Abstract
Background
To establish a risk stratification score to facilitate individualized treatment for patients with advanced epidermal growth factor receptor (EGFR)-mutant non-small cell lung cancer (NSCLC).
Methods
We enrolled 160 advanced EGFR-mutated NSCLC who received first-generation EGFR-tyrosine kinase inhibitor (EGFR-TKI) with or without bevacizumab. Kaplan–Meier curves were used for survival analysis. Univariate and multivariate analyses were used to identify independent prognostic factors associated with progression-free survival (PFS) and overall survival (OS).
Results
There were 107 patients in EGFR-TKI monotherapy (T group) and 53 patients in EGFR-TKI with bevacizumab (A + T group). The median PFS in the A + T group was significantly longer than that in the T group (p = 0.002). No difference in the median OS between the A + T and T groups (p = 0.721). The multivariate analyses showed that Eastern Cooperative Oncology Group performance status (ECOG PS) and the pre-treatment lactate dehydrogenase-albumin ratio (LAR) were independent prognostic factors for PFS and OS. The LAR-ECOG PS (LAPS) score was constructed by combining the pre-treatment LAR and ECOG PS. We defined ECOG PS 2 and high pre-treatment LAR as a score of 1. Then, patients with a total LAPS score of 0 were categorized as low-risk and those with 1–2 scores were classified as high-risk. For patients in low-risk group, there was no significant difference in PFS, OS, objective response rate (ORR), and disease control rate (DCR) among those who received EGFR-TKI with or without bevacizumab. However, patients in high-risk group had a significant benefit in PFS and DCR when treated with EGFR-TKI plus bevacizumab compared to those who received EGFR-TKI alone.
Conclusions
Novel LAPS score may help to facilitate individualized treatment of advanced EGFR-mutated NSCLC receiving EGFR-TKI with or without bevacizumab.
1. Introduction
Lung cancer is a prevalent type of cancer and remains one of the major causes of cancer-associated deaths worldwide [Citation1]. In 2020, more than one-third of lung cancer cases in the world occur in China [Citation1,Citation2]. Non-small cell lung cancer (NSCLC) constitutes 80–85% of all cases[Citation1]. The epidermal growth factor receptor (EGFR) is the most frequently mutated driver gene observed in NSCLC. The incidence of EGFR mutation is approximately 50% in Asian patients[Citation3], and the frequency of EGFR mutation in Chinese population of lung adenocarcinoma is up to 58.7%[Citation4]. The use of EGFR-tyrosine kinase inhibitor (EGFR-TKI) effectively prolongs the survival of patients with EGFR mutation advanced NSCLC, and has become the standard first-line treatment for EGFR-mutated advanced NSCLC [Citation5]. However, not all patients respond well to EGFR-TKI, and most patients develop acquired drug-resistance after undergoing EGFR-TKI treatment [Citation6]. Consequently, an increasing number of scholars are exploring combination therapy to enhance efficacy.
VEGF signaling has an essential role in angiogenesis, and its inhibition exerts a critical effect on the treatment of NSCLC [Citation7,Citation8]. Preclinical studies have shown that simultaneous inhibition of EGFR and VEGF/VEGFR pathways has a synergistic effect in anti-tumor therapy [Citation9]. Bevacizumab is a recombinant humanized monoclonal antibody, which works against angiogenesis by inhibiting VEGF-A to achieve anti-tumor effects. Therefore, it is widely used in the treatment of various malignant tumors in the clinic. Several clinical trials have demonstrated that combination anti-vascular therapy can improve patients’ outcomes compared to EGFR-TKI monotherapy [Citation10–12]. For example, in the NEJ026 study, erlotinib combined with bevacizumab significantly prolonged progression-free survival (PFS) (median PFS: 16.9 months vs. 13.3 months, p = 0.016) but not overall survival (OS) (median OS: 50.7 months vs. 46.2 months, p = 0.97) compared to erlotinib alone in EGFR-mutated advanced NSCLC [Citation10,Citation11]. Nevertheless, it has also been observed that the addition of anti-vascular therapy did not have a significant survival benefit. A meta-analysis by Zhao et al. analyzed ten clinical studies and demonstrated that erlotinib plus bevacizumab failed to significantly prolong OS, ORR, or PFS [Citation13]. Evidently, the addition of bevacizumab did not provide additional benefits to all patients, and on the contrary, it might increase the occurrence of adverse events, as well as the financial burden for patients.
In the era of precision medicine, the importance of personalized treatment is emphasized. Adopting personalized treatment measures for different populations of patients can maximize the benefits. Up to now, there has been no effective risk-scoring system used to guide individualized treatment for patients with advanced EGFR-mutated NSCLC. Hence, this study aimed to construct a simple risk-scoring scheme for advanced EGFR mutation NSCLC, which was of great significance in guiding clinical treatment decisions.
2. Methods
2.1. Patients
This retrospective study enrolled advanced EGFR-mutated NSCLC patients receiving first-generation EGFR-TKI with or without bevacizumab from January 2017 to November 2022. The inclusion criteria were: (1) pathologically confirmed as non-squamous NSCLC; (2) diagnosed with stage IIIB-IV; (3) had EGFR mutation; (4) Eastern Cooperative Oncology Group performance status (ECOG PS) 0–2; (5) received either first-generation EGFR-TKI monotherapy, or combined with bevacizumab. The exclusion criteria were: (1) less than 18 years old or older than 80 years old; (2) had a second primary malignancy; (3) had incomplete clinical data. Finally, a total of 160 patients met the criteria, of whom 107 received EGFR-TKI alone and 53 received EGFR-TKI plus bevacizumab.
2.2. Clinical features
The clinical features included age, gender, smoking history, ECOG PS, clinical stage, number of metastatic organs, metastatic sites (brain, bone, liver metastasis), EGFR mutation subtype (19 deletion, L858R, G719X, L861Q, S768I, T790M), the type of first-generation EGFR-TKI (gefitinib and icotinib) and the pre-treatment lactate dehydrogenase-albumin ratio (LAR). The EGFR mutation was detected by amplification refractory mutation system (ARMS) real-time polymerase chain reaction (PCR) or next-generation sequencing (NGS) from the primary or metastatic tumor. LDH and Alb were collected from peripheral blood within 1 week prior to EGFR-TKI treatment. The pre-treatment LAR was calculated as follows: LAR = LDH/Alb. Advanced EGFR-mutated NSCLC patients who were treated with EGFR-TKI monotherapy were divided into high and low pre-treatment LAR based on the optimal LAR cutoff values (LAR = 5.0) determined by X-tile software[Citation14]. Patients were restaged according to the American Joint Committee on Cancer (AJCC) 8th edition TNM staging system [Citation15].
2.3. Survival outcome
PFS was defined as the period of duration from the first day of treatment with EGFR-TKI to disease progression (defined by Response Evaluation Criteria in Solid Tumors [RECIST], version 1.1), death, or last follow-up. OS was defined as the period of duration from the first day of treatment with EGFR-TKI to death or last follow-up. The last follow-up was in May 2023.
2.4. Evaluation of response
The efficacy response evaluation was based on RECIST version 1.1, including complete responses (CR), partial responses (PR), stable disease (SD), or progressive disease (PD). The overall response rate (ORR) consisted of CR and PR, while the disease control rate (DCR) consisted of CR, PR and SD.
2.5. Statistical analysis
The analysis of categorical variables involved chi-square and Fisher’s exact tests, while that of numerical variables utilized the Wilcoxon test. We estimated survival analyses using Kaplan-Meier curves and compared them with log-rank tests, and incorporated variables with P values < 0.1 in the univariate analysis into the multivariate analysis to identify independent prognostic factors that were associated with PFS and OS. Time-dependent area under the receiver operating characteristic (ROC) curve (AUC) was used to assess the score’s predictive accuracy. All analyses were conducted using SPSS 24.0 (IBM, Armonk, NY, USA), and P values < 0.05 were considered statistically significant.
3. Results
3.1. Patients’ characteristics
A total of 160 patients with advanced EGFR-mutated NSCLC were included in this study, of which 107 patients received EGFR-TKI monotherapy (T group), while 53 received EGFR-TKI plus bevacizumab (A + T group). Baseline characteristics of patients are shown in . Overall, 67 patients (41.9%) were over the age of 65, 87 (54.4%) were female, and 69 (43.1%) had a history of smoking. Most (87.5%) had an ECOG PS of 0–1. Clinical staging for IIIB-IVA and IVB were 106 (66.2%) and 54 (33.8%), respectively. Patients had brain metastasis, bone metastasis, and liver metastasis in 18 (11.2%), 64 (40.0%), and 12 (7.5%) cases, respectively. The majority of patients had EGFR mutations, with 19del (46.9%) and L858R (39.4%).
Table 1. Baseline clinical characteristics.
3.2. Response and survival
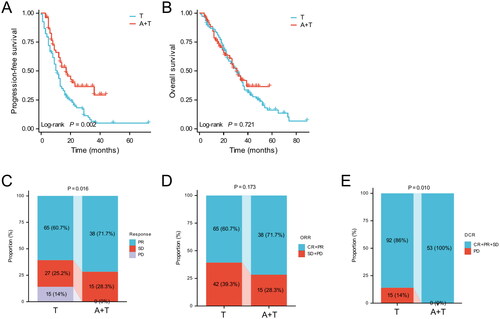
Kaplan-Meier analysis showed that patients in the A + T group had longer median PFS than those in the T group (17 months vs. 11 months; p = 0.002; ). In addition, there was no statistical difference in median OS between the A + T and T groups (31 months vs. 31 months; p = 0.721; ). As shown in patients (60.7%) achieved PR, 27 (25.2%) reached SD, and 15 (14%) were PD in the T group. In contrast, 38 patients (71.7%) achieved PR, 15 (28.3%) reached SD, and none of the patients had PD in the A + T group. There were no patients in either group who achieved CR. The ORR of the A + T group was higher than that of the T group (71.7% vs. 60.7%, p = 0.173, ). The DCR of the A + T group was significantly higher than that of the T group (100% vs. 86%, p = 0.010, ).
3.3. Prognostic factors
Univariate and multivariate Cox regression analysis were used to identify prognostic factors in advanced EGFR-mutated NSCLC treated with EGFR-TKI monotherapy. The univariate analysis indicated that ECOG PS, clinical stage, other EGFR mutations, and pre-treatment LAR were identified as prognostic factors affecting PFS (), while age, ECOG PS, liver metastases, and pre-treatment LAR were identified as prognostic factors affecting OS (all p < 0.05) (). Multivariate analysis revealed that ECOG PS (HR: 3.397, 95%CI: 1.854–6.224, p < 0.001) and pre-treatment LAR (HR: 1.559, 95%CI: 1.007–2.414, p = 0.047) were independent prognostic factors for PFS () and ECOG PS (HR: 20.854, 95%CI: 9.507–45.746, p < 0.001), liver metastasis (HR: 3.317, 95%CI: 1.409-7.806), p = 0.006), and pre-treatment LAR (HR: 2.423, 95%CI: 1.485–3.955, p < 0.001) were independent prognostic factors for OS (), respectively. Kaplan–Meier analysis indicated that among patients who received EGFR-TKIs monotherapy, those with ECOG PS 0-1 and pre-treatment low LAR had significantly better median PFS and OS than those with ECOG PS 2 and pre-treatment high LAR (all p < 0.05, ).
Figure 2. Kaplan–Meier curves of PFS (A) and OS (B) in EGFR-mutated advanced NSCLC receiving EGFR-TKI monotherapy according to ECOG PS. Kaplan–Meier curves of PFS (C) and OS (D) in EGFR-mutated advanced NSCLC receiving EGFR-TKIs monotherapy according to pre-treatment LAR.
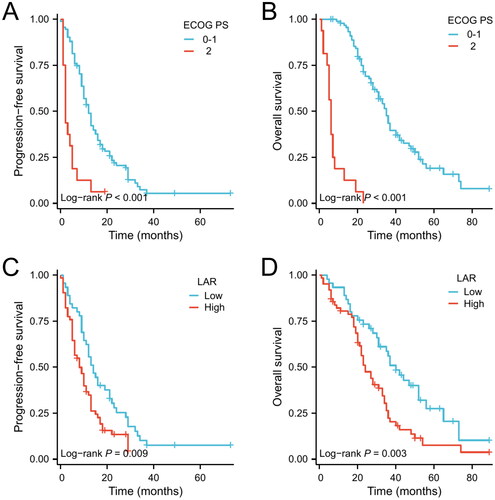
Table 2. Univariate and Multivariate Cox analysis for PFS in advanced EGFR-mutated NSCLC receiving EGFR-TKI monotherapy.
Table 3. Univariate and multivariate analysis for OS in advanced EGFR-mutated NSCLC receiving EGFR-TKI monotherapy.
3.4. LAPS score and risk stratification
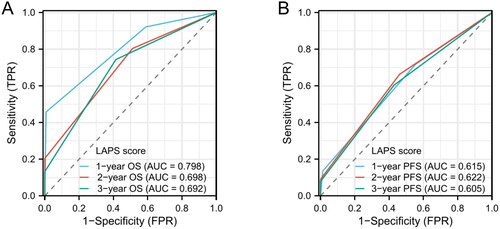
ECOG PS, pre-treatment LAR both were independent prognostic factors for PFS and OS. Then, we defined ECOG PS 2 and high pre-treatment high LAR (LAR > 5) as a score of 1. The LAR-ECOG PS (LAPS) score was constructed by combining the pre-treatment LAR and ECOG PS. Time-dependent ROC curve analysis showed that the AUCs of LAPS score predicting 1-, 2-, and 3-year OS were 0.798, 0.698 and 0.692, while the AUCs of LAPS score predicting 1-, 2-, and 3-year PFS were 0.615, 0.622 and 0.605 ().
Figure 3. Time-dependent ROC curves of LAPS score for predicting 1-, 2-, 3-years OS (A) and P1-, 2-, 3-years PFS (B).
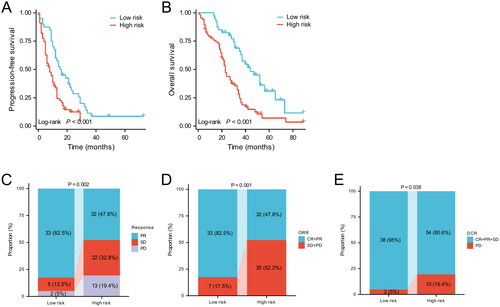
Next, patients with a total LAPS score of 0 were classified as the low-risk group, whereas patients with a total LAPS score of 1–2 were classified as the high-risk group. For patients treated with EGFR-TKI monotherapy, median PFS and OS were significantly better in the low-risk group than in the high-risk group (median PFS: 16.8 months vs. 8 months, p < 0.001; median OS: 44 months vs. 23 months; p < 0.001; ). In addition, there were significant differences in the short-term efficacy between different risk groups. In the low-risk group, 33 (82.5%) patients achieved PR, 5 (12.5%) achieved SD, and 2 (5%) were PD, while in the high-risk group, 32 (47.8%) achieved PR, 22 (32.8%) achieved SD, and 13 (19.4%) were PD (). Both ORR and DCR were significantly higher in the low-risk group than in the high-risk group (ORR: 82.5% vs. 47.8%, p = 0.173; DCR: 95% vs. 80.6%, p = 0.038; ).
3.5. Clinical benefit according to LAPS score
Based on the LAPS score, patients were divided into high and low-risk groups, where in the low-risk group, 40 received EGFR-TKI monotherapy and 16 received EGFR-TKI plus bevacizumab, whereas in the high-risk group, 67 received EGFR-TKI monotherapy and 37 received EGFR-TKI plus bevacizumab (). In the low-risk group (LAPS score 0), there was no significant difference in the short-term efficacy between the T group and T + A groups. The ORR in the T group and A + T groups were 82.5% and 81.2%, respectively (p = 1.000), while the DCR were 95% and 100%, respectively (p = 0.909). In the high-risk group (LAPS score 1-2), the short-term efficacy of the A + T group was better than that of the T group, with the A + T group having better ORR and DCR than the T group (ORR: 67.6% vs. 47.8%, p = 0.052; DCR: 100% vs. 80.6%, p = 0.011).
Table 4. Efficacy response of different treatment modalities according to LAPS score.
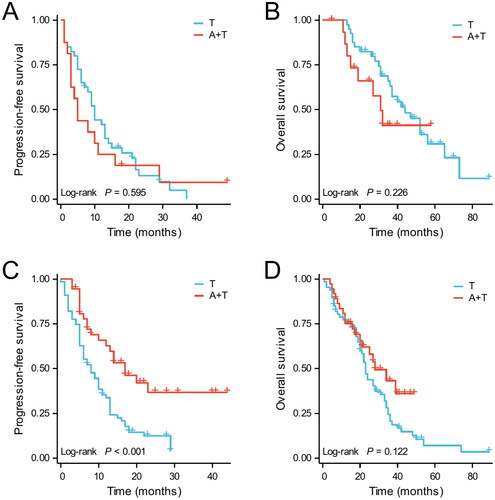
Similarly, the Kaplan–Meier survival analysis was performed, and the results are shown in . In low-risk patients, there was no significant difference in PFS or OS between those who received EGFR-TKI plus bevacizumab and EGFR-TKI monotherapy. In contrast, for high-risk patients, there was a significant PFS benefit in EGFR-TKI combined with bevacizumab compared to EGFR-TKI monotherapy (p < 0.001), but the benefit in OS was not significant (p = 0.122).
4. Discussion
So far, there was no available validated risk score for advanced EGFR mutation NSLCL. Our study was the first to propose the LAPS scoring scheme, which was based on pre-treatment LAR and ECOG PS. The novel risk score was valuable in the clinical treatment decision-making for advanced EGFR-mutated NSCLC.
LAR is a novel biomarker based on the ratio of serum LDH to albumin. Several studies have demonstrated the prognostic value of LAR in various cancers [Citation16–20]. However, no studies have reported the prognostic value of LAR in advanced EGFR-mutated NSCLC. In this study, we were the first to discover that patients with high LAR (LAR > 5) before treatment did not respond well to EGFR-TKIs monotherapy. The LAR was composed of serum LDH and albumin. LDH is a known prognostic factor in patients with a different malignant tumor, and Elevated serum levels of LDH level were associated with a poorer prognosis [Citation21–23]. Results of a retrospective study indicated that changes in serum LDH levels after erlotinib treatment were independently correlated with DCR, PFS and OS [Citation24]. Another study included 199 patients with EGFR mutation-positive advanced NSCLC and likewise confirmed that serum LDH was an indicator of unfavourable clinical outcomes in patients with EGFR mutation-positive advanced NSCLC [Citation25]. Serum albumin level is an important indicator of the patient’s nutritional status. Low serum albumin level usually means poor nutrition in patients. For patients with malignant tumors, albumin is a significant prognostic indicator [Citation26]. Studies have revealed that patients with low Alb levels had a poor prognosis. Conversely, elevated levels of Alb have the potential to extend survival time [Citation27–29]. Then, a novel biomarker, named LAR, is created by combining serum LDH and albumin. Our research findings demonstrated that low LAR prior to treatment was an independent prognostic factor for PFS and OS in advanced EGFR mutation NSCLC. Patients with low LAR prior to treatment had a better prognosis after receiving EGFR-TKI monotherapy. In clinical practice, LAR was a biomarker that was easily measured and was important for clinical treatment decisions in advanced EGFR mutation NSCLC.
Besides, our study also found that ECOG PS was an independent prognostic factor for PFS and OS in advanced EGFR mutation NSCLC. Moreover, an analysis of real-world data from the EGFR-2013-CPHG study demonstrated a significantly increased risk of death in patients with poorer baseline ECOG PS [Citation30]. Another study, retrospectively analyzing 224 patients with EGFR-mutated lung adenocarcinoma treated with EGFR-TKI, similarly showed that ECOG PS ≥2 (OR: 2.189; 95% CI: 1.374–3.437; p < 0.001) had a poor prognosis [Citation31]. Given the above results, we defined ECOG PS 2 and pre-treatment high LAR (LAR > 5) as 1 point, thus constructing a simple LAPS score. Patients with a total score of 0 were categorized as low-risk, while those with a score of 1-2 were grouped as high-risk. Patients who were classified as low-risk, and who received EGFR-TKI monotherapy, showed significantly longer median PFS (16.8 months vs. 8 months, p < 0.001) and OS (44 months vs. 23 months; p < 0.001) than high-risk patients. Moreover, the low-risk group showed significantly higher ORR (82.5% vs. 47.8%; p = 0.173) and DCR (95% vs. 80.6%; p = 0.038) than the high-risk group. Obviously, the high-risk group did not benefit from EGFR-TKI monotherapy, so could combination therapy improve the prognosis of those patients who were in high-risk group?
A growing number of clinical studies have attempted to use combination therapy strategies to improve survival in advanced EGFR-mutated NSCLC. Preclinical studies have demonstrated that EGFR and VEGF share common signaling pathways that synergistically suppress tumors[Citation7]. Subsequently, an open-label, randomized, multicenter phase 3 trial (NEJ026) demonstrated that the median PFS was significantly better in the combination group (erlotinib in combination with bevacizumab) than in the erlotinib monotherapy group (16.9 months vs. 13.3 months, HR = 0.605, p = 0.016) [Citation11]. Our study found similar results to theirs, indicating that the median PFS of the combination bevacizumab group was significantly longer than the EGFR-TKI monotherapy group (median PFS: 17 months vs. 11 months; p = 0.002). Further, we performed a stratified analysis based on the LAPS score and the results revealed that in low-risk (LAPS score 0) patients, the addition of abevacizumab did not bring additional survival benefit, but instead may lead to more adverse events and increased financial burden for patients. In high-risk patients (LAPS score 0–1), combination therapy (EFGR-TKI plus bevacizumab) was associated with better PFS, ORR and DCR compared to EGFR-TKI monotherapy. In this regard, we recommend that patients with pretreatment LAPS score 0 undergo EGFR-TKI monotherapy, while for patients with pretreatment LAPS score 0-1, EGFR-TKI combined with bevacizumab is recommended to improve the prognosis of advanced EGFR-mutated non-small cell lung cancer.
Our study also had several limitations. First, our study was a single-center, retrospective, small-sample study with inevitable selection bias, and future multicenter, prospective clinical studies are needed to validate our findings. Second, our study included patients who received first-generation EGFR-TKI treatment, and further research is needed regarding the benefits of third-generation EGFR-TKI for high-risk populations.
5. Conclusions
A simple LAPS score based on ECOG PS and pre-treatment LAR could facilitate individualized treatment of advanced EGFR-mutated NSCLC. In low-risk patients (LAPS score 0), EGFR-TKI with or without bevacizumab resulted in favorable clinical outcomes. However, in high-risk patients (LAPS score 0-1), EGFR-TKI in combination with bevacizumab has better clinical benefits.
Ethics approval and consent to participate
This study was conducted under the Declaration of Helsinki and approved by the ethics committee of Fujian Medical University Union Hospital. The requirement for informed consent of the patients was waived due to the retrospective nature of this study.
Authors’ contributions
Conceived and designed the analysis: YHW, BD and JHL.
Collected the data: YHW, XHJ and CLL.
Contributed data or analysis tools: XHJ and CLL.
Performed the analysis: YHW and BD.
Wrote the paper: YHW, BD.
Disclosure statement
No potential competing interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):1–10. doi: 10.3322/caac.21660.
- Qiu H, Cao S, Xu R. Cancer incidence, mortality, and burden in China: a time-trend analysis and comparison with the United States and United Kingdom based on the global epidemiological data released in 2020. Cancer Commun. 2021;41(10):1037–1048. doi: 10.1002/cac2.12197.
- Melosky B, Kambartel K, Hantschel M, et al. Worldwide prevalence of epidermal growth factor receptor mutations in non-small cell lung cancer: a meta-analysis. Mol Diagn Ther. 2022;26(1):7–18. doi: 10.1007/s40291-021-00563-1.
- Ma F, Laster K, Dong Z. The comparison of cancer gene mutation frequencies in Chinese and U.S. patient populations. Nat Commun. 2022;13(1):5651. doi: 10.1038/s41467-022-33351-4.
- Gray JE, Okamoto I, Sriuranpong V, et al. Tissue and plasma EGFR mutation analysis in the FLAURA trial: osimertinib versus comparator EGFR tyrosine kinase inhibitor as First-line treatment in patients with EGFR-mutated advanced Non-Small cell lung cancer. Clin Cancer Res. 2019;25(22):6644–6652. doi: 10.1158/1078-0432.CCR-19-1126.
- Westover D, Zugazagoitia J, Cho BC, et al. Mechanisms of acquired resistance to first- and second-generation EGFR tyrosine kinase inhibitors. Ann Oncol. 2018;29(suppl_1):i10–i19. doi: 10.1093/annonc/mdx703.
- Le X, Nilsson M, Goldman J, et al. Dual EGFR-VEGF pathway inhibition: a promising strategy for patients with EGFR-mutant NSCLC. J Thorac Oncol. 2021;16(2):205–215. doi: 10.1016/j.jtho.2020.10.006.
- Frezzetti D, Gallo M, Maiello MR, et al. VEGF as a potential target in lung cancer. Expert Opin Ther Targets. 2017;21(10):959–966. doi: 10.1080/14728222.2017.1371137.
- Schicher N, Paulitschke V, Swoboda A, et al. Erlotinib and bevacizumab have synergistic activity against melanoma. Clin Cancer Res. 2009;15(10):3495–3502. doi: 10.1158/1078-0432.CCR-08-2407.
- Kawashima Y, Fukuhara T, Saito H, et al. Bevacizumab plus erlotinib versus erlotinib alone in japanese patients with advanced, metastatic, EGFR-mutant non-small-cell lung cancer (NEJ026): overall survival analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Respir Med. 2022;10(1):72–82. doi: 10.1016/S2213-2600(21)00166-1.
- Saito H, Fukuhara T, Furuya N, et al. Erlotinib plus bevacizumab versus erlotinib alone in patients with EGFR-positive advanced non-squamous non-small-cell lung cancer (NEJ026): interim analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Oncol. 2019;20(5):625–635. doi: 10.1016/S1470-2045(19)30035-X.
- Yamamoto N, Seto T, Nishio M, et al. Erlotinib plus bevacizumab vs erlotinib monotherapy as first-line treatment for advanced EGFR mutation-positive non-squamous non-small-cell lung cancer: survival follow-up results of the randomized JO25567 study. Lung Cancer. 2021;151:20–24. doi: 10.1016/j.lungcan.2020.11.020.
- Zhao B, Zhang W, Yu D, et al. Erlotinib in combination with bevacizumab has potential benefit in non-small cell lung cancer: a systematic review and meta-analysis of randomized clinical trials. Lung Cancer. 2018;122:10–21. doi: 10.1016/j.lungcan.2018.05.011.
- Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10(21):7252–7259. doi: 10.1158/1078-0432.CCR-04-0713.
- Detterbeck FC, Boffa DJ, Kim AW, et al. The eighth edition lung cancer stage classification. Chest. 2017;151(1):193–203. doi: 10.1016/j.chest.2016.10.010.
- Gao S, Wu M, Chen Y, et al. Actic dehydrogenase to albumin ratio in prediction of unresectable pancreatic cancer with intervention chemotherapy. Future Oncol. 2018;14(14):1377–1386. doi: 10.2217/fon-2017-0556.
- Peng R-R, Liang Z-G, Chen K-H, et al. Nomogram based on lactate dehydrogenase-to-albumin ratio (LAR) and platelet-to-lymphocyte ratio (PLR) for predicting survival in nasopharyngeal carcinoma. J Inflamm Res. 2021;14:4019–4033. volumedoi: 10.2147/JIR.S322475.
- Hu Y, Zhou Y, Cao Y, et al. Nomograms based on lactate dehydrogenase to albumin ratio for predicting survival in colorectal cancer. Int J Med Sci. 2022;19(6):1003–1012. doi: 10.7150/ijms.71971.
- Aday U, Tatli F, Akpulat FV, et al. Prognostic significance of pretreatment serum lactate dehydrogenase-to-albumin ratio in gastric cancer. Contemp Oncol. 2020;24(3):145–149. doi: 10.5114/wo.2020.100219.
- Xie Z, Zhou H, Wang L, et al. The significance of the preoperative lactate dehydrogenase/albumin ratio in the prognosis of colon cancer: a retrospective study. Peer J. 2022;10:e13091. doi: 10.7717/peerj.13091.
- Rotondo FS, Gabril A, Filter M, et al. Lactate dehydrogenase a is a potential prognostic marker in clear cell renal cell carcinoma. Mol Cancer. 2014;13:101. doi: 10.1186/1476-4598-13-101.
- Hermes A, Gatzemeier U, Waschki B, et al. Lactate dehydrogenase as prognostic factor in limited and extensive disease stage small cell lung cancer – a retrospective single institution analysis. Respir Med. 2010;104(12):1937–1942. doi: 10.1016/j.rmed.2010.07.013.
- Danner BC, Didilis VN, Wiemeyer S, et al. Long-term survival is linked to serum LDH and partly to tumour LDH-5 in NSCLC. Anticancer Res. 2010;30(4):1347–1351.
- Fiala O, Pesek M, Finek J, et al. Change in serum lactate dehydrogenase is associated with outcome of patients with advanced-stage NSCLC treated with erlotinib. Anticancer Res. 2016;36(5):2459–2465.
- Ng IK, Kumarakulasinghe NB, Syn NL, et al. Development, internal validation and calibration of a risk score to predict survival in patients with EGFR-mutant non-small cell lung cancer. J Clin Pathol. 2021;74(2):116–122. doi: 10.1136/jclinpath-2020-206754.
- Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J. 2010;9(1):69. doi: 10.1186/1475-2891-9-69.
- Li Q, Kong F, Ma J, et al. Nomograms based on fibrinogen, albumin, neutrophil-Lymphocyte ratio, and carbohydrate antigen 125 for predicting endometrial cancer prognosis. Cancers (Basel). 2022;14(22):5632. doi: 10.3390/cancers14225632.
- Lis CG, Gupta D, Lammersfeld CA, et al. Role of nutritional status in predicting quality of life outcomes in cancer–a systematic review of the epidemiological literature. Nutr J. 2012;11(1):27. 24 doi: 10.1186/1475-2891-11-27.
- Mori S, Usami N, Fukumoto K, et al. The significance of the prognostic nutritional index in patients with completely resected non-small cell lung cancer. PLoS One. 2015;10(9):e0136897. doi: 10.1371/journal.pone.0136897.
- Payen T, Trédaniel J, Moreau L, et al. Real world data of efficacy and safety of erlotinib as first-line TKI treatment in EGFR mutation-positive advanced non-small cell lung cancer: results from the EGFR-2013-CPHG study. Respir Med Res. 2021;80:100795. doi: 10.1016/j.resmer.2020.100795.
- Cha YK, Lee HY, Ahn MJ, et al. Survival outcome assessed according to tumor burden and progression patterns in patients with epidermal growth factor receptor mutant lung adenocarcinoma undergoing epidermal growth factor receptor tyrosine kinase inhibitor therapy. Clin Lung Cancer. 2015;16(3):228–236. doi: 10.1016/j.cllc.2014.11.002.