Abstract
Introduction
Chronotherapeutic interventions for bipolar depression and mania are promising interventions associated with rapid response and benign side effect profiles. Filtering of biologically active short wavelength (blue) light by orange tinted eyewear has been shown to induce antimanic and sleep promoting effects in inpatient mania. We here describe a study protocol assessing acute and long-term stabilizing effects of blue blocking (BB) glasses in outpatient treatment of bipolar disorder.
Patients and Methods
A total of 150 outpatients with bipolar disorder and current symptoms of (hypo)-mania will be randomized 1:1 to wear glasses with either high (99%) (intervention group) or low (15%) (control group) filtration of short wavelength light (<500 nm). Following a baseline assessment including ratings of manic and depressive symptoms, sleep questionnaires, pupillometric evaluation and 48-h actigraphy, participants will wear the glasses from 6 PM to 8 AM for 7 consecutive days. The primary outcome is the between group difference in change in Young Mania Rating Scale scores after 7 days of intervention (day 9). Following the initial treatment period, the long-term stabilizing effects on mood and sleep will be explored in a 3-month treatment paradigm, where the period of BB treatment is tailored to the current symptomatology using a 14-h antimanic schedule during (hypo-) manic episodes (BB glasses or dark bedroom from 6 PM to 8 AM) and a 2-h maintenance schedule (BB glasses on two hours prior to bedtime/dark bedroom) during euthymic and depressive states.
The assessments will be repeated at follow-up visits after 1 and 3 months. Throughout the 3-month study period, participants will perform continuous daily self-monitoring of mood, sleep and activity in a smartphone-based app. Secondary outcomes include between-group differences in actigraphic sleep parameters on day 9 and in day-to-day instability in mood, sleep and activity, general functioning and objective sleep markers (actigraphy) at weeks 5 and 15.
Trial registration
The trial will be registered at www.clinicaltrials.gov prior to initiation and has not yet received a trial reference.
Administrative information
The current paper is based on protocol version 1.0_31.07.23. Trial sponsor: Lars Vedel Kessing.
Introduction
Bipolar disorder (BD) is a severe mental disorder that affects 1-2% of the population. The core BD symptomatology is week or month-long depressive and (hypo)manic episodes separated by periods of euthymia. The treatment of BD consists of acute interventions to treat affective episodes as well as continuous maintenance therapy aimed to minimize residual symptoms and prevent new episodes. Evidence suggests that inter-episode residual symptoms, such as sleep problems, cognitive deficits and mood instability adversely affect patient wellbeing, prognosis and functioning [Citation1–4]. Thus, day-to-day mood variation is associated with increased stress, reduced functioning and quality of life, as well as risk of relapse and hospitalization [Citation2,Citation4,Citation5]. Poor inter-episode sleep is associated with lower functioning and quality of life [Citation3]. Moreover, sleep disturbance has been found to negatively influence pharmacological treatment response in BD patients [Citation6].
Pharmacological mood stabilizers are associated with side effects that can add to the risk of discontinuation and relapse during long-term maintenance treatment, hence the need for new treatments [Citation7,Citation8]. A task force under the International Society for Bipolar Disorder (ISBD) has recently reviewed the current chronotherapeutic modalities that may be additions or alternatives to the current treatment of BD [Citation9]. Preliminary evidence suggests that addition of light and dark therapies to standard interventions for depression and mania may be associated with rapid symptom control during acute episodes, and both interventions display benign side effect profiles [Citation9]. However, stronger evidence for efficacy during acute mania is needed, and specifically, there is a scarcity of evidence for maintenance or prophylactic potential of chronotherapeutic approaches in BD.
Dark therapy (DT) for mania involves patients being exposed to darkness for up to 14 h a day. An antimanic effect of DT was reported in two case reports in the late 1990s [Citation10,Citation11]. The intervention was further explored in a controlled trial by Barbini et al. in 2005, in which 16 inpatients with mania were exposed to DT for 3 days in addition to antimanic treatment as usual (TAU) [Citation12]. The 3-day addition of DT was associated with improved sleep and reductions in manic symptoms, and patients were discharged earlier than control patients receiving TAU only. However, the treatment was poorly tolerated by patients and was associated with practical disadvantages such as maintaining a dark patient room, keeping the patient in the room, the social isolation that followed, etc.
A more tolerable version of DT has been developed using orange-tinted eyewear to eliminate only the daylight signaling portion of light; the shorter wavelengths (<530 nm), that is blue/green, blue and violet light [Citation13,Citation14]. In an inpatient RCT, 32 patients with mania were randomized to glasses with either blue-blocking (BB) or clear (placebo) lenses [Citation15]. The use of BB glasses from 6 PM to 8 AM led to a significant reduction in manic symptoms after 3 days, and the effect increased further throughout the 7-day trial (Cohen’s d 1.86). Participants in the BB group obtained higher sleep efficiency, reduced activity levels, and received less sedative and psychotropic medication during the study period [Citation15,Citation16]. In the first outpatient RCT evaluating the effects of BB treatment on BD-related insomnia in 43 patients, BB treatment was associated with a small advance in chronotype, but not with improved sleep quality [Citation17].
The neurobiological target for BB treatment is a specialized retinal ganglion cell (RGC) strain in the inner retina, the intrinsically photosensitive RGCs (ipRGCs) [Citation18]. The ipRGCs are highly sensitive to short wavelength light (440-470 nm) and extend direct projections to the amygdala and central nuclei in the hypothalamus with regulatory influence on circadian rhythm and sleep, as well as mood-regulatory projections to the lateral habenula [Citation18–20]. Via polysynaptic pathways, the ipRGCs connect with the monoaminergic nuclei of the brainstem. Diffuse blue light stimulus has been shown to activate these nuclei within seconds and subsequently activate the general cortex within 20 min [Citation21]. A role of the ipRGCs in both acute light responses and more longerterm circadian adaptation to changes in the light-dark cycle has been confirmed in clinical and rodent studies [Citation19,Citation22–27]. These effects are partly obtained by direct stimulation of mood regulatory nuclei and indirectly through regulation of circadian rhythm, sleep and melatonin release [Citation19]. A few recent observational studies suggest that affective disorders are associated with slight aberrancies of the ipRGC system [Citation28–32]. A growing number of experimental studies also support a super-sensitivity to the phase-shifting effects of light as a possible trait in bipolar disorder [Citation33]. In sum, there is converging evidence from multiple research disciplines supporting the potential for chronotherapeutic interventions in BD, and a disproportionate lack of well-powered studies on clinical effectiveness. With this study protocol, we aim to fill a knowledge gap on the acute antimanic effects of BB treatment in an outpatient setting and to explore potential longer-term stabilizing effects on mood and sleep, as proposed by the ISBD chronotherapeutic task force [Citation9]. We hypothesize that 7 days treatment with TAU + BB glasses will lead to significant reductions in manic symptoms compared to TAU + low filtration glasses. Secondarily, we hypothesize that the continued use of BB glasses for 3 months will be associated with stabilization of mood, sleep and activity.
Patients and methods
Setting
The study will be performed at the Copenhagen Affective Disorder Research Center (CADIC), Denmark. Patients with BD who are treated at specialized BD outpatient clinics within the Mental Health Center Copenhagen will be included in a 2-arm randomized single-blinded trial of BB or low filtration glasses as an additive to outpatient BD TAU. Inclusion is scheduled to take place from February 2024 to February 2026.
Participants
Outpatients with an existing diagnosis of BD will be eligible for inclusion when demonstrating manic symptoms corresponding to a Young Mania Rating Scale (YMRS) score > 13 and meeting ICD-10 criteria for a hypomanic or manic episode (F31.0, F31.1). Further inclusion criteria will be age between 18 and 60 years and fluency in Danish. Exclusion criteria include unwillingness or inability to adhere to the protocol including the electronic self-monitoring system, severe eye disorder or eye trauma, use of beta blockers (suppresses melatonin release), sleep apnea, restless legs syndrome, REM sleep disorder, narcolepsy, substance abuse, prior/current use of BB glasses, current/planned pregnancy, night shift work and suicidality (Hamilton Depression Rating Scale, HDRS item 3 > 2).
Eligible participants will be identified by the clinical staff at the outpatient clinics. Participants will be referred to the investigator who will provide written and verbal information about the study, assess eligibility and collect consent.
Interventions
Following inclusion to the study, participants will be randomized 1:1 to wearing glasses with either orange-tinted lenses that block nearly 100% of short wavelength light < 500 nm (BB) (intervention group) or clear lenses that block approximately 15% of short wavelength light (low filtration (LF) (control group). Participants will be instructed to wear the glasses from 6 PM to 8 AM for 7 consecutive days, including any waking periods during the night. The glasses are removed during sleep. Participants will be encouraged to maintain a dark bedroom and/or use a sleep mask. Adherence to the use of the glasses will be documented every morning along with the use of medication.
After the initial 7-day intervention, participants will continue to wear the allocated eyewear for a 3-month maintenance period, where the timing of the use of glasses can be changed according to the current symptomatology. When patients are in depressive, euthymic, or mixed state, the glasses are worn daily for 2 h before planned bedtime, continuing with real darkness in the bedroom. The dosage can be increased to the 14-h antimanic period (from 6 PM to 8 AM) if the participant experiences symptoms of hypomania/mania (YMRS >13). Participants will be instructed to contact the study coordinator if they experience emerging symptoms of mania. Moreover, participants will perform continuous daily self-monitoring of mood, sleep and activity in a smartphone-based app as part of TAU [Citation1]. Self-monitoring will also include daily registration of the timing of the use of the glasses in the app. All data will be available to the investigator, who will trace emerging hypomanic/manic episodes and contact the participant to advice a dose increase.
During the study, participants will receive TAU in the outpatient clinics, including mood stabilizers, psychoeducation and supportive interventions [Citation34].
Outcomes and assessments
At the baseline assessment, the investigator will confirm the diagnosis of BD according to ICD-10 criteria by interview based on the Schedules for Clinical Assessment in Neuropsychiatry (SCAN) [Citation35], evaluate comorbidities, and collect routine socio-demographic data (age, sex, marital/cohabitation status, employment status) as well as a psychiatric and somatic history.
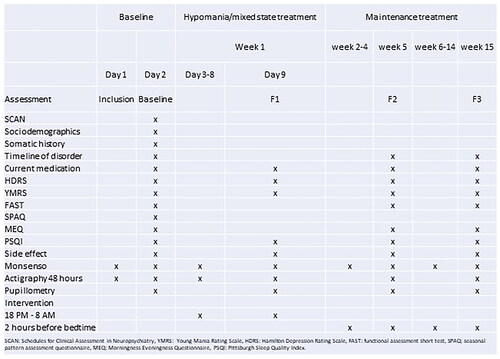
The assessment battery consists of validated instruments to evaluate current manic (YMRS) [Citation36] and depressive (HDRS-17) [Citation37] symptoms, general functioning (Functional assessment short test (FAST)) [Citation38], subjective sleep quality (Pittsburgh Sleep Quality Index (PSQI)) and chronotype (Morningness-Eveningness-Questionnaire (MEQ)). For objective measures of sleep and activity, an actigraphic baseline assessment will be performed for 48 h prior to the intervention, throughout the initial 7-day intervention and repeated after 5 and 15 weeks (48 h). Subjective evaluations of mood, sleep and activity will be performed daily throughout the study using the Monsenso App [Citation1]. A screening for side effects will be performed at baseline and at 9 days, 5 weeks and 15 weeks.
In case participants report increased suicidality (HDRS item 3 > 2) at any of the follow-up assessments, the intervention will be discontinued for that participant. Data up to the discontinuation will be included in the analysis.
For an overview of the timing and frequency of outcome assessments, see .
The primary outcome of the trial is the difference in change in YMRS score between the intervention group and the control group on day 9. The assessments will be conducted by a trained and blinded investigator who participates in regular joint YMRS rating sessions with clinicians and researchers at the Copenhagen Affective Disorder Clinic, Denmark.
Secondary outcomes include between-group differences in objective sleep markers (actigraphy) on day 9 as well as between-group differences in self-reported day-to-day instability in mood, sleep and activity, general functioning (FAST) and objective sleep markers (actigraphy) at weeks 5 and 15.
Tertiary outcomes include between-group differences in self-reported diurnal rhythm (MEQ) and sleep quality (PSQI) at week 15, as well as medicine use and the number and duration of affective episodes and admissions during the study period.
Assessments
Electronic smartphone-based self-monitoring – the Monsenso App
Participants will perform day-to-day self-monitoring with the Monsenso App [Citation1], which is a smartphone-based self-monitoring system that is an integral part of the treatment at the involved outpatient clinics. The Monsenso system includes a web-based interphase that can be accessed by the clinical staff/research team. Participants impute daily data on mood, sleep, activity, medication use, etc. The app has a reminder function that can remind participants to complete the data. Participants impute the timing and length of their sleep and rate their mood and activity level on a 9-point scale (−3 to + 3) where scores from −0.5 to 0.5 reflect normal variations, scores of + 1, + 2 or + 3 correspond to mild, moderate and severe increases, and scores of −1, −2 or −3 correspond to mild, moderate and severe decreases. Based on these ratings, an instability score (IS) can be estimated by applying the root mean square successive difference (rMSSD) method to daily ratings of mood and activity [Citation5].
Actigraphy
An actigraph is a wrist-worn combined accelerometer and light sensor that continuously records movement and ambient light intensity. Previous research has shown that wrist-worn actigraphy is well-tolerated and feasible in manic episodes [Citation15,Citation39]. Activity data can be visualized in a so-called actigram, showing a 24-h time interval on the X-axis and sequential days on the Y-axis. Motion activity registers as a signal: high signal activity corresponds to wakefulness, while absence of signal activity corresponds to sleep. Actigraphy is a validated method of objectively measuring standard sleep parameters such as total sleep time, nightly awakenings and napping (sleep fragmentation), timing of sleep, as well as activity parameters such as intra- and inter-daily variability and average daily and nightly activity counts.
Pupillometry
Chromatic pupillometry is a swift and non-invasive method for recording and quantifying the responsivity of the ipRGC-system to blue light stimulation. A blue light stimulus delivered to the eye elicits a specific extended pupillary constriction that persists beyond the termination of the light stimulus, a response called the post-illumination pupillary response (PIPR). With pupillometry, a monochromatic blue light stimulus is presented to the eye, and the consequent pupillary dynamics are traced with a continuous recording by infrared camera. A measure of PIPR is given as 1 – (pupil diameter after termination of the stimulus relative to the pupil diameter at baseline).
Sample size calculation
Due to the scarcity of studies evaluating BB treatment in BD, a strict sample size calculation is difficult to conduct. From the inpatient RCT on mania [Citation15], an effect size of 1.86 is reported. In an outpatient setting with milder symptomatology and potentially more wavering compliance, we expect to see smaller effects.
With a power of 0.80 at an alpha level of 0.05, a sample of 116 will allow us to detect a minimal detectable difference on the YMRS of 4 with an expected standard deviation of 7. Extending the sample to 150 participants will allow for a 30% dropout. For long-term effects of BB treatment, this sample size will allow us to detect a between-group treatment effect in instability scores for mood and activity of 0.35 with a standard deviation of 0.7.
The BD outpatient clinics treat approximately 300 patients per year, 40% of whom are expected to develop a (hypo)manic episode each year, why it seems plausible to include 150 patients over a 2-year study period.
Randomization and blinding
Patients will be randomized on a 1:1 basis with stratification according to sex and outpatient clinic. Before randomization, participant sex and the given outpatient clinic will be registered to determine the appropriate intervention for the included participant. Study identification numbers will be provided consecutively within each stratum. To avoid unblinding of the investigator, the glasses will be kept in solid, non-transparent cases and handed to the participant in the case, which will not be opened until the participant has left the clinic. The randomization list will be kept in a locked filing cabinet for which the outcome assessor will have no access. The allocation sequence is generated by the "sealed envelope" tool [https://www.sealedenvelope.com/simple-randomiser/v1/lists] and the randomization is done in the Research Electronic Data Capture (REDCap) database.
Assessors will be blinded to the treatment assignment and the blinding will be maintained throughout the study period and data analysis process. At the beginning of each assessment, participants will be instructed not to describe or discuss the color of the lenses with the assessor. In the event that the principal investigator is unblinded, the assessments will be performed by another member of the research team. Because the two types of glasses are of different colors (clear and orange), we cannot obtain full blinding of the participants.
Participants will be informed that we are studying the effect of two different types of light filters without further details, so they will not know for sure, which condition is experimental vs. control. Participants will be discouraged from actively seeking information about sleep glasses during the study. We will assess the integrity of the blinding by asking participants if they believe they received the glasses with high or low filtration lenses at the end of the study.
Data collection, management and analysis
At the eligibility assessment, personal information will be obtained by interview and through access to the electronic patient chart for details. Written informed consent forms will be signed upon inclusion and then kept in a locked filing cabinet. Personal information will be collected. A password-protected list will match participant ID numbers with personal information, and this list will be stored apart from pseudo-anonymized data.
After 10 years, the list will be deleted and consent forms will be maculated, after which all data are fully anonymized. Pseudo-anonymized data will be entered directly into the Research Electronic Data Capture (REDCap) database. The REDCap data system fulfills data management requirements of the Danish law and the General Data Protection Regulation. Named authors will have access to the final dataset. Questionnaires will be sent electronically from REDCap to patients to enable direct replies into the database.
REDCap has a logging module which enables tracking of the entered data. The trial sponsor will perform biannual checks for missing or inconsistent data and for deviations from the protocol regarding the timing of follow-up visits. According to Danish law, there is no requirement for an external data monitoring committee when conducting clinical trials with medical equipment with existing CE (Conformité Européenne) certification as type I medical equipment. No interim analyses are planned.
We will model between-group differences in primary, secondary and tertiary outcomes in linear mixed model analyses using group-by-time interactions and a random intercept for the participant. We will perform sensitivity analyses to assess the effect of clinical or socio-demographic variables that were not balanced at randomization. The main outcome will be analyzed according to the intention to treat principle. Missing data will be handled as missing-at-random. The analyses will be performed in the statistical software package R.
Ethics and dissemination
Informed consent will be obtained from all participants prior to inclusion. The study will be approved by the Research Ethics Committee under the Danish National Center for Ethics. Data will be handled according to the General Data Protection Regulation and the study will be registered in the central registry for research in the Capital Region of Denmark (Privacy). In accordance with the recommendations of the International Committee of Medical Journal Editors, the proposed trial will be registered in www.clinicaltrials.gov before initiation. There is great international interest in new treatment paradigms in BD and the results will be published in international peer-reviewed journals and presented at national and international conferences. The research team works closely with the outpatient clinics and the clinical academic group developing BD treatment in the Capital Region of Denmark, and knowledge dissemination is therefore immediate in this area.
In previous studies, the use of BB glasses has only been associated with minor side effects such as headaches or pain from insufficient fitting of the frames. Thus, we do not expect the treatment to pose a risk to participants.
Discussion
The cornerstone of BD- treatment remains pharmacological treatment with mood stabilizers that effectively treat manic and depressive episodes and prevent relapse. Unfortunately, the medication can be associated with side effects and discontinuation in the long-term regime that is often required [Citation7]. Although the current evidence base is limited, BB-therapy may constitute an effective treatment addition [Citation17,Citation15,Citation16].
The ISBD chronotherapeutic task force has made specific suggestions on methods to expand the evidence base for the chronotherapies [Citation9,Citation40]. The presented study seeks to address some of these suggestions to obtain a strong design. The strengths of the study include a randomized, blinded design, a relevant sample size and the inclusion of both objective and subjective circadian outcomes such as rest-activity and sleep-wake cycle assessment in addition to mood assessment. Moreover, the study is the first to assess the effects of extended DT beyond the acute phase of mania/hypomania. The 3-month follow up period is however not sufficient to directly address potential benefits regarding long-term stabilization and prevention of relapse. Moreover, our design includes patients with a rather mild symptom load and the results may not be fully generalizable to the full range of patients with BD. There are also inherent limitations to a design involving a placebo intervention that cannot be fully blinded. We do not expect this to be a significant issue as the use of sleep glasses or blue blockers has not received great attention in Denmark. We are mindful of the risk of dropout from the low filtration group if participants become aware that they are receiving a placebo or low efficacy treatment. However, we do not expect this to affect our primary acute outcome measured after the initial 9 days.
With this study, we aim to add to the evidence base for a potential antimanic treatment with minimal side effects and rapid response. Moreover, through explorations of several secondary circadian outcomes, we aspire to add to the growing knowledge base on the effects of light interventions and the role of circadian regularity in affective disorders [Citation41].
Authors contributions
HM, KM, IH, TEGH, MFJ, MK, LVK has contributed to the study concept and design. HM has drafted the protocol and the first version of the current manuscript. All authors provided critical revision of the protocol and manuscript for important intellectual content. All authors read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
After publication of the results, anonymized data can be made accessible on request.
Additional information
Funding
References
- Faurholt-Jepsen M, Miskowiak KW, Frost M, et al. Patient-evaluated cognitive function measured with smartphones and the association with objective cognitive function, perceived stress, quality of life and function capacity in patients with bipolar disorder. Int J Bipolar Disord. 2020;8(1):1. doi:10.1186/s40345-020-00205-1.
- Kessing LV, Faurholt-Jepsen M. Mood instability – a new outcome measure in randomised trials of bipolar disorder? Eur Neuropsychopharmacol. 2022;58:39–8. doi:10.1016/j.euroneuro.2022.02.005.
- Slyepchenko A, Allega OR, Leng X, et al. Association of functioning and quality of life with objective and subjective measures of sleep and biological rhythms in major depressive and bipolar disorder. Aust N Z J Psychiatry. 2019;53(7):683–696. doi:10.1177/0004867419829228.
- Stanislaus S, Faurholt-Jepsen M, Vinberg M, et al. Mood instability in patients with newly diagnosed bipolar disorder, unaffected relatives, and healthy control individuals measured daily using smartphones. J Affect Disord. 2020;271:336–344. doi:10.1016/j.jad.2020.03.049.
- Faurholt-Jepsen M, Busk J, Bardram JE, et al. Mood instability and activity/energy instability in patients with bipolar disorder according to day-to-day smartphone-based data – An exploratory post hoc study. J Affect Disord. 2023;334:83–91. doi:10.1016/j.jad.2023.04.139.
- Sylvia LG, Chang WC, Kamali M, et al. Sleep disturbance may impact treatment outcome in bipolar disorder: a preliminary investigation in the context of a large comparative effectiveness trial. J Affect Disord. 2018;225:563–568. doi:10.1016/j.jad.2017.08.056.
- Gutiérrez-Rojas L, Jurado D, Martínez-Ortega JM, et al. Poor adherence to treatment associated with a high recurrence in a bipolar disorder outpatient sample. J Affect Disord. 2010;127(1–3):77–83. doi:10.1016/j.jad.2010.05.021.
- Kessing LV, Søndergård L, Kvist K, et al. Adherence to lithium in naturalistic settings: results from a nationwide pharmacoepidemiological study. Bipolar Disord. 2007;9(7):730–736. doi:10.1111/j.1399-5618.2007.00405.x.
- Gottlieb JF, Benedetti F, Geoffroy PA, et al. The chronotherapeutic treatment of bipolar disorders: a systematic review and practice recommendations from the ISBD task force on chronotherapy and chronobiology. In. Bipolar Disord. 2019;21(8):741–773. doi:10.1111/bdi.12847.
- Wehr TA, Turner EH, Shimada JM, et al. Treatment of rapidly cycling bipolar patient by using extended bed rest and darkness to stabilize the timing and duration of sleep. Biol Psychiatry. 1998;43(11):822–828. doi:10.1016/s0006-3223(97)00542-8.
- Wirz-Justice A, Quinto C, Cajochen C, et al. A rapid-cycling bipolar patient treated with long nights, bedrest, and light. Biol Psychiatry. 1999;45(8):1075–1077. doi:10.1016/S0006-3223(98)00289-3.
- Barbini B, Benedetti F, Colombo C, et al. Dark therapy for mania: a pilot study. Bipolar Disord. 2005;7(1):98–101. doi:10.1111/j.1399-5618.2004.00166.x.
- Henriksen TE, Skrede S, Fasmer OB, et al. Blocking blue light during mania - markedly increased regularity of sleep and rapid improvement of symptoms: a case report. Bipolar Disord. 2014;16(8):894–898. doi:10.1111/bdi.12265.
- Hester L, Dang D, Barker CJ, et al. Evening wear of blue-blocking glasses for sleep and mood disorders: a systematic review. Chronobiol Int. 2021;38(10):1375–1383. doi:10.1080/07420528.2021.1930029.
- Henriksen TE, Skrede S, Fasmer OB, et al. Blue-blocking glasses as additive treatment for mania: a randomized placebo-controlled trial. Bipolar Disord. 2016;18(3):221–232. doi:10.1111/bdi.12390.
- Henriksen TEG, Grønli J, Assmus J, et al. Blue-blocking glasses as additive treatment for mania: effects on actigraphy-derived sleep parameters. J Sleep Res. 2020;29(5):e12984. doi:10.1111/jsr.12984.
- Esaki Y, Takeuchi I, Tsuboi S, et al. A double-blind, randomized, placebo-controlled trial of adjunctive blue-blocking glasses for the treatment of sleep and circadian rhythm in patients with bipolar disorder. Bipolar Disord. 2020;22(7):739–748. doi:10.1111/bdi.12912.
- Hattar S, Kumar M, Park A, et al. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J Comp Neurol. 2006;497(3):326–349. doi:10.1002/cne.20970.
- Legates TA, Altimus CM, Wang H, et al. Aberrant light directly impairs mood and learning through melanopsin-expressing neurons. Nature. 2012;491(7425):594–598. doi:10.1038/nature11673.
- Legates TA, Fernandez DC, Hattar S. Light as a Central modulator of circadian rhythms, sleep and affect. Nat Rev Neurosci. 2014;15(7):443–454. doi:10.1038/nrn3743.
- Vandewalle G, Maquet P, Dijk D-J. Light as a modulator of cognitive brain function. Trends Cogn Sci. 2009;13(10):429–438. doi:10.1016/j.tics.2009.07.004.
- Kawasaki A, Wisniewski S, Healey B, et al. Impact of long-term daylight deprivation on retinal light sensitivity, circadian rhythms and sleep during the antarctic winter. Sci Rep. 2018;8(1):1–12. doi:10.1038/s41598-018-33450-7.
- Lax P, Ortuño-Lizarán I, Maneu V, et al. Photosensitive melanopsin-containing retinal ganglion cells in health and disease: implications for circadian rhythms. Int J Mol Sci. 2019;20(13):3164. doi:10.3390/ijms20133164.
- Münch M, Kourti P, Brouzas D, et al. Variation in the pupil light reflex between winter and summer seasons. Acta Ophthalmol. 2016;94(3):e244–e246. doi:10.1111/aos.12966.
- Münch M, Léon L, Crippa SV, et al. Circadian and wake-dependent effects on the pupil light reflex in response to narrow-bandwidth light pulses. Invest Ophthalmol Vis Sci. 2012;53(8):4546–4555. doi:10.1167/iovs.12-9494.
- Ritter P, Wieland F, Skene DJ, et al. Melatonin suppression by melanopsin-weighted light in patients with bipolar disorder compared to healthy controls. J Psychiatry Neurosci. 2020;45(2):79–87. doi:10.1503/jpn.190005.
- Van Der Meijden WP, Van Someren JL, Te Lindert BHW, et al. Individual differences in sleep timing relate to melanopsin-based phototransduction in healthy adolescents and young adults. Sleep. 2016;39(6):1305–1310. doi:10.5665/sleep.5858.
- Berman G, Muttuvelu D, Berman D, et al. Decreased retinal sensitivity in depressive disorder: a controlled study. Acta Psychiatr Scand. 2018;137(3):231–240. doi:10.1111/acps.12851.
- Laurenzo SA, Kardon R, Ledolter J, et al. Pupillary response abnormalities in depressive disorders. Psychiatry Res. 2016;246:492–499. doi:10.1016/j.psychres.2016.10.039.
- Madsen HØ, Ba-Ali S, Heegaard S, et al. Melanopsin-mediated pupillary responses in bipolar disorder—a cross-sectional pupillometric investigation. Int J Bipolar Disord. 2021;9(1):1–10. doi:10.1186/s40345-020-00211-3.
- McGlashan EM, Coleman MY, Vidafar P, et al. Decreased sensitivity of the circadian system to light in current, but not remitted depression. J Affect Disord. 2019;256:386–392. doi:10.1016/j.jad.2019.05.076.
- Roecklein KA, Rohan KJ, Duncan WC, et al. A missense variant (P10L) of the melanopsin (OPN4) gene in seasonal affective disorder. J Affect Disord. 2009;114(1-3):279–285. doi:10.1016/j.jad.2008.08.005.
- Ritter P, Soltmann B, Sauer C, et al. Supersensitivity of patients With bipolar I disorder to light-Induced phase delay by narrow bandwidth blue light. Biol Psychiatry Glob Open Sci. 2022;2(1):28–35. doi:10.1016/j.bpsgos.2021.06.004.
- Kessing LV, Kyster NB, Bondo-Kozuch P, et al. Effect of specialised versus generalised outpatient treatment for bipolar disorder: the CAG bipolar trial - study protocol for a randomised controlled trial. BMJ Open. 2021;11(10):e048821. doi:10.1136/bmjopen-2021-048821.
- Wing JK, Babor T, Brugha T, et al. SCAN. Arch Gen Psychiatry. 1990;47(6):589–593. doi:10.1001/archpsyc.1990.01810180089012.
- Young RC, Biggs JT, Ziegler VE, et al. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133(5):429–435. doi:10.1192/bjp.133.5.429.
- Hamilton M. A rating scale FOR depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56–62. doi:10.1136/jnnp.23.1.56.
- Rosa AR, Sánchez-Moreno J, Martínez-Aran A, et al. Validity and reliability of the functioning assessment short test (FAST) in bipolar disorder. Clin Pract Epidemiol Ment Health. 2007;3(1):5. doi:10.1186/1745-0179-3-5.
- Krane-Gartiser K, Asheim A, Fasmer OB, et al. Actigraphy as an objective intra-individual marker of activity patterns in acute-phase bipolar disorder: a case series. Int J Bipolar Disord. 2018;6(1):8. doi:10.1186/s40345-017-0115-3.
- Murray G, Gottlieb J, Hidalgo MP, et al. Measuring circadian function in bipolar disorders: empirical and conceptual review of physiological, actigraphic, and self-report approaches. Bipolar Disord. 2020;22(7):693–710. doi:10.1111/bdi.12963.
- Lyall LM, Wyse CA, Graham N, et al. Association of disrupted circadian rhythmicity with mood disorders, subjective wellbeing, and cognitive function: a cross-sectional study of 91 105 participants from the UK biobank. Lancet Psychiatry. 2018;5(6):507–514. doi:10.1016/S2215-0366(18)30139-1.