis and interpretation of the results.
12. Mahima Khatri: Provided guidance and supervision throughout the study, contributed to the study design, methodology development, and interpretation of the results.
13. Satesh Kumar: Provided guidance and supervision throughout the study, contributed to the study design, methodology development, and interpretation of the results.
Supplemental Material
Download MS Word (784.8 KB)Supplemental Material
Download MS Word (14.3 KB)Acknowledmgents
None.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data Availability Statement: The data supporting the findings of this study, titled ‘ Comparative Efficacy and Safety Profile of once-weekly Semaglutide versus once-daily Sitagliptin as an add-on to Metformin in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis’ are available on PubMed and can be accessed via their respective DOI (Digital Object Identifier). Interested researchers can retrieve the data by searching for the article using the provided DOI. Should additional information or data be required for the purpose of verification, replication, or further analysis, the corresponding authors of the study can be contacted.
Their contact details are as follows: Tirath Patel
Institute: American University of Antigua
Email: [email protected] contact: 091-8128250661
Department: Medicine country: Antigua and Barbuda
The authors are committed to promoting transparency and facilitating scientific progress. While the primary data are available on PubMed, the corresponding authors are willing to assist researchers in accessing any additional information that may be necessary to support their work.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Saha A, Samadder A, Nandi S. Stem cell therapy in combination with naturopathy: current progressive management of diabetes and associated complications. Curr Top Med Chem. 2023;23(8):1–14. doi: 10.2174/1568026623666221201150933.
- Kumari K, Kumar R, Memon A, et al. Treatment with testosterone therapy in type 2 diabetic hypogonadal adult males: a systematic review and Meta-Analysis. Clin Pract. 2023; 13(2):454–469. doi: 10.3390/clinpract13020041.
- Kumar S, Khatri M, Memon RA, et al. Effects of testosterone therapy in adult males with hypogonadism and T2DM: a meta-analysis and systematic review. Diabetes Metab Syndr. 2022;16(8):102588. doi: 10.1016/j.dsx.2022.102588.
- ‘Diabetes.‘. World Health Organization, www.who.int/news-room/fact-sheets/detail/diabetes. Accessed 10 July 2023.
- Yu J, Lee SH, Kim MK. Recent updates to clinical practice guidelines for diabetes mellitus. Endocrinol Metab. 2022; 37(1):26–37. doi: 10.3803/EnM.2022.105.
- Alhindi Y, Avery A. The efficacy and safety of oral semaglutide for glycaemic management in adults with type 2 diabetes compared to subcutaneous semaglutide, placebo, and other GLP-1 RA comparators: a systematic review and network meta-analysis. Contemp Clin Trials Commun. 2022; 28:100944. doi: 10.1016/j.conctc.2022.100944.
- Rubino DM, Greenway FL, Khalid U, STEP 8 Investigators., et al. Effect of weekly subcutaneous semaglutide vs daily liraglutide on body weight in adults with overweight or obesity without diabetes: the STEP 8 randomized clinical trial. JAMA. 2022; 327(2):138–150. doi: 10.1001/jama.2021.23619.
- Mahapatra MK, Karuppasamy M, Sahoo BM. Semaglutide, a glucagon like peptide-1 receptor agonist with cardiovascular benefits for management of type 2 diabetes. Rev Endocr Metab Disord. 2022;23(3):521–539. doi: 10.1007/s11154-021-09699-1.
- Jalaludin MY, Deeb A, Zeitler P, et al. Efficacy and safety of the addition of sitagliptin to treatment of youth with type 2 diabetes and inadequate glycemic control on metformin without or with insulin. Pediatr Diabetes. 2022;23(2):183–193. doi: 10.1111/pedi.13282.
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American diabetes association and the european association for the study of diabetes. Diabetes Care. 2015; 38(1):140–149. doi: 10.2337/dc14-2441.
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71.
- Higgins JP, Altman DG, Gøtzsche PC,, et al. The cochrane collaboration’s tool for assessing risk of bias in randomized trials. BMJ. 2011; 343:d5928. doi: 10.1136/bmj.d5928.
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557.
- Ahrén B, Masmiquel L, Kumar H, et al. Efficacy and safety of once-weekly semaglutide versus once-daily sitagliptin as an add-on to metformin, thiazolidinediones, or both, in patients with type 2 diabetes (SUSTAIN 2): a 56-week, double-blind, phase 3a, randomised trial. Lancet Diabetes Endocrinol. 2017; 5(5):341–354. doi: 10.1016/S2213-8587(17)30092-X.
- Ji L, Dong X, Li Y, et al. Efficacy and safety of once-weekly semaglutide versus once-daily sitagliptin as add-on to metformin in patients with type 2 diabetes in SUSTAIN China: a 30-week, double-blind, phase 3a, randomized trial. Diabetes Obes Metab. 2021;23(2):404–414. doi: 10.1111/dom.14232.
- Leiter LA, Carr MC, Stewart M, et al. Efficacy and safety of the once-weekly GLP-1 receptor agonist albiglutide versus sitagliptin in patients with type 2 diabetes and renal impairment: a randomized phase III study. Diabetes Care. 2014;37(10):2723–2730. doi: 10.2337/dc13-2855.
- Stolar MW, Grimm M, Chen S. Comparison of extended release GLP-1 receptor agonist therapy versus sitagliptin in the management of type 2 diabetes. Diabetes Metab Syndr Obes. 2013; 6:435–444. doi: 10.2147/DMSO.S48837.
- Nauck MA, Kahle M, Baranov O, et al. Addition of a dipeptidyl peptidase-4 inhibitor, sitagliptin, to ongoing therapy with the glucagon-like peptide-1 receptor agonist liraglutide: a randomized controlled trial in patients with type 2 diabetes. Diabetes Obes Metab. 2017; 19(2):200–207. doi: 10.1111/dom.12802.
- Scheen AJ. Dipeptidylpeptidase-4 (DPP-4) inhibitors are favourable to glucagon-like peptide-1 (GLP-1) receptor agonists: yes. Eur J Intern Med. 2012;23(2):126–131. doi: 10.1016/j.ejim.2011.10.007.
- Li S, Li H, Wang R, et al. The effect of sitagliptin on obese patients with insulin treatment-induced diabetes mellitus. Eur Rev Med Pharmacol Sci. 2017; 21(15):3490–3495.
- Kodera R, Shikata K, Nakamura A, et al. The glucose-lowering efficacy of sitagliptin in obese Japanese patients with type 2 diabetes. Intern Med. 2017;56(6):605–613. doi: 10.2169/internalmedicine.56.7428.
- Dai D, Mao Y, Jin H, et al. Efficacy and hypoglycemic risk of sitagliptin in obese/overweight patients with type 2 diabetes compared with GLP-1 receptor agonists: a meta-analysis. Medicine (Baltimore). 2019;98(36):e17081. doi: 10.1097/MD.0000000000017081.
- Buse JB, Bode BW, Mertens A, et al. Long-term efficacy and safety of oral semaglutide and the effect of switching from sitagliptin to oral semaglutide in patients with type 2 diabetes: a 52-week, randomized, open-label extension of the PIONEER 7 trial. BMJ Open Diabetes Res Care. 2020; 8(2):e001649. doi: 10.1136/bmjdrc-2020-001649.
- Lavernia F, Blonde L. Clinical review of the efficacy and safety of oral semaglutide in patients with type 2 diabetes compared with other oral antihyperglycemic agents and placebo. Postgrad Med. 2020; 132(sup2):15–25. doi: 10.1080/00325481.2020.1798638.
- Shi FH, Li H, Cui M, et al. Efficacy and safety of once-weekly semaglutide for the treatment of type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Front Pharmacol. 2018; 9:576. doi: 10.3389/fphar.2018.00576.
- Rosenstock J, Allison D, Birkenfeld AL, et al. Effect of additional oral semaglutide vs sitagliptin on glycated hemoglobin in adults with type 2 diabetes uncontrolled with metformin alone or with sulfonylurea: the PIONEER 3 randomized clinical trial. JAMA. 2019; 321(15):1466–1480. doi: 10.1001/jama.2019.2942.
- Wilding JPH, Batterham RL, Calanna S, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989–1002. doi: 10.1056/NEJMoa2032183.
- Rubino D, Abrahamsson N, Davies M, et al. Effect of continued weekly subcutaneous semaglutide vs placebo on weight loss maintenance in adults with overweight or obesity: the STEP 4 randomized clinical trial. JAMA. 2021;325(14):1414–1425. doi: 10.1001/jama.2021.3224.
- Miles KE, Kerr JL. Semaglutide for the treatment of type 2 diabetes mellitus. J Pharm Technol. 2018;34(6):281–289. doi: 10.1177/8755122518790925.