Abstract
Background
Spinal disorders affect millions of people worldwide, and can cause significant disability and pain. The paraspinal muscles, located on either side of the spinal column, play a crucial role in the movement, support, and stabilization of the spine. Many spinal disorders can affect paraspinal muscles, as evidenced by changes in their morphology, including hypertrophy, atrophy, and degeneration.
Objectives
The objectives of this review were to examine the current literature on the relationship between the paraspinal muscles and spinal disorders, summarize the methods used in previous studies, and identify areas for future research.
Methods
We reviewed studies on the morphological characteristics of the paravertebral muscle and discussed their relationship with spinal disorders, as well as the current limitations and future research directions.
Results
The paraspinal muscles play a critical role in spinal disorders and are important targets for the treatment and prevention of spinal disorders. Clinicians should consider the role of the paraspinal muscles in the development and progression of spinal disorders and incorporate assessments of the paraspinal muscle function in clinical practice.
Conclusion
The findings of this review highlight the need for further research to better understand the relationship between the paraspinal muscles and spinal disorders, and to develop effective interventions to improve spinal health and reduce the burden of spinal disorders.
1. Introduction
With the aging of the global population, clinicians worldwide will be required to manage an increasing number of spinal disorders. The elderly population poses a unique challenge to health care systems and spinal physicians [Citation1]. Population-based studies have demonstrated that around 80 to 90% of individuals exhibit disk degeneration by the age of 50 years [Citation2–5]. In addition, adult spinal deformity is highly prevalent in individuals aged older than 65 years, affecting between 32% and 68% of that population [Citation6–8]. Spinal disorders contribute significantly to the incidence of disability and suffering. Globally, the prevalence and burden of musculoskeletal disorders are remarkably high [Citation9,Citation10]. Since 1990, the prevalence of these disorders has dramatically increased, making them a leading cause of global disability-adjusted life years [Citation11]. Spinal disorders encompass a diverse spectrum of musculoskeletal issues that affect the spinal column and its associated structures. Spinal disorders can lead to disability, reduced mobility, and a decreased ability to perform daily activities, which can have a significant impact on an individual’s quality of life. Furthermore, spinal disorders are associated with a high economic burden because of lost productivity, increased healthcare costs, and a significant financial burden on individuals and society [Citation12–19]. Therefore, it is crucial to develop effective prevention and management strategies to minimize their impact on individuals and society.
Scientific research has revealed that the paraspinal muscles are closely associated with some spinal disorders. The paraspinal muscles, or paravertebral muscles, especially the multifidus (MF), erector spinae (ES), and psoas major (PS), are a group of muscles located on either side of the spine and are important in spinal stability and function [Citation20]. Several methods have been used to evaluate the function and morphology of the paraspinal muscles, such as biopsy, electromyography, and radiography. Biopsy can furnish tissue specimens for analysis, elucidating intricate details of muscle tissue but necessitating surgical intervention that may incite trauma and pain [Citation21,Citation22]. Electromyography allows for the appraisal of the interplay between nerves and muscles, but it is more susceptible to extraneous interference [Citation22,Citation23]. Some imaging techniques such as computed tomography (CT), magnetic resonance imaging (MRI), and ultrasound (US) have allowed for a more detailed assessment of the structure and function of the paraspinal muscles in individuals with spinal disorders, with the added benefits of non-invasiveness and obviating the need for surgical intervention [Citation24–26].
Atrophy and degeneration of the paraspinal muscles can be evaluated using MRI. In early adulthood, the size of the paraspinal muscles is correlated with age and sex [Citation27]. The ES in the old group showed a significant decrease in size and an increase in fat composition [Citation28]. The degree of paraspinal muscle degeneration increases with age and an increase in BMI [Citation29–31]. Additionally, abnormalities in the morphological characteristics of the paraspinal muscles have been reported in various spinal disorders [Citation32–35]. Pathological changes in the paraspinal muscles were observed in patients with degenerative changes in lumbar structure [Citation36]. The function and morphology of paraspinal muscles can not only reflect the functional status of the spine in spinal disorders but also provide a reference for the prognosis of disorders [Citation37–40]. This relationship intimates the paraspinal muscles and could play an important role in the diagnosis and treatment of spinal disorders. Until the last century, few studies have described the role of the paraspinal muscles in spinal disorders. However, an increasing number of studies have been conducted on the relationship between paraspinal muscles and spinal disorders in the past decade. The conclusions of studies regarding the correlation between the paraspinal muscles and spinal disorders need to be summarized. Therefore, we have reviewed the morphological characteristics of paraspinal muscles in research, as well as studies related to the relationship between paraspinal muscles and spinal disorders, in order to make a positive impact in clinical practice.
2. Anatomical characteristics of paraspinal muscles
The main function of the paraspinal muscles is to extend the spine and play a role in spinal stabilization. The anatomy of the paraspinal muscles can be divided into three groups: anterior, lateral, and posterior. The anterior group muscles are located in front of the cervical spine and include the longus colli, longus capitis, rectus capitis anterior, and rectus capitis lateralis. The lateral group muscles include the anterior, middle, and posterior scalene muscles in the neck and the quadratus lumborum, PS, and psoas minor muscles in the abdomen. The posterior group muscles include the superficial trapezius, latissimus dorsi, levator scapulae, rhomboid muscles, the intermediate serratus posterior superior, serratus posterior inferior muscles, the deep ES (which includes the iliocostalis, longissimus, and spinalis), transversospinalis (which includes the MF, rotatores, and semispinalis), interspinales, intertransversarii, and levatores costarum muscles. The cervical spine has a remarkably wide range of motion. The cervical paraspinal muscles assist the neck in performing movements such as flexion, extension, lateral flexion, and rotation, ensuring the neck’s flexibility and the coordination of its motion. Deep extensor muscles have significant roles in preserving cervical sagittal balance [Citation41]. The paraspinal muscles in the thoracic and lumbar spine work together to maintain body balance and stabilize the spine. Heightened muscle activity plays a pivotal role in stabilizing intervertebral motion segments during bending and other spinal movements [Citation42]. They also actively participate in movements such as flexion, extension, lateral flexion, and rotation [Citation43].
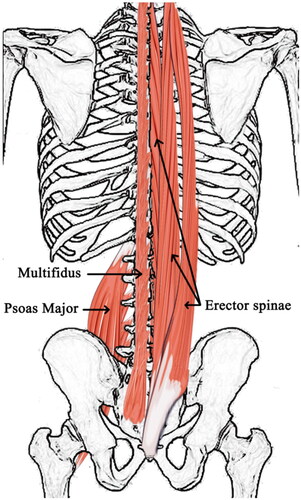
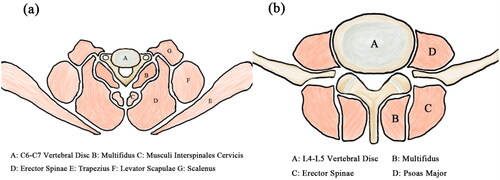
ES, MF, and PS are particularly beneficial for spinal stabilization and many studies have focused on them (). ES originates from the dorsal sacrum, lumbar spinous processes, dorsal crista iliaca, and lumbodorsal fascia. The muscle fascicles are gradually divided into three parallel longitudinal muscle columns from inside to outside. The lateral part is the iliocostalis, middle part is the longissimus, and medial side is the spinalis. They end at the lower edge of the angulus costae, transverse processes of the cervical and thoracic vertebrae, and mastoid processes of the temporal and spinous processes of the cervical and thoracic vertebrae. These were divided into three parts. Iliocostalis includes costocervicalis, iliocostalis thoracis, and iliocostalis lumborum. Longissimus includes longissimus capitis, longissimus cervicis, and longissimus thoracis. Spininalis includes spinalis capitis, spinalis cervicis, and spinalis thoracis. The MF originates from the dorsal sacrum, transverse processes of the lumbar and thoracic vertebrae, and articular process of the fourth to seventh cervical vertebrae. The muscle fascicle crosses two to four vertebrae and ends at the spinous processes of all vertebrae, except the atlas. PS originates from the lateral aspect of the T12 vertebra, the lumbar vertebrae from L1 to L5 and intervertebral discs, and the anterior and inferior margins of all lumbar transverse processes. The muscle fascicles join the iliocostalis muscle inferiorly to form a sinew that passes through the anterior aspect of the iliopectineal eminence and anteromedial aspect of the hip joint capsule and ends at the femoral lesser trochanter [Citation44–49] ().
At the micro level, there are three kinds of pure muscle fibers: type I slow-twitch oxidative, type IIa fast-twitch oxidation/glycolytic, and type IIx fast-twitch glycolytic fibers in paraspinal muscle fibers. Mixed muscle fibers were also discovered in the paraspinal muscles. The metabolic capability and contractile features of muscles are determined by the muscle fiber types [Citation50]. A more fast-twitch glycolytic profile was found in the lumbar spinal musculature of patients with low back pain(LBP) than that of normal individuals [Citation21]. However, a study demonstrated that in individuals with degenerative changes in lumbar structure, macroscopic and microscopic examinations of the muscle in the superficial and deep areas of the MF were the same [Citation51]. Another study found that the ES of patients suffering from LBP contains prominent fewer glycolytic muscle fibers and more oxidative muscle fibers, while in the biopsy samples of the MF, no distinct change in muscle fiber type was found [Citation50]. A study found no emphatic difference between individuals with or without chronic LBP (CLBP) in fiber type composition, not only by numbers but also by the proportional area occupied by fibers [Citation52].
3. Morphological characteristics of the paraspinal muscles
A decrease in muscle mass and an increase in fat infiltration (FI) are common features of muscle degeneration. Both amyotrophy, which is always accompanied by a decreasing cross-sectional surface area (CSA) in the paraspinal muscles, and FI are considered to be important characteristics of the functional decline of the paraspinal muscles. More fat means lower quality in the paraspinal muscles, which could lead to a reduction in spinal function. When it comes to evaluating the morphology of the paraspinal muscles in clinical research, MRI is a common and non-invasive method that can provide valuable information. Some studies that compared MRI and histology have shown a close correlation and good agreement between imaging and histological analysis [Citation53,Citation54]. The two radiographic parameters that are often used are the CSA and FI. CSA reflects the size or mass of the muscle, whereas FI provides information about the fat content within the muscle tissue. Together, these parameters can help identify muscle atrophy, hypertrophy, and degeneration, which can be useful in diagnosing and managing spinal disorders.
3.1. CSA
CSA is a commonly used clinical indicator for evaluating paraspinal muscle degeneration, with a decrease in CSA indicating atrophy of paraspinal muscles. The region of interest (ROI) was determined along the muscle edge when measuring CSA. CSA can be measured using CT, MRI, and US, which can be measured as total CSA or gross CSA and functional CSA (FCSA). The ratio of FCSA to total CSA (FCSA/CSA) was calculated using these parameters. CSA asymmetry, the CSA of the paraspinal muscles relative to the CSA of the vertebral body (rCSA), can also be measured additionally [Citation55–57] ().
Figure 3. The example for measurement of CSA of multifidus (MF), psoas major (PS) and erector spinae(ES)on axial T2 weighted images.
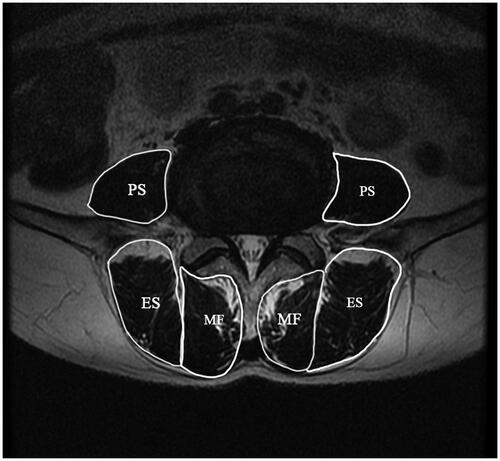
CSA can be used to quantify atrophy and hypertrophy levels of the paraspinal muscles and to evaluate the function of the paraspinal muscles. The total content of lean muscle fibers in the confines of the lumbar paraspinal muscle fascia can be signified by FCSA to measure changes in muscle fibers [Citation58]. Therefore, FCSA/CSA can not only evaluate the atrophy and hypertrophy levels of the paraspinal muscles but also the FI of the paraspinal muscles. Despite some studies concerning muscle fibers and nearby tissues, the concept of FCSA was first proposed by Ranson et al. in 2006 [Citation24,Citation59,Citation60]. rCSA is the ratio of the total CSA of the paraspinal muscles to the CSA of the vertebra in the same segment [Citation61]. It can be used to remove individual differences in muscle volume, so it is sometimes referred to as adjusted CSA (aCSA). The normalized FCSA difference index (CDI) is a type of evaluation index that is also considered as the degree of asymmetric change in the paraspinal muscles [Citation62].
3.2. FI
FI is another commonly used indicator of paraspinal muscle degeneration. CT utilizes density values, whereas MRI employs signal intensity to reflect the degree of FI. FI can be measured using CT, MRI, and US, similar to the CSA. FI can also be classified by MR spectroscopy, which is a non-invasive method that can quantitatively analyze intramyocellular lipids (IMCLs) and extramyocellular lipids (EMCLs). The fatty infiltration rate (FIR or FI%) is the ratio of fat CSA to total CSA, or one minus the ratio of FCSA/CSA of the paraspinal muscles. The data obtained by separating the MR signals of water and fat can be utilized to quantify the fat fraction (FF) of the paraspinal muscles, which is the ratio of the signal intensity from water divided by the sum of the signal intensities from both water and fat [Citation63,Citation64]. In 1984, Dixon et al. introduced a simple method of proton chemical-shift imaging to separate signals from fat and water protons [Citation65]. Subsequently, Buxton et al. introduced the concept of FF in 1986, based on this method [Citation66]. Although there are differences in the calculation methods and results between FIR and FF, both are commonly used to assess FI ().
Table 1. Morphologic parameters, method of measurement and clinical significance of paraspinal muscles.
There are three methods for assessing FI: visual qualitative assessment, semiquantitative assessment, and quantitative assessment. Visual qualitative assessment, which is widely used in clinical practice, is a macroscopic and intuitive method for determining FI occurrence. However, accurate information cannot be provided, which limits its application in scientific research. Visual semiquantitative assessment is a straightforward method that combines qualitative and quantitative approaches for measuring FI. Semi-quantitative methods such as the Goutallier classification system have two obvious advantages: they not only characterize FI effortlessly like visual qualitative assessment but also provide semiquantitative and numerical scales that cannot be obtained by visual assessment merely for FI. If FI could not be measured accurately, the Goutallier classification might have been the best replacement for the quantitative quality of paraspinal muscles [Citation67,Citation68]. Although the semiquantitative method is an excellent measure, it has some inevitable disadvantages. There may be different consequences for experienced and inexperienced assessors. Moreover, the consequences were reported as an ordinal scale with discrete levels instead of consecutive results [Citation57]. In contrast, the quantitative assessment of FI could yield more interesting and consecutive results. It is more suitable for research than clinical practice because it is more time-consuming.
Recently, the application of deep learning methods and artificial intelligence in result analysis has shown impressive achievements in research practices of paraspinal muscles such as convolutional neural networks, population-averaged MRI atlases, and multi-scale iterative random forest classifications. Accordingly, they may be efficient in quantifying the characteristics observed in spinal disorders. In addition, these methods can be used for predictive modeling, determining patient phenotypes through clustering, or building multivariable MRI-based biomarkers, as well as exploratory data analysis and hypothesis generation [Citation25,Citation69–72].
3.3. Others
Muscle thickness is a significant parameter that reflects the size and health status of the muscle and serves as an indicator of paraspinal muscle mass and function. Paraspinal muscle thickness can be measured by measuring the distance between the upper and lower parallel surfaces of the muscle in a transverse section in ultrasonography studies [Citation73]. In clinical research, ultrasound measurement of muscle thickness is often a non-invasive, cost-effective, and user-friendly option.
The volume of the paraspinal muscles can be analyzed in three dimensions by segmenting them and other soft tissues in imaging sections using software processing [Citation74,Citation75]. Three-dimensional measurements can generate high-definition three-dimensional muscle images that can visually display the distribution and trends of the paraspinal muscles. An accurate measurement of the volume of the paraspinal muscles by considering the muscle changes in all three spatial dimensions is provided, thereby avoiding errors in two-dimensional measurements.
A prospective study was conducted to evaluate the quality of the paravertebral muscles using dual-energy X-ray absorptiometry. The results of this method showed a strong positive correlation with the 3D analysis of paravertebral muscle quality using MRI [Citation76]. Recently, some studies demonstrated that Dual-energy CT is regarded as a promising radiographic technique to characterize the FI of the lumbar paraspinal muscles, which includes a prospective investigation [Citation77,Citation78]. The application of dual-energy X-ray absorptiometry may be valuable in the diagnosis osteoporosis and sarcopenia [Citation76,Citation79,Citation80].
4. Paraspinal muscles and Cervical disorders
Paraspinal muscles play a vital role in supporting and stabilizing the cervical regions of the spine. When affected by a disorder or injury, these muscles can result in pain and decreased mobility. Cervical disorders that can affect the paraspinal muscles are common and can result from a range of causes including degenerative conditions, trauma, or poor posture. As a result, accurate diagnosis and effective management of these disorders are crucial for maintaining spinal function and improving quality of life.
4.1. Whiplash-associated disorder
Whiplash-associated disorder (WAD) refers to cervical spinal cord injury caused by abrupt acceleration-deceleration of the cervical vertebrae, which is common in car accidents. Several studies have argued that WAD often causes pathological changes in paraspinal muscles [Citation81–87]. A cross-sectional study indicated that the extent and distribution of FI in the cervical MF and semispinalis cervicis muscle was quantified in individuals with chronic WAD, and a significant FI was found in patients with chronic WAD [Citation81]. This result was corroborated by a prospective study with a larger cohort [Citation82]. Two cross-sectional studies have demonstrated that there are differences in FI in different degrees of whiplash injuries. Larger amounts of FI in the cervical MF were found in individuals with heavy pain-related disability due to whiplash injury than in healthy individuals and those with light or moderate disability due to whiplash injury [Citation83]. Another study confirmed this view and found that the grading method of qualitative FI by different FI frequencies demonstrated eminent predictability for differentiating between patients with light, moderate, and severe WAD [Citation84]. FI of paraspinal muscles may hold potential clinical value in the diagnosis and grading of WAD ().
Table 2. The researches between paraspinal muscles and WAD.
4.2. Cervical spondylosis
Cervical spondylosis is a disorder characterized by degenerative pathological progress mainly due to long-term strain of the cervical spine, prolapse of the intervertebral disc, or thickening of the ligamentum flavum, resulting in compression of the cervical spinal cord, nerve roots, or vertebral arteries, resulting in a series of dysfunctions in the clinical syndrome. FI and amyotrophy of the parespinal muscles are common in individuals with cervical spondylosis. Greater muscle volume and FI in the deep cervical spine extensor muscles were found in individuals with chronic idiopathic neck pain [Citation88]. Two retrospective studies indicated that all the flexor and extensor paraspinal muscles were found to be atrophy in individuals suffering from cervical spondylotic myelopathy (CSM) [Citation89,Citation90]. Even in the adjacent segment, which was not compressed, the degree and FI of the paraspinal muscles in CSM patients were also aggravated [Citation90]. Tamai et al. retrospectively pointed out that as a key part of the stable structure of the spine, the decline in function was closely correlated with the decline in the stability of the spine. Cervical balance and cervical disc degeneration were considered significantly related to paraspinal muscle volume [Citation91]. Yuksel et al. retrospectively pointed out that the CSA of the cervical paraspinal muscles was found to increase in the prophase of cervical disc degeneration, while a reduction in the CSA of all cervical paraspinal muscles was observed with an increase in disc degeneration [Citation92].
The functional status of the paraspinal muscles in patients diagnosed with cervical spondylosis correlated with functional scores, clinical signs, and symptoms. A cross-sectional study suggested that in patients with degenerative cervical myelopathy, the more asymmetric the CSA of the semispinalis capitis, the higher the neck disability index scores. In patients with degenerative cervical myelopathy, a significant increase in muscle FI and CSA asymmetry was demonstrated at the level beneath the compression [Citation93]. Cervical spondylosis and a clinically significant decrease in sensorimotor function were correlated with increasing FI in the MF [Citation94]. Secondary muscle loss and FI could be found in spinal injuries in individuals with CSM [Citation94]. Some cross-sectional studied indicated that severe neck symptoms and cervical imbalance were related to decreased extensor muscle volume and increased FI in patients with cervical spondylosis [Citation41,Citation95,Citation96]. A cross-sectional study of patients with cervical spondylotic radiculopathy found that normal activity of the musculature could be inhibited by FI within the muscle, and FI of the cervical MF could cause postural instability in static standing [Citation97]. In addition, in patients with unilateral cervical radiculopathy, there was myoatrophy of the MF homolateral to the cervical radiculopathy [Citation98]. The aforementioned studies indicate that paraspinal muscles may play a certain compensatory role in the development of cervical spine diseases. However, further high-level research is needed to substantiate this observation ().
Table 3. The researches between paraspinal muscles and cervical spondylosis.
4.3. Other disorders
Morphological changes in the paraspinal muscles have also been identified in non-specific neck pain, cervical spine deformities, and ossification of the posterior longitudinal ligament (OPLL). Two cross-sectional studies suggested that a lower degree of FI in the neck paraspinal muscles indicated a favorable prognosis [Citation99,Citation100]. A larger CSA of the paraspinal muscles was advantageous for patients with chronic non-specific neck pain [Citation100].On the other hand, in OPLL patients, the severity of OPLL might be associated with FI in the deep posterior neck muscles, with greater FI predicting poorer scores on the neck disability index [Citation95]. Currently, there is limited research on the correlation between cervical diseases and paraspinal muscle morphology, which could represent a valuable avenue for future exploration.
5. Paraspinal muscles and thoracic/lumbar disorders
The paraspinal muscles play a critical role in the stability of the thoracic and lumbar spine. Any disorder or injury affecting these muscles can have a significant impact on spinal function and the overall quality of life. Thoracic or lumbar disorders can cause pain, stiffness, and limited mobility. Therefore, early diagnosis and effective management of these disorders are crucial to maintaining spinal function and well-being.
5.1. Adolescent idiopathic scoliosis (AIS)
AIS is the most common type of scoliosis. Changes in the function and structure of the paraspinal muscles are often observed in individuals with AIS. Both MRI and histological analyses have demonstrated a consistent conclusion that there were significant differences in FI between the convex and concave sides of the scoliotic deformity in patients with AIS, in which greater FI was observed at the concavity [Citation101,Citation102].
There was significant paraspinal muscle asymmetry in individuals with mild AIS [Citation103]. Some pathological studies have concluded that at the microscopic level, significant differences were found in endomysial and perimysial fibrosis, as well as fatty involution on both sides of the vertebrae. There was significant asymmetry on both sides of the concave and convex scoliotic curves, although both sides showed great atrophy [Citation104]. More fibrosis and FI were found in the paraspinal muscles on the concave side of the scoliosis apex [Citation105]. Stetkarova et al. compared the relationship between electrophysiological and histological changes in paraspinal muscles and revealed a significant asymmetry in the dispersion of type I fibers, which correlated with an altered function proven by electrophysiological means in the paraspinal muscles and showed predominance of the convexity of the scoliotic curve [Citation106]. The above-mentioned research indicates that paraspinal muscles may be affected during the development of AIS. Additionally, alterations in paraspinal muscle morphology may also influence the progression of AIS. However, the relationship between the two requires further investigation to better guide clinical treatment ().
Table 4. The researches between paraspinal muscles and AIS.
5.2. Degenerative scoliosis (DS)
DS refers to the absence of scoliosis in adolescents and the appearance of scoliosis with increasing age. When lumbar segments are affected, it is called degenerative lumbar scoliosis (DLS). In patients with DS, alteration of the paraspinal muscles has certain characteristics. It has been proposed that the CSAs of the MF and PS were significantly smaller, and the FI of MF and ES at the lower lumbar level was higher than that at the upper level, but the FI of PS at the lower lumbar level was lower than that at the higher level in individuals with DLS, which include a retrospective matched cohort study [Citation107,Citation108].
Asymmetric degeneration of the paraspinal muscles has been prospectively demonstrated in patients with DLS [Citation62]. Some cross-sectional studies provided support for this viewpoint. The CSA difference indices of the apical PS and MF at the curvature level were significantly greater on the convex side than on the concave side, while the mean percentage of FI area was significantly higher on the concave side than on the convex side [Citation32,Citation109]. The atrophy and FI of the MF on the concave side were more severe than those on the convex side [Citation109,Citation110].
Alteration of the paraspinal muscles is closely related to the prognosis of patients with DS. A prospective study indicated a significant decline in quality of life was accompanied by an increase in asymmetric myoatrophy and FI in the MF [Citation62]. Wang et al. retrospectively suggested that degeneration of the paraspinal muscles was an independent factor of screw loosening in patients with DS after surgery [Citation38]. For patients with DS, morphological analysis of paraspinal muscles could be a valuable means to assess the disease’s severity and prognosis, thereby guiding clinical treatment ().
Table 5. The researches between paraspinal muscles and DS.
5.3. Degenerative lumbar spondylolisthesis (DLS)
DLS is primarily characterized by the anterior displacement of one lumbar vertebra relative to the adjacent vertebra. This condition is primarily attributed to degenerative changes in the lumbar structure. Some cross-sectional and retrospective studies have shown the change of paraspinal muscles in DLS patients. In patients with DLS, there was notable atrophy in MF, alongside hypertrophy in ES [Citation111]. The MF atrophy might contribute to lumbar instability, while the ES hypertrophy might act as a compensatory mechanism to address this instability. Paraspinal muscle atrophy could be considered a potential risk factor for DLS [Citation112–114]. MF degeneration in all lumbar segments was segmental in patients, which meant there was a different degree of FI in different lumbar segments, where the FI significantly increased in the lower lumbar spine [Citation61].
Although the paraspinal muscles in patients with isthmic spondylolisthesis (IS) and DLS showed varying degrees of degeneration [Citation114], they might play an important role in maintaining the structure and function of the spine in patients with DLS and IS. Some retrospective research has provided support for this viewpoint. Low FI and high CSA of the paraspinal muscles might have a protective effect against the development of DLS and IS [Citation114]. In addition, larger CSAs of MF and PS might be protective factors to prevent lumbar stability in patients with isthmic spondylolisthesis [Citation115]. Another study supported the notion that the paraspinal muscles had a protective effect in patients with DLS. Patients with severe disability caused by DLS had a significantly reduced absolute CSA of the PS compared with those with mild or moderate disability. Notably, atrophy of the PS might have an adverse effect on the severity of lumbar pathology [Citation116]. The aforementioned research indicates that functional exercises targeting paraspinal muscles may be beneficial in improving the prognosis of patients with DLS ().
Table 6. The researches between paraspinal muscles and DLS.
5.4. Lumbar spinal stenosis (LSS)
LSS is mostly seen in middle-aged and elderly people, and is a common cause of LBP, caused by degeneration of the lumbar disc and thickening of ligaments with age, leading to smaller and compressed perineural spaces, and is a common disorder resulting from degenerative changes in the lumbar disorders. Compared with healthy people, the alteration rules of paraspinal muscles were similar in patients with LSS, but the degeneration, including reduced volume, increased FI, predominantly IMCLs, and bilateral muscle asymmetry, of paraspinal muscles, especially in MF, was more severe [Citation117–119].
In patients diagnosed with LSS, functional status was found to be associated with the size of the MF and PS, as well as with paraspinal FI levels, according to previous studies cited in references. Specifically, higher levels of paraspinal FI were linked to greater disability and lower health-related quality of life, which include a prospective cohort study and three retrospective researches [Citation120–123]. Moreover, LSS patients with high functional performance showed a larger rCSA of the PS and a lower level of FI in the MF [Citation124]. A retrospective study and a cross-sectional study indicated that Patients with symptomatic LSS had greater density and CSA of the ES, and the severity of MF atrophy was positively correlated with the degree of spinal stenosis. Atrophic MF was more pronounced in the stenotic segments of the spine and more severe on the symptomatic side of the spine in patients [Citation33,Citation125]. Additionally, muscle degeneration was found to be more closely related to muscle FI than to muscle volume in LSS patients [Citation75].
Assessing the status of the paraspinal muscles before surgery can assist surgeons in predicting the functional status and recovery of patients. One key factor to consider was the degree of FI in these muscles, as there appears to be a strong correlation between FI and bone nonunion after posterior lumbar interbody fusion [Citation126]. Among the various paraspinal muscle parameters, the pre-operative FI of the MF has been identified as a reliable predictor of patient function [Citation127]. An ambispective cohort study supported the view [Citation128]. Furthermore, a study has emphasized that LSS patients with FI of MF ≥25% had a significantly higher risk of bone nonunion after undergoing posterior lumbar interbody fusion [Citation129]. Severe degeneration of the paraspinal muscles was also a risk factor that could lead to suboptimal surgical outcomes following lumbar short-segment decompression and fusion for LSS [Citation130]. Conversely, a prospective study indicated a healthy pre-operative lumbar MF indicated a better outcome following lumbar spinal decompression [Citation131]. The above-mentioned research highlights the importance of incorporating paraspinal muscle morphological assessment into the clinical evaluation of patients with LSS. This inclusion aids in formulating individualized treatment strategies and predicting prognosis ().
Table 7. The researches between paraspinal muscles and LSS.
5.5. Lumbar disc herniation(LDH)
LDH is a pathological condition in which the nucleus pulposus at the center of the intervertebral disc protrudes through the damaged annulus fibrosus, compressing the surrounding nerve roots and/or spinal cord within the spinal canal. Many studies have documented a strong relationship between intervertebral disc degeneration and morphofunctional changes in the paraspinal muscles.
A cross-section study showed a correlation demonstrated between LDH and paraspinal muscles [Citation132]. Some retrospective studies provide evidence for this viewpoint. Yazici et al. retrospectively researched the correlation between MF degeneration and LDH might be a process of mutual influence and interaction [Citation133]. Sustained compression of spinal nerve roots due to disc herniation could lead to atrophy and degeneration of the corresponding muscle groups [Citation134]. Herniation was found more frequently in individuals with MF atrophy [Citation135]. The percentage of MF atrophy correlated with the grade of disc degeneration [Citation136,Citation137]. In a case-control study, there was a significant correlation documented between functional disability, LBP intensity, and muscle size in LDH patients [Citation138]. Choi et al. retrospectively suggested among middle-aged and elderly individuals with LDH, there were potential risk factors for recurrence, such as younger age, segmental instability, and increased mass of the PS [Citation139]. Zhong et al. retrospectively investigated the correlation between the straight-leg raising(SLR) test and the extent of FI in the lumbar MF among patients with LDH, and found that the SLR could serve as a crucial indicator of lumbar MF dysfunction. Nevertheless, the severity of lumbar MF degeneration could not be assessed solely by the degree of the SLR test [Citation140].
There is some controversy regarding asymmetry in the morphological and functional changes of the paraspinal muscles on the affected versus unaffected side in patients with unilateral LDH. Hodges et al. discovered that the CSA of the MF decreased at the L4 level on the same side as the disc lesion in Animal experiments [Citation141]. Subsequently, Battié et al. retrospectively proposed a different conclusion that the FCSA/CSA was smaller on the side affected by herniation than on the unaffected side, both below and at the level of herniation. At the level below the herniation, the FI in the MF was greater on the herniated side. Interestingly, a greater total CSA of MF was found on the same side as the pathology at the herniation level, contrary to the conclusion of Hodges et al. [Citation142]. Wan et al. retrospectively compared LDH patients with and without LBP and found a significant decrease in the CSA of the MF muscle at multiple levels on the painful side [Citation143]. Fortin et al. found that at any spinal level, there were no significant differences in the CSA, FCSA, or FCSA/CSA of the MF between the two sides. However, more FI was observed in the MF and ES on the side of the LDH at the L5-S1 level. There was no significant asymmetry in the MF at the level above, at the same level, or below the herniated lumbar disc [Citation34]. According to Xiao et al. there were no significant differences in CSA asymmetry at L5-S1 and S1 or in side-to-side variations of FI in patients with unilateral LDH [Citation144]. Yazici et al. conducted a cross-section study and reported a correlation between the FI of the paraspinal muscles and LDH but did not find any correlation with asymmetry [Citation132]. It is important to note that these conclusions may be attributed to differences in the study populations, methods, sample sizes, and other factors. Further research is necessary to confirm the reliability of these findings ().
Table 8. The researches between paraspinal muscles and IDH.
5.6. LBP
LBP is a common clinical symptom dominated by pain on one or both sides of the lumbar area. It has been reported to be a healthy, social, and economic problem related to absenteeism, disability, and a high budget for society [Citation145]. Lumbar muscles play a vital role in the stability and function of the lumbar vertebrae [Citation146]. LBP, one of the common manifestations of low back disorders, is associated with alterations in the paraspinal muscles. A prospective study indicates that the fat content in the MF of CLBP patients was significantly higher than that in asymptomatic volunteers [Citation147]. High-level studies indicated there was a smaller MF which was accompanied by a significant amount of IMCL in the individuals with LBP [Citation147,Citation148]. It was observed that the lumbar MF, which was believed to play a critical role in stability, exhibited atrophy in physically active patients with CLBP [Citation146]. A systematic review suggested there was a negative association observed between the CSA of the lumbar MF and LBP [Citation149]. Age, obesity, and muscle atrophy were correlated with EMCL concentration in MF. IMCL concentration in the MF exhibited a correlation with the intensity of LBP, and the measurement of IMCLs might serve as a distinctive characteristic of CLBP as well as a potential predictor of spinal deformity [Citation147,Citation150,Citation151].
The morphofunction of the paraspinal muscles in patients with LBP is affected by many factors, and different manifestations of LBP are also indicated by these factors. Some cross-section studies demonstrated that [Citation152–154]. A notable inverse relationship between age and CSA of the paraspinal muscles [Citation152]. Compared with their counterparts without LBP, older adults with CLBP exhibited significantly higher levels of intramuscular fat in the MF and reduced ES size [Citation153]. Patients with CLBP might develop muscular asymmetries in the lumbar region of the spine, which were associated with facet asymmetry [Citation154]. Zhao et al. retrospectively demonstrated that young patients experiencing unilateral neurological symptoms due to LDH might be at a higher risk of developing LBP if they exhibit symmetrical atrophy of the bilateral MF [Citation155]. Two cross-sectional studies suggested that morphological changes in the paraspinal muscles might lead to different clinical presentations [Citation26,Citation156]. The degree and manifestation of pain might be influenced by the escalation of disability and functional impairment as potential determinants [Citation156]. There were no differences in lean muscle atrophy and total atrophy between the recurrent LBP, non-continuous CLBP, and continuous CLBP groups. However, increased activity-induced metabolic changes were found in the lumbar muscles in continuous or non-continuous CLBP compared to recurrent LBP [Citation26]. The severity of FI in the paraspinal muscles may serve as a potential indicator for evaluating the occurrence and progression of LBP. Some studies have found that the IMCL in MF was significantly increased in patients with LBP, which included a prospective investigation [Citation157–159]. A cross-sectional study revealed a significant correlation between the FIR of the ES in the upper lumbar spine and the occurrence of LBP [Citation160]. A significant correlation was observed between the severity of FI in the lumbar MF and the reduction in lumbar flexion range of motion [Citation161].
Whether physical activity (PA) can improve CSA and FI of the paraspinal muscles in patients with LBP remains somewhat controversial. A direct and significant association was detected between the physical activity level and CSA of the paraspinal muscles at the L2-L3 and L3-L4 levels [Citation152]. Physical inactivity was correlated with diminished intervertebral disc width, augmented adipose content of the MF, and intensified high-threshold LBP and incapacity in a dose-dependent manner [Citation162]. Conversely, a longitudinal study among a cohort of youthful individuals indicated that PA behavior was not associated with LBP [Citation158]. It has been proposed that the mean CSA of the paraspinal muscles and the extent of FI remained unaltered following bouts of high-intensity physical exertion [Citation40]. Non-specific LBP is a complex condition, and the assessment of these patients should be comprehensive. The morphological characteristics of paraspinal muscles may serve as a crucial parameter for their evaluation. Currently, there is an urgent need to establish an evaluation system for LBP patients, enabling better clinical guidance for this disorder ().
Table 9. The researches between paraspinal muscles and LBP.
6. Surgery
In spinal surgeries, damage to the paraspinal muscles is unavoidable. Patients experiencing postoperative pain following lumbar disc excision were found to have impaired lumbar paraspinal muscle function, particularly in the deep stabilizing muscle [Citation163]. Patients who underwent intervertebral disc excision surgery exhibited comprehensive paraspinal muscle atrophy in the lumbar region, including both surgical and non-surgical sites [Citation164,Citation165].
Different surgical approaches have varying effects on paraspinal muscle damage. Lee et al. prospectively compared the differences between unilateral traditional open approach (OA) and the contralateral muscle-preserving approach (MPA) for laminoplasty, concluding that MPA effectively preserved the volume of cervical extensor muscles and minimized postoperative muscle-skeletal complications [Citation166]. He et al. retrospectively compared the "stand-alone" oblique lateral interbody fusion (OLIF) with regular OLIF, suggesting that compared to OLIF combined with pedicle screw and rod fixation, "stand-alone" OLIF achieved better clinical outcomes [Citation167]. Lin et al. retrospectively assessed the differences between unilateral K-rod dynamic internal fixation and posterior lumbar interbody fusion (PLIF) and observed a significant reduction in paraspinal muscle atrophy and FI in patients undergoing unilateral K-rod dynamic internal fixation in the lumbar spine [Citation168]. A case-control study suggested that compared to conventional laminoplasties, bilateral spinal approaches significantly improved muscle preservation on the open side [Citation169].
Minimally invasive surgery represents the future trend in spinal surgery. In comparison to traditional surgery, minimally invasive procedures typically better protect the paraspinal muscles. Fan et al. compared the differences in treating paraspinal muscle damage between the modified minimally invasive approach (MMIA) and traditional operative approach (TOPA) for one-level instrumented posterior lumbar inter-body fusion (PLIF), and the results indicated that both approaches led to an increase in paraspinal muscle FI. However, the TOPA group showed a more significant increase in FI in the MF and accompanied MF atrophy. In comparison, MMIA significantly alleviated paraspinal muscle damage and reduced the occurrence of LBP [Citation170]. Li et al. prospectively demonstrated minimally invasive PLIF using the cortical bone trajectory technique could minimize its impact on the paraspinal muscles [Citation171]. Liu et al. prospectively compared the differences between modified unilateral laminotomy for bilateral decompression (M-ULBD) and conventional laminoplasty in the treatment of patients with LSS, and the results indicated that the M-ULBD group had a smaller rate of MF atrophy [Citation172]. Yoo et al. retrospectively found in their study that following multilevel minimally invasive transforaminal interbody fusion (MITLIF), there was no significant difference in the decrease of CSA of parapinal muscles in single, double, or triple fusion groups. Clinical outcomes did not show any significant differences either [Citation173]. In the treatment of thoracolumbar fractures, Wiltse approach led to less MF atrophy and FI compared to the traditional open posterior fixation method [Citation174]. Ntilikina et al. retrospectively suggested that compared to open surgery, percutaneous transpedicular thoracolumbar fracture fixation resulted in less paraspinal muscle atrophy [Citation175].
Postoperative morphological changes in paraspinal muscles may influence patient outcomes. Choi et al. retrospectively demonstrated after cage-alone anterior cervical decompression and fusion (ACDF), individuals with larger CSA of the preoperative extensor muscles might have a more favorable effect on bone fusion than those with smaller CSA [Citation176]. Kim et al. retrospectively suggested a smaller rCSA of the preoperative paraspinal muscles was a significant predictor of the development of adjacent segment degeneration after lumbar fusion [Citation177]. The FI of the preoperative MF in patients with degenerative LSS could serve as a predictive indicator for postoperative functional improvement, and high paraspinal muscle FI is closely related to bone non-union [Citation126, Citation129]. A prospective cohort study supported the opinion [Citation128]. The other study suggested that the preoperative FI of the MF could serve as a good predictive indicator for assessing functional improvement in LSS patients [Citation127]. Kumaran et al. built a finite element model and analyzed that the reduction in CSA during lumbar procedures for transforaminal lumbar interbody fusion (TLIF) could lead to changes in spinal component stress in adjacent segments and might result in persistent postoperative LBP [Citation178]. Patients with larger CSA and smaller FI in MF demonstrated better outcomes following lumbar interbody fusion [Citation179].
7. Limitation and prospect
Recent studies have demonstrated that changes in the size, composition, and FI of paraspinal muscles may be associated with various spinal disorders. Changes in the morphological characteristics of the paraspinal muscles have the potential to serve as prognosticators of the severity and progression of spinal disorders. However, there are numerous parameters for evaluating paraspinal muscles, and the value of each parameter requires further verification. Research is also required to determine how to select appropriate parameters for paraspinal muscle assessment. Indeed, higher-level evidence, such as pathological or histological analyses, is needed to demonstrate the relationship between parameters from clinical examination of paraspinal muscles and the occurrence and development of spinal disorders. Furthermore, the controversial conclusions of some studies require reasonable explanations.
In the future, continued research in this area is essential for developing more precise and personalized treatment strategies for spinal disorders. Furthermore, the creation of machine learning algorithms and artificial intelligence has enabled the development of predictive modeling, determining patient phenotypes through clustering, or building multivariable MRI-based biomarkers, as well as exploratory data analysis and hypothesis generation, which may help identify patients at high risk for developing spinal diseases. It has the potential to improve patient outcomes through early intervention and more targeted treatment. By identifying different characteristics of inspection results of paraspinal muscles that are associated with different types of spinal disorders, clinicians can tailor treatment plans to each individual to improve the effectiveness of treatment and ultimately lead to better outcomes for patients.
8. Summary
In conclusion, the paraspinal muscles play a critical role in spinal stability, and their dysfunction is implicated in a variety of spinal disorders. Similarly, spinal disorders can affect the paraspinal muscles, leading to pain, weakness, and decline in function. The development of radiographic techniques has allowed a better understanding of the relationship between the morphological characteristics of the paraspinal muscles and spinal pathologies. In addition to diagnostic applications, radiographic techniques have been used to evaluate the effectiveness of interventions aimed at spinal disorders. The advent of new techniques has provided new approaches for further study of paraspinal muscles. Although some details are still controversial, clinicians and researchers can develop more effective treatment strategies to improve patient outcomes by understanding the potential mechanisms underlying the changes in the morphological characteristics of the paraspinal muscles.
Author contributions
Conceptualization, L.Z.H; writing—original draft preparation, S.M.R; writing—review and editing, S.M.R.; All authors prepared and contributed to the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data sharing is not applicable to this article, as no new data were created or analyzed in this study.
Additional information
Funding
References
- Fehlings MG, Tetreault L, Nater A, et al. The aging of the global population: the changing epidemiology of disease and spinal disorders. Neurosurgery. 2015;77 Suppl 4: 1 1–22. doi: 10.1227/NEU.0000000000000953.
- Brinjikji W, Luetmer PH, Comstock B, et al. Systematic literature review of imaging features of spinal degeneration in asymptomatic populations. AJNR Am J Neuroradiol. 2015;36(4):811–816. doi: 10.3174/ajnr.A4173.
- Theodore N. Degenerative cervical spondylosis. N Engl J Med. 2020;383(2):159–168. doi: 10.1056/NEJMra2003558.
- Teraguchi M, Yoshimura N, Hashizume H, et al. Prevalence and distribution of intervertebral disc degeneration over the entire spine in a population-based cohort: the Wakayama spine study. Osteoarthritis Cartilage. 2014;22(1):104–110. doi: 10.1016/j.joca.2013.10.019.
- Boos N, Weissbach S, Rohrbach H, et al. Classification of age-related changes in lumbar intervertebral discs: 2002 volvo award in basic science. Spine. 2002;27(23):2631–2644. doi: 10.1097/00007632-200212010-00002.
- Diebo BG, Shah NV, Boachie-Adjei O, et al. Adult spinal deformity. Lancet. 2019;394(10193):160–172. doi: 10.1016/S0140-6736(19)31125-0.
- Kebaish KM, Neubauer PR, Voros GD, et al. Scoliosis in adults aged forty years and older: prevalence and relationship to age, race, and gender. Spine. 2011;36(9):731–736. doi: 10.1097/BRS.0b013e3181e9f120.
- Schwab F, Dubey A, Gamez L, et al. Adult scoliosis: prevalence, SF-36, and nutritional parameters in an elderly volunteer population. Spine. 2005;30(9):1082–1085. doi: 10.1097/01.brs.0000160842.43482.cd.
- Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the global burden of disease study 2013. Lancet. 2015;386(9995):743–800.
- Smith E, Hoy DG, Cross M, et al. The global burden of other musculoskeletal disorders: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73(8):1462–1469. doi: 10.1136/annrheumdis-2013-204680.
- March L, Smith EU, Hoy DG, et al. Burden of disability due to musculoskeletal (MSK) disorders. Best Pract Res Clin Rheumatol. 2014;28(3):353–366. doi: 10.1016/j.berh.2014.08.002.
- Hartvigsen J, Hancock MJ, Kongsted A, et al. What low back pain is and why we need to pay attention [J. ]Lancet. 2018;391(10137):2356–2367. doi: 10.1016/S0140-6736(18)30480-X.
- Clark S, Horton R. Low back pain: a major global challenge. Lancet. 2018;391(10137):2302. doi: 10.1016/S0140-6736(18)30725-6.
- Germon T, Clifford D, Lee W, et al. Low back pain. Lancet. 2018;392(10164):2547. doi: 10.1016/S0140-6736(18)32220-7.
- Asher AL, Devin CJ, Archer KR, et al. An analysis from the quality outcomes database, part 2. Predictive model for return to work after elective surgery for lumbar degenerative disease. J Neurosurg Spine. 2017;27(4):370–381. doi: 10.3171/2016.8.SPINE16527.
- Maher C, Underwood M, Buchbinder R. Non-specific low back pain. Lancet. 2017;389(10070):736–747. doi: 10.1016/S0140-6736(16)30970-9.
- Buchbinder R, VAN Tulder M, Öberg B, et al. Low back pain: a call for action. Lancet. 2018;391(10137):2384–2388. doi: 10.1016/S0140-6736(18)30488-4.
- Hoy D, March L, Brooks P, et al. The global burden of low back pain: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73(6):968–974. doi: 10.1136/annrheumdis-2013-204428.
- Hoy D, Geere JA, Davatchi F, et al. A time for action: opportunities for preventing the growing burden and disability from musculoskeletal conditions in low- and Middle-income countries. Best Pract Res Clin Rheumatol. 2014;28(3):377–393. doi: 10.1016/j.berh.2014.07.006.
- Hodges PW, Danneels L. Changes in structure and function of the back muscles in low back pain: different time points, observations, and mechanisms. J Orthop Sports Phys Ther. 2019;49(6):464–476. doi: 10.2519/jospt.2019.8827.
- Mannion AF, Weber BR, Dvorak J, et al. Fibre type characteristics of the lumbar paraspinal muscles in normal healthy subjects and in patients with low back pain. J Orthop Res. 1997;15(6):881–887. doi: 10.1002/jor.1100150614.
- Crossman K, Mahon M, Watson PJ, et al. Chronic low back pain-associated paraspinal muscle dysfunction is not the result of a constitutionally determined "adverse" fiber-type composition. Spine (Phila Pa 1976). 2004;29(6):628–634. doi: 10.1097/01.brs.0000115133.97216.ec.
- Gertken JT, Hunt CH, Chinea NI, et al. Risk of hematoma following needle electromyography of the paraspinal muscles. Muscle Nerve. 2011;44(3):439–440. doi: 10.1002/mus.22138.
- Ranson CA, Burnett AF, Kerslake R, et al. An investigation into the use of MR imaging to determine the functional cross sectional area of lumbar paraspinal muscles. Eur Spine J. 2006;15(6):764–773. doi: 10.1007/s00586-005-0909-3.
- Xiao Y, Fortin M, BATTIé MC, et al. Population-averaged MRI atlases for automated image processing and assessments of lumbar paraspinal muscles. Eur Spine J. 2018;27(10):2442–2448. doi: 10.1007/s00586-018-5704-z.
- Goubert D, DE Pauw R, Meeus M, et al. Lumbar muscle structure and function in chronic versus recurrent low back pain: a cross-sectional study. Spine J. 2017;17(9):1285–1296. doi: 10.1016/j.spinee.2017.04.025.
- MäKI T, Oura P, Paananen M, et al. Longitudinal analysis of paraspinal muscle cross-sectional area during early adulthood – a 10-year follow-up MRI study. Sci Rep. 2019;9(1):19497. doi: 10.1038/s41598-019-56186-4.
- Dallaway A, Hattersley J, Diokno M, et al. Age-related degeneration of lumbar muscle morphology in healthy younger versus older men. Aging Male. 2020;23(5):1583–1597. doi: 10.1080/13685538.2021.1878130.
- Peng X, Li X, Xu Z, et al. Age-related fatty infiltration of lumbar paraspinal muscles: a normative reference database study in 516 chinese females. Quant Imaging Med Surg. 2020;10(8):1590–1601. doi: 10.21037/qims-19-835.
- Engelke K, Ghasemikaram M, Chaudry O, et al. The effect of ageing on fat infiltration of thigh and paraspinal muscles in men. Aging Clin Exp Res. 2022;34(9):2089–2098. doi: 10.1007/s40520-022-02149-1.
- Burian E, Franz D, Greve T, et al. Age- and gender-related variations of cervical muscle composition using chemical shift encoding-based water-fat MRI. Eur J Radiol. 2020;125:108904. doi: 10.1016/j.ejrad.2020.108904.
- Kim H, Lee CK, Yeom JS, et al. Asymmetry of the cross-sectional area of paravertebral and psoas muscle in patients with degenerative scoliosis. Eur Spine J. 2013;22(6):1332–1338. doi: 10.1007/s00586-013-2740-6.
- Abbas J, Slon V, May H, et al. Paraspinal muscles density: a marker for degenerative lumbar spinal stenosis? BMC Musculoskelet Disord. 2016;17(1):422. doi: 10.1186/s12891-016-1282-6.
- Fortin M, LAZáRY À, Varga PP, et al. Paraspinal muscle asymmetry and fat infiltration in patients with symptomatic disc herniation. Eur Spine J. 2016;25(5):1452–1459. doi: 10.1007/s00586-016-4503-7.
- Suzuki K, Hasebe Y, Yamamoto M, et al. Risk factor analysis for fat infiltration in the lumbar paraspinal muscles in patients with lumbar degenerative diseases. Geriatr Orthop Surg Rehabil. 2022;13:21514593211070688. doi: 10.1177/21514593211070688.
- Shahidi B, Hubbard JC, Gibbons MC, et al. Lumbar multifidus muscle degenerates in individuals with chronic degenerative lumbar spine pathology. J Orthop Res. 2017;35(12):2700–2706. doi: 10.1002/jor.23597.
- Yuan L, Zeng Y, Chen Z, et al. Degenerative lumbar scoliosis patients with proximal junctional kyphosis have lower muscularity, fatty degeneration at the lumbar area. Eur Spine J. 2021;30(5):1133–1143. doi: 10.1007/s00586-020-06394-8.
- Wang W, Li W, Chen Z. Risk factors for screw loosening in patients with adult degenerative scoliosis: the importance of paraspinal muscle degeneration. J Orthop Surg Res. 2021;16(1):448. doi: 10.1186/s13018-021-02589-x.
- Kim HJ, Yang JH, Chang DG, et al. Long-Term influence of paraspinal muscle quantity in adolescent idiopathic scoliosis following deformity correction by posterior approach. J Clin Med. 2021;10(20):4790. doi: 10.3390/jcm10204790.
- Berry DB, Padwal J, Johnson S, et al. The effect of high-intensity resistance exercise on lumbar musculature in patients with low back pain: a preliminary study. BMC Musculoskelet Disord. 2019;20(1):290. doi: 10.1186/s12891-019-2658-1.
- Gu Y, Wang C, Hu J, et al. Association between the cervical extensor musculature and the demographic features, symptoms, and sagittal balance in patients with multilevel cervical spondylotic myelopathy. World Neurosurg. 2023;169:e40–e50. doi: 10.1016/j.wneu.2022.10.014.
- Du Rose A, Breen A, Breen A. Relationships between muscle electrical activity and the control of inter-vertebral motion during a forward bending task. J Electromyogr Kinesiol. 2018;43:48–54. doi: 10.1016/j.jelekin.2018.08.004.
- Kong MH, Morishita Y, He W, et al. Lumbar segmental mobility according to the grade of the disc, the facet joint, the muscle, and the ligament pathology by using kinetic magnetic resonance imaging. Spine (Phila Pa 1976). 2009;34(23):2537–2544. doi: 10.1097/BRS.0b013e3181b353ea.
- Creze M, Soubeyrand M, Gagey O. The paraspinal muscle-tendon system: its paradoxical anatomy. PLoS One. 2019;14(4):e0214812. doi: 10.1371/journal.pone.0214812.
- Anderson JS, Hsu AW, Vasavada AN. Morphology, architecture, and biomechanics of human cervical multifidus. Spine (Phila Pa 1976). 2005;30(4):E86–91. doi: 10.1097/01.brs.0000153700.97830.02.
- Willard FH, Vleeming A, Schuenke MD, et al. The thoracolumbar fascia: anatomy, function and clinical considerations. J Anat. 2012;221(6):507–536. doi: 10.1111/j.1469-7580.2012.01511.x.
- Macintosh JE, Valencia F, Bogduk N, et al. The morphology of the human lumbar multifidus. Clin Biomech. 1986;1(4):196–204. doi: 10.1016/0268-0033(86)90146-4.
- Macintosh JE, Bogduk N. The attachments of the lumbar erector spinae. Spine. 1991;16(7):783–792. doi: 10.1097/00007632-199107000-00017.
- Daggfeldt K, Huang QM, Thorstensson A. The visible human anatomy of the lumbar erector spinae. Spine. 2000;25(21):2719–2725. doi: 10.1097/00007632-200011010-00002.
- Agten A, Stevens S, Verbrugghe J, et al. Biopsy samples from the erector spinae of persons with nonspecific chronic low back pain display a decrease in glycolytic muscle fibers. Spine J. 2020;20(2):199–206. doi: 10.1016/j.spinee.2019.09.023.
- Padwal J, Berry DB, Hubbard JC, et al. Regional differences between superficial and deep lumbar multifidus in patients with chronic lumbar spine pathology. BMC Musculoskelet Disord. 2020;21(1):764. doi: 10.1186/s12891-020-03791-4.
- Purushotham S, Stephenson RS, Sanderson A, et al. Microscopic changes in the spinal extensor musculature in people with chronic spinal pain: a systematic review. Spine J. 2022;22(7):1205–1221. doi: 10.1016/j.spinee.2022.01.023.
- Zhu DC, Lin JH, Xu JJ, et al. An assessment of morphological and pathological changes in paravertebral muscle degeneration using imaging and histological analysis: a cross-sectional study. BMC Musculoskelet Disord. 2021;22(1):854. doi: 10.1186/s12891-021-04734-3.
- Zhi-Jun H, Wen-BIN X, Shuai C, et al. Accuracy of magnetic resonance imaging signal intensity ratio measurements in the evaluation of multifidus muscle injury and atrophy relative to that of histological examinations. Spine. 2014;39(10):E623–9. doi: 10.1097/BRS.0000000000000286.
- Fortin M, Gibbons LE, Videman T, et al. Do variations in paraspinal muscle morphology and composition predict low back pain in men?. Scand J Med Sci Sports. 2015;25(6):880–887. doi: 10.1111/sms.12301.
- Kalichman L, Carmeli E, Been E. The association between imaging parameters of the paraspinal muscles, spinal degeneration, and low back pain. Biomed Res Int. 2017;2017:2562957–2562914. doi: 10.1155/2017/2562957.
- Mandelli F, NüESCH C, Zhang Y, et al. Assessing fatty infiltration of paraspinal muscles in patients with lumbar spinal stenosis: goutallier classification and quantitative MRI measurements. Front Neurol. 2021;12:656487. doi: 10.3389/fneur.2021.656487.
- Qu H, Yu LJ, Wu JT, et al. Spine system changes in soldiers after load carriage training in a Plateau environment: a prediction model research. Mil Med Res. 2020;7(1):63. doi: 10.1186/s40779-020-00293-1.
- Kader DF, Wardlaw D, Smith FW. Correlation between the MRI changes in the lumbar multifidus muscles and leg pain. Clin Radiol. 2000;55(2):145–149. doi: 10.1053/crad.1999.0340.
- Parkkola R, RYTöKOSKI U, Kormano M. Magnetic resonance imaging of the discs and trunk muscles in patients with chronic low back pain and healthy control subjects. Spine. 1993;18(7):830–836. doi: 10.1097/00007632-199306000-00004.
- Ding JZ, Kong C, Li XY, et al. Different degeneration patterns of paraspinal muscles in degenerative lumbar diseases: a MRI analysis of 154 patients. Eur Spine J. 2022;31(3):764–773. doi: 10.1007/s00586-021-07053-2.
- Tang Y, Yang S, Chen C, et al. Assessment of the association between paraspinal muscle degeneration and quality of life in patients with degenerative lumbar scoliosis. Exp Ther Med. 2020;20(1):505–511. doi: 10.3892/etm.2020.8682.
- Berry DB, Shahidi B, RODRíGUEZ-Soto AE, et al. Lumbar muscle structure predicts operational postures in Active-Duty marines. J Orthop Sports Phys Ther. 2018;48(8):613–621. doi: 10.2519/jospt.2018.7865.
- Dahlqvist JR, Vissing CR, Hedermann G, et al. Fat replacement of paraspinal muscles with aging in healthy adults. Med Sci Sports Exerc. 2017;49(3):595–601. doi: 10.1249/MSS.0000000000001119.
- Dixon WT. Simple proton spectroscopic imaging. Radiology. 1984;153(1):189–194. doi: 10.1148/radiology.153.1.6089263.
- Buxton RB, Wismer GL, Brady TJ, et al. Quantitative proton chemical-shift imaging. Magn Reson Med. 1986;3(6):881–900. doi: 10.1002/mrm.1910030609.
- Sollmann N, Bonnheim NB, Joseph GB, et al. Paraspinal muscle in chronic low back pain: comparison between standard parameters and chemical shift Encoding-Based Water-Fat MRI. J Magn Reson Imaging. 2022;56(5):1600–1608. doi: 10.1002/jmri.28145.
- Goutallier D, Postel J-M, Bernageau J, et al. Fatty muscle degeneration in cuff ruptures: pre- and postoperative evaluation by CT. Clin Orthop Relat Res. 1994;304(304):78–83.
- Kamiya N, Li J, Kume M, et al. Fully automatic segmentation of paraspinal muscles from 3D torso CT images via multi-scale iterative random Forest classifications. Int J Comput Assist Radiol Surg. 2018;13(11):1697–1706. doi: 10.1007/s11548-018-1852-1.
- Wesselink EO, Elliott JM, Coppieters MW, et al. Convolutional neural networks for the automatic segmentation of lumbar paraspinal muscles in people with low back pain. Sci Rep. 2022;12(1):13485. doi: 10.1038/s41598-022-16710-5.
- Weber KA, Smith AC, Wasielewski M, et al. Deep learning convolutional neural networks for the automatic quantification of muscle fat infiltration following whiplash injury. Sci Rep. 2019;9(1):7973. doi: 10.1038/s41598-019-44416-8.
- Barnard R, Tan J, Roller B, et al. Machine learning for automatic paraspinous muscle area and attenuation measures on low-dose chest CT scans. Acad Radiol. 2019;26(12):1686–1694. doi: 10.1016/j.acra.2019.06.017.
- BELAVý DL, Armbrecht G, Felsenberg D. Real-time ultrasound measures of lumbar erector spinae and multifidus: reliability and comparison to magnetic resonance imaging. Physiol Meas. 2015;36(11):2285–2299. doi: 10.1088/0967-3334/36/11/2285.
- Jolivet E, Daguet E, Pomero V, et al. Volumic patient-specific reconstruction of muscular system based on a reduced dataset of medical images. Comput Methods Biomech Biomed Engin. 2008;11(3):281–290. doi: 10.1080/10255840801959479.
- BOISSIèRE L, Moal B, Gille O, et al. Lumbar spinal muscles and spinal canal study by MRI three-dimensional reconstruction in adult lumbar spinal stenosis. Orthop Traumatol Surg Res. 2017;103(2):279–283. doi: 10.1016/j.otsr.2016.10.025.
- Lee SY, Kim DH, Park SJ, et al. Novel lateral whole-body dual-energy X-ray absorptiometry of lumbar paraspinal muscle mass: results from the SarcoSpine study. J Cachexia Sarcopenia Muscle. 2021;12(4):913–920. doi: 10.1002/jcsm.12721.
- Zi Y, Zhang B, Liu L, et al. Fat content in lumbar paravertebral muscles: quantitative and qualitative analysis using dual-energy CT in correlation to MR imaging. Eur J Radiol. 2022;148:110150. doi: 10.1016/j.ejrad.2021.110150.
- Molwitz I, Leiderer M, Mcdonough R, et al. Skeletal muscle fat quantification by dual-energy computed tomography in comparison with 3T MR imaging. Eur Radiol. 2021;31(10):7529–7539. doi: 10.1007/s00330-021-07820-1.
- Huang CWC, Tseng IJ, Yang SW, et al. Lumbar muscle volume in postmenopausal women with osteoporotic compression fractures: quantitative measurement using MRI. Eur Radiol. 2019;29(9):4999–5006. doi: 10.1007/s00330-019-06034-w.
- Kim M, Chon J, Lee SA, et al. Does unilateral lumbosacral radiculopathy affect the association between lumbar spinal muscle morphometry and bone mineral density?. Int J Environ Res Public Health. 2021;18(24):13155. doi: 10.3390/ijerph182413155.
- Abbott R, Pedler A, Sterling M, et al. The geography of fatty infiltrates within the cervical multifidus and semispinalis cervicis in individuals with chronic whiplash-associated disorders. J Orthop Sports Phys Ther. 2015;45(4):281–288. doi: 10.2519/jospt.2015.5719.
- Smith AC, Albin SR, Abbott R, et al. Confirming the geography of fatty infiltration in the deep cervical extensor muscles in whiplash recovery. Sci Rep. 2020;10(1):11471. doi: 10.1038/s41598-020-68452-x.
- Karlsson A, Leinhard OD, Åslund U, et al. An investigation of fat infiltration of the multifidus muscle in patients with severe neck symptoms associated with chronic Whiplash-Associated disorder. J Orthop Sports Phys Ther. 2016;46(10):886–893. doi: 10.2519/jospt.2016.6553.
- Abbott R, Peolsson A, West J, et al. The qualitative grading of muscle fat infiltration in whiplash using fat and water magnetic resonance imaging. Spine J. 2018;18(5):717–725. doi: 10.1016/j.spinee.2017.08.233.
- Elliott JM, Courtney DM, Rademaker A, et al. The rapid and progressive degeneration of the cervical multifidus in whiplash: an MRI study of fatty infiltration. Spine. 2015;40(12):E694–700. doi: 10.1097/BRS.0000000000000891.
- Rahnama L, Peterson G, Kazemnejad A, et al. Alterations in the mechanical response of deep dorsal neck muscles in individuals experiencing Whiplash-Associated disorders compared to healthy controls: an ultrasound study. Am J Phys Med Rehabil. 2018;97(2):75–82. doi: 10.1097/PHM.0000000000000845.
- Valera-Calero JA, FERNáNDEZ-DE-LAS-PEñAS C, Cleland JA, et al. Ultrasound assessment of deep cervical extensors morphology and quality in populations with whiplash associated disorders: an intra- and inter-examiner reliability study. Musculoskelet Sci Pract. 2022;59:102538. doi: 10.1016/j.msksp.2022.102538.
- Snodgrass SJ, Stanwell P, Weber KA, et al. Greater muscle volume and muscle fat infiltrate in the deep cervical spine extensor muscles (multifidus with semispinalis cervicis) in individuals with chronic idiopathic neck pain compared to age and sex-matched asymptomatic controls: a cross-sectional study. BMC Musculoskelet Disord. 2022;23(1):973. doi: 10.1186/s12891-022-05924-3.
- Thakar S, Mohan D, Furtado SV, et al. Paraspinal muscle morphometry in cervical spondylotic myelopathy and its implications in clinicoradiological outcomes following Central corpectomy: clinical article. J Neurosurg Spine. 2014;21(2):223–230. doi: 10.3171/2014.4.SPINE13627.
- Hou X, Lu S, Wang B, et al. Morphologic characteristics of the deep cervical paraspinal muscles in patients with Single-Level cervical spondylotic myelopathy. World Neurosurg. 2020;134:e166–e71. doi: 10.1016/j.wneu.2019.09.162.
- Tamai K, Grisdela P, JR., Romanu J, et al. The impact of cervical spinal muscle degeneration on cervical sagittal balance and spinal degenerative disorders. Clin Spine Surg. 2019;32(4):E206–e13. doi: 10.1097/BSD.0000000000000789.
- Yuksel Y, Ergun T, Torun E. The relationship between the flexor and extensor muscle areas and the presence and degree of intervertebral disc degeneration in the cervical region. Medicine (Baltimore). 2022;101(42):e31132. doi: 10.1097/MD.0000000000031132.
- Fortin M, Dobrescu O, Courtemanche M, et al. Association between paraspinal muscle morphology, clinical symptoms, and functional status in patients with degenerative cervical myelopathy. Spine (Phila Pa 1976). 2017;42(4):232–239. doi: 10.1097/BRS.0000000000001704.
- Cloney M, Smith AC, Coffey T, et al. Fatty infiltration of the cervical multifidus musculature and their clinical correlates in spondylotic myelopathy. J Clin Neurosci. 2018;57:208–213. doi: 10.1016/j.jocn.2018.03.028.
- Doi T, Ohtomo N, Oguchi F, et al. Association between deep posterior cervical paraspinal muscle morphology and clinical features in patients with cervical ossification of the posterior longitudinal ligament. Global Spine J. 2023;13(1):8–16. doi: 10.1177/2192568221989655.
- Fortin M, Wilk N, Dobrescu O, et al. Relationship between cervical muscle morphology evaluated by MRI, cervical muscle strength and functional outcomes in patients with degenerative cervical myelopathy. Musculoskelet Sci Pract. 2018;38:1–7. doi: 10.1016/j.msksp.2018.07.003.
- Mitsutake T, Sakamoto M, Chyuda Y, et al. Greater cervical muscle fat infiltration evaluated by magnetic resonance imaging is associated with poor postural stability in patients with cervical spondylotic radiculopathy. Spine. 2016;41(1):E8–14. doi: 10.1097/BRS.0000000000001196.
- Yun Y, Lee EJ, Kim Y, et al. Asymmetric atrophy of cervical multifidus muscles in patients with chronic unilateral cervical radiculopathy. Medicine. 2019;98(32):e16041. doi: 10.1097/MD.0000000000016041.
- Ekşi M, Özcan-Ekşi EE, Orhun Ö, et al. Proposal for a new scoring system for spinal degeneration: mo-Fi-Disc. Clin Neurol Neurosurg. 2020;198:106120. doi: 10.1016/j.clineuro.2020.106120.
- Huang Z, Bai Z, Yan J, et al. Association between muscle morphology changes, cervical spine degeneration, and clinical features in patients with chronic nonspecific neck pain: a magnetic resonance imaging analysis. World Neurosurg. 2022;159:e273–e84. doi: 10.1016/j.wneu.2021.12.041.
- Yeung K H, Man GCW, Shi L, et al. Magnetic resonance Imaging-Based morphological change of paraspinal muscles in girls with adolescent idiopathic scoliosis. Spine. 2019;44(19):1356–1363. doi: 10.1097/BRS.0000000000003078.
- Wajchenberg M, Astur N, Fernandes EA, et al. Assessment of fatty infiltration of the multifidus muscle in patients with adolescent idiopathic scoliosis through evaluation by magnetic resonance imaging compared with histological analysis: a diagnostic accuracy study. J Pediatr Orthop B. 2019;28(4):362–367. doi: 10.1097/BPB.0000000000000578.
- Zapata KA, Wang-Price SS, Sucato DJ, et al. Ultrasonographic measurements of paraspinal muscle thickness in adolescent idiopathic scoliosis: a comparison and reliability study. Pediatr Phys Ther. 2015;27(2):119–125. doi: 10.1097/PEP.0000000000000131.
- Shahidi B, Yoo A, Farnsworth C, et al. Paraspinal muscle morphology and composition in adolescent idiopathic scoliosis: a histological analysis. JOR Spine. 2021;4(3):e1169. doi: 10.1002/jsp2.1169.
- Wajchenberg M, Martins DE, Luciano RP, et al. Histochemical analysis of paraspinal rotator muscles from patients with adolescent idiopathic scoliosis: a cross-sectional study. Medicine. 2015;94(8):e598. doi: 10.1097/MD.0000000000000598.
- Stetkarova I, Zamecnik J, Bocek V, et al. Electrophysiological and histological changes of paraspinal muscles in adolescent idiopathic scoliosis. Eur Spine J. 2016;25(10):3146–3153. doi: 10.1007/s00586-016-4628-8.
- Yagi M, Hosogane N, Watanabe K, et al. The paravertebral muscle and psoas for the maintenance of global spinal alignment in patient with degenerative lumbar scoliosis. Spine J. 2016;16(4):451–458. doi: 10.1016/j.spinee.2015.07.001.
- Xia W, Fu H, Zhu Z, et al. Association between back muscle degeneration and spinal-pelvic parameters in patients with degenerative spinal kyphosis. BMC Musculoskelet Disord. 2019;20(1):454. doi: 10.1186/s12891-019-2837-0.
- Xie D, Zhang J, Ding W, et al. Abnormal change of paravertebral muscle in adult degenerative scoliosis and its association with bony structural parameters. Eur Spine J. 2019;28(7):1626–1637. doi: 10.1007/s00586-019-05958-7.
- Sun XY, Kong C, Zhang TT, et al. Correlation between multifidus muscle atrophy, spinopelvic parameters, and severity of deformity in patients with adult degenerative scoliosis: the parallelogram effect of LMA on the diagonal through the apical vertebra. J Orthop Surg Res. 2019;14(1):276. doi: 10.1186/s13018-019-1323-6.
- Wang G, Karki SB, Xu S, et al. Quantitative MRI and X-ray analysis of disc degeneration and paraspinal muscle changes in degenerative spondylolisthesis. J Back Musculoskelet Rehabil. 2015;28(2):277–285. doi: 10.3233/BMR-140515.
- Wang Z, Tian Y, Li C, et al. Radiographic risk factors for degenerative lumbar spondylolisthesis: a comparison with healthy control subjects. Front Surg. 2022;9:956696. doi: 10.3389/fsurg.2022.956696.
- Lee ET, Lee SA, Soh Y, et al. Association of lumbar paraspinal muscle morphometry with degenerative spondylolisthesis. Int J Environ Res Public Health. 2021;18(8):4037. doi: 10.3390/ijerph18084037.
- Li C, Wang L, Wang Z, et al. Radiological changes of paraspinal muscles: a comparative study of patients with isthmic spondylolisthesis, patients with degenerative lumbar spondylolisthesis, and healthy subjects. J Pain Res. 2022;15:3563–3573. doi: 10.2147/JPR.S376575.
- Park JH, Kim KW, Youn Y, et al. Association of MRI-defined lumbar paraspinal muscle mass and slip percentage in degenerative and isthmic spondylolisthesis: a multicenter, retrospective, observational study. Medicine. 2019;98(49):e18157. doi: 10.1097/MD.0000000000018157.
- Wagner SC, Sebastian AS, Mckenzie JC, et al. Severe lumbar disability is associated with decreased psoas cross-sectional area in degenerative spondylolisthesis. Global Spine J. 2018;8(7):716–721. doi: 10.1177/2192568218765399.
- Wang W, Guo Y, Li W, et al. The difference of paraspinal muscle between patients with lumbar spinal stenosis and normal Middle-aged and elderly people, studying by propensity score matching. Front Endocrinol (Lausanne). 2022;13:1080033. doi: 10.3389/fendo.2022.1080033.
- Jiang J, Wang H, Wang L, et al. Multifidus degeneration, a new risk factor for lumbar spinal stenosis: a Case-Control study. World Neurosurg. 2017;99:226–231. doi: 10.1016/j.wneu.2016.11.142.
- Sun D, Wang Z, Mou J, et al. Characteristics of paraspinal muscle degeneration in degenerative diseases of the lumbar spine at different ages. Clin Neurol Neurosurg. 2022;223:107484. doi: 10.1016/j.clineuro.2022.107484.
- Fortin M, LAZáRY À, Varga PP, et al. Association between paraspinal muscle morphology, clinical symptoms and functional status in patients with lumbar spinal stenosis. Eur Spine J. 2017;26(10):2543–2551. doi: 10.1007/s00586-017-5228-y.
- Getzmann JM, Ashouri H, Burgstaller JM, et al. The effect of paraspinal fatty muscle infiltration and cumulative lumbar spine degeneration on the outcome of patients with lumbar spinal canal stenosis: analysis of the lumbar stenosis outcome study (LSOS) data. Spine. 2023;48(2):97–106. doi: 10.1097/BRS.0000000000004477.
- Liu Y, Liu Y, Hai Y, et al. Lumbar lordosis reduction and disc bulge may correlate with multifidus muscle fatty infiltration in patients with single-segment degenerative lumbar spinal stenosis. Clin Neurol Neurosurg. 2020;189:105629. doi: 10.1016/j.clineuro.2019.105629.
- Liu Y, Liu Y, Hai Y, et al. Multifidus muscle fatty infiltration as an index of dysfunction in patients with single-segment degenerative lumbar spinal stenosis: a case-control study based on propensity score matching. J Clin Neurosci. 2020;75:139–148. doi: 10.1016/j.jocn.2020.03.001.
- Chen YY, Pao JL, Liaw CK, et al. Image changes of paraspinal muscles and clinical correlations in patients with unilateral lumbar spinal stenosis. Eur Spine J. 2014;23(5):999–1006. doi: 10.1007/s00586-013-3148-z.
- Xia G, Li X, Shang Y, et al. Correlation between severity of spinal stenosis and multifidus atrophy in degenerative lumbar spinal stenosis. BMC Musculoskelet Disord. 2021;22(1):536. doi: 10.1186/s12891-021-04411-5.
- Han G, Zou D, Liu Z, et al. Fat infiltration of paraspinal muscles as an independent risk for bone nonunion after posterior lumbar interbody fusion. BMC Musculoskelet Disord. 2022;23(1):232. doi: 10.1186/s12891-022-05178-z.
- Wang W, Sun Z, Li W, et al. The effect of paraspinal muscle on functional status and recovery in patients with lumbar spinal stenosis. J Orthop Surg Res. 2020;15(1):235. doi: 10.1186/s13018-020-01751-1.
- Liu Y, Liu Y, Hai Y, et al. Fat infiltration in the multifidus muscle as a predictor of prognosis after decompression and fusion in patients with single-segment degenerative lumbar spinal stenosis: an ambispective cohort study based on propensity score matching. World Neurosurg. 2019;128:e989–e1001. doi: 10.1016/j.wneu.2019.05.055.
- Han G, Zou D, Li X, et al. Can fat infiltration in the multifidus muscle be a predictor of postoperative symptoms and complications in patients undergoing lumbar fusion for degenerative lumbar spinal stenosis? A case-control study. J Orthop Surg Res. 2022;17(1):289. doi: 10.1186/s13018-022-03186-2.
- Zhu W, Sun K, Li X, et al. Symptomatic sagittal imbalance and severe degeneration of paraspinal muscle predispose suboptimal outcomes after lumbar short fusion surgery for degenerative lumbar spinal stenosis. World Neurosurg. 2022;164:e741–e8. doi: 10.1016/j.wneu.2022.05.044.
- Zotti MGT, Boas FV, Clifton T, et al. Does pre-operative magnetic resonance imaging of the lumbar multifidus muscle predict clinical outcomes following lumbar spinal decompression for symptomatic spinal stenosis?. Eur Spine J. 2017;26(10):2589–2597. doi: 10.1007/s00586-017-4986-x.
- Yazici A, Yerlikaya T. The relationship between the degeneration and asymmetry of the lumbar multifidus and erector spinae muscles in patients with lumbar disc herniation with and without root compression. J Orthop Surg Res. 2022;17(1):541. doi: 10.1186/s13018-022-03444-3.
- Liu C, Xue J, Liu J, et al. Is there a correlation between upper lumbar disc herniation and multifidus muscle degeneration? A retrospective study of MRI morphology. BMC Musculoskelet Disord. 2021;22(1):92. doi: 10.1186/s12891-021-03970-x.
- YALTıRıK K, GüDü BO, IŞıK Y, et al. Volumetric muscle measurements indicate significant muscle degeneration in Single-Level disc herniation patients. World Neurosurg. 2018;116:e500–e4. doi: 10.1016/j.wneu.2018.05.019.
- Ekin EE, Kurtul YıLDıZ H, Mutlu H. Age and sex-based distribution of lumbar multifidus muscle atrophy and coexistence of disc hernia: an MRI study of 2028 patients. Diagn Interv Radiol. 2016;22(3):273–276. doi: 10.5152/dir.2015.15307.
- Sun D, Liu P, Cheng J, et al. Correlation between intervertebral disc degeneration, paraspinal muscle atrophy, and lumbar facet joints degeneration in patients with lumbar disc herniation. BMC Musculoskelet Disord. 2017;18(1):167. doi: 10.1186/s12891-017-1522-4.
- Faur C, Patrascu JM, Haragus H, et al. Correlation between multifidus fatty atrophy and lumbar disc degeneration in low back pain. BMC Musculoskelet Disord. 2019;20(1):414. doi: 10.1186/s12891-019-2786-7.
- Naghdi N, Mohseni-Bandpei MA, Taghipour M, et al. Lumbar multifidus muscle morphology changes in patient with different degrees of lumbar disc herniation: an ultrasonographic study. Medicina. 2021;57(7):699. doi: 10.3390/medicina57070699.
- Choi TY, Chang MY, Lee SH, et al. Psoas muscle measurement as a predictor of recurrent lumbar disc herniation: a retrospective blind study. Medicine. 2022;101(26):e29778. doi: 10.1097/MD.0000000000029778.
- Zhong Y, Liu J, Zhou W, et al. Relationship between straight leg-raising test measurements and area of fat infiltration in multifidus muscles in patients with lumbar disc hernation. J Back Musculoskelet Rehabil. 2020;33(1):57–63. doi: 10.3233/BMR-181304.
- Hodges P, Holm AK, Hansson T, et al. Rapid atrophy of the lumbar multifidus follows experimental disc or nerve root injury. Spine. 2006;31(25):2926–2933. doi: 10.1097/01.brs.0000248453.51165.0b.
- BATTIé MC, Niemelainen R, Gibbons LE, et al. Is level- and side-specific multifidus asymmetry a marker for lumbar disc pathology? Spine J. 2012;12(10):932–939. doi: 10.1016/j.spinee.2012.08.020.
- Wan Q, Lin C, Li X, et al. MRI assessment of paraspinal muscles in patients with acute and chronic unilateral low back pain. Br J Radiol. 2015;88(1053):20140546. doi: 10.1259/bjr.20140546.
- Xiao Y, Fortin M, Ahn J, et al. Statistical morphological analysis reveals characteristic paraspinal muscle asymmetry in unilateral lumbar disc herniation. Sci Rep. 2021;11(1):15576. doi: 10.1038/s41598-021-95149-6.
- Saragiotto BT, Maher CG, Yamato TP, et al. Motor control exercise for chronic non-specific low-back pain. Cochrane Database Syst Rev. 2016;2016(1):Cd012004. doi: 10.1002/14651858.CD012004.
- Danneels LA, Vanderstraeten GG, Cambier DC, et al. CT imaging of trunk muscles in chronic low back pain patients and healthy control subjects. Eur Spine J. 2000;9(4):266–272. doi: 10.1007/s005860000190.
- Mengiardi B, Schmid MR, Boos N, et al. Fat content of lumbar paraspinal muscles in patients with chronic low back pain and in asymptomatic volunteers: quantification with MR spectroscopy. Radiology. 2006;240(3):786–792. doi: 10.1148/radiol.2403050820.
- Seyedhoseinpoor T, Taghipour M, Dadgoo M, et al. Alteration of lumbar muscle morphology and composition in relation to low back pain: a systematic review and meta-analysis. Spine J. 2022;22(4):660–676. doi: 10.1016/j.spinee.2021.10.018.
- Ranger TA, Cicuttini FM, Jensen TS, et al. Are the size and composition of the paraspinal muscles associated with low back pain? A systematic review. Spine J. 2017;17(11):1729–1748. doi: 10.1016/j.spinee.2017.07.002.
- Ogon I, Takebayashi T, Takashima H, et al. Magnetic resonance spectroscopic analysis of multifidus muscles lipid content and association with spinopelvic malalignment in chronic low back pain. Br J Radiol. 2017;90(1073):20160753. doi: 10.1259/bjr.20160753.
- Takashima H, Takebayashi T, Ogon I, et al. Analysis of intra and extramyocellular lipids in the multifidus muscle in patients with chronic low back pain using MR spectroscopy. Br J Radiol. 2018;91(1083):20170536. doi: 10.1259/bjr.20170536.
- Cankurtaran D, Yigman ZA, Umay E. Factors associated with paravertebral muscle cross-sectional area in patients with chronic low back pain. Korean J Pain. 2021;34(4):454–462. doi: 10.3344/kjp.2021.34.4.454.
- Sions JM, Elliott JM, Pohlig RT, et al. Trunk muscle characteristics of the multifidi, erector spinae, psoas, and quadratus lumborum in older adults with and without chronic low back pain. J Orthop Sports Phys Ther. 2017;47(3):173–179. doi: 10.2519/jospt.2017.7002.
- Xu WB, Chen S, Fan SW, et al. Facet orientation and tropism: associations with asymmetric lumbar paraspinal and psoas muscle parameters in patients with chronic low back pain. J Back Musculoskelet Rehabil. 2016;29(3):581–586. doi: 10.3233/BMR-160661.
- Zhao X, Liang H, Hua Z, et al. The morphological characteristics of paraspinal muscles in young patients with unilateral neurological symptoms of lumbar disc herniation. BMC Musculoskelet Disord. 2022;23(1):994. doi: 10.1186/s12891-022-05968-5.
- UçAR İ, KARARTı C, CüCE İ, et al. The relationship between muscle size, obesity, body fat ratio, pain and disability in individuals with and without nonspecific low back pain. Clin Anat. 2021;34(8):1201–1207. doi: 10.1002/ca.23776.
- Takashima H, Takebayashi T, Ogon I, et al. Evaluation of intramyocellular and extramyocellular lipids in the paraspinal muscle in patients with chronic low back pain using MR spectroscopy: preliminary results. Br J Radiol. 2016;89(1064):20160136. doi: 10.1259/bjr.20160136.
- Cunningham E, Wedderkopp N, Kjaer P, et al. The relationships between physical activity, lumbar multifidus muscle morphology, and low back pain from childhood to early adulthood: a 12-year longitudinal study. Sci Rep. 2022;12(1):8851. doi: 10.1038/s41598-022-12674-8.
- Singh R, Yadav SK, Sood S, et al. Magnetic resonance imaging of lumbar trunk parameters in chronic low backache patients and healthy population: a comparative study. Eur Spine J. 2016;25(9):2864–2872. doi: 10.1007/s00586-016-4698-7.
- Sasaki T, Yoshimura N, Hashizume H, et al. MRI-defined paraspinal muscle morphology in japanese population: the Wakayama spine study. PLoS One. 2017;12(11):e0187765. doi: 10.1371/journal.pone.0187765.
- Hildebrandt M, Fankhauser G, Meichtry A, et al. Correlation between lumbar dysfunction and fat infiltration in lumbar multifidus muscles in patients with low back pain. BMC Musculoskelet Disord. 2017;18(1):12. doi: 10.1186/s12891-016-1376-1.
- Teichtahl AJ, Urquhart DM, Wang Y, et al. Physical inactivity is associated with narrower lumbar intervertebral discs, high fat content of paraspinal muscles and low back pain and disability. Arthritis Res Ther. 2015;17(1):114. doi: 10.1186/s13075-015-0629-y.
- Bouche KG, Vanovermeire O, Stevens VK, et al. Computed tomographic analysis of the quality of trunk muscles in asymptomatic and symptomatic lumbar discectomy patients. BMC Musculoskelet Disord. 2011;12(1):65. doi: 10.1186/1471-2474-12-65.
- Ghiasi MS, Arjmand N, Shirazi-Adl A, et al. Cross-sectional area of human trunk paraspinal muscles before and after posterior lumbar surgery using magnetic resonance imaging. Eur Spine J. 2016;25(3):774–782. doi: 10.1007/s00586-015-4014-y.
- Lee D, Cha B, Kim J, et al. Paraspinal muscles atrophy on both sides and at multiple levels after unilateral lumbar partial discectomy. Medicine. 2023;102(3):e32688. doi: 10.1097/MD.0000000000032688.
- Lee YS, Lee S, Ko MJ, et al. Preservation of deep cervical extensor muscle volume: comparison between conventional Open-Door and muscle preserving laminoplasty approaches in the same patients. World Neurosurg. 2020;141:e514–e23. doi: 10.1016/j.wneu.2020.05.225.
- He W, He D, Sun Y, et al. Quantitative analysis of paraspinal muscle atrophy after oblique lateral interbody fusion alone vs. combined with percutaneous pedicle screw fixation in patients with spondylolisthesis. BMC Musculoskelet Disord. 2020;21(1):30. doi: 10.1186/s12891-020-3051-9.
- Lin GX, Ma YM, Xiao YC, et al. The effect of posterior lumbar dynamic fixation and intervertebral fusion on paraspinal muscles. BMC Musculoskelet Disord. 2021;22(1):1049. doi: 10.1186/s12891-021-04943-w.
- Guo Q, Xu Y, Fang Z, et al. Clinical and radiological outcomes of two modified open-door laminoplasties based on a novel paraspinal approach for treatment of multilevel cervical spondylotic myelopathy. Spine. 2022;47(6):E222–e32. doi: 10.1097/BRS.0000000000004254.
- Fan SW, Hu ZJ, Fang XQ, et al. Comparison of paraspinal muscle injury in one-level lumbar posterior inter-body fusion: modified minimally invasive and traditional open approaches. Orthop Surg. 2010;2(3):194–200. doi: 10.1111/j.1757-7861.2010.00086.x.
- Li Y, Chen Y, Liu Y, et al. Changes in paraspinal muscles and facet joints after minimally invasive posterior lumbar interbody fusion using the cortical bone trajectory technique: a prospective study. Pain Res Manag. 2022;2022:2690291–2690297. doi: 10.1155/2022/2690291.
- Liu X, Yuan S, Tian Y. Modified unilateral laminotomy for bilateral decompression for lumbar spinal stenosis: technical note. Spine. 2013;38(12):E732–7. doi: 10.1097/BRS.0b013e31828fc84c.
- Yoo JS, Min SH, Yoon SH, et al. Paraspinal muscle changes of unilateral multilevel minimally invasive transforaminal interbody fusion. J Orthop Surg Res. 2014;9(1):130. doi: 10.1186/s13018-014-0130-3.
- Junhui L, Zhengbao P, Wenbin X, et al. Comparison of pedicle fixation by the wiltse approach and the conventional posterior open approach for thoracolumbar fractures, using MRI, histological and electrophysiological analyses of the multifidus muscle. Eur Spine J. 2017;26(5):1506–1514. doi: 10.1007/s00586-017-5010-1.
- Ntilikina Y, Bahlau D, Garnon J, et al. Open versus percutaneous instrumentation in thoracolumbar fractures: magnetic resonance imaging comparison of paravertebral muscles after implant removal. J Neurosurg Spine. 2017;27(2):235–241. doi: 10.3171/2017.1.SPINE16886.
- Choi MK, Kim SB, Park CK, et al. Relation of deep paraspinal muscles’ Cross-Sectional area of the cervical spine and bone union in anterior cervical decompression and fusion: a retrospective study. World Neurosurg. 2016;96:91–100. doi: 10.1016/j.wneu.2016.08.104.
- Kim JY, Ryu DS, Paik HK, et al. Paraspinal muscle, facet joint, and disc problems: risk factors for adjacent segment degeneration after lumbar fusion. Spine J. 2016;16(7):867–875. doi: 10.1016/j.spinee.2016.03.010.
- Kumaran Y, Shah A, Katragadda A, et al. Iatrogenic muscle damage in transforaminal lumbar interbody fusion and adjacent segment degeneration: a comparative finite element analysis of open and minimally invasive surgeries. Eur Spine J. 2021;30(9):2622–2630. doi: 10.1007/s00586-021-06909-x.
- Chen J, Li J, Sheng B, et al. Does preoperative morphology of multifidus influence the surgical outcomes of stand-alone lateral lumbar interbody fusion for lumbar spondylolisthesis?. Clin Neurol Neurosurg. 2022;215:107177. doi: 10.1016/j.clineuro.2022.107177.