Abstract
Aims
To estimate healthcare resource use and direct healthcare costs of Transthyretin Amyloid Cardiomyopathy (ATTR-CM) in Sweden over 12 months across severity stages as defined by the New York Heart Association (NYHA). Secondary to investigate the current diagnostic trajectory for patients with ATTR-CM in Sweden.
Methods
A stratified inclusion of patients with a confirmed diagnosis of ATTR-CM in different NYHA classes. Data was extracted from medical records in two cardiology clinics in Sweden. Healthcare resource use data were retrospectively collected for 12 months.
Results
38 patients were included, of whom 7 were in NYHA class II, 20 in class III and 4 in class IV. The total cost of health care per patient increased from SEK 69,000 (€6800) in NYHA stage II, SEK 219,000 (€21,500) in NYHA stage III, to SEK 638,000 (€62,900) in stage IV, mainly due to an increase in inpatient stays. Mean time (standard deviation, SD) from any cardiac related diagnosis prior to ATTR-CM diagnosis was 3.5 (3.1) years.
Conclusions
Advanced ATTR-CM stages are associated with significant healthcare costs, as patients more often require resource-intensive inpatient care. The current diagnostic trajectory of ATTR-CM in this study was characterized by a diagnostic delay of several years.
KEY MESSAGES
This study shows that both healthcare resource use and healthcare costs increased considerably with a higher degree of ATTR-CM severity.
The diagnostic trajectory of ATTR-CM in this study was characterized by a diagnostic delay of several years.
Greater disease awareness and a lower threshold for screening risk groups for TTR-amyloidosis is prompted to establish an earlier diagnosis.
Introduction
Transthyretin Amyloid Cardiomyopathy (ATTR-CM) is an underdiagnosed and fatal disease that manifests as progressive heart failure [Citation1]. The underlying cause of ATTR-CM is misfolding of the transthyretin (TTR) protein, which makes it aggregate into large and rigid amyloid fibrils [Citation2]. Amyloid infiltration in tissues (amyloidosis) is characterized by stiffness, fibrosis, and cytotoxicity [Citation1,Citation2]. Amyloidosis can cause biventricular wall thickening in the myocardium, leading to diastolic dysfunction and low cardiac output [Citation3].
The natural history of the condition is progressive heart failure, complicated by arrhythmias and conduction system disease [Citation3,Citation4] and is associated with an increased impairment of health-related quality of life [Citation5,Citation6]. Studies have shown that ATTR-CM diagnosis is often delayed for many years after symptoms develop [Citation6]. Early associated diagnoses may include bilateral carpal tunnel syndrome and spinal stenosis [Citation7]. The prognosis of ATTR-CM is generally poor compared to that of heart failure, with a median survival time from diagnosis of 2 to 6 years, depending on the disease type and stage at diagnosis [Citation6,Citation8,Citation9].
ATTR-CM is either acquired by age (the so-called wild-type, ATTRwt) or it is hereditary (variant, ATTRv). In ATTRwt, cardiomyopathy is central and often what leads to diagnosis and there is a strong male predominance. ATTRv is more clinically variable and may also present as primary polyneuropathy (PN) [Citation1]. For ATTRv, genetic variants are endemic, with increased prevalence among populations in specific areas such as Sweden, Japan, Brazil, and Portugal [Citation10,Citation11].
ATTR-CM has traditionally been classified as a rare disease; however, recent data suggest that its prevalence may be substantially higher than previously assumed due to underdiagnosis [Citation12]. This condition is becoming increasingly recognized as a cause of heart failure in older patients with increased myocardial wall thickness [Citation13,Citation14]. The overall prevalence of ATTR-CM has been estimated to be at least 17 per 100,000 in Sweden in 2018 [Citation15]. The incidence appears to be rising, presumably as a result of increased awareness [Citation13], and an aging population [Citation14].
Historical underdiagnosis of ATTR-CM is likely due to the absence of adequate treatment options, lack of non-invasive diagnostic methods, and lack of consensus diagnostic criteria [Citation1,Citation16]. However, new diagnostic algorithms using bone scintigraphy combined with serum free light chain (FLC) assays and electrophoresis to rule out systemic light chain (AL)-amyloidosis have enabled noninvasive diagnostic criteria for ATTR-CM [Citation17]. Furthermore, pharmaceutical treatments that inhibit amyloidosis in ATTR-CM have been developed and improve prospects for patients with this disease [Citation17–20].
Recent studies in Scandinavia have shown that ATTR-CM imposes a considerable burden on the healthcare system [Citation21,Citation22] with more hospitalization and outpatient visits compared to other causes of heart failure [Citation21]. However, studies investigating healthcare resource use and costs in relation to the different stages of heart failure progression are lacking.
The primary objective of this study was to estimate the annual healthcare resource use and associated direct costs for ATTR-CM in Sweden at different stages of heart failure, as defined by the New York Heart Association (NYHA). The secondary objective was to investigate the current diagnostic trajectory for patients with ATTR-CM in Sweden, including the time between the first ATTR-CM related diagnosis and ATTR-CM diagnosis as well as diagnostic procedures.
Some results from the study have previously been presented in a conference poster presentation [Citation23].
Materials and methods
Study design
This study was a non-interventional, cross-sectional analysis of patients with ATTR-CM in Sweden. Data from two cardiology clinics, Karolinska University Hospital in Stockholm and Akademiska University Hospital in Uppsala, were extracted from electronic medical records. Cardiologists experienced in treating patients with ATTR-CM, together with nurses, filled out reported Case Report Forms (CRF) for the included patients.
Inclusion and exclusion criteria
The study population included patients >18 years of age with diagnostic codes ICD-10 E.85.82, ICD-10 E.85.84, or ICD-10 E.85 or I.43 and a confirmed diagnosis of ATTR-CM. Each patient was assigned an index date at their last point of contact (outpatient, inpatient visit, or phone contact). To reflect different practices in Sweden, patients from different clinics were selected. In case more patients were available than the predefined quota per clinic, patients born in uneven years (e.g. 1947, 1949) were selected consecutively to limit the selection bias. In order to reflect a broad spectrum of patients with ATTR-CM, patients born in even years were added based on the NYHA class. The aim was to have a stratified inclusion of patients in different NYHA classes at the index date reflecting NYHA I and II 40%, NYHA III 50%, and NYHA IV 10%. This NYHA distribution was based on the expected distribution of NYHA classes in medical records while ensuring that all NYHA classes were included in the sample.
Exclusion criteria were a first diagnosis of ATTR-CM less than 12 months before the index date, a diagnosis of ATTR-PN, and treatment with tafamidis during the last 12 months of the observational period. Treatment with tafamidis was an exclusion criterion as tafamidis was not approved for ATTR-CM, only ATTR-PN, in Sweden at the time of the study.
Outcomes
Healthcare resource use was collected retrospectively from medical records for 12 months prior to inclusion, which was defined as each patient’s last healthcare visit at the two cardiology clinics. In the CRF, all healthcare contacts in primary, inpatient, and outpatient specialized care (stays, visits and phone/video contacts), treatments, procedures (echocardiography, ambulatory electrocardiography (ECG), pleural effusion, cardioversion, ablation, and pacemaker), and prespecified prescribed hospital-administered drugs (i.e. diuretics, diflunisal, angiotensin-converting enzyme (ACE) inhibitor, beta blocker, and novel oral anticoagulants (NOAC)), were included to ensure capture of all direct healthcare resource use associated with ATTR-CM.
In addition, patient characteristics at the time of diagnosis, including sex, year of birth, ATTR-CM type (i.e. ATTRwt or ATTRv), and NYHA functional classification, were collected from medical records. The diagnostic trajectory in terms of time from the first ATTR-CM related diagnosis to the ATTR-CM diagnosis was captured retrospectively by following the medical records from the first reported amyloidosis-related diagnosis that was registered before or the same year as the ATTR-CM diagnosis. Amyloidosis-related diagnoses were carpal tunnel syndrome, lumbar spinal stenosis, heart failure, sick sinus syndrome, bradycardia, pacemaker, aortic valve disease/transcatheter aortic valve implantation (TAVI), arrhythmia, and atrioventricular (AV) block. Information regarding the diagnostic procedures used at the time of diagnosis of ATTR-CM was also collected, including echocardiography, troponin/NT-proBNP, Na/K/creatinine, biopsy (cardiac or extra cardiac), bone scintigraphy, protein electrophoresis (serum or urine), FLC ratio, cardiac Magnetic Resonance Imaging (MRI), and ambulatory ECG. Information regarding comorbidities at the time of diagnosis was also collected.
Data analyses
Healthcare resources are presented for the total patient group and in subgroups across NYHA stages, as registered at the last healthcare contact. For healthcare contacts with no registered NYHA stage, the last registered NYHA stage was assigned (last value carried forward approach). If no NYHA stage was registered during the 12-month period, the NYHA stage at diagnosis was used. However, if the diagnosis was made more than two years ago, the patient was excluded from the subgroup analysis of NYHA stages.
Healthcare costs were calculated by multiplying the frequency of resource use from the medical records with unit prices from official Swedish price lists and presented as inpatient care costs, specialized outpatient costs, primary care costs, and drug costs. The costs are presented in SEK 2021 (€1 = SEK 10.15).
In the analysis of time from first ATTR-CM related diagnosis to ATTR-CM diagnosis, the diagnoses were presented in groups of extracardiac (carpal tunnel syndrome and lumbar spinal stenosis) and cardiac-related diagnoses (heart failure, sick sinus syndrome, bradycardia, pacemaker, aortic valve disease/TAVI, arrhythmia, and AV-block).
Statistical analyses
The analysis was descriptive. Standard descriptive statistics were computed, including the mean, standard deviation (SD), median, and interquartile range (IQR). Healthcare resource use and costs were calculated for the total study population and for subgroups divided by NYHA stages. Costs were expressed in mean (SD) in the main text but the median and IQR were also presented in the tables since the cost was positively skewed. No statistical tests were performed, as the subgroups were too small.
All analyses were performed using STATA Statistical software, version Stata/IC 14.2 (College Station, Texas, USA).
Results
The study population included 38 patients with an average duration of 3 years since receiving a diagnosis of ATTR-CM. Only one patient had ATTRv, and the remaining 37 had ATTRwt. Most of the patients were male (89%), and the mean age at diagnosis was high (77.7 years) (). Patients index dates ranged from the year 2019 to 2021.
Table 1. Patient characteristics.
Health care resource use
At the last contact, the majority of patients (53%) were in NYHA stage III, seven patients (18%) were NYHA stage II, and four patients (11%) were NYHA stage IV (). Information regarding the NYHA stage was missing for 7 patients, and these were thus not included in the subgroup analyses. At the last contact, the mean age of patients with NYHA stages II, III, and IV was 77.3, 77.5 and 80.8 years respectively.
In total, nine (24%) patients had progressed to another NYHA stage at the time of diagnosis. Of these, three patients switched stage (from stage III to IV) within the period of 12 months for which health care resource use was collected.
The assessment of healthcare resource use showed an overall trend of higher use with more advanced stages of disease ( and Supplementary material). For inpatient care, the average number of stays in hospital per patient increased from 1 in NYHA II to 1.4 in NYHA III to 9 in NYHA IV, which was largely represented by an increase in the number of stays in cardiology clinics. The time in inpatient care increased consistently, from an average of 2.3 d per patient in NYHA II, 7.8 d in NYHA III to 47 d (corresponding to an average of 5 d per stay) in NYHA IV.
Table 2. Inpatient and outpatient health care resource use during the last 12 months across NYHA stages.
For specialist outpatient care, the average total number of care contacts per patient increased from 8 to 11 for NYHA stages II to IV. Among the individual categories of specialist care, the clearest trend of higher resource use with higher NYHA stages was observed for emergency care and cardiology visits, which increased from 0.6 to 2.8 and from 2.1 to 4.3, respectively, between NYHA stages II and IV.
The average number of primary care visits per person increased from 3.4 to 11 between NYHA classes II and IV. There was a particular increase in home visits by nurses or other healthcare personnel among patients in higher NYHA stages.
The use of healthcare procedures increased between NYHA stages II and IV, which was largely represented by more drainage of pleural effusion among patients in the higher stages. The proportion of patients in the total study population who had been prescribed the prespecified drugs at some time during the 12 months-period was 92% for diuretics (37% had intravenous administration and 87% tablets), 63% for NOAC, 53% for betablockers, 34% for ACE inhibitors, and 11% for diflunisal (). The proportion of patients using intravenously administered diuretics increased with higher NYHA stage (14% in stage II, 45% in stage III, and 75% in stage IV), whereas the use of diuretic tablets decreased (100% in stage II, 95% in stage III, and 75% in stage IV).
Table 3. Drug utilization during last 12 months across NYHA stages, n (%).
Healthcare costs
The total health care cost during the 12-month period was approximately SEK 215,000 (€21,200) per patient with ATTR-CM, of which drugs contributed to less than 5%, primary care to approximately 5%, specialist outpatient care to 25%, and inpatient care to 70% ( and ). Consistent with healthcare resource use, healthcare costs increased with higher NYHA stages. The total cost of health care per patient increased from SEK 69,000 (€6800) in NYHA stage II, SEK 219,000 (€21,500) in NYHA stage III, to SEK 638,000 (€62,900) in NYHA stage IV, mainly due to an increase in the number of inpatient stays in cardiology clinics. The cost of inpatient care accounted for 43%, 61%, and 88% of total costs, respectively, in NYHA stages II, III, and IV.
Figure 1. Mean, yearly inpatient, outpatient, primary care, and drug costs across NYHA stages (€2021).
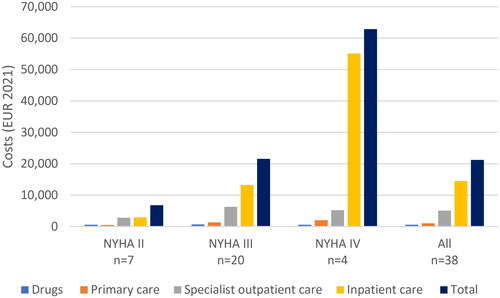
Table 4. Yearly total health care costs across NYHA stages (€2021).
Three out of the 4 patients here categorized as NYHA stage IV have been partly in NYHA stage III and partly in stage IV during the 12-month period (mean 1.8 months in stage IV). Differentiating the healthcare resource use and associated costs accrued in NYHA stage IV and extrapolating those costs to a ‘complete stage IV year’ would yield a total mean annual cost of approximately SEK 1 million.
Diagnostic trajectory
Cardiac-related diagnoses were registered at certain time points for all 38 patients in this study (). Out of the 38 patients, 32 (84%) patients had atrial fibrillation, 17 (45%) had a pacemaker, and 16 (42%) had AV-block. Thirty-one patients (82%) had a cardiac related diagnosis before or the same year as ATTR-CM diagnosis, two patients (5%) had a cardiac related diagnosis registered after the ATTR-CM diagnosis, and for five patients (13%), the date of diagnosis was missing. Among patients with a cardiac-related diagnosis before or the same year as the ATTR-CM diagnosis (n = 32), the average time between the cardiac-related diagnoses and the ATTR-CM diagnosis was 3.5 years (SD 3.1, median 3, IQR 1;5). Extracardiac diagnoses were registered at some time point in 16 patients (42%) (10 patients had lumbar spinal stenosis, and 13 patients had carpal tunnel syndrome). Nine patients (24%) had received extracardiac diagnoses before or the same year as the ATTR-CM diagnosis, and for seven patients (18%) date of diagnosis was missing. Among patients with extracardiac diagnoses registered before or the same year as ATTR-CM diagnosis (n = 9) there was an average time of 4.7 years (SD 5.2, median 4, IQR 1;5) until the diagnosis of ATTR-CM.
Table 5. Cardiac and extra cardiac related diagnoses.
The most common procedures at diagnosis were echocardiography (92%) and troponin/NT-proBNP (89%) and Na/K/creatinine (89%) assays (). The majority had also a biopsy (cardiac 16% or extra cardiac 82%), bone scintigraphy (76%), or protein electrophoresis (serum or urine) (71%), FLC assay was used in half of the patients, whereas MRI and ambulatory ECG were used by less than half of the patients. Twenty-two patients (58%) underwent both bone scintigraphy and biopsy, 18% underwent only bone scintigraphy, and 18% underwent only biopsy. All 19 patients who underwent the FLC assay underwent electrophoresis. Patients without a biopsy (n = 7) underwent bone scintigraphy, FLC assay, and electrophoresis.
Table 6. Diagnostic procedures.
The most common comorbidities reported for the patients were renal failure (21%), diabetes (16%), cancer (11%), and arthrosis (8%).
Discussion
The results from this study showed a clear trend that both healthcare resource use and healthcare costs increased considerably with a higher degree of ATTR-CM severity. Although there were few patients with NYHA stage IV, the results for this group indicated that the progression to stage IV is characterized by a dramatic increase in hospitalization and associated costs.
Moreover, the current diagnostic trajectory of ATTR-CM patients in this study demonstrated that most patients received their ATTR-CM diagnosis 3–4 years after their first ATTR-CM related diagnosis.
Patient inclusion in this study was stratified according to NYHA class, and the data should not be interpreted as representative of the entire cohort of ATTR-CM patients in Sweden. The patients included in this study were elderly, and the majority in NYHA class III to IV, which represents the sicker end of the spectra of this disease. Moreover, in this cohort, atrial fibrillation and bradyarrhythmia, including AV block and pacemaker treatment, were common.
Regarding diagnostic procedures, an overlap between biopsy-based diagnosis and the use of the non-biopsy algorithm was observed; the majority underwent both bone scintigraphy and biopsy. Historically, biopsy was the only means of diagnosis, and the newer non-biopsy algorithm is probably more commonly used today than when these patients were diagnosed.
One important strength of this study is that healthcare resource use and associated direct costs were analysed for different stages of disease progression, the NYHA functional classes. In addition, all types of healthcare resource use are included, such as primary care and healthcare provided by all types of healthcare personnel, hospitalizations, and physician contacts. However, some private clinics that used different electronic medical records were unavailable.
This study has some limitations. The main limitation is the small total population size as well as the small NYHA subgroups, which advocates that the results should be interpreted with caution. In particular, the sample size of patients with NYHA stage IV was small, which may have resulted in overestimated costs. However, the resource use and costs for a stage IV patients may also be underestimated because the data on resource use in stage IV are represented by months both in stage III and stage IV. Nevertheless, the distinction in severity between late stage III (NYHA IIIb) and stage IV is not always straightforward in clinical practice.
Results for the total sample group might not be representative of the whole ATTR-CM population in Sweden. Moreover, due to the small sample sizes, the generalisability of the results per NYHA class is limited, as we do not know to what extent the selected cases are representative of each NYHA class. Nonetheless, our results could be useful, for example in health economic modelling, as there is a lack of publications that report resource use per NYHA class.
The estimation of healthcare costs in this study indicates a high economic burden for this patient group. It is important to note that in most cases the mean costs were greater than median costs indicating that the data were positively skewed. This is however common when analysing costs. Compared to other patients with heart failure in Sweden; the mean cost of approximately SEK 215,000 (€21,200) per ATTR-CM patient per year (all NYHA classes included) is higher than the yearly estimated SEK 122,000 (adjusted to SEK 2021, €12,000) for Swedish patients with heart failure with preserved ejection fraction in a study by Stålhammar et al. for 2005–2007 [Citation24] and the yearly estimated SEK 58,000–134,000 (adjusted to SEK 2021, depending on the first or second year after heart failure diagnosis, €5700–13,200) in a study by Boman et al. for 2010–2012 [Citation25]. However, the relative proportion of costs attributed to hospitalization (70% vs. 80%) was comparable between studies.
The high healthcare cost of ATTR-CM compared to other types of heart failure is also in line with the findings of Lauppe et al. [Citation21], who demonstrated higher healthcare resource use among Scandinavian ATTR-CM patients than among matched heart failure patients without ATTR-CM. The study by Lauppe et al. also demonstrated a large increase in resource use, hospitalizations in particular, over time in patients with ATTR-CM. This was consistent with the trend observed with increasing disease severity. However, in contrast to the 3-year resource use for patients with ATTR-CM presented by Lauppe et al. our study reported a considerably higher use of inpatient care (mean stays/days: 2/11 vs. 1/5) and outpatient care (mean visits: 10 vs. 6) over a period of 12-months. This could be explained by differences in disease severity of patient populations between studies, whereas the patients included in our study had, on average, received their diagnosis 3 years prior to the study year. Lauppe et al. studied resource use for the year of ATTR-CM diagnosis and the three years prior to ATTR-CM diagnosis. Therefore, the data in our study represent later and more severe stages of the disease.
The current diagnostic trajectory of patients with ATTR-CM shown in our study is characterized by an average diagnostic delay of up to 3–4 years from the first ATTR-CM related diagnosis. Diagnostic delays of several years between the first cardiac symptom and ATTR-CM diagnosis have also been demonstrated elsewhere [Citation26]. Furthermore, this diagnostic delay is signified by a patient’s disease burden in terms of frequent hospital visits and poor quality of life [Citation6,Citation27].
Improved disease awareness, early investigation with bone scintigraphy in patients with cardiac disease, and red flags for cardiac amyloidosis could reduce diagnostic delay. Early diagnosis may also contribute to an important reduction in healthcare resource use. A recent Spanish study estimated that early diagnosis and symptomatic treatment of ATTR-CM in heart failure patients could, depending on time horizon, save between €212 and 2900 per patient diagnosed compared to an undiagnosed patient [Citation28]. However, this study did not consider the costs or effects of specific pharmacological ATTR-CM therapies. Early diagnosis would also potentially benefit patients in terms of a haltered decline in quality of life.
In conclusion, this study underlines the importance of increased disease awareness, early diagnosis, appropriate care, and treatment, which potentially can slow or prevent disease progression. This may reduce the disease burden for both patients and the health care system.
Ethics statement
This study was conducted according to the Declaration of Helsinki and approved by the Swedish Ethical Review Authority (Dnr 2020-01722).
Supplemental Material
Download MS Word (16.4 KB)Acknowledgements
We would like to thank Eva Wallgren, BMA, department of medicine Karolinska Institutet, and Pernilla Hallberg, research nurse at the Department of Medical Sciences, Clinical Epidemiology, Uppsala University, for help collecting data from the medical records at the two clinics.
Disclosure statement
FH and JMN have no conflict of interest to declare. KJ and LAL are employed by Pfizer AB. GW conducted the Tafamidis study and has speaker honoraria from Janssen Orion Pharma and Novo Nordisk. PE has Grants: Pfizer; Consultant: Pfizer, Alnylam, BMS, Amicus, Sanofi; Speaker´s honoraria: Pfizer, Alnylam, BMS, Amicus, Orion Pharma, Sanofi.
Data availability statement
Due to ethical restrictions supporting data is not available.
Additional information
Funding
References
- Ruberg FL, Grogan M, Hanna M, et al. Transthyretin amyloid cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73(22):1–8. doi: 10.1016/j.jacc.2019.04.003.
- Saito Y, Nakamura K, Ito H. Molecular mechanisms of cardiac amyloidosis. Int J Mol Sci. 2021;23(1):25.
- Martinez-Naharro A, Hawkins PN, Fontana M. Cardiac amyloidosis. Clin Med. 2018;18(Suppl 2):S30–S35. doi: 10.7861/clinmedicine.18-2-s30.
- Gertz M, Adams D, Ando Y, et al. Avoiding misdiagnosis: expert consensus recommendations for the suspicion and diagnosis of transthyretin amyloidosis for the general practitioner. BMC Fam Pract. 2020;21(1):198. doi: 10.1186/s12875-020-01252-4.
- Nativi-Nicolau J, Judge DP, Hoffman JE, et al. Natural history and progression of transthyretin amyloid cardiomyopathy: insights from ATTR-ACT. ESC Heart Fail. 2021;8(5):3875–3884. doi: 10.1002/ehf2.13541.
- Lane T, Fontana M, Martinez-Naharro A, et al. Natural history, quality of life, and outcome in cardiac transthyretin amyloidosis. Circulation. 2019;140(1):16–26. doi: 10.1161/CIRCULATIONAHA.118.038169.
- Aus Dem Siepen F, Hein S, Prestel S, et al. Carpal tunnel syndrome and spinal canal stenosis: harbingers of transthyretin amyloid cardiomyopathy? Clin Res Cardiol. 2019;108(12):1324–1330. doi: 10.1007/s00392-019-01467-1.
- Gillmore JD, Damy T, Fontana M, et al. A new staging system for cardiac transthyretin amyloidosis. Eur Heart J. 2018;39(30):2799–2806. doi: 10.1093/eurheartj/ehx589.
- Grogan M, Scott CG, Kyle RA, et al. Natural history of Wild-Type transthyretin cardiac amyloidosis and risk stratification using a novel staging system. J Am Coll Cardiol. 2016;68(10):1014–1020. doi: 10.1016/j.jacc.2016.06.033.
- Holmgren G, Costa PM, Andersson C, et al. Geographical distribution of TTR met30 carriers in Northern Sweden: discrepancy between carrier frequency and prevalence rate. J Med Genet. 1994;31(5):351–354. doi: 10.1136/jmg.31.5.351.
- Schmidt HH, Waddington-Cruz M, Botteman MF, et al. Estimating the global prevalence of transthyretin familial amyloid polyneuropathy. Muscle Nerve. 2018;57(5):829–837. doi: 10.1002/mus.26034.
- Bajwa F, O'Connor R, Ananthasubramaniam K. Epidemiology and clinical manifestations of cardiac amyloidosis. Heart Fail Rev. 2022;27(5):1471–1484. doi: 10.1007/s10741-021-10162-1.
- Lauppe RE, Liseth Hansen J, Gerdesköld C, et al. Nationwide prevalence and characteristics of transthyretin amyloid cardiomyopathy in Sweden. Open Heart. 2021;8(2):e001755. doi: 10.1136/openhrt-2021-001755.
- Irabor B, McMillan JM, Fine NM. Assessment and management of older patients with transthyretin amyloidosis cardiomyopathy: geriatric cardiology, frailty assessment and beyond. Front Cardiovasc Med. 2022;9:863179. doi: 10.3389/fcvm.2022.863179.
- Lindmark K, Pilebro B, Sundström T, et al. Prevalence of wild type transtyrethin cardiac amyloidosis in a heart failure clinic. ESC Heart Fail. 2021;8(1):745–749. doi: 10.1002/ehf2.13110.
- Rozenbaum MH, Large S, Bhambri R, et al. Impact of delayed diagnosis and misdiagnosis for patients with transthyretin amyloid cardiomyopathy (ATTR-CM): a targeted literature review. Cardiol Ther. 2021;10(1):141–159. doi: 10.1007/s40119-021-00219-5.
- Garcia-Pavia P, Rapezzi C, Adler Y, et al. Diagnosis and treatment of cardiac amyloidosis: a position statement of the ESC working group on myocardial and pericardial diseases. Eur Heart J. 2021;42(16):1554–1568. doi: 10.1093/eurheartj/ehab072.
- Benbrahim M, Norman K, Sanchorawala V, et al. A review of novel agents and clinical considerations in patients with ATTR cardiac amyloidosis. J Cardiovasc Pharmacol. 2021;77(5):544–548. doi: 10.1097/FJC.0000000000001004.
- Seferovic PM, Ponikowski P, Anker SD, et al. Clinical practice update on heart failure 2019: pharmacotherapy, procedures, devices and patient management. An expert consensus meeting report of the heart failure association of the European Society of Cardiology. Eur J Heart Fail. 2019;21(10):1169–1186. doi: 10.1002/ejhf.1531.
- Kittleson MM, Maurer MS, Ambardekar AV, et al. Cardiac amyloidosis: evolving diagnosis and management: a scientific statement From the American heart association. Circulation. 2020;142(1):e7–e22. doi: 10.1161/CIR.0000000000000792.
- Lauppe R, Liseth Hansen J, Fornwall A, et al. Healthcare resource use of patients with transthyretin amyloid cardiomyopathy. ESC Heart Fail. 2022;9(3):1636–1642. doi: 10.1002/ehf2.13913.
- Pilgaard T, Pedersen MH, Poulsen SH. Diagnostic and lifetime hospital costs of patients suffering from wild-type transthyretin amyloid cardiomyopathy in Denmark. J Med Econ. 2020;23(10):1084–1091. doi: 10.1080/13696998.2020.1789866.
- Hjalte F, Norlin J, Alverbäck-Labberton L, et al. EE434 health care resource use and costs in transthyretin amyloid cardiomyopathy-A swedish medical record review study. Value in Health. 2022;25(12):S141. doi: 10.1016/j.jval.2022.09.680.
- Stålhammar J, Stern L, Linder R, et al. The burden of preserved ejection fraction heart failure in a real-world swedish patient population. J Med Econ. 2014;17(1):43–51. doi: 10.3111/13696998.2013.848808.
- Boman K, Lindmark K, Stålhammar J, et al. Healthcare resource utilisation and costs associated with a heart failure diagnosis: a retrospective, population-based cohort study in Sweden. BMJ Open. 2021;11(10):e053806. doi: 10.1136/bmjopen-2021-053806.
- Alexander KM, Evangelisti A, Witteles RM. Emerging therapies for transthyretin cardiac amyloidosis. Curr Treat Options Cardiovasc Med. 2019;21(8):40.
- Eldhagen P, Lehtonen J, Gude E, et al. Health-related quality of life among transthyretin amyloid cardiomyopathy patients. ESC Heart Fail. 2023;10(3):1871–1882. doi: 10.1002/ehf2.14350.
- Formiga F, García-Pavía P, Martín Sánchez FJ, et al. Health and economic impact of the correct diagnosis of transthyretin cardiac amyloidosis in Spain. Expert Rev Pharmacoecon Outcomes Res. 2021;11:1–7.
