Abstract
Background
Inflammation plays a key role in atherosclerosis development and progression. However, the role of novel inflammatory biomarker pathways, namely the SIRT1-NF-κB-sCD40L, in the etiopathogenesis of human atherosclerosis remains undefined. This study was designed to evaluate the changes and clinical implications of these inflammatory mediators in the plasma of patients with acute myocardial infarction (AMI).
Methods
The peripheral arterial blood of 88 participants (68 patients with AMI and 20 age-matched controls), was drawn prior to performing coronary angiography (CAG). The SIRT1, NF-κB, and sCD40L plasma levels were quantified using ELISA. Spearman’s analysis was used to evaluate the correlation between the three inflammatory markers, while Pearson’s test assessed their potential correlation with cardiac troponin T (TNT) levels. Sensitivity, specificity, and area under the ROC curve (AUC) were calculated as measures of diagnostic accuracy.
Results
Patients with AMI showed higher levels of circulating SIRT1, NF-κB, and sCD40L compared to the age-matched controls (p < 0.05). However, the plasma concentrations of these three inflammatory mediators did not differ between the ST-segment elevation myocardial infarction (STEMI) and non-STEMI (NSTEMI) patients. Additionally, in patients with AMI, the SIRT1 level was positively correlated with NF-κB and sCD40L levels (p < 0.001). Likewise, the levels of SIRT1, NF-κB and sCD40L were positively correlated with TNT levels (p < 0.001). More importantly, the ROC analysis showed that the diagnostic accuracy of AMI was significantly higher when NF-κB or sCD40L level was used in combination with TNT levels (p < 0.05).
Conclusions
The levels of the circulating inflammatory biomarkers, including SIRT1, NF-κB, and sCD40L, were significantly elevated in patients with AMI. These novel biomarkers can improve the diagnostic accuracy of AMI when combined with TNT.
AMI is a potentially lethal CAD and is the leading cause of mortality and morbidity worldwide. Inflammation plays a key role in atherosclerosis development and progression. The levels of the circulating novel inflammatory biomarkers, including SIRT1, NF-κB, and sCD40L, were significantly elevated in patients with AMI.
The SIRT1 level was positively correlated with NF-κB and sCD40L levels in patients with AMI.
The levels of SIRT1, NF-κB and sCD40L were positively correlated with TNT levels.
The ROC analysis showed that the diagnostic accuracy of AMI was significantly higher when NF-κB or sCD40L level was used in combination with TNT levels.
SIRT1/NF-κB/sCD40L axis inhibition is a potential new target for AMI treatment.
KEY MESSAGES
1. Introduction
Acute myocardial infarction (AMI) is a potentially lethal coronary atherosclerotic heart disease (CAD) and is the leading cause of mortality and morbidity worldwide [Citation1]. AMI is classified as ST-segment elevation myocardial infarction (STEMI) and non-STEMI (NSTEMI) depending on the ST segment elevation in the electrocardiogram. Outcomes in these patients remain a challenge despite improved diagnostic (high-sensitivity cardiac TNT) and treatment modalities, such as primary percutaneous coronary intervention (pPCI) [Citation2]. Risk stratification remains problematic, and the identification of novel predictors is crucial for improved outcomes [Citation2]. Presently, an urgent need for identifying novel biomarkers exists, which aids in early diagnosis, precise risk stratification, and personalized treatment, thereby reducing cardiovascular events and improving prognosis.
Atherosclerosis is a chronic immuno-inflammatory disease that involves lipid accumulation in the large arteries [Citation3,Citation4]. The presence of inflammation plays a crucial role in the etiopathogenesis of atherosclerosis, and inflammatory mediators synergistically facilitate the occurrences of acute events [Citation3,Citation5]. Increased inflammatory activity is associated with the progression of stable coronary disease and a higher risk of AMI [Citation5]. Nevertheless, the role of inflammatory mediators in AMI remains unclear, and thus far, routine measurement of inflammatory markers is not supported by the current international guidelines.
Silence information regulator 2-related enzyme 1 (SIRT1), also known as a longevity factor, is known to delay cardiovascular aging, including the development of atherosclerosis, by inhibiting oxidative stress and inflammation [Citation6–8]. The SIRT1 deacetylation targets are key components of the intracellular inflammatory response [Citation9–11]. However, the association of the SIRT1 plasma levels with AMI requires further elucidation.
Transcription factor nuclear factor-κB (NF-κB) is the central regulator of inflammatory reaction, and its activation is closely related to the pathogenesis of atherosclerosis [Citation12–14]. NF-κB is involved in numerous pathological processes of atherosclerosis, including foam cell formation, vascular inflammation, vascular smooth muscle cell proliferation, arterial calcification, and plaque progression [Citation12]. Conversely, inhibition of NF-κB signaling prevents atherosclerosis formation. SIRT1 interacts with NF-κB p65 and inhibits transcription via deacetylation of p65 in response to tumor necrosis factor-alpha (TNF-α) [Citation15–18]. Thus, the SIRT1/NF-κB inflammation axis plays a crucial role in the pathogenesis of atherosclerosis.
Soluble CD40 ligand (sCD40L) represents the activation of the CD40-CD40L signaling pathway, and its upregulation accelerates the progression of chronic inflammatory diseases, such as atherosclerosis [Citation19–24]. sCD40L levels are elevated in CAD [Citation25–28], and it has been proposed as a biomarker of atherothrombosis [Citation23,Citation29]. The expression of CD40 is known to be regulated by the SIRT1/NF-κB pathway [Citation30–33]. The SIRT1-NF-κB-sCD40L pathway, comprising novel inflammatory biomarkers, may play a crucial role in atherosclerosis development and progression.
Therefore, this case-control study was designed to evaluate the plasma levels of the SIRT1/NF-κB/sCD40L axis in patients with AMI. Furthermore, the diagnostic specificity and sensitivity of these novel biomarkers for AMI were analyzed and compared with the classic myocardial injury marker cardiac troponin T (TNT).
2. Materials and methods
2.1. Design and population
This case-control study comprised 88 adults participated who were divided into two groups: the AMI group (n = 68, including STEMI [n = 44] and NSTEMI [n = 24] patients), and an age-matched control group (n = 20) comprising patients without any established cardiovascular disease. The included patients with AMI were recruited at the Second Affiliated Hospital of Shantou University Medical College according to the following inclusion criteria. 1) Patients undergoing AMI Management with or without ST-segment elevation per the ESC guidelines [Citation1,Citation34], i.e. myocardial ischemia with myocardial necrosis, which is defined as cardiac TNT elevation and 2) angiography showing obstructive coronary artery disease. The age-matched control group participants were recruited according to the following inclusion criteria: 1) patients without any established cardiovascular diseases, such as heart failure, previous MI, stroke, or CAD, but presenting with at least one modifiable cardiovascular risk factor, such as smoking habits, hypertension, dyslipidemia, or type 2 diabetes mellitus. The exclusion criteria were: (a) patients with acute and chronic infectious diseases, a history of tumor, or nervous system disorders; (b) patients with acute pulmonary embolism, acute aortic syndrome, peripheral vascular embolism, or stroke; (c) patients with severe liver and kidney failure; (d) those with severe cardiac insufficiency; and (E) patients with a history of recent surgery or trauma.
All individuals provided written informed consent and the study protocol complied with the Declaration of Helsinki. The study was approved by the Medical Ethics Committee of the Second Affiliated Hospital of Shantou University Medical College (permit number: 2018-18).
2.2. Sampling and laboratory analysis
Prior to coronary angiography (CAG), arterial blood samples were collected in clean heparin anticoagulant tubes and centrifuged at 2–8 °C for 15 min at 1000 × g immediately after collection. Subsequently, the separated plasma was centrifuged for an additional 10 min at 10,000 × g for complete platelet separation. Lastly, all plasma samples were stored at -80 °C for further evaluation.
SIRT1 (Catalog Number Q96EB6, USA R&D Systems, Inc.), NF-κB (Catalog Number ab176648, USA Abcam Systems, Inc.) and sCD40L (Catalog Number P29965, USA R&D Systems, Inc.) concentrations were assayed using a commercial Human ELISA kit. All samples were subjected to at least three relatively independent repeated experiments.
TNT levels were measured using a sandwich electrochemiluminescence immunoassay in the Department of Laboratory Medicine (Elecsys TNT, Cobas h232 instrument; Roche Diagnostics). Serum creatinine (SCr), blood urea nitrogen (BUN), and blood lipid levels were also analyzed, and the patient’s sex and relevant medical history, including smoking, hypertension, and diabetes mellitus, were recorded at admission.
2.3. Statistical analysis
Continuous variables were presented as mean ± standard deviation (SD) or median (interquartile range, IQR). Between-group comparisons were performed using a student’s t-test or Mann–Whitney U-test. Categorical variables were expressed as numbers and percentages and compared using the Pearson chi-square test or Fisher’s exact test. Correlation analysis were performed using Pearson’s test or Spearman’s test. Data were analyzed using IBM SPSS statistics v.19 software.
MedCalc 16.8.4 statistical software was used to analyze the diagnostic accuracy of the factors by drawing the receiver operating characteristic curves (ROC) and area under the ROC curve (AUC), and the 95% confidence interval (CI) was described. The DeLong test was used to compare the AUCs. A P value of <0.05 indicated statistical significance.
3. Results
3.1. Participants characteristics
The 88 inpatients were divided into an AMI group (n = 68, including STEMI [n = 44] and NSTEMI [n = 24]) and an age-matched control group (n = 20) based on clinical data and CAG results. The clinical characteristics and biochemical parameters of the participants are shown in and Supplemnetary Material. Patients with AMI showed higher TNT (326.50 [60.75–1361.25] vs. < 40 ng/L, p < 0.001), Hs-CRP (14.86 [2.81–15.49] vs. 3.89 [0.45–4.63] mg/mL, p = 0.02) and SCr (95.89 [77.60–104.40] vs. 81.41 [69.98–93.30] μmol/L, p = 0.04) levels compared to the age-matched control group. There were no significant differences in age, sex, smoking history, presence of hypertension and diabetes mellitus, and blood lipid level between the two groups (p > 0.05).
Table 1. Comparison of the clinical characteristics between patients with AMI and adults with cardiovascular risk factors (age-matched controls).
3.2. Circulating SIRT1, NF-κB, and sCD40L levels
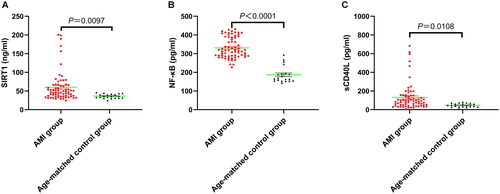
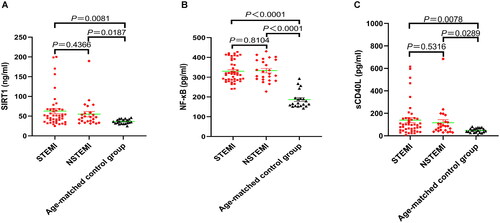
Compared with the age-matched controls, patients with AMI showed higher circulating levels of SIRT1 (60.03 ± 4.80 vs. 36.44 ± 1.32 ng/ml, p = 0.0097; ), NF-κB (331.20 ± 54.81 vs. 186.80 ± 43.71 pg/ml, p < 0.0001; ), and sCD40L (131.30 ± 17.30 vs. 47.63 ± 4.61 pg/ml, p = 0.0108; ). These results are consistent with the changes in Hs-CRP, which is a key inflammatory factor in atherosclerotic cardiovascular diseases. However, a subgroup analysis showed no difference in the plasma concentrations of these three inflammatory mediators between the STEMI and NSTEMI patients (p > 0.05, ). It is worth noting that compared with the age-matched controls, the levels of circulating inflammatory biomarker levels in the SIRT1-NF-κB-sCD40L pathway were significantly elevated both in STEMI and NSTEMI patients (p < 0.05, ).
3.3. Correlation between SIRT1, NF-κB, and sCD40L levels
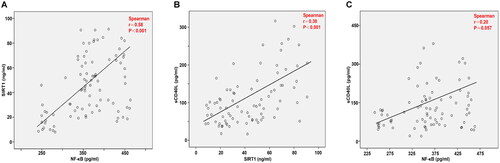
The results of the Spearman’s correlation analysis showed that SIRT1 levels were correlated with NF-κB (r = 0.58, p < 0.001; ) and sCD40L (r = 0.38, p < 0.001; ) levels. Notably, the correlation between NF-κB and sCD40L levels was not significant (r = 0.20, p = 0.057; ).
3.4. Correlation of SIRT1, NF-κB and sCD40L levels with TNT levels
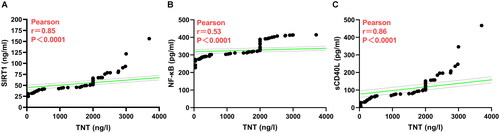
As shown in , the results of Pearson’s correlation analysis showed that SIRT1 (r = 0.85, p < 0.0001; ), NF-κB (r = 0.53, p < 0.0001; ) and sCD40L (r = 0.86, p < 0.0001; ) levels were positively correlated with TNT levels. The above results indicate that the elevated levels of these inflammatory cytokines contribute to the diagnosis of AMI.
3.5. Diagnostic accuracy of TNT alone and in combination with NF-κB and sCD40L
For evaluating the usefulness of these inflammatory mediators as potential biomarkers for identifying patients with AMI, ROC curves were constructed. In , the AUCs of NF-κB and sCD40L for predicting AMI occurrence were 0.97 (95% CI: 0.92–0.99) and 0.76 (95% CI: 0.66–0.85), respectively. The AUCs for these two inflammatory mediators were >0.75; thereby suggesting their utility as biomarkers. Compared with the AUC of TNT (0.95, 95% CI: 0.88–0.99), the laboratory gold standard for diagnosing AMI, NF-κB showed comparable sensitivity and specificity to TNT (Z-score: 0.85, p = 0.39); however, the diagnostic value of sCD40L was significantly lower than TNT (Z-score: 3.36, p = 0.0008).
Figure 5. Receiver operating characteristic (ROC) curves of potential biomarkers for the diagnosis of AMI. The ROC analysis and area under the ROC curve (AUC) are shown for NF-κB and sCD40L alone and combined with TNT.
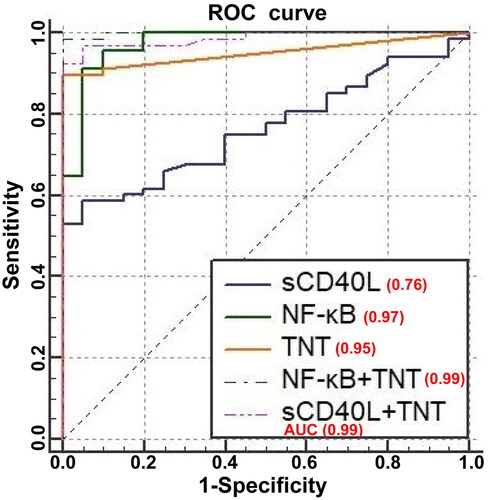
The results of the logistic regression analysis showed that compared with using TNT alone, NF-κB combined with TNT (NF-κB + TNT) had a significantly increased AUC (0.99, 95% CI: 0.96–1.00; p = 0.0107), thereby indicating a higher diagnostic value of NF-κB + TNT than that of TNT alone. Similarly, the AUC (0.99, 95% CI: 0.93–0.99; p = 0.0240) of sCD40L combined with TNT (sCD40L + TNT) was also significantly increased compared to TNT alone, suggesting a higher diagnostic value of sCD40L + TNT compared to TNT alone.
4. Discussion
To the best of our knowledge, the present study is the first to explore the changes in the circulating inflammatory mediators SIRT1, NF-κB, and sCD40L levels in patients with AMI. Three important findings were noted in this study. 1) the plasma concentrations of SIRT1, NF-κB, and sCD40L were measured before pPCI and their levels were significantly increased compared to the age-matched controls. 2) SIRT1 changes were positively correlated with NF-κB and sCD40L level changes; nevertheless, the correlation between the NF-κB and sCD40L levels was statistically insignificant. 3) The levels of SIRT1, NF-κB and sCD40L were positively correlated with TNT levels. 4) NF-κB and TNT had comparable sensitivity and specificity, and their combination significantly improved the diagnostic value of AMI. The inflammatory mediators comprising the SIRT1/NF-κB/sCD40L axis have possible use as novel biomarkers for AMI, and targeted regulation of this axis may reduce acute cardiovascular event incidences and improve patient prognosis.
SIRT1 is a nicotinamide adenine dinucleotide (NAD+) dependent class III deacetylase [Citation35,Citation36]. The biological effects of SIRT1, such as longevity regulation, delaying cardiovascular aging, including atherosclerosis formation, by inhibiting oxidative stress and inflammation, can be attributed to its deacetylation function [Citation6–8,Citation35,Citation36]. SIRT1 is downregulated in atherosclerotic plaques in patients and atherosclerotic animal models [Citation37–40]. Overexpression of SIRT1 (endothelial cell-specific) is protective against atherosclerosis [Citation41]; however, SIRT1 knockdown (smooth muscle cell-specific) has shown increased atherosclerosis formation in ApoE knockout mice [Citation38]. Treatment of ApoE knockout mice with SRT3025, a small molecule activator of SIRT1, showed significant atherosclerosis reduction [Citation42]. However, transgenic mice with systemically overexpressed SIRT1 showed more severe dyslipidemia and atherosclerosis when put under an atherogenic diet [Citation43]. This indicates that SIRT1 can have anti-atherosclerotic and pro-atherosclerotic effects. In the present study, the circulating SIRT1 level was significantly increased in patients with AMI. However, this result is in contrast to that of Breitenstein et al. who found reduced SIRT1 expression in the peripheral blood monocytes of patients with CAD [Citation39]. These differences may be attributed to the different types of CAD, and AMI belongs is a type of unstable CAD. Previous studies have confirmed that inflammation, oxidative stress, and aging can increase SIRT1 expression [Citation44–46], which could be a compensatory mechanism against the adverse effects of ischemia. Additionally, heart-specific overexpression of SIRT1 in mice showed that moderate expression (up to 7.5-fold) of SIRT1 retards aging and protects the heart from oxidative stress, whereas high levels (12.5-fold) of SIRT1 increase oxidative stress and apoptosis [Citation45]. This indicates that SIRT1’s cardioprotective effect is related to its expression level in the heart.
SIRT1 interacts with the NF-κB p65 and inhibits the transcription of p65 through deacetylation [Citation15–18]. NF-κB is a central regulator of inflammatory reaction, and its activation is closely related to the pathogenesis of atherosclerosis [Citation12–14]. Furthermore, it is involved in all stages of atherosclerosis, including early atherosclerotic lesion formation, plaque progression, and advanced atherosclerosis [Citation14]. NF-κB promotes the expression of multiple proinflammatory (TNF-α, IL-1β, and IL-6) and proadhesive genes (MCP1, ICAM-1, and VCAM-1) in endothelial cells [Citation12–14], thereby inducing endothelial dysfunction, which is a crucial initial factor in early atherosclerotic lesion formation. Recently, Karunakaran et al. reported that RIPK1 primarily drives NF-κB-dependent inflammation activation in early atherosclerotic lesion formation [Citation47]. Conversely, studies on ApoE knockout mice have shown that endothelial cell-specific inhibition of NF-κB reduces and stabilizes atherosclerotic plaques [Citation48,Citation49]. Furthermore, clinical NF-κB1 promoter gene polymorphism or mutation has been reported to be closely related to CAD susceptibility and prognosis [Citation50–52]. Subsequent meta-analyses further supported this conclusion [Citation53,Citation54], suggesting that the NF-κB signaling pathway, via its pro-inflammatory mechanisms, is related to CAD pathogenesis and progress. Consistent with these results, we found that the NF-κB plasma concentration in patients with AMI was higher than that in the age-matched controls. The results of Spearman’s correlation analysis showed that the SIRT1 level was positively correlated with NF-κB. The levels of SIRT1 and NF-κB were positively correlated with TNT levels. Moreover, NF-κB and TNT were found to have comparable sensitivity and specificity and their combination could significantly improve the diagnostic accuracy of AMI.
The expression of CD40 can be regulated by the SIRT1/NF-κB pathway [Citation30–33]. sCD40L represents the activation of the CD40-CD40L signaling pathway, and its upregulation can accelerate chronic inflammatory disease progress [Citation23]. Accumulating evidence indicates that the CD40-CD40L system plays a crucial role in atherosclerosis occurrence and development [Citation19–24]. The effect of CD40L on the size of atherosclerotic lesions remains controversial in animal models [Citation55]. Some studies have shown that blocking CD40L via gene knockout or antibodies, can reduce the lesion size [Citation56–58]; whereas, other studies found no effect on the lesion size [Citation59,Citation60]. Fortunately, recent studies have confirmed that CD40L cell-specific knockout can reduce experimental atherosclerosis. Bosmans et al. found that myeloid-specific CD40-deficient reduces atherosclerosis by preventing macrophage pro-inflammatory polarization [Citation61]. Additionally, T cell-specific and dendritic-cell-specific CD40L deficiency also reduces atherosclerosis in mice [Citation29]. Nevertheless, platelet-specific CD40L deficiency has not been found to have any effect on atherosclerotic plaque burden and is only involved in atherothrombosis [Citation29]. These newly recent consistently confirm that CD40L has cell-specific roles in atherosclerosis.
Likewise, the clinical correlation between sCD40L and atherosclerosis remains controversial. sCD40L is elevated in CAD [Citation25–28], and its levels are significantly higher in patients with acute coronary syndrome than in patients with stable angina [Citation62]. Moreover, the sCD40L level is positively correlated with multi-vessel lesions and high-burden thrombus formation in the coronary artery [Citation63]. Nevertheless, some studies found no correlation between sCD40L and CAD. de Lemos et al. found a negligible correlation between sCD40L and hyperlipidemia and no correlation between sCD40L and subclinical atherosclerosis [Citation64]. Notably, a study published by Gergei et al. found no correlation between sCD40L levels and 1-year cardiovascular mortality [Citation65]. The significant correlation between sCD40L levels and cardiovascular mortality was only identified in a subgroup analysis of patients with CAD and heart failure with preserved ejection fraction [Citation65]. Briefly, most of the existing clinical studies have shown that the sCD40L level is closely related to CAD, especially in acute cardiovascular events and unstable atherosclerotic plaques. sCD40L has been considered as a biomarker of atherothrombosis [Citation23,Citation29], which may be attributed to its key role in platelet activation and thrombosis [Citation66,Citation67]. Consistent with the above results, the present study also found that the sCD40L plasma levels were significantly elevated in patients with AMI. The results of the Spearman’s correlation analysis showed that the SIRT1 level was positively correlated with sCD40L. Moreover, the levels of sCD40L were positively correlated with TNT levels. The diagnostic accuracy of sCD40L alone is lower than that of TNT. However, a combination of sCD40L and TNT had significantly improved diagnostic value.
This had some limitations. Firstly, to rule out the effect of iodine-containing contrast agents on inflammatory mediators, this study only selected peripheral arterial blood samples from subjects before CAG and did not dynamically observe changes in the SIRT1/NF-κB/sCD40L axis. Secondly, due to strict screening, the sample size was relatively small, and this was a single-center study. Therefore, it is necessary to establish a large sample database; conduct multicenter, randomized, controlled trials; and obtain more data to form an evaluation consensus.
5. Conclusion
To the best of our knowledge, this study is the first to demonstrate that the levels of circulating inflammatory biomarkers of the SIRT1/NF-κB/sCD40L axis were significantly elevated in patients with AMI. The levels of SIRT1, NF-κB and sCD40L were positively correlated with TNT levels. When combined with TNT, these novel biomarkers can improve the diagnostic accuracy of AMI. Targeted regulation of inflammatory mediators or blocking related pathways can reduce cardiovascular events in patients with AMI, thereby improving patient prognosis. Therefore, SIRT1/NF-κB/sCD40L axis inhibition is a potential new target for AMI treatment.
Acknowledgements
The authors would like to thank ZYEdit Limited for the linguistic editing and proofreading of the manuscript.
Ethics approval and consent to participate
The study was approved by the Medical Ethics Committee of the Second Affiliated Hospital of Shantou University Medical College (permit number: 2018-18). All subjects provided a written consent form.
Consent for publication
Not applicable.
Authors’ contributions
C. Chen and W. Yu designed this program. M. Zheng and W. Wang conducted the data collection and analysis. M. Zheng produced the manuscript which was checked by W. Yu. All authors read and approved the final manuscript.
Supplemental Material
Download MS Excel (22.2 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Ibanez B, James S, Agewall S, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European society of cardiology (ESC). Eur Heart J. 2018;39(2):1–10. doi: 10.1093/eurheartj/ehx393.
- Pouralijan Amiri M, Khoshkam M, Salek RM, et al. Metabolomics in early detection and prognosis of acute coronary syndrome. Clin Chim Acta. 2019;495:43–53. doi: 10.1016/j.cca.2019.03.1632.
- Björkegren JLM, Lusis AJ. Atherosclerosis: recent developments. Cell. 2022;185(10):1630–1645. doi: 10.1016/j.cell.2022.04.004.
- Kong P, Cui ZY, Huang XF, et al. Inflammation and atherosclerosis: signaling pathways and therapeutic intervention. Signal Transduct Target Ther. 2022;7:131.
- Henein MY, Vancheri S, Longo G, et al. The role of inflammation in cardiovascular disease. Int J Mol Sci. 2022;23(21):12906. doi: 10.3390/ijms232112906.
- D’Onofrio N, Servillo L, Balestrieri ML. SIRT1 and SIRT6 signaling pathways in cardiovascular disease protection. Antioxid Redox Signal. 2018;28(8):711–732. doi: 10.1089/ars.2017.7178.
- Zhang MJ, Zhou Y, Chen L, et al. SIRT1 improves VSMC functions in atherosclerosis. Prog Biophys Mol Biol. 2016;121(1):11–15. doi: 10.1016/j.pbiomolbio.2016.02.003.
- Stein S, Matter CM. Protective roles of SIRT1 in atherosclerosis. Cell Cycle. 2011;10(4):640–647. doi: 10.4161/cc.10.4.14863.
- Hwang JW, Yao H, Caito S, et al. Redox regulation of SIRT1 in inflammation and cellular senescence. Free Radic Biol Med. 2013;61:95–110. doi: 10.1016/j.freeradbiomed.2013.03.015.
- Yang Y, Liu Y, Wang Y, et al. Regulation of SIRT1 and its roles in inflammation. Front Immunol. 2022;13:831168. doi: 10.3389/fimmu.2022.831168.
- Singh V, Ubaid S. Role of silent information regulator 1 (SIRT1) in regulating oxidative stress and inflammation. Inflammation. 2020;43(5):1589–1598. doi: 10.1007/s10753-020-01242-9.
- Baker RG, Hayden MS, Ghosh S. NF-κB, inflammation, and metabolic disease. Cell Metab. 2011;13(1):11–22. doi: 10.1016/j.cmet.2010.12.008.
- Niu N, Xu S, Xu Y, et al. Targeting mechanosensitive transcription factors in atherosclerosis. Trends Pharmacol Sci. 2019;40(4):253–266. doi: 10.1016/j.tips.2019.02.004.
- Li W, Jin K, Luo J, et al. NF-κB and its crosstalk with endoplasmic reticulum stress in atherosclerosis. Front Cardiovasc Med. 2022;9:988266. doi: 10.3389/fcvm.2022.988266.
- Kauppinen A, Suuronen T, Ojala J, et al. Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell Signal. 2013;25(10):1939–1948. doi: 10.1016/j.cellsig.2013.06.007.
- Barroso E, Eyre E, Palomer X, et al. The peroxisome proliferator-activated receptor β/δ (PPARβ/δ) agonist GW501516 prevents TNF-α-induced NF-κB activation in human HaCaT cells by reducing p65 acetylation through AMPK and SIRT1. Biochem Pharmacol. 2011;81(4):534–543. doi: 10.1016/j.bcp.2010.12.004.
- Kong P, Yu Y, Wang L, et al. circ-Sirt1 controls NF-κB activation via sequence-specific interaction and enhancement of SIRT1 expression by binding to miR-132/212 in vascular smooth muscle cells. Nucleic Acids Res. 2019;47(7):3580–3593. doi: 10.1093/nar/gkz141.
- Sun HJ, Xiong SP, Cao X, et al. Polysulfide-mediated sulfhydration of SIRT1 prevents diabetic nephropathy by suppressing phosphorylation and acetylation of p65 NF-κB and STAT3. Redox Biol. 2021;38:101813. doi: 10.1016/j.redox.2020.101813.
- Mach F, Schönbeck U, Sukhova GK, et al. Functional CD40 ligand is expressed on human vascular endothelial cells, smooth muscle cells, and macrophages: implications for CD40-CD40 ligand signaling in atherosclerosis. Proc Natl Acad Sci U S A. 1997;94(5):1931–1936. doi: 10.1073/pnas.94.5.1931.
- Laman JD, de Smet BJ, Schoneveld A, et al. CD40-CD40L interactions in atherosclerosis. Immunol Today. 1997;18(6):272–277. doi: 10.1016/s0167-5699(97)80022-9.
- Mach F, Schönbeck U, Libby P. CD40 signaling in vascular cells: a key role in atherosclerosis? Atherosclerosis. 1998;137 Suppl: S89–S95. doi: 10.1016/s0021-9150(97)00309-2.
- Schönbeck U, Libby P. CD40 signaling and plaque instability. Circ Res. 2001;89(12):1092–1103. doi: 10.1161/hh2401.101272.
- Antoniades C, Bakogiannis C, Tousoulis D, et al. The CD40/CD40 ligand system: linking inflammation with atherothrombosis. J Am Coll Cardiol. 2009;54(8):669–677. doi: 10.1016/j.jacc.2009.03.076.
- Bosmans LA, Bosch L, Kusters PJH, et al. The CD40-CD40L dyad as immunotherapeutic target in cardiovascular disease. J Cardiovasc Transl Res. 2021;14(1):13–22. doi: 10.1007/s12265-020-09994-3.
- Marx N, Imhof A, Froehlich J, et al. Effect of rosiglitazone treatment on soluble CD40L in patients with type 2 diabetes and coronary artery disease. Circulation. 2003;107(15):1954–1957. doi: 10.1161/01.CIR.0000069272.06194.91.
- Tayebjee MH, Lip GY, Tan KT, et al. Plasma matrix metalloproteinase-9, tissue inhibitor of metalloproteinase-2, and CD40 ligand levels in patients with stable coronary artery disease. Am J Cardiol. 2005;96(3):339–345. doi: 10.1016/j.amjcard.2005.03.072.
- Lee WL, Lee WJ, Chen YT, et al. The presence of metabolic syndrome is independently associated with elevated serum CD40 ligand and disease severity in patients with symptomatic coronary artery disease. Metabolism. 2006;55(8):1029–1034. doi: 10.1016/j.metabol.2006.03.013.
- Pereira-da-Silva T, Ferreira V, Castelo A, et al. Soluble CD40 ligand expression in stable atherosclerosis: a systematic review and meta-analysis. Atherosclerosis. 2021;319:86–100. doi: 10.1016/j.atherosclerosis.2020.12.011.
- Lacy M, Bürger C, Shami A, et al. Cell-specific and divergent roles of the CD40L-CD40 axis in atherosclerotic vascular disease. Nat Commun. 2021;12(1):3754. doi: 10.1038/s41467-021-23909-z.
- Lin QQ, Yan CF, Lin R, et al. SIRT1 regulates TNF-α-induced expression of CD40 in 3T3-L1 adipocytes via NF-κB pathway. Cytokine. 2012;60(2):447–455. doi: 10.1016/j.cyto.2012.05.025.
- Yang L, Zhang J, Yan C, et al. SIRT1 regulates CD40 expression induced by TNF-α via NF-ĸB pathway in endothelial cells. Cell Physiol Biochem. 2012;30(5):1287–1298. doi: 10.1159/000343318.
- Wang W, Bai L, Qiao H, et al. The protective effect of fenofibrate against TNF-α-induced CD40 expression through SIRT1-mediated deacetylation of NF-κB in endothelial cells. Inflammation. 2014;37(1):177–185. doi: 10.1007/s10753-013-9728-6.
- Lin Q, Geng Y, Zhao M, et al. MiR-21 regulates TNF-α-induced CD40 expression via the SIRT1-NF-κB pathway in renal inner medullary collecting duct cells. Cell Physiol Biochem. 2017;41(1):124–136. doi: 10.1159/000455981.
- Collet JP, Thiele H, Barbato E, et al. 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42(14):1289–1367. doi: 10.1093/eurheartj/ehaa575.
- Grootaert MOJ, Bennett MR. Sirtuins in atherosclerosis: guardians of healthspan and therapeutic targets. Nat Rev Cardiol. 2022;19(10):668–683. doi: 10.1038/s41569-022-00685-x.
- Kane AE, Sinclair DA. Sirtuins and NAD(+) in the development and treatment of metabolic and cardiovascular diseases. Circ Res. 2018;123(7):868–885. doi: 10.1161/CIRCRESAHA.118.312498.
- Thompson AM, Wagner R, Rzucidlo EM. Age-related loss of SirT1 expression results in dysregulated human vascular smooth muscle cell function. Am J Physiol Heart Circ Physiol. 2014;307(4):H533–541. doi: 10.1152/ajpheart.00871.2013.
- Gorenne I, Kumar S, Gray K, et al. Vascular smooth muscle cell sirtuin 1 protects against DNA damage and inhibits atherosclerosis. Circulation. 2013;127(3):386–396. doi: 10.1161/CIRCULATIONAHA.112.124404.
- Breitenstein A, Wyss CA, Spescha RD, et al. Peripheral blood monocyte Sirt1 expression is reduced in patients with coronary artery disease. PLOS One. 2013;8(1):e53106. doi: 10.1371/journal.pone.0053106.
- Rodella LF, Favero G, Rossini C, et al. Aging and vascular dysfunction: beneficial melatonin effects. Age. 2013;35(1):103–115. doi: 10.1007/s11357-011-9336-z.
- Zhang QJ, Wang Z, Chen HZ, et al. Endothelium-specific overexpression of class III deacetylase SIRT1 decreases atherosclerosis in apolipoprotein E-deficient mice. Cardiovasc Res. 2008;80(2):191–199. doi: 10.1093/cvr/cvn224.
- Miranda MX, van Tits LJ, Lohmann C, et al. The Sirt1 activator SRT3025 provides atheroprotection in Apoe-/- mice by reducing hepatic Pcsk9 secretion and enhancing Ldlr expression. Eur Heart J. 2015;36(1):51–59. doi: 10.1093/eurheartj/ehu095.
- Qiang L, Lin HV, Kim-Muller JY, et al. Proatherogenic abnormalities of lipid metabolism in SirT1 transgenic mice are mediated through creb deacetylation. Cell Metab. 2011;14(6):758–767. doi: 10.1016/j.cmet.2011.10.007.
- Akhmedov A, Camici GG, Reiner MF, et al. Endothelial LOX-1 activation differentially regulates arterial thrombus formation depending on oxLDL levels: role of the Oct-1/SIRT1 and ERK1/2 pathways. Cardiovasc Res. 2017;113(5):498–507. doi: 10.1093/cvr/cvx015.
- Alcendor RR, Gao S, Zhai P, et al. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ Res. 2007;100(10):1512–1521. doi: 10.1161/01.RES.0000267723.65696.4a.
- Chan SH, Chu PM, Kao CL, et al. Oleic acid activates MMPs up-regulation through SIRT1/PPAR-γ inhibition: a probable linkage between obesity and coronary arterial disease. J Biochem. 2016;160(4):217–225. doi: 10.1093/jb/mvw028.
- Karunakaran D, Nguyen MA, Geoffrion M, et al. RIPK1 expression associates with inflammation in early atherosclerosis in humans and can be therapeutically silenced to reduce NF-κB activation and atherogenesis in mice. Circulation. 2021;143(2):163–177. doi: 10.1161/CIRCULATIONAHA.118.038379.
- Gareus R, Kotsaki E, Xanthoulea S, et al. Endothelial cell-specific NF-kappaB inhibition protects mice from atherosclerosis. Cell Metab. 2008;8(5):372–383. doi: 10.1016/j.cmet.2008.08.016.
- Song D, Fang G, Mao SZ, et al. Selective inhibition of endothelial NF-κB signaling attenuates chronic intermittent hypoxia-induced atherosclerosis in mice. Atherosclerosis. 2018;270:68–75. doi: 10.1016/j.atherosclerosis.2018.01.027.
- Luo JY, Liu F, Fang BB, et al. NFKB1 gene mutant was associated with prognosis of coronary artery disease and exacerbated endothelial mitochondrial fission and dysfunction. Oxid Med Cell Longev. 2022;2022:9494926–9494913. doi: 10.1155/2022/9494926.
- Lai H, Chen Q, Li X, et al. Association between genetic polymorphism in NFKB1 and NFKBIA and coronary artery disease in a Chinese Han population. Int J Clin Exp Med. 2015;8:21487–21496.
- Lai HM, Li XM, Yang YN, et al. Genetic variation in NFKB1 and NFKBIA and susceptibility to coronary artery disease in a Chinese Uygur population. PLOS One. 2015;10(6):e0129144. doi: 10.1371/journal.pone.0129144.
- Wang Y, Wu B, Zhang M, et al. Significant association between rs28362491 polymorphism in NF-κB1 gene and coronary artery disease: a meta-analysis. BMC Cardiovasc Disord. 2020;20(1):278. doi: 10.1186/s12872-020-01568-0.
- Chen QJ, Lai HM, Zhao L, et al. Association between the NFKB1-94ins/del ATTG polymorphism (rs28362491) and coronary artery disease: a systematic review and meta-analysis. Genet Test Mol Biomarkers. 2016;20(3):105–111. doi: 10.1089/gtmb.2015.0242.
- Michel NA, Zirlik A, Wolf D. CD40L and its receptors in atherothrombosis-an update. Front Cardiovasc Med. 2017;4:40. doi: 10.3389/fcvm.2017.00040.
- Mach F, Schönbeck U, Sukhova GK, et al. Reduction of atherosclerosis in mice by inhibition of CD40 signalling. Nature. 1998;394(6689):200–203. doi: 10.1038/28204.
- Schönbeck U, Sukhova GK, Shimizu K, et al. Inhibition of CD40 signaling limits evolution of established atherosclerosis in mice. Proc Natl Acad Sci U S A. 2000;97(13):7458–7463. doi: 10.1073/pnas.97.13.7458.
- Bavendiek U, Zirlik A, LaClair S, et al. Atherogenesis in mice does not require CD40 ligand from bone marrow-derived cells. Arterioscler Thromb Vasc Biol. 2005;25(6):1244–1249. doi: 10.1161/01.ATV.0000161420.55482.ef.
- Lutgens E, Gorelik L, Daemen MJ, et al. Requirement for CD154 in the progression of atherosclerosis. Nat Med. 1999;5(11):1313–1316. doi: 10.1038/15271.
- Lutgens E, Cleutjens KB, Heeneman S, et al. Both early and delayed anti-CD40L antibody treatment induces a stable plaque phenotype. Proc Natl Acad Sci U S A. 2000;97(13):7464–7469. doi: 10.1073/pnas.97.13.7464.
- Bosmans LA, van Tiel CM, Aarts S, et al. Myeloid CD40 deficiency reduces atherosclerosis by impairing macrophages’ transition into a pro-inflammatory state. Cardiovasc Res. 2023;119(5):1146–1160. doi: 10.1093/cvr/cvac084.
- Peng DQ, Zhao SP, Li YF, et al. Elevated soluble CD40 ligand is related to the endothelial adhesion molecules in patients with acute coronary syndrome. Clin Chim Acta. 2002;319(1):19–26. doi: 10.1016/s0009-8981(02)00014-1.
- Zhao W, Zhang F, Li Z, et al. Soluble CD40 ligand is associated with angiographic severity of coronary artery disease in patients with acute coronary syndrome. Chin Med J. 2014;127:2218–2221.
- de Lemos JA, Zirlik A, Schönbeck U, et al. Associations between soluble CD40 ligand, atherosclerosis risk factors, and subclinical atherosclerosis: results from the Dallas heart study. Arterioscler Thromb Vasc Biol. 2005;25(10):2192–2196. doi: 10.1161/01.ATV.0000182904.08513.60.
- Gergei I, Kälsch T, Scharnagl H, et al. Association of soluble CD40L with short-term and long-term cardiovascular and all-cause mortality: the Ludwigshafen Risk and cardiovascular health (LURIC) study. Atherosclerosis. 2019;291:127–131. doi: 10.1016/j.atherosclerosis.2019.09.004.
- Kannan M, Ahmad F, Saxena R. Platelet activation markers in evaluation of thrombotic risk factors in various clinical settings. Blood Rev. 2019;37:100583. doi: 10.1016/j.blre.2019.05.007.
- Al-Tamimi AO, Yusuf AM, Jayakumar MN, et al. SARS-CoV-2 infection induces soluble platelet activation markers and PAI-1 in the early moderate stage of COVID-19. Int J Lab Hematol. 2022;44(4):712–721. doi: 10.1111/ijlh.13829.