Abstract
Backgrounds
The Naples prognosis score (NPS) is a novel prognostic biomarker-based immune and nutritional status and that can be used to evaluate prognosis. Our study aimed to investigate the prognostic role of NPS in SCLC patients.
Methods
Patients treated with chemoradiotherapy were retrospectively analyzed between June 2012 and August 2017. We divided patients into three groups depending on the NPS: group 0, n = 31; group 1, n = 100; and group 2, n = 48, and associations between clinical characteristics and NPS group were analyzed. The univariable and multivariable Cox analyses were used to evaluate the prognostic value of clinicopathological characteristics and laboratory indicators for overall survival (OS) and progression-free survival (PFS).
Results
Data from 179 patients were analyzed. Treatment modality (p < 0.001) and serum CEA (p = 0.03) were significantly different among the NPS groups. The age, sex, smoking status, KPS, Karnofsky performance score (KPS), disease extent, and number of metastatic sites were not correlated with NPS (all p > 0.05). KPS, disease extent, prophylactic cranial irradiation, treatment response and NPS Group were associated with OS. In addition, KPS, disease extent, prophylactic cranial irradiation, treatment response and NPS Group were associated with PFS. Multivariate analysis results showed that NPS was identified as an independent prognostic factor for OS (Group 1: hazard ratio [HR] = 2.704, 95% confidence interval [CI] = 1.403-5.210; p = 0.003; Group 2: HR = 5.154, 95% CI = 2.614-10.166; p < 0.001) and PFS (Group 1: HR = 2.018, 95% CI = 1.014-4.014; p = 0.045; Group 2: HR = 3.339, 95% CI = 1.650-6.756; p = 0.001).
Conclusions
NPS is related to clinical outcomes in patients with SCLC.
Key Messages
Despite the high clinical curative effect to radiation therapy and chemotherapy in SCLC, most patients subsequently experience tumor recurrence or metastasis.
Whether NPS has prognostic values in SCLC has not been investigated to date.
NPS is related to clinical outcomes in patients with SCLC.
NPS as an innovative scoring system, can improves prediction of survival in SCLC patients.
Introduction
Lung cancer (LC), one of the high morbidity and mortality malignant tumors, seriously threatens people health. Small cell lung cancer (SCLC) is poorly differentiated neuroendocrine carcinoma, which is accounting for about 15% of LC cases [Citation1]. SCLC is divided into limited-stage SCLC (LS-SCLC) and extensive-stage SCLC (ES-SCLC) [Citation2], and which has the characteristics of rapid tumor proliferation, abundant neoangiogenesis and high aggressiveness [Citation3]. Despite the high clinical curative effect to radiation therapy and chemotherapy, most patients subsequently experience tumor recurrence or metastasis [Citation4]. Despite the considerable efforts for SCLC patients, the 5-year survival rate in SCLC patients is <5%-7% [Citation5]. The pathogenesis of SCLC is complexly regulated by multiple mechanisms and there are no reliable biomarkers for predicting prognosis [Citation6]. Hence, further research is needed to fully explore the prognostic markers and implement individualized precision therapy.
Accumulating evidence suggests that host inflammation play an important role in tumor cell biology. Due to the multiple roles of inflammation in tumor invasion, angiogenesis, and distant metastasis, a series of host inflammation- related biomarkers are intrinsically identified as prognostic indicators [Citation7,Citation8]. Neutrophil-to-lymphocyte ratio (NLR), lymphocyte-to-monocyte ratio (LMR), platelet-to-lymphocyte ratio (PLR), systemic immune-inflammation index (SII) and C-reactive protein (CRP) appear to offer utility as the predictors of tumor prognosis with reliable analysis [Citation9–13]. In addition, nutritional status is also an important indicator to evaluate the host immune capacity and can be used to predict outcomes, such as the prognostic nutritional index (PNI), the modified Glasgow Prognostic Score (mGPS), and the Controlling Nutritional Status (CONUT) [Citation14–16]. Inflammation and nutritional status related-biomarkers have been proven to be of prognostic value, but alone is often limited and unreliable. Multidimensional prognostic factors together may be a new direction in the era of precision medicine.
A novel prognostic system, Naples prognostic score (NPS) incorporates several inflammation and nutritional biomarkers including the NLR, LMR, serum albumin, and total cholesterol. NPS represent patients’ whole status and has been predominantly used in original research. Its prognostic value was validated by a prospective clinical trial of gastric cancer [Citation17] Furthermore, NPS has been reported to be strongly associated with outcomes in patients with esophageal cancer [Citation18],pancreatic cancer [Citation19], and colorectal cancer [Citation20]. However, whether NPS has prognostic values in SCLC has not been investigated to date.
Hence, we were interested to investigate the role of NPS in SCLC patients. The aim of this study was to validate the relationships between the clinicopathological characteristics and NPS group, using this novel score, to investigate whether NPS is associated with overall survival (OS) and progression-free survival (PFS) in patients with SCLC.
Materials and methods
Patients
A retrospective study enrolled 179 patients with SCLC from between June 2012 and August 2017 at our institution. For inclusion criteria, Karnofsky Performance Scores (KPS) ≥70; patients with histologically confirmed SCLC; patient had no prior anti-cancer treatment; complete clinical recorded and follow-up data. For exclusion criteria, patients with infection or pneumonia disease, patients with chronic inflammatory or autoimmune disease were excluded.
Clinical data on the following clinicopathological variables was obtained from electronic medical records of our hospital information system: age, sex, smoking status, Karnofsky performance score (KPS), disease extent, number of metastatic sites, treatment modality, prophylactic cranial irradiation, cycles of chemotherapy, treatment response. The laboratory variables of CEA, NSE, CYFRa21-1, neutrophil, lymphocyte, monocyte, serum albumin and total cholesterol were obtained within 1 week before treatment.
Naples prognostic score group definition
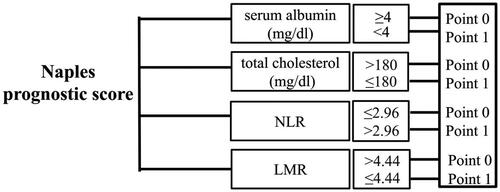
According to galizia et al.’s study method in gastric and colorectal cancers [Citation17,Citation21], NPS was calculated from serum albumin, total cholesterol, NLR, and LMR. Serum albumin ≥4 mg/dL, total cholesterol > 180 mg/dL, NLR < 2.96, or LMR > 4.44 was scored as 0, while serum albumin <4 mg/dL, total cholesterol ≤ 180 mg/dL, NLR ≥ 2.96, or LMR ≤ 4.44 was scored as 1 (). Patients were divided into three groups according to NPS score: group 0 patients with a score of 0; group 1, patients with a score of 1 or 2; and group 2, patients with a score of 3 or 4. NLR and LMR were calculated as previous reported [Citation22].
Statistical analyses
Statistical analyses were performed using the SPSS20.0 and GraphPad Prism software (version 5.0). The follow-up data was obtained by electronic medical records or telephone follow-up. Follow-up examinations were performed at regular intervals: 2 monthly for the first year, 3-6 monthly during the following years. OS was calculated from the date of diagnosis until the date of death from any cause or the last date that the patient was known to be alive. PFS was defined as the period between the date of diagnosis and the date of confirmed disease progression. Spearman correlation analyses were used to estimate the correlations between serum albumin, total cholesterol, NLR, and LMR. The associations between categorical variables were analyzed with Fisher’s exact test or chi-square test. Survival curves were analyzed using the Kaplan-Meier method, and differences of survival distributions were compared by log-rank tests. Univariable and multivariable Cox analyses were performed to determine independent predictive factors of OS and PFS. In general, two-tailed P-value < 0.05 was considered statistically significant.
Results
Patients characteristics
The baseline characteristics for 179 patients are shown in . The median age was 62 (interquartile range: 54-68) years, 80 (44.7%) patients were female and 99 (55.3%) were male. According to the 8th AJCC, 65 (53.3%), and 114 (63.7%) patients were in limited-stage (LS) disease and extensive-stage (ES) disease, respectively. Approximate half of the patients (n = 132, 73.4%) were in good general condition with KPS score of 90-100. 112 (62.6%) patients presented with never-smoking and 67 (37.4%) patients presented with smoking status. The sites of metastasis in SCLC patients included brain, liver, adrenal gland, bone. All of the laboratory biomarkers are summarized in .
Table 1. Patients characteristics.
Table 2. The laboratory biomarkers.
Relationships between NPS and clinical characteristics
The patients were categorized into three groups: NPS group 0, NPS group 1 and NPS group 2. There were 31 (17.3%), 100 (55.9%), and 48 (26.8%) patients in group 0, group 1, and group 2, respectively. The relationships between NPS and clinical characteristics are shown in . On receiver operating characteristic (ROC) analysis, the NPS was found to have the largest area under the curve (AUC = 0.702; 95% confidence interval [CI], 0.606-0.761; p < 0.001; sensitivity: 39.8%; specificity: 90.8%; Youden Index: 0.306) (). NPS was significantly associated with treatment modality (p < 0.001) and serum CEA (p = 0.03). However, no significant statistical relationship was found between age, sex, smoking status, KPS, disease extent, number of metastatic sites, prophylactic cranial irradiation, cycles of chemotherapy, treatment response, CEA, NSE and CYFRa21-1 (all p > 0.05).
Figure 2. ROC Curve of NPS for recurrence prediction. Abbreviations: Receiver operating characteristic (ROC); Naples prognostic score (NPS).
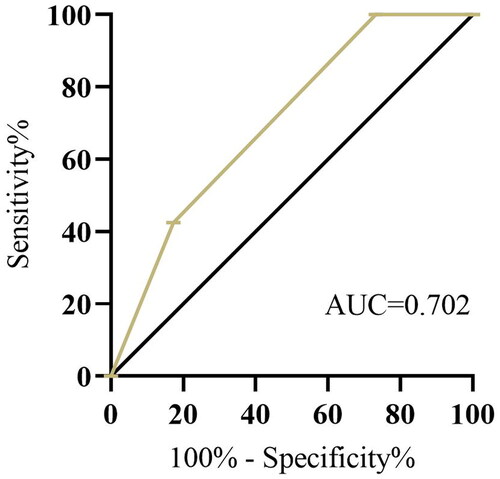
Table 3. Relationship between NPS and clinicopathological characteristics.
Relationships among serum albumin, total cholesterol, NLR and LMR
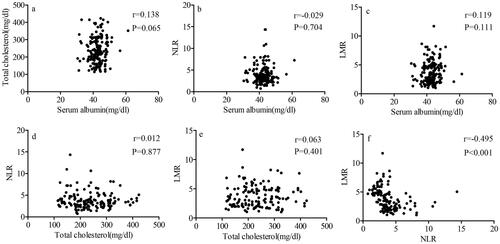
The relationships among serum albumin, total cholesterol, NLR and LMR are shown in . The results revealed that negative correlations were found between NLR and LMR (r = −0.495, p < 0.001). Besides, no significant correlations were found between cholesterol and NLR (r = 0.012, p = 0.877), cholesterol and LMR (r = 0.063, p = 0.401), serum albumin and total cholesterol (r = 0.138, p = 0.065), serum albumin and NLR (r = −0.029, p = 0.704), serum albumin and LMR (r = 0.119, p = 0.111).
Figure 3. Correlations between serum albumin, total cholesterol, NLR, and LMR. a serum albumin and total cholesterol. b serum albumin and NLR. c serum albumin and LMR. d total cholesterol and NLR. e total cholesterol and LMR. f NLR and LMR. Abbreviations: neutrophil to lymphocyte ratio (NLR), lymphocyte to monocyte ratio (LMR).
Patient response and outcome
Of the included patients, 120 (67%) patients received chemoradiotherapy, and 59 (33%) patients only received chemotherapy without thoracic radiation therapy. For radiation therapy, patient was treated with intensity modulated radiation therapy (IMRT). Chemotherapy regimens used cisplatin/carboplatin combined with etoposide. Results showed that complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD) were observed in 38 (21.2%), 30 (16.8%), 50 (27.9%) and 61 (34.1%) cases, respectively. The median follow-up time was 36 months (5-59 months). Median OS for whole enrolled patients was 15 months (95% CI: 14-17 months), while median PFS was 10 months (95% CI: 8-12 months). Among the 179 SCLC patients, 103 of them experienced tumor recurrence or metastasis, and 118 of them died.
Kaplan-Meier survival analysis
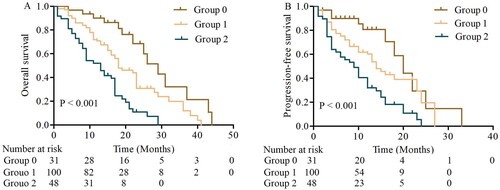
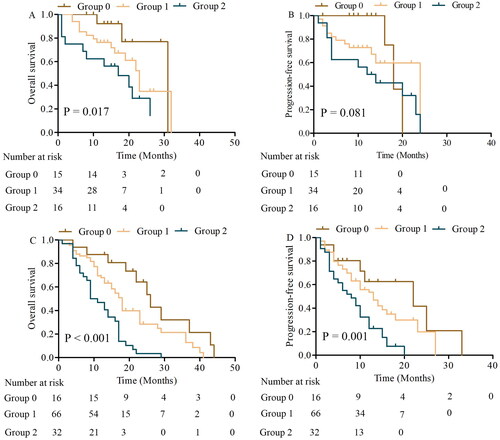
We used the Kaplan-Meier analysis to evaluate the survival differences among the NPS groups. As shown in , patients in NPS group 2 and group 1 had significantly poorer OS compared to those in NPS group 0 (). In addition, patients in NPS group 0 had significantly better PFS compared to those in NPS group 1 and group 2 (). We further performed subgroup analysis in LS and ES, the OS of patients with NPS group 0, 1, and 2 had significant differences in LS (p = 0.017), but PFS had no statistical significance (p = 0.081). The ES patients in groups 1 and 2, were significantly worse than the OS and PFS of those in group 0 (p < 0.001, OS; p = 0.001, PFS) ().
Univariable and multivariable Cox analyses of survival
Univariable Cox analysis shown that KPS, disease extent, prophylactic cranial irradiation, response, and NPS were significantly correlated with OS and PFS (). Five variables were further investigated in multivariable Cox analyses. Multivariable Cox analysis indicated that NPS was independent prognostic factors for OS (Group 1: hazard ratio [HR] = 2.704, 95% CI = 1.403-5.210; p = 0.003; Group 2: HR = 5.154, 95% CI = 2.614-10.166; p < 0.001) and PFS (Group 1: HR = 2.018, 95% CI = 1.014-4.014; p = 0.045; Group 2: HR = 3.339, 95% CI = 1.650-6.756; p = 0.001). In addition, treatment response was also independent prognostic factors for OS (p = 0.029) and PFS (p = 0.036) ().
Table 4. Univariate analysis about impact of variables on OS and PFS.
Table 5. Multivariate analysis about impact of variables on OS and PFS.
Discussion
This study represents the first study aimed at evaluating potential prognostic role of NPS for OS and PFS in patients with SCLC. The results demonstrated that NPS is significantly associated with clinical outcomes, and it is an independent prognostic factor for OS and PFS in SCLC patients.
The relationship between inflammation and cancer was first described by Virchow [Citation23]. Scientists conducted various researches about the mechanism of inflammation and cancer to further clarify potential relationship. Various studies have suggested that inflammation promotes tumor cell proliferation, angiogenesis, migration, invasion, and resistance of chemotherapy and radiation by promoting growth factors and inhibiting apoptosis [Citation24–27]. Peripheral inflammatory cells, such as neutrophils, monocytes, and lymphocytes, are associated with the prognosis of different types of cancers. The interaction between inflammation and cancer cells is reciprocal. Cancer cells can produce G-CS), IL-6, and other cytokines, leading to an increased neutrophil count [Citation28]. Meanwhile, neutrophils modulates tumor development, immune escape and metastasis by releasing inflammatory factors, such as NF-kB, MMP-9, and IL-8 [Citation29,Citation30]. Monocytes regulate tumor microenvironment and create an environment of chronic oxidative stress to promote tumor angiogenesis, inflammatory response and distant metastases [Citation31,Citation32]. Lymphocytes is an important component of anticancer immunity response, immune defenses and immune surveillance, as well as lymphocytes are implicated in the killing of cancer cells and increased sensitivity to chemotherapy and radiation [Citation33–35]. NLR and LMR as inflammatory scoring systems, which have been investigated the role of prognostic value in malignant tumors. Guo et al. conducted a separate meta-analysis and confirmed that patients with high NLR had poor OS and high recurrence risk [Citation36]. Similarly, Zhang et al. showed that high NLR was significantly related to poor survival [Citation37]. Go et al. showed that a low LMR was an unfavourable prognostic factor for OS and PFS [Citation38]. A study of 268 Spain patients with NSCLC proved that LMR was independently associated with DFS (p = 0.001) and OS (p = 0.007) [Citation39].
There is increasingly evidence that not only inflammation indictors but also host nutritional status, particularly nutritional-related biomarkers, are crucial for tumor growth, angiogenesis, and tumor progression [Citation40]. Hypoalbuminemia has been confirmed as a marker of malnutrition. At the same time, it can be as systemic inflammation secreting some pro-inflammatory substances, reduces the concentration of serum albumin [Citation41]. In addition, hypoalbuminemia impairs the mobility of cell surface receptors and reduces free oxygen radicals scavenging [Citation42]. Jin et al. found that serum albumin levels was a significant independent prognostic factor for tumor recurrence in NSCLC patients who had undergone complete resection (p = 0.005) [Citation43].Lei et al. had shown that lower serum albumin levels are predictive of worse OS and PFS [Citation44]. In addition, lipid metabolism may be related to the tumor development and angiogenesis. Cholesterol is involved in key cellular signaling pathways, which promote malignant transformation via modulation of cytoskeleton alteration, cell polarity, and angiogenesis [Citation45].The total cholesterol is identified to be related to tumor development and outcomes in gastric cancer [Citation46]. Combining serum albumin, total cholesterol, NLR and LMR may better reflect the inflammation and nutritional status of patients and enhance performance in prognostic stratification.
NPS as a novel indicator, incorporating serum albumin, total cholesterol, NLR and LMR, which is available tool for precision predictor of prognosis from multiple perspectives. Li et al. showed that NPS is an independent prognostic factor for OS and PFS in grade 2 or 3 endometrial cancer [Citation47]. Miyamoto et al. concluded that patients with high NPS had significantly shorter OS than those in NPS group 0 and 1 (p = 0.012 and 0.022) and multivariate analysis identified the NPS as an independent prognostic factor for OS (HR = 1.574; p = 0.004) [Citation20]. In the study performed by Li et al. 457 patients with operable NSCLC cases was enrolled, which has been shown that increase in NPS was found to be significantly associated with unfavorable OS and DFS of NSCLC [Citation48]. In the study performed by Galizia et al. only NPS was selected as an independent significant predictor for OS (p = 0.024) and DFS (p = 0.009) among the different scoring systems [Citation17]. Yang et al. reported that NPS was a novel and multidimensional prognostic scoring system with favorable predictive performance in 133 osteosarcoma patients [Citation49]. Lieto et al. found that assessment of NPS in gastric cancer patients treating with multimodal treatment may be valuable in modulating immune-nutritional conditions in order to improve patient’s reaction against the tumor [Citation50]. Therefore, NPS may represent different risk grades, suggesting that patients in different groups have different prognosis.
In present study, NPS were grouped in three groups and method is consistent with the previous studies reported [Citation17,Citation21,Citation50]. Furthermore, the patients with different NPS groups in our study were associated with treatment modality (p < 0.001) and serum CEA (p = 0.03). The significant association among serum albumin, total cholesterol, NLR and LMR were first observed in SCLC. The results revealed that significant correlations were found between NLR and LMR (r = −0.495, p < 0.001). Our study included patients at LS and ES of SCLC and the results indicated that NPS was identified as an independent prognostic factor for OS (Group 1: HR= 2.704, 95% CI = 1.403-5.210; p = 0.003; Group 2: HR = 5.154, 95% CI = 2.614-10.166; p < 0.001) and PFS (Group 1: HR = 2.018, 95% CI = 1.014-4.014; p = 0.045; Group 2: HR = 3.339, 95% CI = 1.650-6.756; p = 0.001). Early reducing inflammation and improving nutritional status might benefit for cancer patient outcome. Identification of patient inflammation and nutritional status could help us determine prognostic stratification and individualized therapy in the future.
Our study had certain limitations. First, our study is the limited number of sample size from a single-center and retrospective analysis; Second, although we relatively strict eligibility criteria, some potential confounding influence remained. Patients treated with different treat strategies may have different clinical outcomes. This is the potential factor. Third, despite the clinical significance of NPS in SCLC had been shown in our study, the significance in all lung cancer remained unclear. Fourth, other inflammatory, chemokines, or cytokines indicators involved in tumor-specific pathophysiological processes were not explored the correlations with NPS. Finally, the value of NPS was measured at a single time point before treatment. A prospective dynamic analysis on the value of NPS in SCLC was considered to be the future research directions.
Conclusion
In summary, we used a prediction model based on NPS and evaluated the prognostic values in SCLC. We found that NPS model is more reliable and accurate than other independent prediction biomarkers based on clinicopathological characteristics. NPS could be a novel scoring system and was an independent prognostic factor in patients with SCLC. NPS acts as a comprehensive index of nutritional and inflammatory status, we are looking forward to conduct prospective studies to further validate our findings.
Authors contributions
Conception and design of the study: GBL, ZCL, JFL and ZZW; acquisition of clinical data: JFL, ZCL, HLL and SJJ; analysis and interpretation of the data: JFL and SJJ; manuscript drafting and revision: GBL, ZZW, JFL and SJJ. All authors read and approved the final manuscript.
Acknowledgements
Ethics approval and consent to participate: The procedures in this study were approved by the affiliated Suzhou Hospital of Nanjing Medical University Institutional Review Board.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Dingemans AC, Fruh M, Ardizzoni A, et al. Small-cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2021;32(7):1–11. doi: 10.1016/j.annonc.2021.03.207.
- Sen T, Gay CM, Byers LA. Targeting DNA damage repair in small cell lung cancer and the biomarker landscape. Transl Lung Cancer Res. 2018;7(1):50–68. doi: 10.21037/tlcr.2018.02.03.
- Alvarado-Luna G, Morales-Espinosa D. Treatment for small cell lung cancer, where are we now?-a review. Transl Lung Cancer Res. 2016;5(1):26–38.
- Guo D, Jing W, Zhu H, et al. Clinical value of carcinoembryonic antigen for predicting the incidence of brain metastases and survival in small cell lung cancer patients treated with prophylactic cranial irradiation. Cancer Manag Res. 2018;10:3199–3205. doi: 10.2147/CMAR.S175043.
- Stratigos M, Matikas A, Voutsina A, et al. Targeting angiogenesis in small cell lung cancer. Transl Lung Cancer Res. 2016;5(4):389–400. doi: 10.21037/tlcr.2016.08.04.
- Tsoukalas N, Aravantinou-Fatorou E, Baxevanos P, et al. Advanced small cell lung cancer (SCLC): new challenges and new expectations. Ann Transl Med. 2018;6(8):145. doi: 10.21037/atm.2018.03.31.
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013.
- Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205.
- Feng JF, Huang Y, Chen QX. Preoperative platelet lymphocyte ratio (PLR) is superior to neutrophil lymphocyte ratio (NLR) as a predictive factor in patients with esophageal squamous cell carcinoma. World J Surg Oncol. 2014;12:58. doi: 10.1186/1477-7819-12-58.
- Guo D, Jin F, Jing W, et al. Incorporation of the SUVmax measured from FDG PET and neutrophil-to-lymphocyte ratio improves prediction of clinical outcomes in patients with locally advanced non-small-cell lung cancer. Clin Lung Cancer. 2019;20(6):412–419. doi: 10.1016/j.cllc.2019.06.008.
- Guo D, Zhang J, Jing W, et al. Prognostic value of systemic immune-inflammation index in patients with advanced non-small-cell lung cancer. Future Oncol. 2018;14(25):2643–2650. doi: 10.2217/fon-2018-0285.
- Li KJ, Xia XF, Su M, et al. Predictive value of lymphocyte-to-monocyte ratio (LMR) and neutrophil-to-lymphocyte ratio (NLR) in patients with oesophageal cancer undergoing concurrent chemoradiotherapy. BMC Cancer. 2019;19(1):1004. doi: 10.1186/s12885-019-6157-4.
- Liu Z, Shi H, Chen L. Prognostic role of pre-treatment C-reactive protein/albumin ratio in esophageal cancer: a meta-analysis. BMC Cancer. 2019;19(1):1161. doi: 10.1186/s12885-019-6373-y.
- Harimoto N, Yoshizumi T, Inokuchi S, et al. Prognostic significance of preoperative controlling nutritional status (CONUT) score in patients undergoing hepatic resection for hepatocellular carcinoma: a multi-institutional study. Ann Surg Oncol. 2018;25(11):3316–3323. doi: 10.1245/s10434-018-6672-6.
- Sonehara K, Tateishi K, Komatsu M, et al. Modified glasgow prognostic score as a prognostic factor in patients with extensive disease-small-cell lung cancer: a retrospective study in a single institute. Chemotherapy. 2019;64(3):129–137. doi: 10.1159/000502681.
- Yang Y, Gao P, Song Y, et al. The prognostic nutritional index is a predictive indicator of prognosis and postoperative complications in gastric cancer: a meta-analysis. Eur J Surg Oncol. 2016;42(8):1176–1182. doi: 10.1016/j.ejso.2016.05.029.
- Galizia G, Auricchio A, de Vita F, et al. Inflammatory and nutritional status is a predictor of long-term outcome in patients undergoing surgery for gastric cancer. Validation of the Naples prognostic score. Ann Ital Chir. 2019;90:404–416.
- Kano K, Yamada T, Yamamoto K, et al. The impact of pretherapeutic Naples prognostic score on survival in patients with locally advanced esophageal cancer. Ann Surg Oncol. 2021;28(8):4530–4539. doi: 10.1245/s10434-020-09549-5.
- Nakagawa N, Yamada S, Sonohara F, et al. Clinical implications of Naples prognostic score in patients with resected pancreatic cancer. Ann Surg Oncol. 2020;27(3):887–895. doi: 10.1245/s10434-019-08047-7.
- Miyamoto Y, Hiyoshi Y, Daitoku N, et al. Naples prognostic score is a useful prognostic marker in patients with metastatic colorectal cancer. Dis Colon Rectum. 2019;62(12):1485–1493. doi: 10.1097/DCR.0000000000001484.
- Galizia G, Lieto E, Auricchio A, et al. Naples prognostic score, based on nutritional and inflammatory status, is an independent predictor of long-term outcome in patients undergoing surgery for colorectal cancer. Dis Colon Rectum. 2017;60(12):1273–1284. doi: 10.1097/DCR.0000000000000961.
- Li A, He K, Guo D, et al. Pretreatment blood biomarkers predict pathologic responses to neo-CRT in patients with locally advanced rectal cancer. Future Oncol. 2019;15(28):3233–3242. doi: 10.2217/fon-2019-0389.
- Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–545. doi: 10.1016/S0140-6736(00)04046-0.
- Apte RS, Chen DS, Ferrara N. VEGF in signaling and disease: beyond discovery and development. Cell. 2019;176(6):1248–1264. doi: 10.1016/j.cell.2019.01.021.
- Centurione L, Aiello FB. DNA repair and cytokines: TGF-beta, IL-6, and thrombopoietin as different biomarkers of radioresistance. Front Oncol. 2016;6:175. doi: 10.3389/fonc.2016.00175.
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi: 10.1016/j.cell.2010.01.025.
- Shaverdian N, Wang J, Levin-Epstein R, et al. Pro-inflammatory state portends poor outcomes with stereotactic radiosurgery for brain metastases. Anticancer Res. 2016;36(10):5333–5337. doi: 10.21873/anticanres.11106.
- Wu L, Saxena S, Awaji M, et al. Tumor-Associated neutrophils in cancer: going pro. Cancers (Basel). 2019;11(4):564. doi: 10.3390/cancers11040564.
- Houghton AM, Rzymkiewicz DM, Ji H, et al. Neutrophil elastase-mediated degradation of IRS-1 accelerates lung tumor growth. Nat Med. 2010;16(2):219–223. doi: 10.1038/nm.2084.
- Lerman I, Hammes SR. Neutrophil elastase in the tumor microenvironment. Steroids. 2018;133:96–101. doi: 10.1016/j.steroids.2017.11.006.
- Laviron M, Combadiere C, Boissonnas A. Tracking monocytes and macrophages in tumors with live imaging. Front Immunol. 2019;10:1201. doi: 10.3389/fimmu.2019.01201.
- Ronca R, Benkheil M, Mitola S, et al. Tumor angiogenesis revisited: regulators and clinical implications. Med Res Rev. 2017;37(6):1231–1274. doi: 10.1002/med.21452.
- Droin N, Hendra JB, Ducoroy P, et al. Human defensins as cancer biomarkers and antitumour molecules. J Proteomics. 2009;72(6):918–927. doi: 10.1016/j.jprot.2009.01.002.
- Goedegebuure RSA, Harrasser M, de Klerk LK, et al. Pre-treatment tumor-infiltrating T cells influence response to neoadjuvant chemoradiotherapy in esophageal adenocarcinoma. Oncoimmunology. 2021;10(1):1954807. doi: 10.1080/2162402X.2021.1954807.
- Mohammed ZM, Going JJ, Edwards J, et al. The role of the tumour inflammatory cell infiltrate in predicting recurrence and survival in patients with primary operable breast cancer. Cancer Treat Rev. 2012;38(8):943–955. doi: 10.1016/j.ctrv.2012.04.011.
- Guo W, Lu X, Liu Q, et al. Prognostic value of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio for breast cancer patients: an updated meta-analysis of 17079 individuals. Cancer Med. 2019;8(9):4135–4148. doi: 10.1002/cam4.2281.
- Zhang Y, Feng YC, Zhu HG, et al. The peripheral blood neutrophil-to-lymphocyte ratio is a prognostic predictor for survival of EGFR-mutant nonsmall cell lung cancer patients treated with EGFR-TKIs. Medicine (Baltimore). 2018;97(30):e11648. doi: 10.1097/MD.0000000000011648.
- Go SI, Kim RB, Song HN, et al. Prognostic significance of the lymphocyte-to-monocyte ratio in patients with small cell lung cancer. Med Oncol. 2014;31(12):323. doi: 10.1007/s12032-014-0323-y.
- Ramos R, Macia I, Navarro-Martin A, et al. Prognostic value of the preoperative lymphocyte-to-monocyte ratio for survival after lung cancer surgery. BMC Pulm Med. 2021;21(1):75. doi: 10.1186/s12890-021-01446-1.
- Huang X, Hu H, Zhang W, et al. Prognostic value of prognostic nutritional index and systemic immune-inflammation index in patients with osteosarcoma. J Cell Physiol. 2019;234(10):18408–18414. doi: 10.1002/jcp.28476.
- Tokunaga R, Sakamoto Y, Nakagawa S, et al. CONUT: a novel independent predictive score for colorectal cancer patients undergoing potentially curative resection. Int J Colorectal Dis. 2017;32(1):99–106. doi: 10.1007/s00384-016-2668-5.
- Oliver MF. Serum cholesterol–the knave of hearts and the joker. Lancet. 1981;2(8255):1090–1095. doi: 10.1016/s0140-6736(81)91286-1.
- Jin Y, Zhao L, Peng F. Prognostic impact of serum albumin levels on the recurrence of stage I non-small cell lung cancer. Clinics (Sao Paulo). 2013;68(5):686–693. doi: 10.6061/clinics/2013(05)17.
- Lei J, Wang Y, Guo X, et al. Low preoperative serum ALB level is independently associated with poor overall survival in endometrial cancer patients. Future Oncol. 2020;16(8):307–316. doi: 10.2217/fon-2019-0732.
- Mandal CC, Rahman MM. Targeting intracellular cholesterol is a novel therapeutic strategy for cancer treatment. J Cancer Sci Ther. 2014;6(12):510–513. doi: 10.4172/1948-5956.1000316.
- Kang R, Li P, Wang T, et al. Apolipoprotein E epsilon 2 allele and low serum cholesterol as risk factors for gastric cancer in a Chinese Han population. Sci Rep. 2016;6:19930. doi: 10.1038/srep19930.
- Li Q, Cong R, Wang Y, et al. Naples prognostic score is an independent prognostic factor in patients with operable endometrial cancer: results from a retrospective cohort study. Gynecol Oncol. 2021;160(1):91–98. doi: 10.1016/j.ygyno.2020.10.013.
- Li S, Wang H, Yang Z, et al. Naples prognostic score as a novel prognostic prediction tool in video-assisted thoracoscopic surgery for early-stage lung cancer: a propensity score matching study. Surg Endosc. 2021;35(7):3679–3697. doi: 10.1007/s00464-020-07851-7.
- Yang Q, Chen T, Yao Z, et al. Prognostic value of pre-treatment Naples prognostic score (NPS) in patients with osteosarcoma. World J Surg Oncol. 2020;18(1):24. doi: 10.1186/s12957-020-1789-z.
- Lieto E, Auricchio A, Tirino G, et al. Naples prognostic score predicts tumor regression grade in resectable gastric cancer treated with preoperative chemotherapy. Cancers (Basel). 2021;13(18):4676. doi: 10.3390/cancers13184676.