Abstract
Background
This study aimed to compare the efficacy and safety of different treatment modalities for previously untreated advanced EGFR-mutated non-squamous non-small-cell lung cancer (NSCLC).
Methods
This retrospective study included 196 advanced EGFR-mutated non-squamous NSCLC. 107 received EGFR-tyrosine kinase inhibitor (EGFR-TKI) monotherapy (T), 53 received EGFR-TKI + bevacizumab (T + A), and 36 received EGFR-TKI + bevacizumab + chemotherapy (T + A + C). The endpoints included progression-free survival (PFS), overall survival (OS), objective response rate (ORR) and adverse events (AEs).
Results
The median PFS was 27 months in the T + A + C group, 17 months in the T + A group, and 10 months in the T group. The multivariate analysis showed lower disease progression in the T + A + C group (HR, 0.377; 95% CI, 0.224–0.634; p < .001). Subgroup analysis showed that the T + A + C group did significantly improve PFS in patients with metastatic organs ≥2, brain metastases, liver metastases, and EGFR 19del compared to T + A group. No significant improvement in OS in the T + A + C group versus the T + A group, but a significant benefit in the subgroup of patients with metastatic organs ≥2. We also performed a subgroup analysis of the T + A + C group versus the T group, which similarly showed that the T + A + C group had better PFS than the T group in most subgroups, and the T + A + C group significantly improved OS in patients with metastatic organ ≥2 and liver metastases compared with the T group. The ORR was significantly higher in the T + A + C group than A + T and T groups (83.3% vs 71.7% vs 60.7%, p = .033). In safety, the T + A + C group had a higher incidence of AEs, but the majority was grade 1–2. The most frequent AEs of grade ≥ 3 were leukopenia (8.3%) and increased aminotransferase (8.3%) in the T + A + C group.
Conclusions
First-generation EGFR-TKI plus bevacizumab plus chemotherapy was a promising strategy for advanced EGFR-mutated non-squamous NSCLC.
1. Background
Lung cancer remains one of the most common malignancies and is the leading cause of cancer-related deaths [Citation1]. Non-small cell lung cancer (NSCLC), the most common histological subtype, accounts for approximately 80–85% of all lung cancer cases [Citation2]. Despite remarkable progress in the diagnosis and management of NSCLC, a considerable number of patients were diagnosed at an advanced stage, with a 5-year survival rate of merely about 16% [Citation3]. The epidermal growth factor receptor (EGFR) gene is the most common driver oncogene in NSCLC, with up to 50% of Asian lung adenocarcinoma patients [Citation4]. Subsequently, targeted therapies such as EGFR-tyrosine kinase inhibitor (EGFR-TKI) have dramatically altered the treatment landscape for patients with advanced EGFR mutation NSCLC [Citation5]. Several studies demonstrated that EGFR-TKI had a significant effect on improving the survival of advanced NSCLC patients with EGFR-positive, compared to conventional chemotherapy [Citation6,Citation7]. EGFR-TKI therapy was recommended as the first-line standard of care for advanced NSCLC with EGFR mutations [Citation8].
Although EGFR-TKI have shown a great clinical benefit, similar to chemotherapeutic agents, drug-resistance is still inevitable [Citation9,Citation10]. Hence, an increasing number of studies have been striving to use combination therapy, in order to improve their efficacy and impede the onset of resistance. The JO25567 trial indicated that erlotinib plus bevacizumab had a longer median progresssion-free survival (PFS) than erlotinib monotherapy (16.0 months vs. 9.7 months, p = .0015), but no difference in median overall survival (OS) [Citation11]. The Phase III clinical trial (NEJ026) also demonstrated a higher PFS in the combination group compared to the single-agent group (16.9 months vs. 13.3 months, p = .016) [Citation12]. The NEJ009 phase III clinical trial, which assessed the efficacy of gefitinib with or without chemotherapy as the first-line treatment for EGFR-mutated metastatic NSCLC, demonstrated that the group receiving combination chemotherapy exhibited a greater overall response rate (84% vs. 67%, p < .001), PFS (20.9 months vs. 11.9 months, p < .001), and OS (50.9 vs. 38.8 months, p < .021) [Citation13]. EGFR-TKI in combination with bevacizumab or chemotherapy has been shown to have a PFS benefit, but no significant improvement in OS was reported in most trials.
So far, there have been few reports on the combination of EGFR-TKI plus bevacizumab and chemotherapy. This study exploratively analyzed the efficacy and safety of first-generation EGFR-TKI plus bevacizumab plus chemotherapy versus EGFR-TKI alone or with bevacizumab for previously untreated patients with advanced EGFR-mutated non-squamous NSCLC.
2. Methods
2.1. Patients
This retrospective study included patients diagnosed with advanced EGFR-mutated non-squamous NSCLC, who received either first-generation EGFR-TKI monotherapy, or first-generation EGFR-TKI plus bevacizumab, or first-generation EGFR-TKI plus bevacizumab plus chemotherapy at our institution from January 2017 to November 2022. The inclusion criteria were: (1) pathologically confirmed as non-squamous NSCLC. (2) diagnosed with stage IIIB–IV; (3) Eastern Cooperative Oncology Group performance status (ECOG PS) 0–2. The exclusion criteria were: (1) less than 18 years or older than 80 years; (2) had a second primary malignancy; (3) previously received other treatments (surgery, chemotherapy, radiotherapy and immunotherapy); (4) had incomplete clinical data. All patients in the study were staged clinically, using the American Joint Committee on Cancer (AJCC) 8th edition TNM staging system [Citation14]. This study was conducted under the Declaration of Helsinki and approved by the ethics committee of Fujian Medical University Union Hospital. The requirement for informed consent of the patients was waived due to the retrospective nature of this study.
2.2. Data collections
The clinical information included age, gender, smoking history, ECOG PS, histology, clinical stage, number of metastatic organs, sites of metastases (brain, bone, liver), EGFR mutation, survival time, treatment response, acquired T790M mutation, and any adverse events (AEs) experienced during treatment. The last follow-up was conducted in May 2023.
2.3. Treatment regimen
Patients in the T group received gefitinib (250 mg once daily) or icotinib (125 mg 3 times a day) alone. Patients in the T + A group were given gefitinib (250 mg once daily) or icotinib (125 mg 3 times a day) plus bevacizumab (7.5 mg/kg d1) 21 d for one cycle. Patients in the T + A + C group were given gefitinib (250 mg once daily) or icotinib (125 mg 3 times a day), bevacizumab (7.5 mg/kg d1), pemetrexed (500 mg/m2 d1), and carboplatin (AUC 5 d1) for one cycle of 21 d, followed by pemetrexed plus bevacizumab maintenance after four cycles
2.4. Outcomes
The primary endpoint included progression-free survival (PFS). The PFS was measured as the duration between the initiation of EGFR-TKI therapy to the time of disease progression, death due to any cause, or the last follow-up. Meanwhile, the secondary endpoints included overall survival (OS), objective response rate (ORR) and disease control rate (DCR). The OS was defined as the duration between EGFR-TKI therapy to death for any reason or last follow-up. ORR was defined as the percent of patients who achieved a complete or partial response. DCR was the sum of the percent of patients achieving complete or partial response, or stable disease. Response assessment was conducted using Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 [Citation15].
2.5. Adverse events
We collected adverse events (AEs) that occurred during treatment through the record system. The AEs included dermatological, hematological, hepatic, gastrointestinal, renal and cardiovascular toxicities. The severity of AEs was assessed based on the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. To enable accurate capture of adverse events, the occurrence of adverse events and their grading were independently verified by two experienced clinicians, and another experienced clinician was invited to adjudicate on the presence of controversial findings.
2.6. Statistical analysis
Categorical variables were analyzed using either the chi-square test or Fisher’s exact test. For survival analysis, Kaplan-Meier curves were estimated and compared using the log-rank test. Variables with a p-value <0.1 in the univariate analysis were included in the multivariable analysis to identify independent prognostic factors related to PFS and OS. The logistic regression analysis was employed to identify predictors of ORR. All analyses used SPSS 24.0 (IBM, Armonk, NY, USA) and p-values less than 0.05 were deemed statistically significant.
3. Result
3.1. Patient characteristics
The study enrolled 196 advanced non-squamous NSCLC patients who had EGFR mutation. The clinical characteristics were listed in . The cohort was divided into three groups: 107 patients received first-generation EGFR-TKI monotherapy (T), 53 patients received first-generation EGFR-TKI plus bevacizumab (T + A), and 36 patients received first-generation EGFR-TKI plus bevacizumab plus chemotherapy (T + A + C). In total, 77 (39.3%) were ≥65 years, 93 (47.4%) were male, and 84 (42.9%) had a smoking history. The majority of the patients (88.3%) had an ECOG PS of 0–1. According to the genetic testing results, 91 cases (46.4%) exhibited EGFR 19Del and 81 cases (41.3%) exhibited EGFR 21L858R.
Table 1. The baseline characteristics of patients.
3.2. Clinical outcome and efficacy analysis
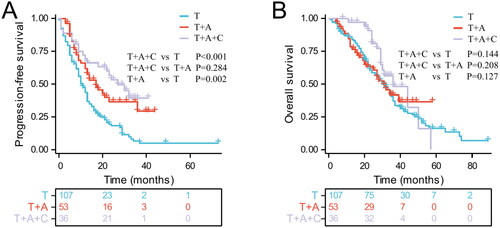
Up to May 2023, 143 of 196 patients had progression-free survival events (94 [87.9%] in the T group, 30 [56.6%] in the T + A group, and 19 [52.8%] in the T + A + C group). The median PFS was 27, 17, and 11 months for the T + A + C, T + A, and T groups, respectively (T + A + C vs. T: p < .001; T + A + C vs. T + A: p = 0.284; T + A vs. T: p = .002, ). A total of 123 patients experienced overall survival events (81 [75.7%] in the T group, 26 [49.1%] in the T + A group, and 16 [44.4%] in the T + A + C group). The T+ A + C group had a median OS of 36 months, while the T + A group and T group had a median OS of 31 months each (p > .05, ). The T + A + C group appeared to have a higher OS than T + A and T groups, but there was no significant difference.
Figure 1. Kaplan-Meier curves for progression-free survival (A) and overall survival (B) in patients with advanced EGFR-mutated non-small cell lung cancer in different treatment groups.
As shown in , 133 (67.9%) experienced PR, 48 (24.5%) had SD, and 15 (7.7%) experienced PD. No patients achieved CR. The ORR and DCR were 67.9% and 90.8%, respectively in the whole cohort. The ORR was significantly higher in the T + A + C group than in A + T and T groups (83.3% vs 71.7% vs 60.7%, p = .033). The DCR was 100%, 100%, 86% in T + A + C, T + A, T groups (p = .015). Of the 132 patients who underwent genetic testing after first-line treatment failure, 64 (48.5%) were positive for T790M mutations, and the T + A + C group showed a lower rate of acquired T790M mutation than the T + A and T groups. (36.8% vs 46.7% vs 50.0%, p = .574).
Table 2. Treatment response and acquired T790M mutation.
3.3. Subgroup analysis
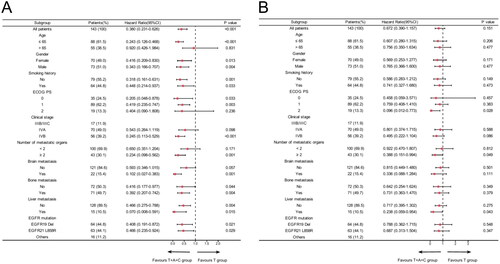
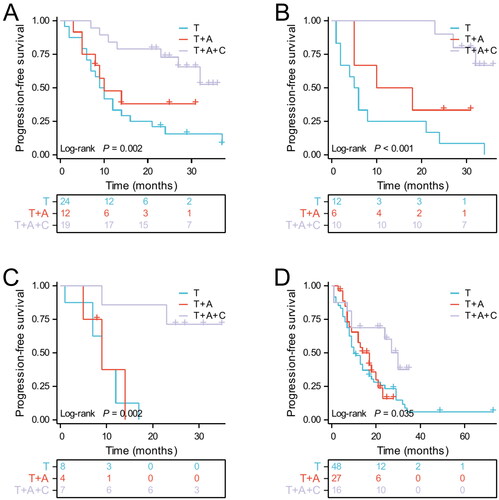
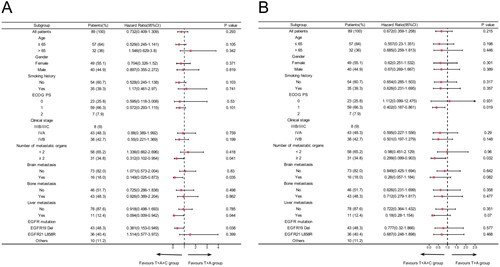
We further performed subgroup analysis based on clinical characteristics. The results suggested that PFS was superior in the T + A group than in the T group in most subgroups, including age ≤65, men, with or without smoking history, ECOG PS 0, stage IVB, the number of metastatic organs <2, without brain metastases and liver metastases, with or without bone metastases, EGFR L858R and other mutations. However, the T + A group did not significantly prolong PFS in patients with metastatic organs ≥2, brain metastases, liver metastases, and EGFR 19del compared to the T group (). Secondly, overall survival HRs favoured the T + A group over the T group in most subgroups, although these differences were not statistically significant (). Next, a subgroup analysis of the T + A + C group versus the T + A group was performed, and the results suggested that T + A + C group did significantly improve PFS in patients with metastatic organs ≥2, brain metastases, liver metastases, and EGFR 19del compared to T + A group (). Furthermore, no significant improvement in OS in the T + A + C group versus the T + A group, but a significant benefit in the subgroup of patients with metastatic organs ≥2 (). We also performed a subgroup analysis of the T + A + C group versus the T group, which similarly showed that the T + A + C group had better PFS than the T group in most subgroups (), and the T + A + C group significantly improved OS in patients with metastatic organ ≥2 and liver metastases compared with the T group (). Finally, Kaplan-Meier curves showed that the T + A + C group could significantly prolong PFS for patients with metastatic organs ≥2, brain metastases, liver metastases, and EGFR 19del than the T + A and T groups ().
Figure 2. Subgroup analysis of the T + A group versus the T group for progression-free survival (A) and overall survival (B) based on baseline characteristics.
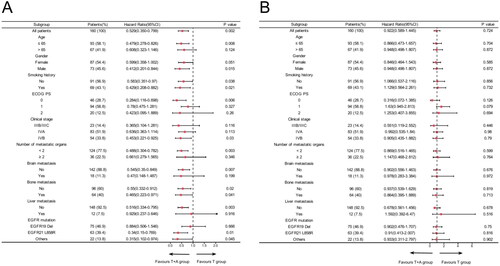
Figure 3. Subgroup analysis of the T + A + C group versus the T + A group for progression-free survival (A) and overall survival (B) based on baseline characteristics.
3.4. Safety and adverse events
and provided a summary of all adverse events that occurred in different treatment groups. The incidence of any grade adverse events varied significantly across three groups, with 69.4% in the T + A + C group, 50.9% in the T + A group, and 39.3% in the T group (p = .006). Most of the AEs were grade 1–2, with an incidence of 47.2% in the T + A + C group, 43.4% in the T + A group, and 33.6% in the T group (p = .253). Grade 3 or higher adverse events occurred in 8 of 36 patients (22.2%) in the T + A + C group, 4 of 53 patients (7.5%) in the T + A group, and 6 of 107 patients (5.6%) in the T group (p = .010). The most frequent adverse events of grade 3 or higher were leukopenia (8.3%) and increased aminotransferase (8.3%) in the T + A + C group, increased aminotransferase (3.8%) in the T + A group, and rash (2.8%) and diarrhea (2.8%) in the T group. No death-related adverse events were observed in any groups.
Table 3. Comparison of adverse events in different treatment groups.
Table 4. Common adverse events in different treatment groups.
3.5. Risk factors affecting PFS
Univariate and multivariate cox regression analysis used to identify prognostic factors that affect PFS (). The univariate Cox regression analysis revealed a significant correlation between PFS with ECOG PS, and treatment pattern. Variables with a p-value <.1 in the univariate analysis were selected for multivariate analysis, which identified ECOG PS (p < .05) and treatment pattern (p < 0.05) as independent prognostic factors influencing PFS.
Table 5. Univariate and multivariate cox analysis of PFS.
3.6. Risk factors affecting OS
We conducted univariate and multivariate Cox regression analyses to identify prognostic factors that affect OS (Supplementary Table S1). The univariate Cox analysis indicated that age, ECOG PS, and liver metastasis were significant prognostic factors affecting OS (all p < .05). In multivariate analysis, ECOG PS was identified as independent prognostic factors significantly influencing OS (p < .05).
3.7. Risk factors affecting ORR
Clinical factors were analyzed through the logistic regression analysis to identify the predictors of ORR (Supplementary Table S2). Univariate logistic regression analysis revealed that ECOG PS and treatment pattern impacted the ORR at a significance level of p < .05. The multivariate logistic regression analysis further showed that patients with a high ECOG PS (ECOG 1: OR: 0.147, 95% CI: 0.048 − 0.448, p = .001; ECOG 2: OR: 0.016, 95%CI: 0.003 − 0.075, p < 0.001) had a low ORR. Conversely, patients in the T + A + C group (OR: 4.047, 95% CI 1.373 − 11.939, p = .011) demonstrated high ORR.
4. Discussion
The emergence of targeted therapy significantly prolonged survival for advanced EGFR-mutated NSCLC. It was reported that first-generation EGFR-TKI increased the median PFS to 9.2–13.1 months compared to traditional chemotherapy [Citation7,Citation16–18]. However, the benefit of EGFR-TKI alone was limited. Combining EGFR-TKI with anti-vascular therapy or chemotherapy had demonstrated beneficial outcomes. Nonetheless, little was known about EGFR-TKI plus anti-vascular therapy and chemotherapy. To our knowledge, our study was the first to retrospectively analyze the effectiveness and safety of first-generation EGFR-TKI plus bevacizumab plus chemotherapy compared to first-generation EGFR-TKI alone or with bevacizumab in patients with advanced EGFR-mutated non-squamous NSCLC. The results showed that the multi-combination therapy significantly improved PFS (median PFS up to 27 months) especially for patients with metastatic organs ≥ 2, brain metastases, liver metastases, and EGFR 19del. Although no significant improvement in OS was observed, but a significant benefit in the subgroup of patients with metastatic organs ≥ 2. In safety, the multi-combination therapy had a higher incidence of AEs, but the majority was grade 1–2. These results indicated that the combination of first-generation EGFR-TKI, bevacizumab and chemotherapy was a promising strategy for advanced EGFR-mutated non-squamous NSCLC.
EGFR-TKI combined with anti-vascular therapy (‘A + T’), which has proven its efficacy in several clinical studies. In a phase II study (JO25567), the combination of erlotinib and bevacizumab as first-line treatment for patients with EGFR-mutated NSCLC had better PFS than erlotinib alone (median PFS 16.0 months vs. 9.7 months, p = .0015) [Citation11]. The clinical benefit of the ‘A + T’ treatment modality was confirmed in a phase III study (NEJ026), which showed an improvement in PFS in the A + T group compared to the T group (16.9 months vs. 13.3 months, p = .016) [Citation19]. The median PFS observed in our study was 17.0 months in the first-generation EGFR-TKI plus bevacizumab and 11 months in the first-generation EGFR-TKI alone, which was similar to the JO25567 and NEJ026 study. Subgroup analysis suggested that PFS was superior in the T + A group than in the T group in most subgroups. Subgroup analysis of the NEJ026 study similarly showed no progression-free survival benefit to the addition of bevacizumab for patients with asymptomatic central nervous system metastasis, but a benefit for patients with Leu858Arg mutation [Citation19]. But there was another study (ARTEMIS-CTONG1509) discovered that first-line erlotinib in combination with bevacizumab significantly improved PFS in patients with metastatic EGFR-mutated NSCLC, including those with brain metastases [Citation20]. The mechanism by which the combination of EGFR-TKI and bevacizumab in improving efficacy is unclear. It is possible that the effect of bevacizumab-mediated VEGF blockade on physiological changes in tumor vasculature leads to increased intra-tumor EGFR-TKI drug concentrations and thus increased antitumor activity [Citation21].
In general, EGFR-TKI combined with anti-vascular therapy resulted in progression-free survival benefit, but not in all subgroups of patients. Our research indicated that first-generation EGFR-TKI plus bevacizumab did not significantly prolong PFS in patients with metastatic organs ≥ 2, brain metastases, liver metastases, and EGFR 19del compared to EGFR-TKI alone. Therefore, we hypothesize that the addition of chemotherapy may improve the prognosis of this subgroup of patients. EGFR-TKI plus chemotherapy has been validated in multiple clinical studies. Preclinical studies suggested a synergistic effect when pemetrexed was combined with EGFR-TKI [Citation22]. In a randomized phase II trial exploring the efficacy of gefitinib, with and without pemetrexed for advanced NSCLC, it was found that combination therapy significantly improved PFS [Citation23]. The results of another clinical study (NEJ009) also indicated that the median PFS in the combination chemotherapy group was 20.9 months, which was longer than that in the EGFR-TKI monotherapy group (11.2 months) [Citation13]. At present, there were few reports on the efficacy of the EGFR-TKI plus bevacizumab and chemotherapy. Then, we retrospectively analyzed the efficacy and safety of the first-generation EGFR-TKI plus bevacizumab plus chemotherapy. The results of this study suggested that the first-generation EGFR-TKI plus bevacizumab plus chemotherapy did significantly improve PFS in patients with metastatic organs ≥ 2, brain metastases, liver metastases, and EGFR 19del. This result illustrated that the addition of chemotherapy was beneficial for the control of liver and brain metastases or multiple organ metastases.
Then, we further explored the effect on OS in different treatment modalities. Similar to previous clinical trials, EGFR-TKI plus bevacizumab did not significantly prolong OS compared to EGFR-TKI alone [Citation19]. In our study, the addition of chemotherapy to EGFR-TKI plus bevacizumab appeared to increase OS, but there was no significant difference. This result may be related to insufficient follow-up time, and second-line treatment has a certain degree of impact on OS. In addition, it was interesting to find in subgroup analysis that for patients with metastatic organs ≥ 2, there was a significant benefit in OS for patients receiving first-generation EGFR-TKI plus bevacizumab plus chemotherapy.
Almost all of patients would develop acquired resistance to EGFR-TKI inevitably [Citation7,Citation16,Citation17,Citation24–26]. One of the most prominent mechanisms of resistance was T790M resistance mutations. A real-world study that retrospectively analyzed the frequency of T790M mutations up to 52.8% after EGFR-TKI treatment [Citation27]. In a multinational study (RELAY), the rate of acquired T790M mutations was similar in the ramucirumab plus erlotinib and erlotinib alone groups (43% vs. 47%, p = .849) [Citation28]. In our study, the results were similar to previous research. The first-generation EGFR-TKI with or without bevacizumab had comparable T790M mutation rates, with 50% and 50.6%, respectively. But for the first-generation EGFR-TKI plus bevacizumab and chemotherapy, it had a relatively low rate of T790M mutation, at 36.8%. In terms of safety, the multi-combination therapy had more adverse events compared to the other two groups, but mainly grade 1–2. Although multi-combination therapy was associated with an increased incidence of grade 3 and higher leukopenia and hepatic dysfunction, these adverse events were tolerable and manageable in combination with adjuvant leukoproliferation or hepatoprotective agents during treatment. Furthermore, there were no death-related adverse events. Therefore, first-generation EGFR-TKI plus bevacizumab and chemotherapy was effective and relatively safe and controllable.
Finally, this study had some limitations. Firstly, it was a single-center clinical study with a relatively small sample size and inevitable selective bias, and a large, multicenter prospective study was required to confirm our results. Secondly, more follow-up was necessary to ascertain the survival benefit of the combination therapy, since the available data on overall survival was not adequate. Moreover, our study only included patients treated with first-generation EGFR-TKI, and it had remained unknown whether the treatment modality of third-generation EGFR-TKI in combination with anti-vascular and chemotherapy has a superior survival benefit and deserves to be explored subsequently.
5. Conclusions
First-generation EGFR-TKI plus bevacizumab plus chemotherapy was an effective and tolerable first-line treatment for advanced EGFR-mutant non-squamous NSCLC patients, especially for patients with metastatic organs ≥ 2, brain metastases, liver metastases, and EGFR 19del.
Authors’ contributions
Conceived and designed the analysis: YHW, BD, and JHL.
Collected the data: YHW and CLL.
Contributed data or analysis tools: XHJ, YQL and NY.
Performed the analysis: YHW, BD, NY, and YJZ.
Wrote the paper: YHW and BD.
Ethical approval
This study was conducted under the Declaration of Helsinki and approved by the ethics committee of Fujian Medical University Union Hospital. The requirement for informed consent of the patients was waived due to the retrospective nature of this study.
Supplemental Material
Download MS Word (19.6 KB)Disclosure statement
No potential competing interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):1–11. doi: 10.3322/caac.21660.
- Chen Z, Fillmore CM, Hammerman PS, et al. Non-small-cell lung cancers: a heterogeneous set of diseases. Nat Rev Cancer. 2014;14(8):535–546. doi: 10.1038/nrc3775.
- Huo J, Xu Y, Sheu T, et al. Complication rates and downstream medical costs associated with invasive diagnostic procedures for lung abnormalities in the community setting. JAMA Intern Med. 2019;179(3):324–332. doi: 10.1001/jamainternmed.2018.6277.
- Pennell NA, Neal JW, Chaft JE, et al. SELECT: a phase II trial of adjuvant erlotinib in patients with resected epidermal growth factor receptor-mutant non-small-cell lung cancer. J Clin Oncol. 2019;37(2):97–104. doi: 10.1200/JCO.18.00131.
- Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553(7689):446–454. doi: 10.1038/nature25183.
- Fukuoka M, Wu YL, Thongprasert S, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol. 2011;29(21):2866–2874. doi: 10.1200/JCO.2010.33.4235.
- Shi YK, Wang L, Han BH, et al. First-line icotinib versus cisplatin/pemetrexed plus pemetrexed maintenance therapy for patients with advanced EGFR mutation-positive lung adenocarcinoma (CONVINCE): a phase 3, open-label, randomized study. Ann Oncol. 2017;28(10):2443–2450. doi: 10.1093/annonc/mdx359.
- Gelatti ACZ, Drilon A, Santini FC. Optimizing the sequencing of tyrosine kinase inhibitors (TKIs) in epidermal growth factor receptor (EGFR) mutation-positive non-small cell lung cancer (NSCLC). Lung Cancer. 2019;137:113–122. doi: 10.1016/j.lungcan.2019.09.017.
- Nagano T, Tachihara M, Nishimura Y. Mechanism of resistance to epidermal growth factor Receptor-Tyrosine kinase inhibitors and a potential treatment strategy. Cells. 2018;7(11):212. doi: 10.3390/cells7110212.
- Westover D, Zugazagoitia J, Cho BC, et al. Mechanisms of acquired resistance to first- and second-generation EGFR tyrosine kinase inhibitors. Ann Oncol. 2018;29(Suppl 1):i10–i19. doi: 10.1093/annonc/mdx703.
- Seto T, Kato T, Nishio M, et al. Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): an open-label, randomised, multicentre, phase 2 study. Lancet Oncol. 2014;15(11):1236–1244. doi: 10.1016/S1470-2045(14)70381-X.
- Kawashima Y, Fukuhara T, Saito H, et al. Bevacizumab plus erlotinib versus erlotinib alone in japanese patients with advanced, metastatic, EGFR-mutant non-small-cell lung cancer (NEJ026): overall survival analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Respir Med. 2022;10(1):72–82. doi: 10.1016/S2213-2600(21)00166-1.
- Hosomi Y, Morita S, Sugawara S, et al. Gefitinib alone versus gefitinib plus chemotherapy for Non-Small-Cell lung cancer with mutated epidermal growth factor receptor: NEJ009 study. J Clin Oncol. 2020;38(2):115–123. doi: 10.1200/JCO.19.01488.
- Detterbeck FC, Boffa DJ, Kim AW, et al. The eighth edition lung cancer stage classification. Chest. 2017;151(1):193–203. doi: 10.1016/j.chest.2016.10.010.
- Schwartz LH, Litiere S, de Vries E, et al. RECIST 1.1-Update and clarification: from the RECIST committee. Eur J Cancer. 2016;62:132–137. doi: 10.1016/j.ejca.2016.03.081.
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239–246. doi: 10.1016/S1470-2045(11)70393-X.
- Wu YL, Zhou C, Liam CK, et al. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer: analyses from the phase III, randomized, open-label, ENSURE study. Ann Oncol. 2015;26(9):1883–1889. doi: 10.1093/annonc/mdv270.
- Maemondo M, Inoue A, Kobayashi KS, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380–2388. doi: 10.1056/NEJMoa0909530.
- Saito H, Fukuhara T, Furuya N, et al. Erlotinib plus bevacizumab versus erlotinib alone in patients with EGFR-positive advanced non-squamous non-small-cell lung cancer (NEJ026): interim analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Oncol. 2019;20(5):625–635. doi: 10.1016/S1470-2045(19)30035-X.
- Zhou Q, Xu CR, Cheng Y, et al. Bevacizumab plus erlotinib in Chinese patients with untreated, EGFR-mutated, advanced NSCLC (ARTEMIS-CTONG1509): a multicenter phase 3 study. Cancer Cell. 2021;39(9):1279–1291 e1273. doi: 10.1016/j.ccell.2021.07.005.
- Dickson PV, Hamner JB, Sims TL, et al. Bevacizumab-induced transient remodeling of the vasculature in neuroblastoma xenografts results in improved delivery and efficacy of systemically administered chemotherapy. Clin Cancer Res. 2007;13(13):3942–3950. doi: 10.1158/1078-0432.CCR-07-0278.
- Li T, Ling YH, Goldman ID, et al. Schedule-dependent cytotoxic synergism of pemetrexed and erlotinib in human non-small cell lung cancer cells. Clin Cancer Res. 2007;13(11):3413–3422. doi: 10.1158/1078-0432.CCR-06-2923.
- Cheng Y, Murakami H, Yang PC, et al. Randomized phase II trial of gefitinib with and without pemetrexed as first-line therapy in patients with advanced nonsquamous non-small-cell lung cancer with activating epidermal growth factor receptor mutations. J Clin Oncol. 2016;34(27):3258–3266. doi: 10.1200/JCO.2016.66.9218.
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735–742. doi: 10.1016/S1470-2045(11)70184-X.
- Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11(2):121–128. doi: 10.1016/S1470-2045(09)70364-X.
- Inoue A, Kobayashi K, Maemondo M, et al. Updated overall survival results from a randomized phase III trial comparing gefitinib with carboplatin-paclitaxel for chemo-naive non-small cell lung cancer with sensitive EGFR gene mutations (NEJ002). Ann Oncol. 2013;24(1):54–59. doi: 10.1093/annonc/mds214.
- Wu SG, Chiang CL, Liu CY, et al. An observational study of acquired EGFR T790M-Dependent resistance to EGFR-TKI treatment in lung adenocarcinoma patients in Taiwan. Front Oncol. 2020;10:1481. doi: 10.3389/fonc.2020.01481.
- Nakagawa K, Garon EB, Seto T, et al. Ramucirumab plus erlotinib in patients with untreated, EGFR-mutated, advanced non-small-cell lung cancer (RELAY): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(12):1655–1669. doi: 10.1016/S1470-2045(19)30634-5.