Abstract
Introduction: Bipolar disorder (BD) is a prevalent and disabling mental disorder characterized by disrupted circadian rhythms and impaired neurocognitive features, both of which fall under the major domains of Research Domain Criteria (RDoC). However, there is limited evidence regarding the interaction between circadian rhythms and long-term neurocognitive functioning. Therefore, this longitudinal cohort study protocol aims to explore whether circadian rhythm can predict changes in neurocognitive functioning over time in patients with BD.
Methods: This study adopts a longitudinal cohort design, aiming to recruit 100 BD patients in either depressive or remitted states. Participants will undergo evaluations from clinical, circadian rhythm, and neurocognitive perspectives at baseline, 6-month, and 12-month follow-ups, involving questionnaires, actigraphy, and computed neurocognitive tests. We will examine both cross-sectional and longitudinal associations between participants’ circadian rhythm patterns and neurocognitive functioning. Statistical analyses will employ Spearman correlation and mixed regression models.
Discussion: We anticipate that circadian rhythms may serve as predictors of neurocognitive functioning changes. The findings of this study could offer supplementary insights into BD pathophysiology, potential treatment targets, and prediction.
Trial Registration: This study has been registered with the Chinese Clinical Trial Registry under the registration code ChiCTR2200064922 on 21st October 2022.
Introduction
Bipolar disorder (BD) is characterized by alternating manic/hypomanic episodes and depressive episodes, with an incidence of about 1% − 4% and suicide mortality 20-30 times higher than that in the general population [Citation1,Citation2]. As a significant biological feature, disrupted circadian rhythms have been highlighted by the International Society for Bipolar Disorders (ISBD). Among BD patients, delayed and disrupted chronotypes, changes in circadian rhythm gene expression, and abnormalities in cortisol and melatonin levels are commonly observed, showing correlations with prognosis, social functioning, and lithium response [Citation3–6]. Considering that many studies have utilized subjective measurements, such as rhythm questionnaires and sleep diaries, objective measurements appear more reliable, including actigraphy, polysomnography, and the concentration of correlated hormones, especially melatonin and cortisol in serum, saliva, hair, and urine [Citation7]. However, despite actigraphy being highly recommended for circadian rhythm evaluation, its limited application in long-term BD studies due to a scarcity of prospective designs has been noted [Citation8].
Moreover, neurocognitive impairment, a distinctive feature of BD, has garnered significant attention due to its impact on the quality of life and long-term rehabilitation, contributing to BD being ranked as the second greatest cause of days out of role [Citation1,Citation9]. Commonly affected cognitive domains in BD encompass working memory, sustained attention, and executive functions [Citation10]. While Lancet reviews have almost universally suggested that BD patients exhibit neuroprogressive characteristics resembling schizophrenia, previous longitudinal studies have yielded uncertain conclusions, highlighting the heterogeneity of cognitive phenotypes [Citation1,Citation2]. As BD staging and phenotyping of rhythmic and cognitive states gain popularity, the Research Domain Criteria (RDoC) has separately incorporated constructs related to circadian rhythms, attention, and working memory into arousal and regulatory systems, as well as cognitive systems, encouraging investigations into neurocircuitry and advancing future disorder classifications [Citation11,Citation12].
Interestingly, exploring circadian rhythm influence on neurocognitive functioning seems feasible with potential pathophysiological inspirations [Citation13]. Recent studies focusing on Alzheimer’s and other neurodegenerative diseases have speculated on the involvement of seasonal cognitive plasticity and circadian rhythm disruption in neurocognitive functioning changes [Citation14–17]. Additionally, a few pieces of evidence also support such an association with mental disorders [Citation18]. For instance, rats subjected to chronic jet lag simulation demonstrated enhanced depressive behaviors and cognitive deficits [Citation19]. Large-scale UK Biobank data revealed an association between disrupted circadian rhythmicity in mood disorders and cognitive function [Citation20]. Moreover, the ‘desynchronization effect’ resulting from evening chronotypes and circadian disruption might render individuals vulnerable to cognitive and psychiatric challenges [Citation21]. Furthermore, certain molecules are believed to play a role in both circadian rhythm disruption and neurocognitive impairment, mediated through mechanisms like immune-inflammatory activities [Citation22,Citation23].
Regrettably, up to this point, no study has been found that focuses on the long-term clinical correlation between circadian rhythm and neurocognitive functioning in BD patients. Exploring such a correlation holds utmost importance because, if the speculation proves true, 1) it could aid in identifying patients with a worse cognitive prognosis through circadian rhythm evaluation, enabling early intervention when necessary; 2) it may open up new possibilities for treating neurocognitive impairment in BD, for example, from a rhythmic perspective, such as brain synchronization; 3) it would provide stronger evidence supporting the pivotal role of circadian rhythm in BD pathophysiology [Citation24].
Therefore, this study protocol was designed to bridge the current research gaps by investigating the predictability of neurocognitive functioning through circadian rhythm in BD. The objective of this study is to assess whether circadian rhythm could predict the long-term change in neurocognitive functioning among BD patients. It is hypothesized that circadian rhythm could predict the long-term change in neurocognitive functioning.
Methods and analysis
Setting and recruitment
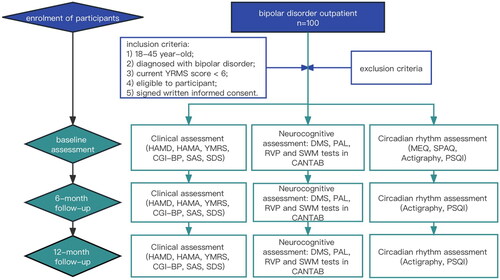
This study follows a longitudinal cohort design, as depicted in , with participant evaluations scheduled at baseline, 6-month follow-up, and 12-month follow-up after recruitment. Participants who miss the 6-month follow-up will still be eligible for the 12-month follow-up assessment. Ethical approval was obtained from the Research Ethics Committee of the First Affiliated Hospital of Chongqing Medical University (Approval Number: 20227001). Furthermore, this study has been registered with the Chinese Clinical Trial Registry under the registration code ChiCTR2200064922 on 21 October 2022. The study will be conducted in accordance with the Declaration of Helsinki [Citation25].
Recruitment will be conducted at the Psychiatric Outpatient Department of The First Affiliated Hospital of Chongqing Medical University in China, which is one of the largest hospitals with an esteemed reputation in southwest China. Recruitment advertisements will be disseminated online, providing contact information for interested patients. Additionally, researchers will proactively contact potential participants based on electronic medical records and expert referrals. Participants will not receive any economic compensation, except for assistance with making outpatient appointments. The recruitment period will span from January 2023 to July 2023. Participants will have the autonomy to withdraw from the study, with reasons and dates recorded. No specific replacement plan is in place for participants who choose to drop out.
The inclusion criteria are as follows: 1) aged 18–45 years old, either female or male; 2) diagnosed with bipolar disorder according to the Diagnostic and Statistical Manual of Mental Disorders-5 (DSM-5), which will be confirmed by researchers using Structured clinical interview for DSM-5, research version (SCID-5-RV) [Citation26,Citation27]; 3) currently in depressive or remitted states, as indicated by a YRMS score < 6; 4) eligible to complete the required examinations; 5) signed written informed consent to participate in this study.
The exclusion criteria are as follows: 1) presence of other diseases that could severely impact circadian rhythm or neurocognitive functioning, such as amnesia, stroke, sleep apnea, obesity, hyperthyroidism, diabetes, brain injury, and cortisol or melatonin related endocrinopathy; 2) living conditions that might influence circadian rhythm or neurocognitive functioning, including engaging in shift work more than once a week, residing across time zones with a time difference exceeding two hours and a shifting frequency of more than once a month, and being in the perinatal or lactation period; 3) concurrent severe psychoactive substance use, defined as consuming alcohol more than twice a week, smoking over 20 cigarettes a day, or using psychedelics or other illegal substances; 4) currently experiencing strong suicidal ideation or attempt, or exhibiting high-risk impulsive, violent, or self-injurious behaviors necessitating immediate hospitalization or constant supervision; 5) having undergone electroconvulsive therapy within the last 6 months prior to recruitment.
The participants should not receive electroconvulsive therapy during the study; otherwise, their follow-up will be terminated. No other specific treatment requirements are in place.
Assessment
Schedule of the enrolment and assessment was listed in .
Table 1. Schedule of enrolment and assessment.
Clinical assessment
Sociodemographic variables will be collected at baseline, including age, sex, height, weight, body mass index (BMI), ethnicity, education level, family history of mental illness, economic situation, handedness, history of tobacco and alcohol use and marriage status. We will also record the duration of illness, age of onset, subtypes of bipolar disorder, history of suicidal ideation and attempt, and history of psychotic symptoms.
For mood symptom monitoring at baseline, 6-month follow-up and 12-month follow-up, we will use the 17-item Hamilton Depression Scale (HAMD-17) and 14-item Hamilton Anxiety Scale (HAMA-14) which are interviewer-rating scales for depression and anxiety; Zung’s Self-Rating Depression Scale (SDS) and Zung’s Self-Rating Anxiety Scale (SAS) which are self-rating scales for depression and anxiety; and Young Mania Rating Scale (YMRS) which is an interviewer-rating scale for mania [Citation28–33].
Other evaluation includes the Clinical Global Impressions-Bipolar Version (CGI-BP). CGI-BP is an interviewer-rating scale for disease severity and treatment efficacy [Citation34].
Circadian rhythm assessment
The circadian rhythm variable consists of two components: subjective questionnaires and objective behavioral data. The questionnaires used include the Morningness Eveningness Questionnaire (MEQ), the Pittsburgh Sleep Quality Index (PSQI), and the Seasonal Pattern Assessment Questionnaire (SPAQ). The MEQ is a 19-item self-rating scale used to evaluate chronotype, with scores indicating evening, intermediate, or morning types [Citation35]. The PSQI is an 18-item self-rating scale that assesses sleep quality and disturbances, and a validated Chinese version will be used [Citation36,Citation37]. The SPAQ is a widely used questionnaire for evaluating seasonal effects on mood and behavior. It includes a general seasonality score (GSS) that can be used to screen for seasonal affective disorder (SAD) [Citation38,Citation39]. Except for the SPAQ, all the aforementioned scales and questionnaires have already been validated or translated into Chinese versions.
On the day of evaluation, participants will be asked to wear a wrist-worn actigraph device (wGT3X-BT, v1.9.2, ActiGraph LLC) for 14 consecutive days and nights. The actigraph’s flashing light will be disabled throughout the monitoring period. The device will continuously record physical activity, sleep/wake information and sedentary time using a sampling frequency of 100 Hz. Participants will be instructed to continuously wear the actigraph except during activities such as showering, swimming, or any activity that would consistently expose the device to water for more than 30 min. Data retrieval and analyses will be performed using the ActiLife software program (version 6.13.4) with 60-second epochs [Citation40]. The collected raw data will be stored and analyzed using publicly available algorithms. Non-wear time will be defined as 60 consecutive minutes of zeros with a 2-minute spike tolerance based on the default Wear Time Validation algorithm provided by Actilife 6.13.4. Any non-wearing epochs will be excluded from the analysis without any data imputation [Citation41,Citation42]. For a day to be deemed valid, it must have a minimum of 20 h of wearing time [Citation42]. A participant will be considered valid if they have a minimum of 7 valid days, including at least 5 weekdays and 2 weekend days [Citation35].
The circadian rhythms of sleep-wake activity will be calculated using Cole-Kripke Algorithm in Actilife [Citation43,Citation44]. We will retrieve sleep parameters such as Sleep Onset (SO), Total Sleep Time (TST), Wake after Sleep Onset (WASO) and Sleep Efficiency (SE). SO means the first minute that the algorithm scores ‘asleep’. TST equals to the total number of minutes scored as ‘asleep’. WASO means the total number of minutes the subject was awake after SO occurred. SE represents the number of sleep minutes divided by the total number of minutes the subject was in bed (i.e. the difference between the In-Bed and Out Bed time) [Citation44].
The circadian rhythm parameters of physical activity will be calculated using the specialized GGIR package for R software [Citation45]. Non-parametric circadian rhythm analysis will be employed. We aim to determine the intradaily variability (IV), interdaily stability (IS) and relative amplitude (RA) of the rest-activity rhythms [Citation43,Citation46,Citation47]. IV refers to the consolidation of rest-activity states within a day, where higher values indicate greater fragmentation of the rhythm with more transitions between rest and active states [Citation45]. IS describes the consistency of rest-activity patterns across different days, with higher values indicating greater stability [Citation48]. RA represents the difference in activity between the most active 10-hour period (M10) and the least active 5-hour period (L5) and serves as a marker of amplitude strength [Citation46]. Time of sedentary and moderate-to-vigorous physical activities (MVPA) will also be calculated based on validated activity cut-points in Actilife [Citation35,Citation49,Citation50].
Neurocognitive assessment
The primary outcome will be a comprehensive cognitive composite score. Neurocognitive functioning will be evaluated using the Cambridge Neuropsychological Test Automated Battery (CANTAB), developed by Cambridge Cognition in Cambridge, UK (www.camcog.com) [Citation51]. The tests have undergone validation in studies involving healthy human volunteers as well as various patient groups in the fields of behavioral and psychopharmacological research [Citation52,Citation53]. These tests will be administered on the same day as the actigraph wearing. The selected tests, including Delayed Matching to Sample (DMS), Paired Associates Learning (PAL), Rapid Visual Information Processing (RVP), and Spatial Working Memory (SWM), assess different cognitive domains [Citation54–56]. DMS and PAL evaluate visual memory, RVP measures sustained attention, and SWM reflects executive function. By converting the results of these four tests into Z-scores, the scores of each participant on different cognitive domains can be derived, and a composite cognitive functioning score can be calculated [Citation57,Citation58].
Sample size calculation
The sample size for this study was determined based on the primary hypothesis. To calculate the sample size, a review of relevant literature was conducted, and the PASS.15 computer software was used. Due to limited available reports, data from a previous study that evaluated changes in SWM between errors after 1 month of olanzapine treatment in 15 unipolar/bipolar depressive patients was used. In that study, the between errors at baseline and 1-month olanzapine treatment were 34 ± 24 and 21 ± 21, respectively (Mean ± Standard Deviation) [Citation59].
With an assumed power of 0.80 and an alpha level of 0.05, it was determined that a cohort size of 41 at follow-up would achieve 80% power to detect a difference of −13 between the null hypothesis (both baseline and 12-month follow-up means of SWM errors are 34) and the alternative hypothesis (mean of 12-month follow-up is 21). The estimated standard deviations were 21 and 21 at baseline and 12-month follow-up, respectively, using a two-sample t-test [Citation54]. Considering a potential dropout rate of 50% and the decision not to replace dropout participants, a total of 81 patients at baseline were deemed necessary. Finally, given the large number of outpatient receptions, we plan to recruit 100 participants.
Therefore, the study aimed to recruit a sample size of 100 patients at baseline to account for potential dropouts and achieve the desired statistical power.
Statistical analysis
SPSS (version 26) will be used for the analysis of this study. Histograms will be used to determine the normality or approximate normality of the data. This will be achieved by combining them with the Shapiro-Wilk test [Citation60]. Descriptive analyses will be performed to examine participant and disease characteristics, as well as data from clinical, circadian rhythm, and neurocognitive functioning assessments. Missing value analyses will be conducted with multiple imputation or expectation maximization methods to handle any missing data.
Spearman correlations will be employed to compare subjective and objectively assessed circadian rhythm patterns. Subjective assessments include MEQ, SPAQ, and PSQI results, while objective assessments will primarily rely on behavioral data.
Longitudinal analyses will involve calculating changes in SAS, SDS, HAMD, HAMA, and YMRS scores, and correlating these difference scores (baseline minus 6-month follow-up, baseline minus 12-month follow-up) with circadian rhythm parameters for each participant using Spearman correlation. Mixed regression models will be used to determine if changes in circadian rhythm significantly predict changes in neurocognitive functioning from baseline to 6-month follow-up and 12-month follow-up [Citation61]. Stepwise adjustment of covariates will be employed to construct several regression models to explore the robustness of the relationship between circadian rhythm and neurocognitive functioning. Adjustments will be made for other factors that may influence these associations, such as disease duration, age, gender, current medication use, education level, and severity of emotional symptoms.
Anticipated results
We anticipate that the circadian rhythm could predict the change of neurocognitive functioning among BD patients. More evidence of circadian rhythm importance for BD could be provided if the assumption holds.
Discussion
The importance of circadian rhythm in BD has always been emphasized as BD is somehow rhythmic by itself in clinical oscillations among depressive, hypomanic/manic, and intermittent episodes [Citation62]. The large genome-wide association study (GWAS) has found significant changes in gene expression around circadian rhythm pathways in BD population [Citation63]. We hypothesized that disrupted circadian rhythm also plays a role in neurocognitive functioning impairment in BD. Currently, limited evidence could be cited to support such an opinion. Mice deficient in Vipr2, which participate in internal rhythm synchronization, exhibit cognitive deficits resembling schizophrenia [Citation64,Citation65]. Circadian clock-deficient cryptochrome knockout mice present with cognitive dysfunction and elevated anxiety [Citation66]. Poor sleep quality is found related to poorer neuropsychological functioning in bipolar I disorder [Citation67]. With RDoC highlighting circadian rhythm and cognitive evaluation in arousal/modulatory system and cognitive system separately, we can’t help speculating the role of circadian rhythm in neurocognitive impairment of BD, where we seek to better understand the correlation of circadian rhythm and neurocognitive functioning [Citation11].
Still, we have not seen any study investigating the long-term neurocognitive predictability by circadian rhythm in BD patients. Thus, we designed such a study protocol where we included subjective rhythm questionnaires and objective actigraph data to represent the circadian rhythm. We plan to test our hypothesis from both cross-sectional and prospective perspectives. Specifically, to detect if the disrupted circadian rhythm is cross-sectionally correlated with worse neurocognitive functioning, if the subjectively measured circadian rhythm is comparable to objectively measured circadian rhythm, and if the circadian rhythm is predictive of long-term neurocognitive functioning changes.
This study is novel and intriguing due to the following advantages. Firstly, It is the first study protocol to establish the correlation between daily and seasonal circadian rhythms and neurocognitive functioning among BD patients. Secondly, it uses reliable yet less utilized techniques for evaluation accuracies, such as actigraph and CANTAB tests, with a combination of commonly recognized questionnaires for rhythm and mood evaluations. Thirdly, it is a prospective study lasting 12 months that fits in RDoC matrix, which could efficiently fill in the gap of long-term actigraph and cognitive functioning studies. Our study bears the potential to provide stronger evidence than a cross-sectional design and could enlighten similar designs guided by RDoC recommendations [Citation8].
Our study also has some limitations. Initially, we do not explore the melatonin and cortisol levels fluctuations, which is the strongest indication of circadian rhythm [Citation68]. Since we are not able to provide a laboratory condition for such repetitive samples, we are afraid that outpatients could not complete such a complicated measurement outside the hospital. Also, we did not use a sleep diary out of consideration that BD patients lack motivation with no economic compensation. Fortunately, there are some evidences supporting the comparability of cortisol and melatonin tests and sleep diary by actigraph data [Citation69].
Secondly, this study is not a randomized controlled study, and a lot of facts might add bias to this study. This is a single-center study, and most patients will be recruited from our Bipolar Disorder Specialist Clinic, which is less presentative of real-world situations and might decrease the diversity of treatment procedures and medications. Moreover, to strengthen the validity and reliability of data, we minimized the amount of self-rating questionnaires, thus impeding more involvement of useful questionnaires such as the Athens Insomnia Scale, Biological Rhythms Interview of Assessment in Neuropsychiatry, 32-item Hypomania Checklist, Patient Health Questionnaire-9 and General Anxiety Disorder-7 [Citation60,Citation70–73]. Nevertheless, we argue that this is only an exploratory study. More strict study conditions could be set and adapted based on this small-sample study results.
Finally, there is little information for predicting our results due to lack of enough relevant studies. Therefore, the statistical methods might differ from our original design and should be adapted with our ultimate results.
In summary, this is a 12-month longitudinal cohort study protocol. We aimed to find evidence for circadian rhythm influence on neurocognitive functioning on BD patients. We hypothesize that circadian rhythm could predict the change of neurocognitive functioning. Our findings may provide additional information for BD pathophysiology, treatment targets and prediction. Larger and more homogeneous sample with more integrate design could be considered in future study.
Ethics approval and consent to participate
The study received approval from the Research Ethics Committee of the First Affiliated Hospital of Chongqing Medical University (Approval Number: 20227001). We will conduct this study in accordance with the principles outlined in the Declaration of Helsinki. Informed consent will be obtained from all participants and/or their legal guardian(s).
Consent for publication
Before recruitment, we will obtain signed written informed consent from all participants, granting permission for the publication of study findings.
Authors’ contributions
HRL contributed to the conception of the study and played a major role in writing the manuscript. XQW, YLZ, and JYL designed the case report form for participants. RQH and ZZ designed the statistical analyses. QL and XXZ designed the framework of assessment. WD designed the neurocognitive testing procedures. JY and QHL provided valuable suggestions during the study conception. All authors have read and approved the final manuscript.
Acknowledgements
We extend our gratitude to Professor Huaqing Meng for his invaluable advice in designing this study.
Disclosure statement
The authors declare that they have no competing interests.
Data availability statement
Not applicable.
Additional information
Funding
References
- McIntyre RS, Berk M, Brietzke E, et al. Bipolar disorders. Lancet. 2020;396(10265):1–9. doi: 10.1016/S0140-6736(20)31544-0.
- Grande I, Berk M, Birmaher B, et al. Bipolar disorder. Lancet. 2016;387(10027):1561–1572. doi: 10.1016/S0140-6736(15)00241-X.
- Slyepchenko A, Allega OR, Leng X, et al. Association of functioning and quality of life with objective and subjective measures of sleep and biological rhythms in major depressive and bipolar disorder. Aust N Z J Psychiatry. 2019;53(7):683–696. doi: 10.1177/0004867419829228.
- McCarthy MJ, Wei H, Nievergelt CM, et al. Chronotype and cellular circadian rhythms predict the clinical response to lithium maintenance treatment in patients with bipolar disorder. Neuropsychopharmacology. 2019;44(3):620–628. doi: 10.1038/s41386-018-0273-8.
- Sanghani HR, Jagannath A, Humberstone T, et al. Patient fibroblast circadian rhythms predict lithium sensitivity in bipolar disorder. Mol Psychiatry. 2021;26(9):5252–5265. doi: 10.1038/s41380-020-0769-6.
- Esaki Y, Obayashi K, Saeki K, et al. Association between circadian activity rhythms and mood episode relapse in bipolar disorder: a 12-month prospective cohort study. Transl Psychiatry. 2021;11(1):525. doi: 10.1038/s41398-021-01652-9.
- Geoffroy PA, Palagini L. Biological rhythms and chronotherapeutics in depression. Prog Neuropsychopharmacol Biol Psychiatry. 2021;106:110158. doi: 10.1016/j.pnpbp.2020.110158.
- Scott J, Colom F, Young A, et al. An evidence map of actigraphy studies exploring longitudinal associations between rest-activity rhythms and course and outcome of bipolar disorders. Int J Bipolar Disord. 2020;8(1):37. doi: 10.1186/s40345-020-00200-6.
- Alonso J, Petukhova M, Vilagut G, et al. Days out of role due to common physical and mental conditions: results from the WHO world mental health surveys. Mol Psychiatry. 2011;16(12):1234–1246. doi: 10.1038/mp.2010.101.
- Keramatian K, Torres IJ, Yatham LN. Neurocognitive functioning in bipolar disorder: what we know and what we don’t. Dialogues Clin Neurosci. 2021;23(1):29–38. doi: 10.1080/19585969.2022.2042164.
- Insel T, Cuthbert B, Garvey M, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167(7):748–751. doi: 10.1176/appi.ajp.2010.09091379.
- Guglielmo R, Miskowiak KW, Hasler G. Evaluating endophenotypes for bipolar disorder. Int J Bipolar Disord. 2021;9(1):17. doi: 10.1186/s40345-021-00220-w.
- McCarthy MJ, Gottlieb JF, Gonzalez R, et al. Neurobiological and behavioral mechanisms of circadian rhythm disruption in bipolar disorder: a critical multi-disciplinary literature review and agenda for future research from the ISBD task force on chronobiology. Bipolar Disord. 2022;24(3):232–263. doi: 10.1111/bdi.13165.
- Lim ASP, Gaiteri C, Yu L, et al. Seasonal plasticity of cognition and related biological measures in adults with and without Alzheimer disease: analysis of multiple cohorts. PLOS Med. 2018;15(9):e1002647. doi: 10.1371/journal.pmed.1002647.
- Kumar D, Sharma A, Taliyan R, et al. Orchestration of the circadian clock and its association with Alzheimer’s disease: role of endocannabinoid signaling. Ageing Res Rev. 2022;73:101533. doi: 10.1016/j.arr.2021.101533.
- Nassan M, Videnovic A. Circadian rhythms in neurodegenerative disorders. Nat Rev Neurol. 2022;18(1):7–24. doi: 10.1038/s41582-021-00577-7.
- Hoyt KR, Obrietan K. Circadian clocks, cognition, and Alzheimer’s disease: synaptic mechanisms, signaling effectors, and chronotherapeutics. Mol Neurodegener. 2022;17(1):35.
- Ruiz-Gayo M, Olmo ND. Interaction between circadian rhythms, energy metabolism, and cognitive function. Curr Pharm Des. 2020;26(20):2416–2425. doi: 10.2174/1381612826666200310145006.
- Horsey EA, Maletta T, Turner H, et al. Chronic jet lag simulation decreases hippocampal neurogenesis and enhances depressive behaviors and cognitive deficits in adult male rats. Front Behav Neurosci. 2019;13:272. doi: 10.3389/fnbeh.2019.00272.
- Lyall LM, Wyse CA, Graham N, et al. Association of disrupted circadian rhythmicity with mood disorders, subjective wellbeing, and cognitive function: a cross-sectional study of 91 105 participants from the UK biobank. Lancet Psychiatry. 2018;5(6):507–514. doi: 10.1016/S2215-0366(18)30139-1.
- Taillard J, Sagaspe P, Philip P, et al. Sleep timing, chronotype and social jetlag: impact on cognitive abilities and psychiatric disorders. Biochem Pharmacol. 2021;191:114438. doi: 10.1016/j.bcp.2021.114438.
- Gaebler AJ, Finner-Prevel M, Sudar FP, et al. The interplay between vitamin D, exposure of anticholinergic antipsychotics and cognition in schizophrenia. Biomedicines. 2022;10(5):1096. doi: 10.3390/biomedicines10051096.
- Maruani J, Anderson G, Etain B, et al. The neurobiology of adaptation to seasons: relevance and correlations in bipolar disorders. Chronobiol Int. 2018;35(10):1335–1353. doi: 10.1080/07420528.2018.1487975.
- Grover S, Nguyen JA, Reinhart RMG. Synchronizing brain rhythms to improve cognition. Annu Rev Med. 2021;72:29–43. doi: 10.1146/annurev-med-060619-022857.
- World Medical Association. World medical association declaration of Helsinki: ethical principles for medical research involving human subjects. Jama. 2013;310(20):2191–2194.
- Association AP. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, D.C.: American Psychiatric Association; 2013.
- Association AP. Structured clinical interview for DSM-5, research version (SCID-5-RV). Washington, D.C.: American Psychiatric Association 2015.
- Zung WW. A self-rating depression scale. Arch Gen Psychiatry. 1965;12:63–70. doi: 10.1001/archpsyc.1965.01720310065008.
- Zung WW. A rating instrument for anxiety disorders. Psychosomatics. 1971;12(6):371–379. doi: 10.1016/S0033-3182(71)71479-0.
- Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6(4):278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x.
- Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32(1):50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x.
- Young RC, Biggs JT, Ziegler VE, et al. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429.
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56–62. doi: 10.1136/jnnp.23.1.56.
- Spearing MK, Post RM, Leverich GS, et al. Modification of the clinical global impressions (CGI) scale for use in bipolar illness (BP): the CGI-BP. Psychiatry Res. 1997;73(3):159–171. doi: 10.1016/S0165-1781(97)00123-6.
- O’Brien CM, Duda JL, Kitas GD, et al. Correlates of sedentary behaviour and light physical activity in people living with rheumatoid arthritis: protocol for a longitudinal study. Mediterr J Rheumatol. 2018;29(2):106–117. doi: 10.31138/mjr.29.2.106.
- Buysse DJ, Reynolds CF, 3rd, Monk TH, et al. The pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4.
- Liu X, Tang M. Reliability and validity of the Pittsburgh sleep quality index. Chin Psychiatry. 1996;29:29103–29107.
- N R, G B, T W. Seasonal pattern assessment questionnaire. Bethesda, USA: National Institute of Mental Health:; 1984.
- Reynaud E, Berna F, Haffen E, et al. Validity and usage of the seasonal pattern assessment questionnaire (SPAQ) in a French population of patients with depression. Bipolar Disorders and Controls. J Clin Med. 2021;10(9):1897.
- Neishabouri A, Nguyen J, Samuelsson J, et al. Quantification of acceleration as activity counts in ActiGraph wearable. Sci Rep. 2022;12(1):11958. doi: 10.1038/s41598-022-16003-x.
- van Hees VT, Gorzelniak L, Dean León EC, et al. Separating movement and gravity components in an acceleration signal and implications for the assessment of human daily physical activity. PLOS One. 2013;8(4):e61691. doi: 10.1371/journal.pone.0061691.
- Oliva V, Fanelli G, Zamparini M, et al. Patterns of antipsychotic prescription and accelerometer-based physical activity levels in people with schizophrenia spectrum disorders: a multicenter, prospective study. Int Clin Psychopharmacol. 2023;38(1):28–39. doi: 10.1097/YIC.0000000000000433.
- Van Someren EJ, Swaab DF, Colenda CC, et al. Bright light therapy: improved sensitivity to its effects on rest-activity rhythms in Alzheimer patients by application of nonparametric methods. Chronobiol Int. 1999;16(4):505–518. doi: 10.3109/07420529908998724.
- Cole RJ, Kripke DF, Gruen W, et al. Automatic sleep/wake identification from wrist activity. Sleep. 1992;15(5):461–469. doi: 10.1093/sleep/15.5.461.
- McGowan NM, Goodwin GM, Bilderbeck AC, et al. Circadian rest-activity patterns in bipolar disorder and borderline personality disorder. Transl Psychiatry. 2019;9(1):195. doi: 10.1038/s41398-019-0526-2.
- Meyer N, Faulkner SM, McCutcheon RA, et al. Sleep and circadian rhythm disturbance in remitted schizophrenia and bipolar disorder: a systematic review and meta-analysis. Schizophr Bull. 2020;46(5):1126–1143. doi: 10.1093/schbul/sbaa024.
- Rock P, Goodwin G, Harmer C, et al. Daily rest-activity patterns in the bipolar phenotype: a controlled actigraphy study. Chronobiol Int. 2014;31(2):290–296. doi: 10.3109/07420528.2013.843542.
- Mayeli A, LaGoy AD, Smagula SF, et al. Shared and distinct abnormalities in sleep-wake patterns and their relationship with the negative symptoms of schizophrenia spectrum disorder patients. Mol Psychiatry. 2023.doi: 10.1038/s41380-023-02050-x.
- Santos-Lozano A, Santín-Medeiros F, Cardon G, et al. Actigraph GT3X: validation and determination of physical activity intensity cut points. Int J Sports Med. 2013;34(11):975–982. doi: 10.1055/s-0033-1337945.
- Tudor-Locke C, Leonardi C, Johnson WD, et al. Accelerometer steps/day translation of moderate-to-vigorous activity. Prev Med. 2011;53(1–2):31–33. doi: 10.1016/j.ypmed.2011.01.014.
- Robbins TW, James M, Owen AM, et al. Cambridge neuropsychological test automated battery (CANTAB): a factor analytic study of a large sample of normal elderly volunteers. Dementia. 1994;5(5):266–281.
- Rock PL, Roiser JP, Riedel WJ, et al. Cognitive impairment in depression: a systematic review and meta-analysis. Psychol Med. 2014;44(10):2029–2040. doi: 10.1017/S0033291713002535.
- Roca M, Del Amo AR, Riera-Serra P, et al. Suicidal risk and executive functions in major depressive disorder: a study protocol. BMC Psychiatry. 2019;19(1):253. doi: 10.1186/s12888-019-2233-1.
- Sawalha J, Cao L, Chen J, et al. Individualized identification of first-episode bipolar disorder using machine learning and cognitive tests. J Affect Disord. 2021;282:662–668. doi: 10.1016/j.jad.2020.12.046.
- Li X, Deng W, Xue R, et al. Auditory event-related potentials, neurocognition, and global functioning in drug naive first-episode schizophrenia and bipolar disorder. Psychol Med. 2023;53(3):785–794. doi: 10.1017/S0033291721002130.
- Frias A, Dickstein DP, Merranko J, et al. Longitudinal cognitive trajectories and associated clinical variables in youth with bipolar disorder. Bipolar Disord. 2017;19(4):273–284. doi: 10.1111/bdi.12510.
- Averill IRE, Beaglehole B, Douglas KM, et al. Activation therapy for the treatment of inpatients with depression - protocol for a randomised control trial compared to treatment as usual. BMC Psychiatry. 2019;19(1):52. doi: 10.1186/s12888-019-2038-2.
- Miskowiak KW, Burdick KE, Martinez-Aran A, et al. Methodological recommendations for cognition trials in bipolar disorder by the international society for bipolar disorders targeting cognition task force. Bipolar Disord. 2017;19(8):614–626. doi: 10.1111/bdi.12534.
- Lazowski LK, Townsend B, Hawken ER, et al. Sleep architecture and cognitive changes in olanzapine-treated patients with depression: a double blind randomized placebo controlled trial. BMC Psychiatry. 2014;14:202. doi: 10.1186/1471-244X-14-202.
- Lavin-Gonzalez P, Bourguignon C, Crescenzi O, et al. Inactograms and objective sleep measures as means to capture subjective sleep problems in patients with a bipolar disorder. Bipolar Disord. 2020;22(7):722–730. doi: 10.1111/bdi.12903.
- Goshen A, Goldbourt U, Benyamini Y, et al. Association of diet quality with longevity and successful aging in Israeli adults 65 years or older. JAMA Netw Open. 2022;5(6):e2214916. doi: 10.1001/jamanetworkopen.2022.14916.
- Moreira J, Geoffroy PA. Lithium and bipolar disorder: impacts from molecular to behavioural circadian rhythms. Chronobiol Int. 2016;33(4):351–373. doi: 10.3109/07420528.2016.1151026.
- Mullins N, Forstner AJ, O’Connell KS, et al. Genome-wide association study of more than 40,000 bipolar disorder cases provides new insights into the underlying biology. Nat Genet. 2021;53(6):817–829. doi: 10.1038/s41588-021-00857-4.
- Chaudhury D, Loh DH, Dragich JM, et al. Select cognitive deficits in vasoactive intestinal peptide deficient mice. BMC Neurosci. 2008;9:63. doi: 10.1186/1471-2202-9-63.
- Walker WH, 2nd, Walton JC, DeVries AC, et al. Circadian rhythm disruption and mental health. Transl Psychiatry. 2020;10(1):28. doi: 10.1038/s41398-020-0694-0.
- De Bundel D, Gangarossa G, Biever A, et al. Cognitive dysfunction, elevated anxiety, and reduced cocaine response in circadian clock-deficient cryptochrome knockout mice. Front Behav Neurosci. 2013;7:152. doi: 10.3389/fnbeh.2013.00152.
- Menkes MW, Andrews CM, Burgess HJ, et al. Sleep quality and neuropsychological functioning in bipolar I disorder. J Affect Disord. 2021;293:133–140. doi: 10.1016/j.jad.2021.06.022.
- Melo MCA, Abreu RLC, Linhares Neto VB, et al. Chronotype and circadian rhythm in bipolar disorder: a systematic review. Sleep Med Rev. 2017;34:46–58. doi: 10.1016/j.smrv.2016.06.007.
- Moon JH, Cho CH, Son GH, et al. Advanced circadian phase in mania and delayed circadian phase in mixed mania and depression returned to normal after treatment of bipolar disorder. EBioMedicine. 2016;11:285–295. doi: 10.1016/j.ebiom.2016.08.019.
- Kanagarajan K, Gou K, Antinora C, et al. Morningness-eveningness questionnaire in bipolar disorder. Psychiatry Res. 2018;262:102–107. doi: 10.1016/j.psychres.2018.02.004.
- Spitzer RL, Kroenke K, Williams JB, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092–1097. doi: 10.1001/archinte.166.10.1092.
- Giglio LM, Magalhães PV, Andreazza AC, et al. Development and use of a biological rhythm interview. J Affect Disord. 2009;118(1–3):161–165. doi: 10.1016/j.jad.2009.01.018.
- Angst J, Adolfsson R, Benazzi F, et al. The HCL-32: towards a self-assessment tool for hypomanic symptoms in outpatients. J Affect Disord. 2005;88(2):217–233. doi: 10.1016/j.jad.2005.05.011.