Abstract
Background
Developmental dysplasia of the hip (DDH) is a disorder of hip development that leads to dysplasia, subluxation, or total hip dislocation. Early detection of DDH is important, and early initiation of abduction treatment is key to successful correction of the hip joint. However, mild forms of DDH, including hip instability without complete dislocation, have good spontaneous healing potential, and a watchful waiting strategy in mild DDH has been found to be safe. In this study, we aimed to evaluate the cost differences between different treatment strategies for DDH.
Material and methods
Data were collected retrospectively from the medical records of all children diagnosed with diagnosis and treatment of DDH in Tampere University hospital between 1998 and 2018. In total, 948 patients were included in the study. Patients who underwent casting or operative treatment (n = 48) were excluded from the analysis. All Ortolani positive children were subjected to early abduction treatment. Children with Ortolani negative DDH were subjected to either watchful waiting or early abduction treatment, based on the clinicians’ decision. The regression model estimates for the number of clinical visits with and without ultrasound examination were assessed together with cost reports from Tampere University Hospital for the calculation of savings per patient in spontaneous recovery.
Results
Alpha angles at one month of age (p < 0.001) and treatment method (p < 0.001) affected the number of clinical visits and ultrasound examinations during the treatment follow-up. A low alpha angle predicted closer follow-up, and children with spontaneous recovery had lower numbers of clinical visits and ultrasound examinations than children in abduction treatment. Spontaneous recovery was found to result in approximately 375€/patient savings compared to successful abduction treatment.
Conclusion
With correct patient selection, a watchful waiting strategy is cost-effective in treating mild developmental dysplasia of the hip, considering the high percentage of spontaneous recovery.
KEY MESSAGES
Watchful waiting strategy should be implemented to clinical practice when treating mild DDH as it seems safe and cost effective.
Introduction
Developmental dysplasia of the hip (DDH) varies in severity from mild immaturity of the hip joint to the most severe form of DDH, in which the hip is completely dislocated and not reducible. Ortolani and Barlow’s signs are widely used by clinicians to describe the severity of a condition [Citation1,Citation2]. The true incidence of DDH is unknown because the screening methods vary globally. The incidence of 1–2/1000 newborns has been detected in clinical screening, but with ultrasound screening programs of the newborn, the incidence of all the mildest forms included is reported to be as high as 5–7% [Citation3–6].
It seems, that universal ultrasound screening programs might add overtreatment of the condition without reducing the late diagnosed cases of DDH, and thus universal ultrasound screening of DDH is not recommended by the literature [Citation7–10]. Selective secondary ultrasound screening in infants with clinically abnormal hips and/or known risk factors for DDH is recommended [Citation8,Citation11–13], although a recently published review did not find ultrasound screening of children with risk factors to reduce the number of late cases of DDH, surgery, or complications [Citation10].
Early detection of DDH is important for the successful treatment of this condition [Citation14,Citation15]. Conservative treatment with abduction is widely accepted as the first-line treatment for Ortolani-positive dislocation. Globally, Pavlik Harness, which has shown high success rates, is the most commonly used method for abduction treatment [Citation16,Citation17]. Mild forms of DDH exhibit excellent spontaneous recovery rates [Citation2,Citation14, Citation18]. It has been documented that it is safe to wait for spontaneous recovery for four to six weeks in Barlow-positive, Ortolani-negative DDH [Citation8,Citation19,Citation20].
Abduction treatment is effective and has an excellent success rate. Woodacre et al. reported that the cost of successful abduction treatment is around 700 €/patient but casting or operation multiples the costs per patient [Citation21].
We wanted to evaluate the costs of successful abduction treatment further to determine if there are any factors affecting the number of clinical visits or dynamic ultrasounds performed, thereby affecting the costs of abduction treatment. Our hypothesis was that children in watchful waiting strategy have fewer number of clinical controls and ultrasounds compared to early abduction treatment and thereby it might lead to savings in the treatment costs due to fewer controls and less imaging studies needed. To the best of our knowledge, there have been no previous studies on this subject.
Material and methods
Data were collected retrospectively from the medical records of all children with DDH diagnosis, according to the World Health Organization International Classification of Diseases and Health Related Problems 9th and 10th revisions (ICD-9 and ICD-10), codes 7543.0–7543.5 (ICD-9), and Q65.0-Q65.5 (ICD-10), treated in Tampere University Hospital in the years 1998–2018. Only children whose treatment was started and completed at the Tampere University Hospital were included. In total, 948 patients were included in the study. Children who underwent surgery or spica casting (n = 48) were excluded from analysis.
A positive family history was defined as any first-degree relative (parent or sibling) having a history of DDH. As information on family history was missing in a high number of cases (n = 335), we decided to include children with missing information in the missing information of family history subgroup in the multivariable model of all children, as we did not want to exclude this high number of patients, but we still wanted to include family history in the multivariable analysis.
Regarding risk factors’ association with the need of abduction after an observation period in a group of children initially selected in to watchful waiting, we only included the children with the information of all the risk factors (sex, breech presentation and family history) in to the analysis. We controlled the results including also the children with missing information about family history in to the analysis.
The first clinical status of the hip was defined as Ortolani positive or negative by a pediatric surgeon or pediatric surgery resident.
Alpha angles were measured according to Graf’s method by pediatric radiologists.
There were three possible treatment options: early abduction treatment (abduction started within the first two weeks of life), spontaneous recovery (no abduction treatment), and delayed abduction treatment (abduction treatment started approximately at the age of one month). Children with an Ortolani-positive DDH were treated with early abduction. Children with mild DDH (Ortolani negative, clinically mild hip instability without complete dislocation) were either treated with early abduction or selected for a watchful waiting protocol. The choice of treatment strategy (watchful waiting or early abduction) was made by a pediatric surgeon or a pediatric surgery resident and was mainly based on the clinical presentation, but having risk factors of DDH and parents’ opinion about treatment were also taken account. The first ultrasound was mainly performed at the age of one month, after which sonographic findings, together with the clinical status, guided the treatment and follow-up. The abnormal ultrasound findings at approximately 4–6 weeks of age (range 16 – 89 days) lead to abduction treatment in children initially selected in to watchful waiting strategy. Abduction treatment ended after reaching normal findings in dynamic ultrasound (stable hip joint and alpha angle 60 degrees or more).
Statistical analysis
Data were analyzed using IBM SPSS Statistics for Windows version 23 (IBM). The deviation of normality was checked for the number of clinical visits and ultrasounds as well as for alpha angles using histograms and tests of normality (Kolmogorov-Smirnov and Shapiro-Wilk tests). Neither of these variables passed the formal normality tests; therefore, the Mann-Whitney test was used for binominal variables and the Kruskal-Wallis test for multinomial variables for statistical analysis in the bivariate models. The Spearman correlation- test was used between two continuous parameters (alpha angles and numbers of controls).
We studied risk factors’ associations with needing abduction after observation period in a group of children selected into the watchful waiting protocol in cross-tabulations with chi-square statistics. We then controlled the results in multivariable analysis in binomial regression model.
We studied the associations of risk factors (sex, breech presentation and positive family history), treatment (early abduction, spontaneous recovery or delayed abduction) and alpha angles (as a continuous parameter) with the number of clinical visits without hip ultrasound and including hip ultrasound with multivariable analysis using a linear regression model. The raw residuals were checked for the number of clinical visits, and the distribution of the residuals was considered symmetrical, indicating the reliability of the selected method.
The costs of clinical visits, including ultrasound examination (400€) and clinical visits without ultrasound (125€) were verified from the latest cost reports of Tampere University Hospital. These prices include all the costs related to the clinical visit (for example, radiologists’ statements and harness initiation by pediatric surgeons) but not the cost of the harness itself.
Using the estimates from the multivariable model and the latest cost report of Tampere University Hospital, we calculated the savings per patient that could be achieved with spontaneous recovery.
Ethical approvement
The study was carried out according to Finnish national and European Union legislation and guidelines. The Regional Ethics Committee of the Expert Responsibility area of Tampere University Hospital accepted the study. The need for patients’ written consent was deemed unnecessary by the Regional Ethics Committee of the Expert Responsibility area of Tampere University Hospital as we did not contact the families to conduct this retrospective study.
Results
Of the 900 children, the majority (71.8%) were girls. Ortolani-positive dislocations were detected in 361 patients (40.1%). In total, 640 children (71.1%) underwent abduction treatment. Of the treated children, 482 (75.3%) had an early start of abduction treatment within the first two weeks of life. Majority of these children (98.8%) were treated within the first week of life. Overall, 418 children (45.9%) were subjected to a watchful waiting strategy. Of these, 159 (38.0%) had delayed abduction treatment and 259 (62.0%) recovered spontaneously. Mean observation time before delayed abduction was 35 days (range 16–89 days), and majority (77.4%) of the children in delayed abduction were observed for four to six weeks before abduction. Altogether 19 children (11.9%) had shorter observation period (16–26 days) and 17 (10.6%) children had longer observation period (over six weeks) before the treatment initiation. The patient demographics are presented in .
Table 1. Patient demographics.
Girls were more likely to need abduction treatment after an observation period than boys (p = 0.023). Positive family history (p = 0.818) or breech presentation (p = 0.551) were not associated with the need of abduction after observation. We controlled the results including the children with missing information about family history in to the multivariable analysis, which only made the association, regarding girls needing abduction after observation more often than boys, stronger (p = 0.005). See the results in .
Table 2. Risk factors effect on needing abduction treatment after observation period.
Mean of 2.0 clinical visits without ultrasound (median 1.9, range 0–15) and 2.4 (median 2.36, range 0–6) with ultrasound were performed per patient, respectively. Using the mean values and costs of clinical visits without ultrasound examination and ultrasound examination, the mean cost of treatment was calculated to be 2 × 125€+ 2.4 × 400€= 1210€per patient.
In the multivariate analysis, treatment (p < 0.001) and alpha angles (p < 0.001) were associated with the number of clinical visits without ultrasound (p < 0.001), as well as with the number of clinical visits including ultrasound (p < 0.001). Lower alpha angles were strongly associated with a higher number of clinical visits including ultrasound (p < 0.001) and without ultrasound examinations (p < 0.001). Children who recovered spontaneously had fewer clinical visits with ultrasound (p < 0.001) and without ultrasound examination (p < 0.001) than children who required abduction treatment with early or delayed onset ( and ).
Table 3. Risk factors effect on the numbers of clinical visits without ultrasound examination.
Table 4. Risk factors effect on the numbers of clinical visits with ultrasound examination.
Using the estimates from the multivariable models and the costs of clinical visits with and without hip ultrasound examination, we calculated the estimated savings for a successful watchful waiting strategy. The children who recovered spontaneously had approximately 1.4 clinical controls without ultrasound examination and 0.5 clinical controls, including ultrasound examination, less than the children in early abduction treatment (p < 0.001). Children in the late abduction treatment had 0.7 clinical controls without ultrasound less (p < 0.001) and 0.2 clinical controls including ultrasound examination more (p < 0.001) than children in the early treatment group. Clinical control, including ultrasound examination in the pediatric surgery department costs 400 € and without ultrasound 125€. Therefore, the savings of spontaneous recovery compared to early abduction treatment were 0.5 × 400€+ 1.4 × 125€= 375 €/patient. Late abduction treatment brought savings of 0.7 × 125€ − 0.2 × 400€= 7.5 €/patient, compared to early treatment.
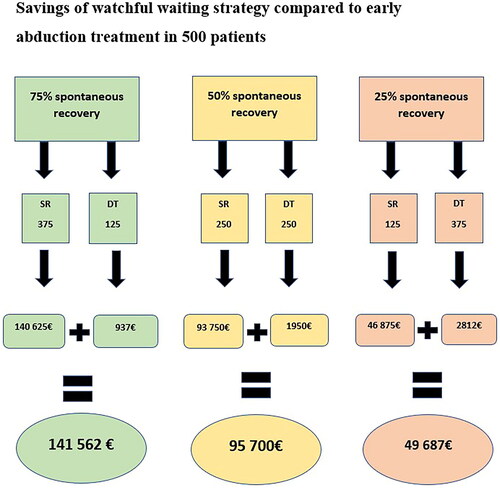
In our centre, the patients in the watchful waiting group demonstrated a 62.0% spontaneous recovery rate. Using these data, the savings achieved using the watchful waiting strategy for this selected patient group (n = 418) instead of immediate abduction treatment were ∼ 98 376 €(0.62 × 418 × 375€+0.38 × 418 × 7,5€). As variable spontaneous recovery rates of DDH during watchful waiting have been reported, we demonstrated the possible savings of a watchful waiting strategy in 500 patients with variable spontaneous recovery rates. Savings were calculated for the spontaneous recovery of 75%, 50%, and 25% of the children. The savings are shown in .
Discussion
As expected, we found that children with low alpha angles at the age of one month required close follow-up in their treatment. The low alpha angles also evidently lead to more expensive treatment because of close follow-up. In Tampere University Hospital, a watchful waiting strategy is a treatment option for children with mild DDH (Ortolani negative) based on the first clinical examination by a pediatric surgeon or pediatric surgery resident within the first 14 days of life. After approximately one month of age, the dynamic ultrasound examination by a pediatric radiologist with alpha angles and clinical status of the hips by a pediatric surgeon or pediatric surgery resident guide the treatment. Previous studies have found that in cases of mild DDH, four to six weeks waiting for treatment onset seems safe, as it does not seem to increase the risk of operative treatment or the duration of subsequent treatment [Citation19,Citation20,Citation22]. However, as we have reported earlier, watchful waiting strategy creates a risk for delay in the recovery of the hip joint anatomy due to later treatment initiation [Citation23]. According to our results, girls were more likely to need abduction treatment after observation period than boys. We have earlier found, that girl sex might increase the risk of late recovery [Citation23] and more severe clinical presentation of DDH [Citation24]. Our finding in the present study further supports our earlier findings. Girl sex is a well-known risk factor of DDH [Citation25,Citation26]. Our findings indicate that girl sex might have an effect on the recovery of DDH, as the observation period did not lead to normalization of alpha angles as often than in boys.
When considering the benefits of the watchful waiting strategy over the early abduction treatment in mild DDH, the cost differences of the strategies are also important to evaluate. More clinical visits and ultrasounds needed during follow-up also indicate a more expensive treatment. In the present study, we found that providing the possibility of spontaneous recovery can significantly reduce the costs of the treatment, depending on the percentage of children recovering spontaneously. The severity of the condition was taken account by having alpha angles in the multivariable model, and despite that the watchful waiting strategy seemed cost effective as the numbers of clinical controls and ultrasound examinations were lower in spontaneous recovery group and they were not significantly higher in delayed abduction group compared to early abduction group.
As earlier studies have pointed out, mild DDH has an excellent spontaneous recovery rate in the first weeks of life, ranging from 40% to 97%, depending on the study [Citation5,Citation14,Citation19,Citation27]. Since reported spontaneous recovery rates vary, we decided to demonstrate the possible savings in 500 patients if 25%, 50%, and 75% of the children in the watchful waiting strategy ended up needing abduction treatment after the observation period. The true savings of watchful waiting strategies depends on the percentage of spontaneous recovery. The higher the spontaneous recovery rate, the better the savings achieved using the watchful waiting strategy as a part of the treatment protocol. To our knowledge, no previous studies have considered the possible savings of watchful waiting strategy in mild DDH. Our research further encourages clinicians to use watchful waiting strategies in mild DDH, as it seems safe and could reduce expenses compared to the strategy in which all the mild forms of DDH are treated with early abduction. With appropriate patient selection, a watchful waiting strategy seems to be cost-effective. In this study, we only included children with successful conservative treatment, as we wanted to evaluate the cost of successful abduction treatment and/or spontaneous recovery. We did not include patients who underwent casting and/or operative treatment (n = 48, 0.051%), and it is necessary to further study the factors that increase the risk of failure of abduction treatment to avoid multiple surgeries and long hospital stays. However, in our unpublished study, we found that patient selection for a watchful waiting strategy at our hospital was safe and successful.
The mean cost of conservative treatment of DDH in our data was approximately 1200€/patient, which is markedly higher than the costs reported by Woodacre et al. reported (601£∼700€) in their study. However, the authors of the study did not report how many clinical controls and ultrasound examinations their patients had in the treatment, which makes it difficult to reliably compare the costs. The treatment costs vary between hospitals due to differences in treatment protocols, manufacturers and healthcare systems. The idea of watchful waiting in DDH is mainly based on two publications by Rosendahl et al. [Citation22] and Laborie et al. [Citation12]. It is reasonable that hospitals in the world in addition to ours base their watchful waiting protocol to these studies describing a period of waiting until about 6 weeks age and then, based on a single control visit with ultrasound, decision for abduction treatment, if necessary. The protocols of abduction treatment include much more variation worldwide. However, harness/splint adjustments in clinical controls in every 2–4 weeks are mandatory due to rapid growth of newborns. Some authors also recommend ultrasound every 2–4 weeks in harness treatment [Citation28]. This means that in all cases, the patients treated with abduction will have more control visits compared to those successfully improved during the watchful waiting. The exact amount of savings might not be generalizable due to different protocols but having savings due to fewer control visits is.
We did not include the costs of Pavlik harness in our calculated savings/patient, as we did not find the exact price of the harness in the Tampere University Hospital cost report. At Tampere University Hospital, we used and washed the same harness multiple times, which decreased the cost of the harness treatment. Woodacre et al. reported the harness cost to be approximately 40€/patient (35£), which can be added to our calculated savings, making the overall savings over 400€/patient in spontaneous recovery compared to early harness treatment.
The retrospective collection of our data is a limitation, as the information regarding family history of DDH and alpha angles was incomplete. We did not find associations between a positive family history and the number of clinical visits and ultrasound examinations. However, we have previously found that a positive family history seems to predispose children to the most severe cases of DDH [Citation23,Citation24]; therefore, children with a family history of DDH might also need closer follow-up than children without a family history. The incomplete data of alpha angles affected the multivariable model, as some children were excluded from the model. However, we wanted to include the alpha angles in the analysis, as they clearly affect the treatment and follow-up of DDH. Despite incomplete data, we still found differences in the number of clinical visits for different treatment strategies. Children were selected for watchful waiting strategies according to the clinical status of the hips. Due to the retrospective study design, the exact reasons behind clinicians’ treatment decisions regarding children with mild hip instability are not clear, and several issues (such as parents’ opinion, and risk factors of DDH) might have affected to it. However, as in our protocol Ortolani positive dislocations get treated early, only the children with clinically mild hip dysplasia were possibly selected to watchful waiting. With clearer protocol, subjecting all the clinically mild DDH in watchful waiting (as suggested in the literature [Citation8,Citation12,Citation22]), the spontaneous recovery rates might be even higher than in our study. However, our protocol is more flexible as it gives an option of early treatment also to children with clinically mild hip dysplasia. Despite the modified protocol, we still made savings as a number of children recovered spontaneously and the delayed treatment did not add controls. Selective ultrasound screening with a clinical screening program is a common practice and is also used in our hospital, as universal ultrasound screening might lead to over-diagnosis and over-treatment of DDH [Citation7,Citation10,Citation11,Citation13,Citation29,Citation30]. Our earlier study [Citation24] found that selective ultrasound screening in our hospital was successful, as the incidence of late cases of DDH (0.27/1000) and operatively treated cases of DDH (0.46/1000) were low, and in line with a recently published meta-analysis around the subject [Citation10].
Conclusions
With appropriate patient selection, a watchful waiting strategy in mild DDH might reduce the costs of treatment, considering the high percentage of spontaneous recovery in these children.
Authors contributions
Author V.L. wrote the main manuscript text and prepared the tables. Authors H.L., J.V-P., M.H., R-L.V. and A.H. reviewed the manuscript and helped with the writing. Authors V.L. and M.H. did the analysis of the data. Authors V.L. and K.B. collected the data from the medical records.
Acknowledgements
We thank the Finnish Cultural Foundation and the Päivikki and Sakari Sohlberg Foundation for their support of the study (grant to Dr. Anna Hyvärinen). We thank the Vappu Uuspää Foundation and Tampere city research foundation for their support in the study (grant to Vilma Lankinen).
Disclosure statement
VL, R-LV, MH, KB, JV, HL, and AH do not have any competing interests.
Data availability statement
The data are not publicly available because of patient confidentiality. The data are available from the corresponding author upon request.
Additional information
Funding
References
- Lehmann HP, Hinton R, Morello P, et al. Hip in conjunction with the C on QIS on DD of the. Developmental dysplasia of the hip practice guideline: technical report. Pediatrics. 2000;105(4):1–8. doi:10.1542/peds.105.4.e57.
- Vaquero-Picado A, González-Morán G, Garay EG, et al. Developmental dysplasia of the hip: update of management. EFORT Open Rev. 2019;4(9):548–556. doi:10.1302/2058-5241.4.180019.
- Peled E, Eidelman M, Katzman A, et al. Neonatal incidence of hip dysplasia: ten years of experience. Clin Orthop Relat Res. 2008;466(4):771–775. doi:10.1007/s11999-008-0132-8.
- Bialik V, Bialik GM, Blazer S, et al. Developmental dysplasia of the hip: a new approach to incidence. Pediatrics. 1999;103(1):93–99. Jan doi:10.1542/peds.103.1.93.
- Mulpuri K, Song KM, Gross RH, et al. The American academy of orthopaedic surgeons evidence-based guideline on detection and nonoperative management of pediatric developmental dysplasia of the hip in infants up to six months of age. J Bone Joint Surg. 2015;97(20):1717–1718. doi:10.2106/JBJS.O.00500.
- Lange AE, Lange J, Ittermann T, et al. Population-based study of the incidence of congenital hip dysplasia in preterm infants from the survey of neonates in pomerania (SNiP). BMC Pediatr. 2017;17(1):78. doi:10.1186/s12887-017-0829-5.
- Rosendahl K, Markestad TL. Ultrasound screening for developmental dysplasia of the hip in the neonate: the effect on treatment rate and prevalence of late cases. Pediatrics. 1994;94(1):47–52.
- Shorter D, Hong T, Osborn DA. Cochrane review: screening programmes for developmental dysplasia of the hip in newborn infants. Evid Based Child Health. 2013;8(1):11–54. doi:10.1002/ebch.1891.
- Olsen SF, Blom HC, Rosendahl K. Introducing universal ultrasound screening for developmental dysplasia of the hip doubled the treatment rate. Acta Paediatr. 2018;107(2):255–261. doi:10.1111/apa.14057.
- Kuitunen I, Uimonen MM, Haapanen M, et al. Incidence of neonatal developmental dysplasia of the hip and late detection rates based on screening strategy: a systematic review and meta-analysis. JAMA Netw Open. 2022;5(8):e2227638–e2227638. doi:10.1001/jamanetworkopen.2022.27638.
- Clarke NMP, Reading IC, Corbin C, et al. Twenty years experience of selective secondary ultrasound screening for congenital dislocation of the hip. Arch Dis Child. 2012;97(5):423–429. doi:10.1136/archdischild-2011-301085.
- Laborie LB, Markestad TJ, Davidsen H, et al. Selective ultrasound screening for developmental hip dysplasia: effect on management and late detected cases. A prospective survey during 1991-2006. Pediatr Radiol. 2014;44(4):410–424. doi:10.1007/s00247-013-2838-3.
- Rosendahl K, Toma P. Ultrasound in the diagnosis of developmental dysplasia of the hip in newborns. The European approach. A review of methods, accuracy and clinical validity. Eur Radiol. 2007;17(8):1960–1967. doi:10.1007/s00330-006-0557-y.
- Barlow TG. Early diagnosis and treatment of congenital dislocation of the hip. J Bone Joint Surg Br. 1962;44-B(2):292–301. doi:10.1302/0301-620X.44B2.292.
- Homer CJ, Baltz RD, Hickson GB, et al. Clinical practice guideline: early detection of developmental dysplasia of the hip. Pediatrics. 2000;105(4 I):896–905.
- Kokavec M, Makai F, Olos M, et al. Pavlik’s method: a retrospective study. Arch Orthop Trauma Surg. 2006;126(2):73–76. doi:10.1007/s00402-005-0086-1.
- Scott JM, Viktor B. Pavlik: the man and his method. J Pediatr Orthop. 2003;23(3):342–346. https://pubmed.ncbi.nlm.nih.gov/12724597/
- Young JR, Anderson MJ, O’Connor CM, et al. Team approach: developmental dysplasia of the hip. JBJS Rev. 2020;8(9):e20.00030. https://pubmed.ncbi.nlm.nih.gov/32890048/
- Cook KA, Schmitt M, Ingram M, et al. Pavlik harness initiation on barlow positive hips: can we wait? J Orthop. 2019;16(5):378–381. doi:10.1016/j.jor.2019.03.012.
- Larson JE, Patel AR, Weatherford B, et al. Timing of pavlik harness initiation: can we wait? J Pediatr Orthop. 2019;39(7):335–338. doi:10.1097/BPO.0000000000000930.
- Woodacre T, Dhadwal A, Ball T, et al. The costs of late detection of developmental dysplasia of the hip. J Child Orthop. 2014;8(4):325–332. doi:10.1007/s11832-014-0599-7.
- Rosendahl K, Dezateux C, Fosse KR, et al. Immediate treatment versus sonographic surveillance for mild hip dysplasia in newborns. Pediatrics. 2010;125(1):e9–16. doi:10.1542/peds.2009-0357.
- Bakti K, Lankinen V, Helminen M, et al. Clinical and sonographic improvement of developmental dysplasia of the hip: analysis of 948 patients. J Orthop Surg Res. 2022;17(1):538. doi:10.1186/s13018-022-03432-7.
- Lankinen V, Helminen M, Bakti K, et al. Known risk factors of the developmental dysplasia of the hip predicting more severe clinical presentation and failure of pavlik harness treatment. BMC Pediatr. 2023;23(1):148. doi:10.1186/s12887-023-03935-0.
- De Hundt M, Vlemmix F, Bais JMJ, et al. Risk factors for developmental dysplasia of the hip: a meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2012;165(1):8–17. doi:10.1016/j.ejogrb.2012.06.030.
- Ortiz-Neira CL, Paolucci EO, Donnon T. A meta-analysis of common risk factors associated with the diagnosis of developmental dysplasia of the hip in newborns. Eur J Radiol. 2012;81(3):e344–e351. European Journal of Radiology. doi:10.1016/j.ejrad.2011.11.003.
- Wedge JH, Wasylenko MJ. The natural history of congenital dislocation of the hip: a critical review. Clin Orthop Relat Res. 1978;(137):154–162. doi:10.1097/00003086-197811000-00024.
- Kelley SP, Feeney MM, Maddock CL, et al. Expert-based consensus on the principles of pavlik harness management of developmental dysplasia of the hip. Jbjs Oa. 2019;4(4):e0054. doi:10.2106/JBJS.OA.18.00054.
- Barrera CA, Cohen SA, Sankar WN, et al. Imaging of developmental dysplasia of the hip: ultrasound, radiography and magnetic resonance imaging. Pediatr Radiol. 2019;49(12):1652–1668. doi:10.1007/s00247-019-04504-3.
- Boeree NR, C NM. Ultrasound imaging and secondary screening for congenital dislocation of the hip. J Bone Joint Surg Br. 1994;76(4):525–533. doi:10.1302/0301-620X.76B4.8027133.