Abstract
Objectives
This study aimed to clarify the effectiveness and safety of two different infusion durations of cyclophosphamide (CTX) plus granulocyte colony-stimulating factor (G-CSF) for peripheral blood stem cell mobilization in patients with newly diagnosed multiple myeloma (NDMM).
Methods
One hundred and fifty-six consecutive NDMM patients receiving CTX plus G-CSF mobilization and autologous stem cell transplantation during the period of September 2008 to May 2020 were selected for retrospective analysis. According to differences in prolonged infusion time of CTX, they were divided into a 24-h group (24-h continuous infusion) and a control group (4-6 h of infusion). Mobilization and safety of infusion were analyzed. Flow cytometry was used to detect the peripheral blood CD34+ cell count. Multivariate analysis was performed to determine the factors influencing the number of CD34+ cells.
Results
The mean CD34+ cell counts collected in 24-h and control groups were 6.78 (interquartile range [IQR] 3.59-11.69) and 4.48 (IQR 2.39-6.30) ×106/kg, respectively (p < 0.001). Meanwhile, the target number of CD34+ cells/kg (defined as ≥4 × 106/kg) was collected from 51 (75%) of cases in 24-h group vs. 45 (51%) in the control group (p = 0.002). Multivariate analysis identified the independence of CTX infusion time as a factor influencing the target number of CD34+ cells/kg [odds ratio OR, 4.045; 95% CI: 1.630-10.038, p = 0.003]. The post-transplantation time to neutrophil engraftment was 10 (IQR 9-11) in 24-h group and 11 (IQR 10-12) in control group (p < 0.001). Finally, no statistical differences were identified between groups in terms of hematologic and non-hematologic toxicities.
Conclusions
For patients with NDMM, 24-h continuous infusion of CTX plus G-CSF contributes to improved mobilization efficiency and equivalent toxicity as a stem cell mobilization regimen.
1. Introduction
Multiple myeloma (MM), characterized by end-organ damage, renal impairment, hypercalcemia, anemia, and bony lytic lesions, is a clonal plasma cell neoplasm with a rising incidence since 1990 [Citation1]. Autologous stem cell transplantation (ASCT), since its first randomized trial in patients with MM [Citation2], has been shown to significantly prolong overall survival compared to traditional chemotherapy. Therefore, ASCT has become an essential component of the management of transplant-eligible MM patients. However, with therapies for MM emerging dramatically over the past couple of decades, including the development and introduction of novel drugs and their combinations, the response rate and depth of patients with MM have improved significantly [Citation3], followed by a debate about the value of ASCT as the first-line therapy for MM. Therefore, several large-scale randomized trials have been conducted to explore the role of ASCT in MM, with results confirming progression-free survival (PFS) and overall survival benefits of ASCT in newly diagnosed MM (NDMM) [Citation4,Citation5].
Based on the above, ASCT remains the mainstay of treatment for young and transplant-eligible MM patients in the current era of novel agents [Citation2,Citation6]. ASCT after high-dose chemotherapy is an important option for transplant-eligible MM patients and can significantly improve patient outcomes [Citation7]. In addition, tandem ASCT is currently recommended for patients with MM and high-risk disease based on the presence of cytogenetic abnormalities [Citation8]. Successful ASCT is increasingly dependent on the harvest of large quantities of peripheral blood stem cells (PBSCs) [Citation9,Citation10]. Adequate CD34+ hematopoietic stem cell collection is critical for successful implantation after ASCT, and high collection volumes are associated with faster hematologic recovery, reduced risk of infection, and improved overall survival [Citation11].
Cytokines such as granulocyte colony-stimulating factor (G-CSF), either alone or in combination with chemotherapy, and the CXCR4 inhibitor plerixafor, are typically used for PBSC mobilization in MM [Citation9]. Compared with G-CSF-based mobilization, chemo-mobilization, with cyclophosphamide (CTX) plus G-CSF being the most commonly used, has been demonstrated to enhance CD34+ cell counts while reducing the number of apheresis and is hypothesized to exert putative anti-tumor effects [Citation12–15]. In these patients, the failure rate of SC mobilization with these preparations is 5-15% [Citation16]. This concern is particularly relevant for patients previously exposed to multicycle lenalidomide during induction therapy, as the drug appears to hinder stem cell mobilization (SCM), at least when G-CSF is used alone as a mobilization agent [Citation17–19]. Moreover, chemo-mobilization has the disadvantages of serious treatment-related side effects and the need for hospitalization [Citation12,Citation14]. While G-CSF in combination with plerixafor has been shown to be effective [Citation20], plerixafor administration is associated with high costs, which has led to its restriction to preemptive regimens for patients at high risk of activity failure. Therefore, the optimal protocol for efficient collection of hematopoietic stem cells remains a challenge.
To further improve the efficiency and safety of CTX-based mobilization, several clinical trials have been carried out, demonstrating the efficacy of low-dose CTX and a relationship between reduced CTX doses with lower toxicity [Citation21,Citation22]. These effective alternatives seem pivotal for individuals requiring tandem or salvage ASCT [Citation23]. In order to provide a reference for individualized therapies, we present a retrospective analysis comparing the mobilization efficacy and safety of “24-h continuous infusion” versus “short time infusion (4-6 h)” CTX in NDMM cases with CTX plus G-CSF mobilization and ASCT at our institution.
2. Material and methods
2.1. Patient selection
One hundred and fifty-six consecutive patients with MM who underwent mobilization with CTX + G-CSF and stem cell harvest at our institution between September 2008 and May 2020 were enrolled in this retrospective study. The first 88 patients were mobilized by “short time infusion” CTX + G-CSF from September 2008 to March 2015 (control group), while the subsequent 68 patients were mobilized by “24-h continuous infusion” CTX + G-CSF from April 2015 to May 2020 (24-h group). The inclusion criteria were as follows: (1) All patients received induction chemotherapy containing bortezomib, followed by ASCT; (2) None of the patients surveyed had a previous history of radiotherapy other than induction chemotherapy, and our previous studies demonstrated that lenalidomide with more than three courses affected stem cell collection [Citation24]. Exclusion criteria: Patients excluded were those who (1) used lenalidomide; (2) used plerixafor because it was only used in the 24-h group; (3) underwent front-line tandem ASCT; (4) had a comorbid condition, which, in the view of the investigators, indicated an increased risk of treatment complications; and (5) those who had prior autologous or allogeneic transplantation.
2.2. Mobilization regimens and leukapheresis
All patients underwent PBSC mobilization using the CTX + G-CSF regimen. They received 3.0 g/m2 of CTX on day 1, in addition to standard hydration, furosemide, mesna, and antiemetic prophylaxis before chemotherapy. Two different infusion times of CTX were used for PBSC at our center. The infusion time of CTX, initially 4-6 h, has been changed to 24 h since April 2015. On day 2, 5 μg/kg of G-CSF was administered subcutaneously. Routine blood tests were initiated on day 3 and continued daily until the last leukapheresis. G-CSF administration was postponed in the presence of leukocytosis (> 30 × 109 white blood cells/L) until the white blood cell count fell below 20 × 109/L. Renal function and electrolyte levels were monitored on day 3. Standard supportive care was performed in accordance with institutional guidelines [Citation25].
Apheresis was scheduled to commence when the patient’s leukocyte count was restored to > 2 × 109/L or the monocyte proportion recovered to 20%. Peripheral blood mononuclear cells were obtained using the COM.TEC Apheresis System (Fresenius KABI, Bad Homburg, Germany), following the manufacturer’s protocol. The primary objective of stem cell collection was a CD34+ cell count of at least 2.0 × 106/kg. Patients who were unable to mobilize their PBPCs successfully underwent bone marrow harvesting or G-CSF remobilization.
2.3. Flow cytometry analysis
According to the International Society for Hematotherapy and Graft Engineering guidelines [Citation26], peripheral blood CD34+ cells were analyzed using BD FACSCanto II (BD Bioscience, Franklin Lakes, NJ). The final product was stored in 10% dimethyl sulfoxide (DMSO) and cryopreserved at a controlled rate in ultra-low-temperature liquid nitrogen.
2.4. Response and safety evaluation
Efficacy, which was rated as very good partial response (VGPR), partial response (PR), and stable disease (SD), was evaluated based on the IMWG 2009 efficacy evaluation criteria [Citation27]. VGPR: Serum and urine M-component detectable by immunofixation but not by electrophoresis or ≥ 90% or greater reduction in serum M-component plus urine M-component < 100 mg per 24 h; PR: ≥ 50% reduction in serum M protein and reduction in 24-h urinary M protein by ≥ 90% or to < 200 mg per 24 h; SD: not meeting criteria for VGPR, PR, or progressive disease.
The National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 4.0, a common toxicity grading system, was used to grade the intensity of adverse events (AEs).
2.5. High-dose chemotherapy and transplantations
Eighty of the patients received intravenous melphalan as a conditioning regimen at a dose of 200 mg/m2 in two daily doses administered on days −2 and −1, whereas in 8 patients with creatinine clearance < 50 mL/min, melphalan was adjusted to 140 mg/m2. Due to the limited availability of melphalan in China after January 30, 2015, the conditioning regimen for 66 patients was modified to the CVB regimen, as detailed below. 0.8 mg/kg of Busulfan was administered every 6 h from day −8 to day −6. Etoposide (10 mg/kg) was given on days −5 and −4. CTX (50 mg/kg) was administered on day −3 and day −2. Two patients were treated with 200 mg/m2 of melphalan plus 1.3 mg/m2 of bortezomib as a conditioning regimen. Post-transplantation growth factor support (G-CSF 5 μg/kg), together with other routine supportive treatments, were applied. The time to neutrophil engraftment was counted as the first day of three consecutive days with a neutrophil count of ≥ 0.5 × 109/L in the absence of G-CSF application. Similarly, the time to platelet engraftment was defined as the first day of 7 consecutive days with a platelet count of ≥ 20 × 109/L without platelet transfusion.
2.6. Study endpoint and statistical analysis
The primary endpoint was the target number of CD34+ cells/kg (defined as ≥ 4 × 106/kg). SPSS v26 (SPSS Inc., Chicago, IL, USA) was used for data analysis, with the results expressed as median (interquartile range [IQR], Q1-Q3) or frequency (percentage). To identify statistical significance, chi-squared or Fisher’s exact tests were used for categorical variables and Mann-Whitney U tests for continuous variables. In the multivariate analysis, a logistic regression model was used to adjust for significant variables of interest. In the logit model, the Box-Tidwell test was performed to assess the linear assumptions of the continuous variables. The Pearson correlation coefficient was used to assess multicollinearity by checking the Variance Inflation Factor in a multivariate regression model. All data included in the logistic regression model were complete. A two-tailed p < 0.05 indicates the presence of statistical significance.
3. Results
3.1. Demographic characteristics of patients
The two groups showed similar demographic characteristics including age, sex, type of myeloma, serum creatinine, serum calcium, hemoglobin, plasma cell percentage, international staging system, and response after induction. The proportion of patients receiving the PAD induction regimen (Bortezomib + Doxorubicin + Dexamethasone) before mobilization was higher in the 24-h group (99%) than in the control group (61%). Meanwhile, the median number of apheresis was 2 (IQR 2-3) in the 24-h group and 1 (IQR 1-2) in the other (p < 0.001), as shown in .
Table 1. Patients’ demographic features.
3.2. SCM efficacy
To explore whether 24-h continuous infusion of CTX could enhance mobilization efficiency, we compared the variables between the 24-h group and the control group at our center. Data describing the SCM efficacy in the two subgroups are summarized in . The median CD34+ cell counts collected in 24-h and control groups were 6.78 × 106/kg and 4.48 × 106/kg, respectively (p < 0.001). Successful mobilization, defined as a minimum count of 2 × 106 CD34+ cells/kg retrieved, was achieved in 62 (91%) in the 24-h group and 71 (81%) in the control group (p = 0.067). The target number of CD34+ cells/kg (defined as ≥4 × 106/kg required for tandem and another transplantation) was 51 (75%) in the 24-h group vs. 45 (51%) in the other (p = 0.002).
Table 2. Stem cell mobilization efficacy.
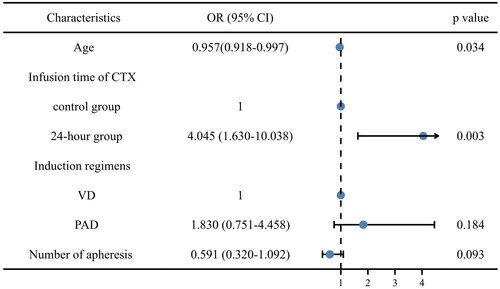
In the multivariate analysis including age at diagnosis, the infusion time of CTX (reference: 24-h), induction regimens (reference: VD), number of apheresis, and infusion time of CTX were identified to be the independent facilitating factors for the target number of CD34+ cells/kg (OR 4.045, 95% CI 1.630-10.038, p = 0.003) ().
3.3. Toxicity
This study also evaluated the hematological and extra-hematological toxicities of CTX-based SCM. The extra-hematological toxicity analysis focused on grade 3/4 AEs, such as nausea or emesis, abdominal pain or diarrhea, pneumonia, mucositis, septicemia, seizure, and hemorrhagic cystitis. Interestingly, no significant differences in AEs were identified between the two subgroups. In addition, septicemia, seizures, and hemorrhagic cystitis occurred only in the control group.
As for hematological toxicity, a lower leukocyte count was determined in the 24-h group (0.33 × 109/L) compared with the control group (0.54 × 109/L) (p = 0.012). However, no significant difference in the rate of neutropenic fever was observed. This might be explained by the shorter neutropenia duration in the 24-h group (median 2, IQR 1-3) as in the control group (median 3, IQR 2-4, p = 0.038). In contrast, statistical significance was absent between the two subgroups in terms of the proportion of patients receiving erythrocyte and platelet transfusions. The toxicity analysis results are presented in .
Table 3. Toxicity during stem cell mobilization.
3.4. Hematologic recovery after ASCT
Seven (10%) and 73 (83%) patients in the 24-h and control groups received standard melphalan (200 mg/m2), respectively. In addition, the CVB regimen was used by 55 (81%) patients in the 24-h group and 11 (13%) patients in the control group. Moreover, 6 (9%) and 2 (2%) patients received dose-reduced melphalan (140 mg/m2) in the 24-h and control groups, respectively. Two patients (2%) in the control group received bortezomib plus melphalan as a conditioning regimen. The two subgroups did not differ significantly in the median CD34+ cell count. Furthermore, the 24-h group had quicker neutrophil engraftment than the other (p < 0.001). While platelet engraftment was similar between the two subgroups (p = 0.272). Hematologic recovery after ASCT analysis is shown in .
Table 4. Hematologic recovery after ASCT.
4. Discussion
To date, prolonging the survival of patients with MM remains a serious clinical challenge, particularly in high-risk patients [Citation28]. In an era of new agents, high-dose chemotherapy and ASCT, which have been shown to improve patient survival, remain the standard of care for transplant-eligible MM patients [Citation29,Citation30]. As indicated by several phase III studies, double ASCT, such as tandem transplant and salvage transplant, is superior to single ASCT, providing more overall survival and progression-free survival benefits for the transplant-eligible population [Citation4,Citation31–33]. Meanwhile, as lenalidomide has become the first-line therapy for individuals eligible for transplantation, concerns about its influence on mobilization continue to be raised [Citation24,Citation34]. Therefore, collecting sufficient CD34+ cells is vital to perform two or more transplants.
CTX combined with G-CSF is a common mobilization regimen for CD34+ cell collection, leading to more effective mobilization and controversial anti-myeloma effects than the G-CSF-alone regimen [Citation12–14,Citation35]. Even after lenalidomide pre-treatment, CTX is still beneficial [Citation36]. Numerous clinical trials investigated different CTX dosages, covering low (1.5–2.5 g/m2), middle (4 g/m2), and high (7 g/m2) doses. The results revealed no significant differences in mobilization efficacy across CTX dosages, whereas larger doses were associated with increased toxicities [Citation21,Citation36–38]. Prolonged infusion times of CTX with sustained low concentrations of activated metabolites have been shown to be more effective than short periods of high peak concentrations [Citation39,Citation40]. Thus, our center changed the infusion time of CTX in the mobilization regimen to 24 h from March 2015. We retrospectively reviewed 156 NDMM patients who received the CTX plus G-CSF mobilization regimen and ASCT at our center from 2008 to 2020.
First, our data demonstrated that mobilization with CTX in the 24-h group induced a higher CD34+ cell count retrieval compared to the control group with short infusion time (6.78 × 106/kg vs. 4.48 × 106/kg; p < 0.001). It is worth noting that the median number of CD34+ cells collected in our study was lower than that in some prior studies, which might be due to the fact that the dosage of G-CSF in the mobilization regimen was 10 µg/kg in previous studies [Citation41,Citation42]. Nonetheless, a higher proportion of collected CD34+ cells (2 × 106 cells/kg) was observed in the 24-h group herein. In addition, 75% of patients reached the target threshold of CD34+ cells (≥4 × 106 cells/kg) in the 24-h group, versus 51% in the other (p = 0.002). Nevertheless, the median number of apheresis sessions was 2 (IQR 2-3) in the 24-h group and 1 (IQR 1-2) in the control group (p < 0.001). Due to the inherent limitations of retrospective research, and to mitigate the effect of the number of apheresis sessions on the results, we compared CD34+ cell counts collected on each day of apheresis. Although the number of CD34+ cells collected from a single apheresis did not differ between the two subgroups, we still observed the yield of CD34+ stem cells on day 2 of apheresis, with a higher maximum CD34+ cell yield of single apheresis in the 24-h group. Moreover, CTX infusion time was identified by multivariate analysis as an independent factor for the target number of CD34+ cells/kg (OR 4.045, 95% CI 1.630-10.038, p = 0.003). This result indicates an increased likelihood of double transplants or more in NDMM patients in the 24-h group.
Second, infectious complications are common with CTX given that it causes neutropenia, which commonly leads to mobilization failure [Citation21]. In our retrospective study, the 24-h group had a lower leukocyte nadir but a shorter neutropenia duration than the control group, which was associated with a similar risk of neutropenic fever. Meanwhile, the two subgroups did not differ significantly in non-hematologic toxicity and other hematologic toxicities. Despite the fact that hemorrhagic cystitis, septicemia, and seizures were observed only in control patients, it is conceivable that the entire spectrum of toxicities was not captured, given the retrospective nature of our study and the fact that no such data was collected. In addition, non-hematologic toxicities, such as nausea, vomiting, and diarrhea, cannot be accurately measured or compared.
Finally, our results showed that the 24-h group had quicker neutrophil engraftment after transplantation than the control group, with a similar amount of transplanted CD34+ cells. As our previous research showed, a one-day delay in neutrophil recovery in the control group was probably caused by different approaches due to conditioning regimen changes at our institution [Citation43].
The major limitation of this study is its retrospective nature. Although both groups were equally matched, the proportion of patients receiving PAD as an induction regimen and the number of leukapheresis cases were higher in the 24-h group. Because of this discrepancy, a multivariate analysis was performed, which revealed no impact on the outcome. However, pharmacokinetic analysis of CTX has not been performed.
5. Conclusion
In conclusion, this study showed that patients with NDMM treated with a 24-h infusion of CTX plus G-CSG regimen exhibited a higher PBSC mobilization capacity, without significant increase in infection or morbidity. A 24-h infusion of CTX-based SCM offers an attractive option over the costly plerixafor. Nevertheless, larger prospective studies are warranted before a final recommendation can be made.
Author contributions
The authors’ contributions to the paper are as follows: conception and design of the work; Yanjuan Li and Juan Li; analysis and interpretation of the data: Yanjuan Li and Junru Liu; drafting of the paper: Yanjuan Li and Juan Li; data collection and critical revision for intellectual content: Yanjuan Li, Junru Liu, Beihui Huang, Meilan Chen, Jingli Gu, and Juan Li; and final approval of the version to be published: Yanjuan Li and Juan Li. All authors agree to be accountable for all aspects of this study.
Ethical approval
This study was conducted in accordance with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of the First Affiliated Hospital of Sun Yat-sen University (December 28, 2021, No.807).
Informed consent statement
Patients were not required to provide informed consent to the study because the analysis used anonymous clinical data that were obtained after each patient agreed to treatment by written consent.
Acknowledgements
We thank the research staff for recruiting patients for the study and for their involvement in data collection and management.
Disclosure statement
The author declare no competing interests.
Data availability statement
The authors confirm that data supporting the findings of this study are available within the article.
References
- Cowan AJ, Allen C, Barac A, et al. Global burden of multiple myeloma: a systematic analysis for the global burden of disease study 2016. JAMA Oncol. 2018;4(9):1–9. doi:10.1001/jamaoncol.2018.2128.
- Attal M, Harousseau J-L, Stoppa A-M, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. N Engl J Med. 1996;335(2):91–97. doi:10.1056/NEJM199607113350204.
- Kumar SK, Dispenzieri A, Lacy MQ, et al. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia. 2014;28(5):1122–1128. doi:10.1038/leu.2013.313.
- Cavo M, Gay F, Beksac M, et al. Autologous haematopoietic stem-cell transplantation versus bortezomib–melphalan–prednisone, with or without bortezomib–lenalidomide–dexamethasone consolidation therapy, and lenalidomide maintenance for newly diagnosed multiple myeloma (EMN02/HO95): a multicentre, randomised, open-label, phase 3 study. Lancet Haematol. 2020;7(6):e456–e68. doi:10.1016/S2352-3026(20)30099-5.
- Attal M, Lauwers-Cances V, Hulin C, et al. Lenalidomide, bortezomib, and dexamethasone with transplantation for myeloma. N Engl J Med. 2017;376(14):1311–1320. doi:10.1056/NEJMoa1611750.
- Child JA, Morgan GJ, Davies FE, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348(19):1875–1883. doi:10.1056/NEJMoa022340.
- Kumar SK, Callander NS, Adekola K, et al. Multiple myeloma, version 3.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2020;18(12):1685–1717. doi:10.6004/jnccn.2020.0057.
- Gagelmann N, Eikema D-J, Koster L, et al. Tandem autologous stem cell transplantation improves outcomes in newly diagnosed multiple myeloma with extramedullary disease and high-risk cytogenetics: a study from the Chronic Malignancies Working Party of the European Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2019;25(11):2134–2142. doi:10.1016/j.bbmt.2019.07.004.
- Mohty M, Hübel K, Kröger N, et al. Autologous haematopoietic stem cell mobilisation in multiple myeloma and lymphoma patients: a position statement from the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2014;49(7):865–872. doi:10.1038/bmt.2014.39.
- Giralt S, Costa L, Schriber J, et al. Optimizing autologous stem cell mobilization strategies to improve patient outcomes: consensus guidelines and recommendations. Biol Blood Marrow Transplant. 2014;20(3):295–308. doi:10.1016/j.bbmt.2013.10.013.
- Moreb JS, Byrne M, Shugarman I, et al. Poor peripheral blood stem cell mobilization affects long-term outcomes in multiple myeloma patients undergoing autologous stem cell transplantation. J Clin Apher. 2018;33(1):29–37. doi:10.1002/jca.21556.
- Chua CC, Lim HY, Chai KL, et al. Peripheral blood stem cell mobilisation with G-CSF alone versus G-CSF and cyclophosphamide after bortezomib, cyclophosphamide and dexamethasone induction in multiple myeloma. Bone Marrow Transplant. 2018;53(9):1116–1123. doi:10.1038/s41409-018-0152-2.
- Tanimura A, Hirai R, Nakamura M, et al. Improved progression-free and event-free survival in myeloma patients undergoing PBSCH receiving a cyclophosphamide + G-CSF regimen than G-CSF alone. Int J Hematol. 2018;107(5):559–567. doi:10.1007/s12185-018-2408-4.
- Gertz M, Kumar S, Lacy M, et al. Comparison of high-dose cy and growth factor with growth factor alone for mobilization of stem cells for transplantation in patients with multiple myeloma. Bone Marrow Transplant. 2009;43(8):619–625. doi:10.1038/bmt.2008.369.
- Wang L, Xiang H, Yan Y, et al. Comparison of the efficiency, safety, and survival outcomes in two stem cell mobilization regimens with cyclophosphamide plus G-CSF or G-CSF alone in multiple myeloma: a meta-analysis. Ann Hematol. 2021;100(2):575–573. doi:10.1007/s00277-020-04376-w.
- Ozsan GH, Micallef IN, Dispenzieri A, et al. Hematopoietic recovery kinetics predicts for poor CD34+ cell mobilization after cyclophosphamide chemotherapy in multiple myeloma. Am J Hematol. 2012;87(1):1–4. doi:10.1002/ajh.22179.
- Mazumder A, Kaufman J, Niesvizky R, et al. Effect of lenalidomide therapy on mobilization of peripheral blood stem cells in previously untreated multiple myeloma patients. Leukemia. 2008;22(6):1280–1281. doi:10.1038/sj.leu.2405035.
- Paripati H, Stewart A, Cabou S, et al. Compromised stem cell mobilization following induction therapy with lenalidomide in myeloma. Leukemia. 2008;22(6):1282–1284. doi:10.1038/sj.leu.2405100.
- Popat U, Saliba R, Thandi R, et al. Impairment of filgrastim-induced stem cell mobilization after prior lenalidomide in patients with multiple myeloma. Biol Blood Marrow Transplant. 2009;15(6):718–723. doi:10.1016/j.bbmt.2009.02.011.
- Afifi S, Adel N, Devlin S, et al. Upfront plerixafor plus G-CSF versus cyclophosphamide plus G-CSF for stem cell mobilization in multiple myeloma: efficacy and cost analysis study. Bone Marrow Transplant. 2016;51(4):546–552. doi:10.1038/bmt.2015.322.
- Winkelmann N, Desole M, Hilgendorf I, et al. Comparison of two dose levels of cyclophosphamide for successful stem cell mobilization in myeloma patients. J Cancer Res Clin Oncol. 2016;142(12):2603–2610. doi:10.1007/s00432-016-2270-9.
- Hamadani M, Kochuparambil ST, Osman S, et al. Intermediate-dose versus low-dose cyclophosphamide and granulocyte colony-stimulating factor for peripheral blood stem cell mobilization in patients with multiple myeloma treated with novel induction therapies. Biol Blood Marrow Transplant. 2012;18(7):1128–1135. doi:10.1016/j.bbmt.2012.01.005.
- Mikhael J, Ismaila N, Cheung MC, et al. Treatment of multiple myeloma: ASCO and CCO Joint Clinical Practice Guideline. J Clin Oncol. 2019;37(14):1228–1263. doi:10.1200/JCO.18.02096.
- Xu L, Liu J, Huang B, et al. Comparison of efficacy, safety, patients’ quality of life, and doctors’ occupational stress between lenalidomide-based and bortezomib-based induction in patients with newly diagnosed multiple myeloma. Cancer Med. 2021;10(5):1656–1667. doi:10.1002/cam4.3762.
- Peterson DE, Bensadoun R-J, Lalla RV, et al. Supportive care treatment guidelines: value, limitations, and opportunities presented at seminars in oncology. 2011;38:367–73. Elsevier. doi:10.1053/j.seminoncol.2011.03.005.
- Sutherland DR, Anderson L, Keeney M, et al. The Ishage guidelines for CD34+ cell determination by flow cytometry. J Hematother. 1996;5(3):213–226. doi:10.1089/scd.1.1996.5.213.
- Kyle R, Rajkumar SV. Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia. 2009;23(1):3–9. doi:10.1038/leu.2008.291.
- Rajkumar SV. Multiple myeloma: every year a new standard? Hematol Oncol. 2019;37(Suppl 1):62–65. doi:10.1002/hon.2586.
- Devarakonda S, Efebera Y, Sharma N. Role of stem cell transplantation in multiple myeloma. Cancers (Basel). 2021;13(4):863. doi:10.3390/cancers13040863.
- Kumar SK, Buadi FK, Rajkumar SV. Pros and cons of frontline autologous transplant in multiple myeloma: the debate over timing. Blood J Am Soc Hematol. 2019;133(7):652–659. doi:10.1182/blood-2018-08-825349.
- Cavo M, Goldschmidt H, Rosinol L, et al. Double vs single autologous stem cell transplantation for newly diagnosed multiple myeloma: long-term follow-up (10-years) analysis of randomized phase 3 studies. Blood. 2018;132(Supplement 1):124–124. doi:10.1182/blood-2018-99-112899.
- Gonsalves W, Gertz M, Lacy M, et al. Second auto-SCT for treatment of relapsed multiple myeloma. Bone Marrow Transplant. 2013;48(4):568–573. doi:10.1038/bmt.2012.183.
- Giralt S, Garderet L, Durie B, et al. American Society of Blood and Marrow Transplantation, European Society of Blood and Marrow Transplantation, Blood and Marrow Transplant Clinical Trials Network, and International Myeloma Working Group Consensus Conference on salvage hematopoietic cell transplantation in patients with relapsed multiple myeloma. Biol Blood Marrow Transplant. 2015;21(12):2039–2051. doi:10.1016/j.bbmt.2015.09.016.
- Musto P, Simeon V, Grossi A, et al. Predicting poor peripheral blood stem cell collection in patients with multiple myeloma receiving pre-transplant induction therapy with novel agents and mobilized with cyclophosphamide plus granulocyte-colony stimulating factor: results from a Gruppo Italiano Malattie Ematologiche Dell’adulto Multiple Myeloma Working Party Study. Stem Cell Res Ther. 2015;6(1):64. doi:10.1186/s13287-015-0033-1.
- Uy GL, Costa LJ, Hari PN, et al. Contribution of chemotherapy mobilization to disease control in multiple myeloma treated with autologous hematopoietic cell transplantation. Bone Marrow Transplant. 2015;50(12):1513–1518. doi:10.1038/bmt.2015.190.
- Silvennoinen R, Anttila P, Säily M, et al. A randomized phase ii study of stem cell mobilization with cyclophosphamide + G-CSF or G-CSF alone after lenalidomide-based induction in multiple myeloma. Bone Marrow Transplant. 2016;51(3):372–376. doi:10.1038/bmt.2015.236.
- Jantunen E, Putkonen M, Nousiainen T, et al. Low-dose or intermediate-dose cyclophosphamide plus granulocyte colony-stimulating factor for progenitor cell mobilisation in patients with multiple myeloma. Bone Marrow Transplant. 2003;31(5):347–351. doi:10.1038/sj.bmt.1703840.
- Fitoussi O, Perreau V, Boiron J, et al. A comparison of toxicity following two different doses of cyclophosphamide for mobilization of peripheral blood progenitor cells in 116 multiple myeloma patients. Bone Marrow Transplant. 2001;27(8):837–842. doi:10.1038/sj.bmt.1702879.
- Klein HO, Wickramanayake PD, Christian E, et al. Therapeutic effects of single-push or fractionated injections or continuous infusion of oxazaphosphorines (cyclophosphamide, ifosfamide, ASTA Z 7557). Cancer. 1984;54(S1):1193–1203. doi:10.1002/1097-0142(19840915)54:1+<1193::AID-CNCR2820541317>3.0.CO;2-Z.
- Voelcker G, Wagner T, Wientzek C, et al. Pharmacokinetics of “activated” cyclophosphamide and therapeutic efficacies. Cancer. 1984;54(S1):1179–1186. doi:10.1002/1097-0142(19840915)54:1+<1179::AID-CNCR2820541315>3.0.CO;2-P.
- Jang JE, Cheong J-W, Kim S-J, et al. Selection of a mobilization regimen for multiple myeloma based on the response to induction therapy: granulocyte-colony stimulating factor (G-CSF) alone versus high-dose cyclophosphamide plus G-CSF. Leuk Lymphoma. 2016;57(6):1389–1397. doi:10.3109/10428194.2015.1102240.
- Jung S-H, Park H, Ahn J-S, et al. Efficacy of stem cell mobilization in patients with newly diagnosed multiple myeloma after a CTD (cyclophosphamide, thalidomide, and dexamethasone) regimen. Int J Hematol. 2013;97(1):92–97. doi:10.1007/s12185-012-1237-0.
- Gu J, Li J, Liu J, et al. High dose melphalan (HDM) is superior to cyclophosphamide plus etoposide and busulfan (CVB) as the conditioning regimen in autologous stem cell transplantation for multiple myeloma. Zhonghua xue ye xue za zhi = Zhonghua Xueyexue Zazhi. 2019;40:732–737.