Abstract
Objectives
The scope of lateral neck lymph node dissection (LND) in papillary thyroid carcinoma (PTC) remains controversial. Our research aimed to explore the value of central lymph node metastasis (CLNM) in frozen sections for predicting neck lateral lymph node metastasis (NLLNM) and to guide clinical surgeons in performing surgical lymph node dissection.
Patients
A total of 275 patients with PTC with suspected ‘Cervical lymph node metastasis (LNM, including CLNM and NLLNM)’ underwent unilateral or bilateral thyroidectomy and an intraoperative frozen diagnosis of central lymph nodes (LNs), as well as central and neck lateral LND. Validity indices and consistency of central LNs in frozen sections were calculated. In total, 216 patients then met the inclusion criteria and were enrolled in the follow-up study. The clinical and pathological data of the patients were retrospectively analyzed. The relationship between the number, metastatic diameter, and the ratio of CLNM to NLLNM was investigated.
Results
CLNM in frozen and paraffin-embedded sections was associated with NLLNM. Univariate and multivariate analyses revealed the following risk factors for NLLNM metastasis: maximum diameter, total number, and ratio of metastatic LNs. A significant result was obtained when a cut-off value of 2.050 mm for the maximum metastatic diameter, 5.5 in the total number, and 0.5342 for the CLNM ratio level was used. Interaction term analyses showed that the association between the number of CLNM and NLLNM differed according to maximum diameter.
Conclusion
Central LNs in frozen sections accurately predicted NLLNM. In patients with PTC with >5 CLNMs, ≥2 and ≤5 CLNMs and maximum metastatic diameter > 2 mm, neck lateral LND should be considered. Our findings will facilitate the identification of patients who are likely to benefit from extended lateral neck LND.
Introduction
Thyroid cancer is the most prevalent tumor of the endocrine system, and papillary thyroid carcinoma (PTC) is the most common pathological type observed in younger females [Citation1,Citation2]. PTC is characterized by early regional lymph node metastasis (LNM); about 80% of cases also have central lymph node metastasis (CLNM), and up to 60% have neck lateral lymph node metastasis (NLLNM) [Citation3,Citation4]. The incidence of regional LNM is very high in PTC and is associated with a higher risk of disease recurrence [Citation5,Citation6]. Surgical dissection of nodal-positive lymph nodes (LNs) in PTC improves patient survival and reduce recurrence [Citation7]. Therefore, it is generally believed that therapeutic cervical lymph node dissection is indicated in patients with PTC with clinically evident cervical LNM [Citation8,Citation9]. According to the American Thyroid Association (ATA) guidelines, therapeutic lymph node dissection (LND) should be performed in patients with biopsy-proven metastatic LNs [Citation10]. Because PTC has a strong propensity for CLNM, preoperative ultrasonography (US) can adequately diagnoses only about half of patients with CLNM [Citation11]. Furthermore, the combination of US with tumor size, adjacent anterior capsule, distance to the lower pole, and CDFI US features have been shown to improve the identification of CLNM [Citation12]. Patients with PTC with BRAFV600E mutation are more likely to have CLNM features [Citation13]. Central lymph node dissection (CLND) eliminates both macroscopic and microscopic metastatic sites [Citation14–16]; therefore, CLND is considered necessary for patients with suspected LNM.
However, neck lateral lymph node dissection (NLLND) is not well-defined for occult NLLNM in patients with PTC. The role and extent of NLLND in patients with PTC remain controversial [Citation8]. At present, some authors believe that preventive NLLND in the test area may be applicable to patients with PTC with LNM in the neck lateral area, as determined by biopsy [Citation17,Citation18], because the low LNM rate in the neck lateral compartment and NLLND increase the risk of damage to the recurrent laryngeal nerve and parathyroid gland [Citation19]. However, other authors believe that LND of the central and lateral neck facilitates accurate clinical staging and can help avoid a second operation after NLLNM in patients [Citation20,Citation21]. Extra surgery is not only difficult in these patients but also increases the risk of complications. Therefore, appropriate and timely management of cervical LND is very important for patients with PTC and may be helpful in improving survival, decreasing regional recurrence, and avoiding overtreatment.
LNM in the neck lateral has been more often associated with CLNM [Citation21,Citation22]. CLND for frozen section analysis is both safe and feasible because it can be performed easily and safely [Citation23]. It is performed before thyroidectomy and is unaffected by the presence of the thyroid gland. This study aimed to explore the value of CLNM in frozen sections to predict NLLNM and identify risk factors for NLLNM. This study retrospectively analyzed the clinicopathological data of 275 patients with PTC who underwent unilateral or bilateral thyroidectomy and CLND, followed by modified radical neck node dissection. The characteristics and correlations of LNM in the central and lateral neck regions of the frozen and paraffin-embedded sections were obtained. This finding provides a clinical basis for surgeons to decide whether NLLND should be performed before or during surgery.
Material and methods
Patients
In total, 275 patients with PTC were consecutively enrolled in this retrospective study between March 2018 and November 2020 at the Department of Endocrine and Breast Surgery of the First Hospital of Chongqing Medical University. All the patients underwent unilateral or bilateral thyroid lobe excision with CLND (unilateral or bilateral) or modified radical neck node dissection (MRND, unilateral or bilateral). All patients underwent pre-operative US and preoperative diagnosis of PTC using US-guided fine-needle aspiration (FNA) cytology. The inclusion criteria were as follows: (1) patients were undergoing first-time thyroidectomy; (2) patients had a suspicion of cervical LNM by high-resolution pre-operative neck ultrasonography (all images should be retrievable); (3) PTC diagnosed by high-resolution ultrasonography and confirmed by fine-needle aspiration (FNA) cytology before surgery; (4) BRAFV600E mutation analysis; and (5) consent to participate. The exclusion criteria were as follows: (1) patients with confirmed lung or other distant metastases on enhanced computed tomography (CT) scans of the thorax and (2) patients with insufficient medical records.
To analyze the relationship between CLNM and NLLNM prediction, intraoperative frozen diagnosis of central LNs was performed in 275 patients with suspected cervical LNM who underwent thyroidectomy and neck dissection. To further study the impact of CLNM on NLLNM, we excluded patients without LNM and those with only NLLNM. Finally, 216 patients with PTC were analyzed in the follow-up study. The clinicopathological data of the patients were collected, with special consideration given to the following LNM-related factors: the maximum diameter of the foci in the metastatic LNs was measured from the largest metastatic focus, not the size of the LNs itself; the number of metastatic LNs; the number of retrieved LNs; the metastatic LNs ratio, which was defined as the number of metastatic LNs divided by the number of retrieved LNs; and any extranidal extension (ENE). The patients were divided into two groups: (1) 125 patients with CLNM and NLLNM were placed in the positive group, and (2) 91 patients with CLNM were placed in the negative group ().
Ultrasonography evaluation and preoperative diagnosis
High-resolution ultrasonography (US) and was performed to evaluate the tumor characteristics and cervical LNM. Two experienced sonologists evaluated the US images. Ultrasonography-guided fine-eedle aspiration (FNA) cytology was used for preoperative diagnosis. All ultrasound-guided FNA cytological findings were evaluated by two experienced pathologists.
BRAFV600E mutation analysis
Detection of BRAFV600E mutations was performed using Ultrasonography-guided FNA cytological specimens, as described in previous studies. First, to amplify exon 15 of BRAF, which contains the V600E mutation, we established polymerase chain reaction (PCR) conditions and primers. Genomic DNA was extracted from the cytological specimens using a QIAGEN Kit, following the manufacturer’s instructions.
Intraoperative diagnosis of frozen sections
Intraoperative diagnosis of frozen tissue sections was performed to evaluate tumor clinicopathology and central LNs status, including the presence or absence of metastases in the central LNs. All the preoperative frozen sections were evaluated by two experienced pathologists. LNs were sectioned at their maximum diameter (maximum diameter <5 mm) or at an interval of 2 mm (if maximum diameter >5 mm). Experienced pathologists can effectively control the error of CLNM diagnosis through the maximum diameter of sectioned LNs (maximum diameter <5 mm) or sectioned LNs with intervals of 2 mm, which was used to ensure reliable results.
Statistical analysis
All data were analyzed using SPSS Statistics for Windows version 23.0 (SPSS), and statistical significance was defined as p < .05. Measurement data are expressed as mean ± SD or median and range. Categorical data are summarized as percentages. Data were compared using the χ2 test or Fisher’s exact test. Logistic regression analysis was used for multivariate analysis. A consistency check was performed to calculate kappa values. A receiver operating characteristic (ROC) curve was used to evaluate the predictive capabilities. Interaction terms were used to investigate the association between the number of CLNM and NLLNM that differed according to the maximum diameter.
Results
In total, 1195 patients with PTC were preoperatively analyzed using ultrasonography and ultrasound-guided FNA cytology and treated by our experienced surgeons between March 2018 and November 2020. 716 patients have a suspicion of cervical LNM by ultrasonography, the percentage of suspicion of cervical lymph node metastasis by ultrasonography is 59.9% (716/1195), of which only 39.2% (469/1195) are suspicion of central LNM by ultrasound, 20.6% (247/1195) are suspicion of both central and neck lateral LNM. 247 patients with suspicion of both central and neck lateral LNM were consecutively enrolled in this study. Considering that the relatively low sensitivity of ultrasonography in detecting cervical LNM [Citation11], and US features of tumors (including size, adjacent anterior capsule, distance to the lower pole and CDFI) and BRAFV600E mutation is associated with cervical LNM and recurrence of PTC [Citation12,Citation24]. Consequently, 275 individuals had a thyroidectomy, central and lateral LN sectioning, and frozen-section identification of central LNs during the procedure, along with including 28 patients who had suspicions of central LNM and US signs of tumors. Among 220 patients with cervical LNM, the detection rate of cervical LNM using the US was 80% (220/275). Of 216 patients with CLNM, 91 had simple CLNM. Of the 125 patients with both central and lateral LNM, four patients had only NLLNM. Ultimately, 55 patients did not have LNM, and LNM in the central region related to LNM in the lateral region (p < .0001). The sensitivity, specificity, false-negative rate, false-positive rate, and accuracy rate of CLNM for predicting NLLNM were 96.90%, 37.67%, 3.10%, 62.33%, and 65.45%, respectively ().
Table 1. Correlation between CLNM and NLLNM in paraffin-embedded sections.
Since central LNs diagnosis by frozen section analysis is both safe and feasible, CLNM in frozen sections for predicting NLLNM can be performed easily and safely. First, we compared the central LNs in frozen sections with those in paraffin sections to evaluate the validity and consistency of our method. Of the 216 patients with CLNM, 210 were diagnosed using intraoperative frozen-section analysis. CLNM in frozen sections was significantly related to CLNM in paraffin sections (p < .0001). The sensitivity, specificity, false-negative rate, false-positive rate, and accuracy rates were 97.20%, 96.72%, 2.80%, 3.28%, and 97.09% (). In a consistency check, the kappa value was 0.918 (p < .0001), which was much higher than 0.8, indicating very good consistency between the frozen section and paraffin section analyses ().
Table 2. Validity of frozen section diagnosis of CLNM.
Table 3. Consistency of central LNM in frozen sections with central LNM in paraffin section.
In the analysis of CLNM in frozen sections for predicting NLLNM, the Chi-square test showed that CLNM in frozen sections was related to NLLNM in paraffin sections (p < .0001). The sensitivity, specificity, false-negative rate, false-positive rate, and accuracy rate were 96.80%, 42.00%, 3.20%, 58.00%, and 66.90% (). There was a similar sensitivity and false-negative rate of CLNM in frozen and paraffin sections for predicting NLLNM ( and ).
Table 4. Validity Of frozen section diagnosis of CLNM for predicting NLLNM.
After excluding patients without CLNM or with only NLLNM, 216 patients were included in the follow-up study. Patients were classified into two groups (CLNM, Only central lymph node metastasis, n = 91; and CLNM + NLLNM, central lymph node metastasis and neck lateral lymph node metastasis, n = 125) according to the presence or absence of NLLNM. Their clinicopathological characteristics are summarized in . The patients had a median age of 37 years, and comprised 69 males and 147 females. The diameter of the primary tumor was 0.4–2.9 cm (mean, 2.7 cm) in 216 patients. Based on the location of the primary tumor, they were divided into unilateral and bilateral groups consisting of 148 and 68 patients, respectively. Of these, 72 were multifocal, and 144 were non-multifocal. Among all patients, five had extrathyroidal extension and 62 had Hashimoto’s thyroiditis.
Table 5. Clinicopathologic characteristics of patients (n = 216).
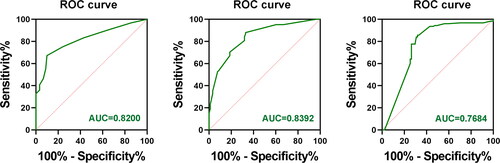
The pathological features of the two groups are compared in . Univariate analysis showed that sex, age, tumor size, Hashimoto’s thyroiditis, extrathyroid extension, bilaterality, and BRAF V600E gene mutations (all p > .05) were not statistically significant. It is worth noting that there was a trend toward a higher frequency of multifocality in the study group, but this did not reach statistical significance (p = .083). Similarly, there was a trend toward a higher frequency of extrathyroid extension, but the difference was not statistically significant (p = .054). The study group showed significant differences in the maximum number of metastatic foci of LNs, number of metastatic LNs, and ratio of metastatic LNs, lymph node yielded (LNY) (p < .05). And there also was a significant relationship between tumor diameter and lymph node metastasis (p < .05). These are the potential risk factors for NLLNM (). Similarly, our multivariate analysis reported the following risk factors for NLLNM: tumor size >1.0 cm, maximum metastatic LN foci diameter of 2.050 mm (AUC = 0.8392), distribution of metastatic LN foci size (>2.0 mm), and number of metastatic LNs of 5.5 (AUC = 0.8200), distribution of number of metastatic LNs (≥5) ( and ). These variables were considered as risk factors for NLLNM in this study.
Table 6. Comparison of the clinicopathologic features between the two groups with and without NLLNM.
Table 7. Validity of ROC curves for total number of CLNM, CLNM focus size, CLNM ratio to predict NLLNM.
We conducted ROC curve analysis to identify the metastatic diameter of CLNM, total number of CLNM, and CLNM ratio cutoff values that predicted NLLNM with the highest Yodon indices. The ROC curves of the metastatic diameter of CLNM, total number of CLNMs, and CLNM ratio did not intersect with the reference line, which may be risk factors for NLLNM. The AUC the area under the ROC curve (AUC) between the prediction model and variables were statistically significant (p < .001). This result was seen as a sensitivity/specificity of CLNM focus size of 88.0%/67.0%, respectively (AUC, 0.8392; SE, 0.027; p < .001). A significant result was obtained when a cutoff value of 2.050 mm was applied to the CLNM focus size level. The sensitivity and specificity were 67.2% and 90.1%, respectively (AUC, 0.8200; SE, 0.028; p < .001). A significant result was obtained when a cut-off value of 5.5 was applied to the total number of CLNMs. This result was seen as a sensitivity/specificity of CLNM ratio of 85.6%/70.3%, respectively (AUC, 0.7684; SE, 0.036; p < .001). A significant result was obtained when a cutoff value of 0.5342 was applied to the CLNM ratio ( and ).
Figure 2. ROC curves for total number of CLNM, CLNM focus size, and CLNM ratio to predict NLLNM. AUC: Area under the ROC curve. The Yodon index = sensitivity + specificity − 1; this value is equal to the difference between the ordinate and the abscissa of a point on the ROC curve, and the corresponding predictive index value of this point is the cut-off value. The maximum Yodon index corresponding to the predictive index value had the best ability to distinguish between the CLNM and NLLNM groups.
As shown in , the association between the total number of CLNM and NLLNM showed a significant interaction with the maximum metastatic diameter (p < .0001). Therefore, these analyses were stratified according to the total number and maximum metastatic diameter. When the number of CLNM was less than or equal to five, and more than or equal to two, the maximum metastatic diameter was more than 2 mm (macrometastasis, as shown in ); the greater the NLLNM (p < .001). Regarding the number of CLNM, the number of CLNM was greater than five; whether the diameter was more than 2 mm or less than 2 mm, the number of CLNM was related to NLLNM.
Figure 3. HE Staining for maximum metastatic CLNM in frozen paraffin-embedded sections. HE staining (× 100 and × 400).
Table 8. Associations of total number of CLNM with NLLNM: maximum metastatic diameter.
Discussion
In recent years, PTC has become one of the most common types of cancer [Citation1]. Although most PTCs have high differentiation and a low degree of malignant carcinoma, and patients have excellent prognoses (The 20-year survival rate is over 90%), the condition always involves early cervical LN metastasis [Citation25], especially for thyroid cancer with cervical LNM suspected by US and BRAF mutation in patients with PTC. Even in clinically LN-negative PTC, occult cervical LN metastasis is frequently confirmed by pathological examination [Citation26,Citation27]. Although it is traditionally accepted that nodal metastasis does not directly affect survival rates, regional nodal metastasis is an important prognostic factor closely related to tumor recurrence [Citation28,Citation29]. For patients with LNM, timely LND facilitates accurate clinical staging and prevents a second operation [Citation17]. LNY in central and lateral neck dissection is associated with papillary thyroid cancer recurrence in the central and lateral neck [Citation30], LNY reflects the quality of the neck dissection. In our study, the LNY in the lateral neck was higher than in the central neck, due to the follow-up time is relatively short in our study, and it is necessary to extend the follow-up time to observe the relation between LNY and the prognosis of PTC. So, we will continue to follow up on the patient’s condition and will focus on the relation between LNY and the prognosis of PTC in the future. Detection of serum Tg level and thyroid function, ultrasound and SPECT/CT scan were regularly performed in follow-up patients with low-risk recurrence and metastasis. According to the American Thyroid Association (ATA) guidelines, adioiodine-131 (131I) therapy be performed for patients with a high risk of recurrence and metastasis (such as tumor size ≥ 2 cm, have NLLNM or distant metastasis). Radioiodine-131 (131I) therapy that can successfully ablate thyroid cancer cells in metastatic lesions, which is conducive to unrecognized micro metastatic lymph nodes preoperatively or incomplete removal of metastatic lymph nodes during surgery for PTC [Citation31]. Therefore, proper surgical treatment, compartment-oriented central and lateral LND and appropriate radioiodine-131 (131I) therapy should not only completely remove the tumor, reduce the recurrence of tumors after surgery, improve the survival rate of patients, but also reduce surgical complications.
Cervical lymph node dissection includes both CLND and NLLND [Citation32]. Approximately 20-90% of patients with PTC have local LNM at diagnosis [Citation33]. Previous study had shown that the presence of palpable cervical LNM in the PTC is uncommon. Especially, papillary thyroid microcarcinomas have a few presents with palpable LNM. In our study, palpable LNM was thought to be present in 37 (13.5%) of the 275 patients (Data not showed). Palpable LNM is related to the size and location of metastatic LNs. The lymph node micrometastatic site and cervical deep regions of LNM is difficult to touch. Based on the evaluation of experienced surgeons, the common palpable LNM is mostly located in the neck lateral of II, III and IV area, and the size of the positive LNs is usually greater than 1 cm. So, ultrasound detection is one of the common methods for preliminarily determining LNM. However, Some CLNM is not easily detected by preoperative ultrasound, particularly micrometastases [Citation34,Citation35], and the sensitivity of US-evaluated cervical LNM is approximately 60% [Citation11]. Due to the relatively low sensitivity of ultrasonography in detecting cervical lymph LNM. Tumor size is one of the main factors that affects lymph node metastasis [Citation36,Citation37]. In our study, the incidence of NLLNM in patients with a tumor size > 1.0 cm was significantly higher than that in patients with a tumor size ≤1 cm. A recent study showed that US features of tumors (including size, adjacent anterior capsule, distance to the lower pole, and CDFI) were potentially beneficial for identifying CLNM [Citation12]. Some studies have shown that the BRAFV600E mutation is associated with cervical LNM and PTC [Citation24]. We combined the evaluation of the US features of tumors interpreted by two experienced sonologists and BRAFV600E mutation to determine suspected ‘cervical LNM’. CLND can significantly reduce the risk of local recurrence and the incidence of NLLNM and is conducive to accurate clinical staging [Citation38]. Therefore, CLND, especially when cervical LNM is suspected, is of great significance for improving the surgical outcome. Guidelines suggest that the central LNs should be routinely dissected during the first surgery in patients with PTC [Citation10]. However, routine LND increases the risk of damage to the recurrent laryngeal nerves and parathyroid glands. Experienced thyroid specialists can effectively control complications associated with central LNs resection [Citation39]. Central LNs can be resected in the same surgical field with minimal trauma. Even if there is lateral lymph node metastasis in the neck, there is no need to clean the central area, thus reducing surgical complications and difficulty [Citation39,Citation40].
We routinely performed bilateral CLND and intraoperative freezing in patients with PTC confirmed via pathological examination during surgery. All operations were performed by experienced surgeons to ensure safety and minimize the risk of morbidities or complications. In this study, we first used intraoperative frozen section analysis to detect CLNM and explored the diagnostic accuracy of central LNs in frozen sections by comparing the results to those of paraffin sections, and then calculated their validity and consistency. The sensitivity, specificity, false negative rate, false positive rate, and accuracy rate were 7.20%, 96.72%, 3.28%%, 2.80%, and 97.09%, respectively. Six cases were undetected by frozen section analysis, including three cases that were misdiagnosed due to the lack of experience of the pathologist in interpreting these types of sections and three cases that were diagnosed due to impalpable micrometastasis after paraffin repair. Two cases could not be detected by paraffin section analysis because of impalpable micrometastases after paraffin repair. The Kappa value was 0.918 (p < .0001), which was much higher than 0.8, indicating a very good consistency of CLNM between the frozen and paraffin sections. Experienced pathologists can effectively control the error in the diagnosis of CLND through the maximum diameter of sectioning of LNs (maximum diameter <5.0 mm) or by sectioning LNs at an interval of 2.0 mm (maximum diameter >5.0 mm), which was the technique used to make our results more reliable. This is similar to the findings of a previous study on the frozen section analysis of ipsilateral central LNs, which showed that frozen pathology had an overall accuracy of 90% for predicting ipsilateral CLNM [Citation38].
More than 60% of patients with NLLNM were identified using preventive NLLND. LNM of tumors is a regular and gradual process, usually ‘primary focus - central LNs - ipsilateral neck lateral LNs - contralateral neck lateral LNs - distant metastasis’ [Citation22,Citation41]. Therefore, LNM in the neck usually occurs first in the central region, and skip metastases directly to the lateral regions of the neck are rare. In this study, only four cases of skip metastases were observed. There is no consensus regarding the predictors of NLLNM. Some authors believe that multifocality and extrathyroid extension are related to LNM [Citation42,Citation43]; in particular, multifocality has been shown to be significantly related to NLLNM [Citation42]. In this study, although multifocality and extrathyroidal extension were not found to be risk factors for NLLNM, there was a trend toward a higher frequency of multifocality and extrathyroidal extension in NLLNM. However, considering the small sample size and the lack of basic research support, further in-depth research with a larger number of cases is required to confirm these findings. Among the risk factors, the CLNM is more likely to be related to the NLLNM, and patients with CLNM are more likely to have NLLNM [Citation4,Citation21]. Our study found that the incidence of NLLNM in patients with CLNM was 57.87% (125/216), much higher than that in patients without CLNM, and that the incidence of NLLNM was 2.52% (4/159), a statistically significant difference (χ2 = 48.571, p < .0001). Therefore, CLNM has a high predictive value for NLLNM and is helpful in guiding NLLND.
To better decide whether to perform NLLND, CLNM in frozen sections can be performed easily and safely to predict NLLNM [Citation44,Citation45]. We conclude that frozen sections of central LNs can serve as surrogate diagnostic tools for NLLNM metastasis. The sensitivity, specificity, false-negative rate, false-positive rate, and accuracy rate were 96.80%, 42.00%, 3.20%, 48.00%, and 66.90%, respectively. There was a similar sensitivity and false-negative rate of CLNM in frozen and paraffin-embedded sections for predicting NLLNM, indicating that CLNM in paraffin-embedded sections can replace CLNM in frozen sections for predicting NLLNM. Current diagnostic methods for predicting NLLNM include high-resolution neck ultrasonography, enhanced computed tomography (CT), and lymph node tracers such as carbon nanoparticles, which cannot detect LN micrometastases [Citation39–42]. Thus, central LNs in frozen sections may be better predictors of lateral neck LNMs than other methods.
Previous studies have shown that the greater the number of metastatic central LNs, the greater the maximum metastatic foci of central LNs and the higher the likelihood of NLLNM [Citation43,Citation44]. In this study, we found that the maximum metastatic foci of LNs, the number of metastatic LNs, and the ratio of metastatic LNs in the central region were significantly related to NLLNM and concluded that these were predictors of NLLNM. For risk factors of structural recurrence, the 2015 American Thyroid Association (ATA) added other LN-related risk factors in the initial risk stratification system, such as the maximal size of the metastatic LN foci, 2.0 mm being the low-to-intermediate risk group, 3.0 mm being the intermediate-to-high risk group, and in terms of number of metastases, five for the low-to-intermediate risk group [Citation9,Citation45]. We showed that the ROC curves of the metastatic diameter of CLNM, the total number of CLNMs, and CLNM ratio did not intersect with the reference line, which may be risk factors for NLLNM. The ROC curve analysis to identify the CLNM ratio cutoff value was 0.5342; therefore, if the ratio of the metastatic diameter of the LN was greater than 0.5, NLLNM was more likely to occur. In addition, the cut-off value of the maximum metastatic diameter of the CLNM was 2.050 mm, and the total number of CLNM was 5.5. In this study, most patients with CLNM had only micrometastases (<2.0 mm), while most patients with occult NLLNM had nodal metastases of >2.0 mm (macrometastasis); the number of CLNM was greater than five, and the incidence of NLLNM was significantly higher than that of CLNM. We further tested whether there was an interaction between the number and maximum metastatic diameter of CLNM and NLLNM. Interaction terms showed that the number of CLNM less than or equal to five and greater than or equal to two and maximum metastatic diameter > 2.0 mm, or the number of CLNM of is greater than five was associated with NLLNM. Thus, when the number of CLNM is more than or equal to two and the CLNM has macrometastases (>2.0 mm), or the number of CLNM is greater than five, NLLND is recommended.
CLNM, whether frozen or embedded in paraffin sections, has a predictive effect on NLLNM. Frozen section analysis in CLND is a safe and rapid procedure. Owing to its effectiveness and consistency, it can accurately provide a basis for NLLND. When the number of metastatic LNs in the central region is five or the number of metastatic LNs is less than five, but the diameter of the metastatic LNs is more than 2 mm, we strongly recommend performing NLLNM. Thus, patients can benefit from an extended LND and avoid the risks and costs associated with excessive LND.
Authors contributions
Xinliang Su and Shanshan Yu designed the research study; Li Peng, Ying Xue, Xiaoya Zheng, and Chun Huang performed the research; Su XL and Shanshan Yu contributed new analytical tools; Li Peng and Shanshan Yu analyzed the data and wrote the manuscript; all authors have read and approved the final manuscript.
Institutional review board statement
This study was reviewed and approved by the Ethics Committee of First Affiliated Hospital of Chongqing Medical University. Approval Code: 2022-K501. This study complies with the requirements of the Helsinki Declaration. The research content and process of the project followed international and national ethical requirements for biomedical research.
Informed consent statement
Patients were not required to provide informed consent because the analysis used anonymous clinical data that were obtained after each patient agreed to treatment by written consent.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data is available upon request.
Additional information
Funding
References
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):1–11. doi:10.3322/caac.21660.
- Azadnajafabad S, Saeedi Moghaddam S, Mohammadi E, et al. Global, regional, and national burden and quality of care index (QCI) of thyroid cancer: a systematic analysis of the global burden of disease study 1990–2017. Cancer Med. 2021;10(7):2496–2508. doi:10.1002/cam4.3823.
- Zhao H, Huang T, Li H. Risk factors for skip metastasis and lateral lymph node metastasis of papillary thyroid cancer. Surgery. 2019;166(1):55–60. doi:10.1016/j.surg.2019.01.025.
- Zhou J, Li DX, Gao H, et al. Relationship between subgroups of central and lateral lymph node metastasis in clinically node-negative papillary thyroid carcinoma. World J Clin Cases. 2022;10(12):3709–3719. doi:10.12998/wjcc.v10.i12.3709.
- Leboulleux S, Rubino C, Baudin E, et al. Prognostic factors for persistent or recurrent disease of papillary thyroid carcinoma with neck lymph node metastases and/or tumor extension beyond the thyroid capsule at initial diagnosis. J Clin Endocrinol Metab. 2005;90(10):5723–5729. doi:10.1210/jc.2005-0285.
- Park JP, Roh JL, Lee JH, et al. Risk factors for central neck lymph node metastasis of clinically noninvasive, node-negative papillary thyroid microcarcinoma. Am J Surg. 2014;208(3):412–418. doi:10.1016/j.amjsurg.2013.10.032.
- Yan XQ, Ma ZS, Zhang ZZ, et al. The utility of sentinel lymph node biopsy in the lateral neck in papillary thyroid carcinoma. Front Endocrinol. 2022;13:937870. doi:10.3389/fendo.2022.937870.
- Wang Y, Guan Q, Xiang J. Nomogram for predicting central lymph node metastasis in papillary thyroid microcarcinoma: a retrospective cohort study of 8668 patients. Int J Surg. 2018;55:98–102. doi:10.1016/j.ijsu.2018.05.023.
- Silver Karcioglu A, Iwata AJ, Pusztaszeri M, et al. The American thyroid association (ATA) integrates molecular testing into its framework for managing patients with anaplastic thyroid carcinoma (ATC): update on the 2021 ATA ATC guidelines. Cancer Cytopathol. 2022;130(3):174–180. doi:10.1002/cncy.22519.
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1–133. doi:10.1089/thy.2015.0020.
- Liu Z, Xun X, Wang Y, et al. MRI and ultrasonography detection of cervical lymph node metastases in differentiated thyroid carcinoma before reoperation. Am J Transl Res. 2014;6:147–154.
- Liu Y, Huang J, Zhang Z, et al. Ultrasonic characteristics improve prediction of Central lymph node metastasis in cN0 unifocal papillary thyroid cancer. Front Endocrinol (Lausanne). 2022;13:870813. doi:10.3389/fendo.2022.870813.
- Virk RK, Van Dyke AL, Finkelstein A, et al. BRAFV600E mutation in papillary thyroid microcarcinoma: a genotype-phenotype correlation. Mod Pathol. 2013;26(1):62–70. doi:10.1038/modpathol.2012.152.
- Machens A, Hauptmann S, Dralle H. Lymph node dissection in the lateral neck for completion in Central node-positive papillary thyroid cancer. Surgery. 2009;145(2):176–181. doi:10.1016/j.surg.2008.09.003.
- Roh JL, Park JY, Kim JM, et al. Use of preoperative ultrasonography as guidance for neck dissection in patients with papillary thyroid carcinoma. J Surg Oncol. 2009;99(1):28–31. doi:10.1002/jso.21164.
- Lang BH, Ng CP, Au KB, et al. Does preoperative neutrophil lymphocyte ratio predict risk of recurrence and occult central nodal metastasis in papillary thyroid carcinoma? World J Surg. 2014;38(10):2605–2612. doi:10.1007/s00268-014-2630-z.
- Lo CY. Lymph node dissection for papillary thyroid carcinoma. Methods Mol Biol. 2022;2534:57–78. doi:10.1007/978-1-0716-2505-7_5.
- Issa K, Stevens MN, Sun Y, et al. A retrospective study of lymph node yield in lateral neck dissection for papillary thyroid carcinoma. Ear Nose Throat J. 2022;101(7):456–462. doi:10.1177/0145561320967339.
- Jo YJ, Choi HR, Park SH, et al. Extent of thyroid surgery for clinically node-negative papillary thyroid carcinoma with confirmed nodal metastases after prophylactic Central neck dissection: a 15-year experience in a single center. Ann Surg Treat Res. 2020;99(4):197–204. doi:10.4174/astr.2020.99.4.197.
- Lee CW, Gong G, Roh JL. Intraoperative diagnosis of central compartment lymph node metastasis predicts recurrence of patients with papillary thyroid carcinoma and clinically node-negative lateral neck and may guide extent of initial surgery. World J Surg. 2015;39(1):194–202. doi:10.1007/s00268-014-2800-z.
- Zhan S, Luo D, Ge W, et al. Clinicopathological predictors of occult lateral neck lymph node metastasis in papillary thyroid cancer: a meta-analysis. Head Neck. 2019;41(7):2441–2449. doi:10.1002/hed.25762.
- Eltelety AM, Terris DJ. Neck dissection in the surgical treatment of thyroid cancer. Endocrinol Metab Clin North Am. 2019;48(1):143–151. doi:10.1016/j.ecl.2018.11.004.
- Kim MJ, Kim HJ, Park CS, et al. Frozen section analysis of central lymph nodes in papillary thyroid cancer: the significance in determining the extent of surgery. Gland Surg. 2022;11(4):640–650. doi:10.21037/gs-22-15.
- Chen Y, Sadow PM, Suh H, et al. BRAF(V600E) is correlated with recurrence of papillary thyroid microcarcinoma: a systematic review, multi-institutional primary data analysis, and meta-analysis. Thyroid. 2016;26(2):248–255. doi:10.1089/thy.2015.0391.
- Lang B, Lo CY, Chan WF, et al. Restaging of differentiated thyroid carcinoma by the sixth edition AJCC/UICC TNM staging system: stage migration and predictability. Ann Surg Oncol. 2007;14(5):1551–1559. doi:10.1245/s10434-006-9242-2.
- Lee KE, Chung IY, Kang E, et al. Ipsilateral and contralateral central lymph node metastasis in papillary thyroid cancer: patterns and predictive factors of nodal metastasis. Head Neck. 2013;35(5):672–676. doi:10.1002/hed.23016.
- Kim WW, Park HY, Jung JH. Surgical extent of central lymph node dissection in clinically node-negative papillary thyroid cancer. Head Neck. 2013;35(11):1616–1620. doi:10.1002/hed.23197.
- Kim TY, Hong SJ, Kim JM, et al. Prognostic parameters for recurrence of papillary thyroid microcarcinoma. BMC Cancer. 2008;8(1):296. doi:10.1186/1471-2407-8-296.
- Ito Y, Miyauchi A, Jikuzono T, et al. Risk factors contributing to a poor prognosis of papillary thyroid carcinoma: validity of UICC/AJCC TNM classification and stage grouping. World J Surg. 2007;31(4):838–848. doi:10.1007/s00268-006-0455-0.
- Heaton CM, Chang JL, Orloff LA. Prognostic implications of lymph node yield in central and lateral neck dissections for well-differentiated papillary thyroid carcinoma. Thyroid. 2016;26(3):434–440. doi:10.1089/thy.2015.0318.
- Yu F, Wu W, Zhang L, et al. Cervical lymph node metastasis prediction of postoperative papillary thyroid carcinoma before 131I therapy based on clinical and ultrasound characteristics. Front Endocrinol. 2023;14:1122517. doi:10.3389/fendo.2023.1122517.
- Song Y, Xu G, Wang T, et al. Indications of super selective neck dissection in patients with lateral node metastasis of papillary thyroid carcinoma. Otolaryngol Head Neck Surg. 2022;166(5):832–839. doi:10.1177/01945998211038318.
- Lim YC, Choi EC, Yoon YH, et al. Central lymph node metastases in unilateral papillary thyroid microcarcinoma. Br J Surg. 2009;96(3):253–257. doi:10.1002/bjs.6484.
- Marshall CL, Lee JE, Xing Y, et al. Routine pre-operative ultrasonography for papillary thyroid cancer: effects on cervical recurrence. Surgery. 2009;146(6):1063–1072. doi:10.1016/j.surg.2009.09.027.
- Wong KP, Lang BH. The role of prophylactic central neck dissection in differentiated thyroid carcinoma: issues and controversies. J Oncol. 2011;2011:127929–127912. doi:10.1155/2011/127929.
- Mukherjee A, Arnav S, Agarwal S, et al. Prophylactic central node dissection in differentiated thyroid cancer: a prospective tertiary care center experience. Cancer Treat Res Commun. 2020;25:100228. doi:10.1016/j.ctarc.2020.100228.
- Zhao H, Li H. Meta-analysis of ultrasound for cervical lymph nodes in papillary thyroid cancer: diagnosis of central and lateral compartment nodal metastases. Eur J Radiol. 2019;112:14–21. doi:10.1016/j.ejrad.2019.01.006.
- Zhou L, Li H, Liang W, et al. Pretracheal-laryngeal lymph nodes in frozen section predicting contralateral paratracheal lymph nodes metastasis. Eur J Surg Oncol. 2020;46(10 Pt A):1829–1834. doi:10.1016/j.ejso.2020.06.048.
- Alsubaie KM, Alsubaie HM, Alzahrani FR, et al. Prophylactic central neck dissection for clinically node-negative papillary thyroid carcinoma. Laryngoscope. 2022;132(6):1320–1328. doi:10.1002/lary.29912.
- Holostenco V, Khafif A. The upper limits of central neck dissection. JAMA Otolaryngol Head Neck Surg. 2014;140(8):731–735. doi:10.1001/jamaoto.2014.972.
- Liu C, Xiao C, Chen J, et al. Risk factor analysis for predicting cervical lymph node metastasis in papillary thyroid carcinoma: a study of 966 patients. BMC Cancer. 2019;19(1):622. doi:10.1186/s12885-019-5835-6.
- Papaioannou C, Lamnisos D, Kyriacou K, et al. Lymph node metastasis and extrathyroidal extension in papillary thyroid microcarcinoma in Cyprus: suspicious subcentimeter nodules should undergo FNA when multifocality is suspected. J Thyroid Res. 2020;2020:3567658.
- Zhao L, Sun X, Luo Y, et al. Clinical and pathologic predictors of lymph node metastasis in papillary thyroid microcarcinomas. Ann Diagn Pathol. 2020;49:151647. doi:10.1016/j.anndiagpath.2020.151647.
- Lim YS, Choi SW, Lee YS, et al. Frozen biopsy of Central compartment in papillary thyroid cancer: quantitative nodal analysis. Head Neck. 2013;35(9):1319–1322. doi:10.1002/hed.23129.
- Santrac N, Markovic I, Medic Milijic N, et al. Sentinel lymph node biopsy in medullary thyroid microcarcinomas. Endocr J. 2020;67(3):295–304. doi:10.1507/endocrj.EJ19-0409.