Abstract
Objective
Dupilumab has been approved for the treatment of severe asthma with type 2 inflammation by inhibiting interleukin (IL)-4 and IL-13 signaling. However, dupilumab-induced hypereosinophilia (HE) has been reported and should not be ignored. The aim of this study was to investigate the efficacy of dupilumab in Chinese patients with severe asthma, whether HE affects its efficacy, and the possible risk factors for HE.
Methods
20 patients with severe asthma who received dupilumab treatment for at least 12 months in the First Affiliated Hospital of Guangzhou Medical University from 2019 to 2022 were included. We compared clinical data and laboratory tests results before dupilumab treatment and at 4 and 12 months after treatment. Based on whether dupilumab treatment triggers HE defined as blood eosinophil count (BEC) ≥ 1.5 × 109 cells/L, the patients were allocated into non-HE and HE groups.
Results
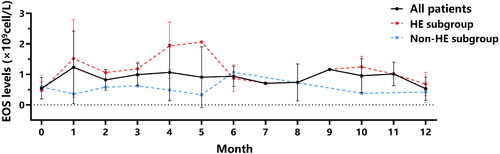
The patients showed a significant increase in asthma control test (ACT) scores, a decrease in the number of exacerbations, a decrease in the proportion of patients taking an oral corticosteroid (OCS) and in the dose, and a significant improvement in the pulmonary function parameters FEV1/FVC (%) and FEV1 (% predicted) after 4 and 12 months of treatment with dupilumab. For type 2 inflammatory biomarkers, the levels of fractional concentration of exhaled nitric oxide (FeNO), sputum eosinophil count percentage (SEC%) and total immunoglobulin E (TIgE) decreased significantly, whereas BEC were higher after 4 months of treatment, but returned to baseline levels after 12 months. 8 patients (40%) developed asymptomatic HE after dupilumab, and the efficacy was not significantly different between the HE and non-HE groups. The earliest BEC elevation appeared at 1 month after treatment, but most of them declined after 6 months, and basically returned to the baseline level around 12 months of treatment. In addition, we further found that when patients had FeNO ≥ 60 ppb, food allergens positive and combined eosinophilic otitis media (EOM), their BEC increased significantly more than that of the control group after 4 months as well as 12 months of treatment.
Conclusions
This study demonstrated that dupilumab was efficacious in Chinese patients with severe asthma, and some patients developed asymptomatic, self-limited HE, which did not affect its efficacy. Additionally, FeNO ≥60 ppb, food allergens positive, and co-morbidities with EOM may be the risk factors for developing HE.
Introduction
Bronchial asthma affects approximately 300 million people worldwide, with 3–10% of patients suffering from severe and uncontrolled asthma. Many patients with severe asthma require long-term OCS treatment or hospitalization, which results in poor quality of life, high healthcare costs, and many side effects [Citation1,Citation2]. Based on the major immune-inflammatory pathways involved, the most common endotypes of severe asthma are now categorized as T2-high and T2-low. A multicenter study of adults with severe asthma in China found that about 75% of patients were classified as T2-high endotype [Citation3]. T2-high asthma is characterized by type 2 inflammation, which is driven by T helper 2 cells (Th2) or group 2 innate lymphoid cells (ILC2) and is characterized by high levels of cytokines such as IL-4, IL-5 and IL-13, accompanied by accumulation of inflammatory cells such as eosinophils and mast cells in the lung tissues, and mucus production as the main pathophysiological manifestations [Citation4].
In recent years, novel monoclonal antibody therapies targeting Th2 inflammatory receptors and mediators have emerged [Citation5]. Dupilumab is a human monoclonal antibody that binds to the IL-4 receptor alpha subunit (IL-4Rα) and inhibits IL-4 and IL-13 signaling [Citation6]. Multiple randomized, placebo-controlled trials have demonstrated the efficacy of dupilumab in patients with type 2-mediated inflammatory diseases such as chronic rhinosinusitis with nasal polyps (CRSwNP), severe asthma, prurigo nodularis, atopic dermatitis (AD) and eosinophilic esophagitis [Citation7–13]. The LIBERTY ASTHMA QUEST phase III clinical trial demonstrated that dupilumab significantly reduced the exacerbations rate and improved lung function and asthma control in patients with severe asthma [Citation14]. However, hypereosinophilia (HE), defined as BEC ≥ 1.5 × 109 cells/L, occurs in approximately 4%–25% of patients during dupilumab treatment [Citation15]. HE caused by dupilumab treatment is usually transient and does not cause clinical symptoms. However, there are occasional cases of severe complications after dupilumab treatment such as eosinophilic pneumonia and eosinophilic granulomatosis with polyangiitis (EGPA) [Citation16–18]. HE caused by dupilumab typically begins in the 4th week of treatment and returns to baseline around the 24th week, but approximately 14% of patients experience HE lasting for 6 months or more [Citation8,Citation19].
It is currently unclear which patients are at risk for HE after the use of dupilumab and whether HE reduces the efficacy of dupilumab. In this study, we reviewed 20 patients with severe asthma treated with dupilumab. Firstly, the patients were divided into HE and non-HE groups according to whether HE occurred during treatment, and the clinical data and laboratory test results of the two groups were compared before dupilumab treatment and at 4 and 12 months after treatment, so as to determine whether HE affects its efficacy. Next, subgroup analyses were performed based on the different clinical characteristics of the patients at baseline to compare the post-treatment BEC levels of the patients between the different subgroups in order to identify risk factors that may lead to elevated BEC in the patients.
Materials and methods
Study design
This is a single-center, retrospective study. 20 patients with severe asthma were enrolled. All the patients completed at least 12 months of dupilumab treatment at the First Affiliated Hospital of Guangzhou Medical University between January 2019 and May 2022. All patients were > 18 years of age and all were diagnosed with asthma according to the Chinese Guidelines for the Diagnosis and Treatment of Asthma or the Global Initiative of Asthma guidelines. Severe asthma is defined as the need to add one or more of the following control medications despite daily inhaled high-dose ICS: long-acting beta-agonists, long-acting muscarinic antagonists, leukotriene receptor antagonists, or daily systemic glucocorticoids.
Study methods
Dupilumab was subcutaneously injected at an initial dose of 600 mg (two 300 mg injections), with a maintenance dose of 300 mg once every 2 weeks. HE appearing during treatment has been ruled out as a secondary factor that may lead to HE in patients, such as parasites and fungal infections, and myeloid or spray neoplasms with platelet derived growth factor receptor alpha and beta (PDGFRA and PDGFRB, respectively).
The percentage of sputum eosinophil count (SEC%), BEC, FeNO, percentage of forced expiratory volume in the first second (FEV1) to forced vital capacity (FVC) (FEV1/FVC%), percentage of forced expiratory volume in the first second to the expected value (FEV1pred%), asthma control test scores, TIgE, asthma exacerbations, and OCS indicators before dupilumab treatment (baseline), and after 4 and 12 months of treatment were recorded. Patients were divided into HE and non-HE groups according to whether HE occurred during treatment. These groups were divided into subgroups according to baseline characteristics of gender, age (≥45 or < 45 years), history of treatment with biological agents, laboratory indicators (baseline BEC, baseline FeNO, baseline pulmonary function level), allergen index including foodborne (eggs, milk, fish, wheat, and shrimp), fungal (Aspergillus fumigatus, Penioillum notatum, and Cladosporium) and inhalation allergenicity (Dermatophagoides pteronyssinus, Dermatophagoides farinae, Blattella germanica, and dander), and concomitant type 2 inflammatory diseases that included CRSwNP, EOM, AD, and aspirin exacerbated respiratory disease (AERD). The study was conducted in accordance with the ethical principles of the Declaration of Helsinki. All patients who participated in this clinical observation were aware of the content of the study and signed a consent form. This study was approved by the Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University.
Data analyses
Continuous variables were expressed as mean (± standard deviation) or median (quartile). Categorical variables were expressed as cases (n) and percentage (%). Pairwise comparisons were performed using a paired t-test or nonparametric Wilcoxon test. The Mann-Whitney U test was used to assess whether the efficacy of dupilumab (number of acute episodes and lung function) was significantly different between the two groups. The Mann-Whitney U test was used to assess whether the median BEC changed at the month 4 and 12 of treatment (including absolute value and percentage change). The median BEC count and percentage change at the month 4 and 12 were tested based on the difference between patient BEC and baseline. p < .05 was statistically significant. All analyses were performed using SPSS version 25.0.
Results
Patients general information
As shown in , twenty patients (14 males, 70%) with severe asthma were included in the study. The mean age of patients was 46.63 years. 8 patients tested positive for specific IgE, of which 4 and 6 were positive for food allergens and inhaled allergens, respectively. Regarding comorbidities, 11 (55%), 5 (25%), 2 (10%), and 5 (25%) patients had CRSwNP, AD, AERD, and EOM, respectively. In terms of treatment, 10 (50%) patients had been treated with other biologics and 15 (75%) were on OCS, while the median dose was 12.50 mg. Furthermore, the baseline BEC level of patients treated with dupilumab was predominantly in the (0.5–1.0) × 109cells/L range in 9 patients (45%). None of the patients had a baseline BEC level higher than 1.5 × 109cells/L. The mean baseline BEC in 20 patients was (0.54 ± 0.35) × 109cells/L. In addition, there were more patients with type 2 inflammatory diseases such as CRSwNP, AD, and EOM in the HE group than in the non-HE group, as well as higher use of OCS than in the non-HE group.
Table 1. Baseline demographic and clinical characteristics of the patients.
Evaluation of therapeutic efficacy
Dupilumab significantly reduced the number of exacerbations and reduced and/or eliminated the use of OCS. After 4 and 12 months of treatment, ACT scores improved from 17.90 ± 2.77 at baseline to 21.35 ± 1.95 and 22.53 ± 1.87, respectively, and the number of exacerbations decreased from 0.85 ± 0.67 times/4 months to 0.30 ± 0.47 times/4 months and 0.11 ± 0.32 times/4 months, respectively. Meanwhile, the mean OCS dosage decreased from 13.63 ± 11.28 mg/d at baseline to 5.25 ± 8.15 mg/d and 2.37 ± 4.45 mg/d. The number of patients dependent on OCS was reduced from 15 to 9 and 6 after 4 and 12 months of treatment, respectively. In addition, dupilumab treatment improved lung function and reduced type 2 inflammatory biomarkers as well. However, we found that after 4 months of treatment, patients had a significant increase in BEC levels, from 0.54 ± 0.35 × 109cell/L to 1.07 ± 0.92 × 109cell/L. In contrast, the SEC (%) was significantly lower than at baseline. However, the BEC levels roughly returned to baseline levels at the end of 12 months of treatment ().
Table 2. Comparison of curative effect before and after treatment with dupilumab.
Dupilumab did not become less efficacious in asthma patients because of the presence of HE in patients. A total of 8 patients who developed BEC ≥1.5 × 109cell/L during follow-up were defined as the HE group and the rest as the non-HE group. Our results revealed that the number of exacerbations, ACT scores, and pulmonary function parameters in the HE group were not significantly different from those in the non-HE group, and there was also no significant difference between the two groups in terms of the reduction of FeNO, TIgE, and sputum eosinophils induced by dupilumab ().
Table 3. Comparison of curative effect before and after treatment with dupilumab between HE and non-HE group.
Analysis of HE-related events during dupilumab treatment
Temporal distribution of HE
As shown in , BEC increased in the first month of dupilumab treatment, peaked after 4 to 5 months of treatment in the HE group, and gradually decreased after approximately 6 months of treatment. BEC tended to return to baseline levels after 12 months of dupilumab treatment.
Possible risk factors of HE
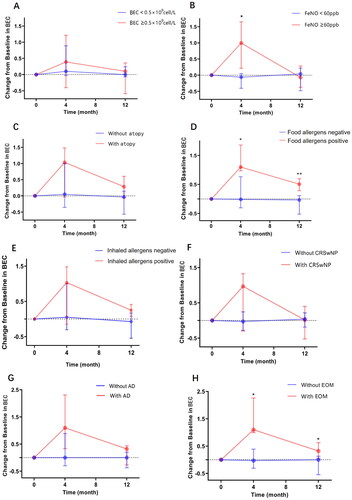
We next performed subgroup analyses based on the different clinical characteristics of the patients at baseline, comparing the BEC levels of the different subgroups after treatment to look for risk factors that might have led to an increase in the BEC of patients. As shown in and Supplementary Table S1, after 4 months of dupilumab treatment, the median increase in BEC was significantly higher in the food allergy positive group than in the food allergy negative group, and this significant difference persisted until 12 months after treatment. Similarly, patients with EOM exhibited a median increase in BEC after 4 months of treatment compared to baseline and after 12 months. Interestingly, patients with baseline FeNO ≥ 60 ppb also exhibited a significantly higher increase in median BEC after 4 months of treatment compared with baseline, whereas this difference disappeared after 12 months of treatment.
Figure 2. Subgroup analysis according to different conditions at baseline to compare changes in BEC after 4 and 12 months of dupilumab treatment. *p < .05, **p < .01, ***p < .001. BEC: blood eosinophil counts; FeNO: fractional exhaled nitric oxide; CRSwNP: chronic rhinosinusitis with nasal polyps; AD: atopic dermatitis; EOM: Eosinophilic otitis media.
Discussion
Dupilumab is widely used in the treatment of severe asthma, moderate-to-severe AD, and CRSwNP by inhibiting IL-4 and IL-13 signaling, and thus plays a key role in suppressing type 2 inflammation. The LIBERTY ASTHMA QUEST trial confirmed the efficacy of dupilumab in asthmatics with BEC ≥ 0.15 × 109 cells/L or FeNO ≥ 25 ppb, and was more pronounced at BEC ≥ 0.3 × 109 cells/L [Citation8]. One study further found that for asthma patients with a baseline BEC >0.5 × 109 cell/L, they showed a favourable response to dupilumab and it appeared that as baseline type 2 inflammatory biomarkers increased, dupilumab improved patients’ lung function and level of asthma control more significantly [Citation20]. In the present study, the mean values of type 2 inflammatory markers such as BEC (0.54 ± 0.35) × 109 cell/L, FeNO (57.85 ± 26.42) ppb, and TIgE (564.16 ± 469.46 kU/L) of the patients at baseline were higher than that of the LIBERTY ASTHMA QUEST study of BEC (0.35 ± 0.37) × 109 cell/L, FeNO (34.97 ± 32.85) ppb and TIgE (432.40 ± 746.46) kU/L, suggesting that they may respond better to dupilumab.
HE induced by dupilumab treatment of asthma was mostly transient and not accompanied by clinical symptoms, and the presence of HE did not affect its efficacy, and the BEC of the patients in the HE group basically recovered to the baseline level at the end of 12 months of treatment. It was found that the presence of HE during dupilumab treatment for AD may lead to a reduction in its efficacy and may cause allergy-related complications [Citation21,Citation22]. However, in this study, the above did not occur in dupilumab-treated asthma patients, and the results showed no significant differences in the number of exacerbations, lung function, or improvement in type 2 inflammation between the HE and non-HE groups. Meanwhile, the levels of BEC could be reduced to (0.50 ± 0.36) × 109 cell/L at the full 12 months of treatment, essentially roughly the same as baseline (0.54 ± 0.35) × 109 cell/L. Wechsler et al. reviewed 11 clinical trials of dupilumab and similarly found that dupilumab-induced HE was transient and usually unrelated to clinical symptoms or efficacy effects, and that the BEC declined to or below baseline over time [Citation23].
HE induced by dupilumab rarely requires discontinuation and/or addition of systemic glucocorticoids. In their recommendations for the management of HE induced by dupilumab, Caminati et al. suggest that patients presenting with a BEC >1.5 × 109 cell/L need to be followed up more frequently to once a month, and that if the patient’s BEC level is further elevated to ≥3.0 × 109 cell/L, assessment of organ damage needs to be initiated, and that if evidence of organ damage occurs, discontinuation of dupilumab and systemic glucocorticoids may be attempted, and reintroduction of dupilumab may be attempted when the patient’s BEC level falls below 1.5 × 109 cell/L and the patient’s condition is reassessed [Citation24]. In this study, 2 patients developed BEC ≥3.0 × 109 cell/L after dupilumab treatment. Eosinophilic pneumonia was suspected in patient 1, so dupilumab was suspended and 20 mg/d of OCS was administered, after which his BEC decreased rapidly and remained stable. Re-treatment with dupilumab was then given, and although the patient experienced subsequent fluctuations in BEC, he did not develop clinical symptoms. HE with clinical symptoms is reported to be extremely rare, mostly eosinophilic pneumonia or EGPA. Wechsler et al. found that among 4666 patients on dupilumab, the number of patients who developed HE with comorbid clinical symptoms was only 7, and all of these occurred in patients with asthma and/or CRSwNP [Citation23]. In this study, patient 1 who developed HE with clinical symptoms was asthma combined with CRSwNP, suggesting that airway disease (asthma, CRSwNP) may be a risk factor for patients to develop HE. Elevated BEC caused by dupilumab may be due to two reasons, one of which is that blockade of the IL-4/13 pathway reduces migration of eosinophils and accumulation in blood by inhibiting eotaxin-3, VCAM-1, and TARC, but without simultaneously inhibiting eosinophilopoiesis in bone marrow [Citation15]; secondly, because glucocorticoids inhibit eosinophils, patients may also experience elevated BEC when OCS is reduced during dupilumab administration [Citation25]. Patient 2 had his OCS dose reduced from 40 mg to 30 mg 1 month after dupilumab treatment due to significant improvement in symptoms, which may have contributed to his elevated BEC, and when the OCS dose was re-increased, this patient’s BEC level was immediately and effectively controlled, and therefore no further elevation of BEC was observed when the OCS dose was slowly and carefully lowered in the subsequent follow up visits.
Our results revealed that patients with combined EOM or food allergen positive had higher increases in BEC after dupilumab than controls at month 4 and month 12. In a single-centre retrospective study of dupilumab-treated AD patients, patients in the food allergy-positive group were found to have a 39% increase in mean BEC levels compared to baseline after 4 months of treatment with dupilumab, compared to only a 5% increase in the negative group [Citation26]. In this study, patients in the food allergens positive group experienced a median BEC increase of 1.10 × 109 cells/L from baseline with dupilumab, which was much higher than the negative group’s increase of −0.02 × 109 cells/L. Similarly, patients with comorbid EOM also experienced a more significant level of BEC elevation after 4 months of dupilumab use compared with the negative group. No cohort studies of dupilumab treatment for EOM have been published. However, there are no cohort studies of dupilumab in the treatment of EOM, but case reports by Iino [Citation27] and Shimizu [Citation28] found that 4 patients had elevated BEC and much greater than baseline levels after treatment with dupilumab.
The present study was limited to be a retrospective study with a small number of enrolled cases, which does not exclude that the non-HE group had an elevated BEC greater than 1.5 × 109 cells/L that was not recorded during the follow-up period, and prospective, large-sample studies are still needed in the future to investigate the risk factors for the emergence of an elevated BEC in patients with severe asthma using dupilumab.
Conclusions
In this single-center study we found good initial efficacy of dupilumab in the treatment of severe asthma. Although some patients may develop asymptomatic HE, the BEC of the majority of patients returned to baseline at the completion of 12 months of treatment. In addition, subgroup analysis revealed that patients with FeNO ≥60 ppb, food allergens positive, and co-morbidities with EOM were more likely to have elevated BEC after dupilumab treatment. Biologics are currently an important therapeutic measure for the control of severe asthma, and further exploration of risk factors and possible mechanisms of HE during dupilumab use is warranted in the future.
Authors contributions
QZ and JX designed the study. YL, ZD and JW completed completed subject enrollment. CO, XC and YL performed the statistical analysis. YL, ZD and JW drafted the manuscript. QZ and JX edited and revised the manuscript. All authors contributed to the critical revision of the manuscript and approved the final version.
Ethical approval
All participants or their parents provided oral and written informed consent for participating in this study, and in conformity with the ethics approval by the First Affiliated Hospital of Guangzhou Medical University ethics committee (Medical Research Ethics Review No. 35, 2018).
Consent form
The funders had no roles in study design, data collection, data analysis, interpretation and writing of the report. QZ and JX had full access to the data in the study and had final responsibility for the decision to submit it for publication.
Supplemental Material
Download MS Word (17.1 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The original data used and/or analysed during this study are available from the corresponding author JX (email: [email protected]) or QZ (email: [email protected]) on reasonable request.
Additional information
Funding
References
- Stern J, Pier J, Litonjua AA. Asthma epidemiology and risk factors. Semin Immunopathol. 2020;42(1):1–9. doi: 10.1007/s00281-020-00785-1.
- García-Marcos L, Asher MI, Pearce N, et al. The burden of asthma, hay fever and eczema in children in 25 countries: GAN phase I study. Eur Respir J. 2022;60(3):2102866. doi: 10.1183/13993003.02866-2021.
- Deng Z, Jin M, Ou C, et al. Eligibility of C-BIOPRED severe asthma cohort for type-2 biologic therapies. Chin Med J. 2023;136(2):230–232. doi: 10.1097/CM9.0000000000002556.
- Pavord I, Bahmer T, Braido F, et al. Severe T2-high asthma in the biologics era: european experts’ opinion. Eur Respir Rev. 2019;28(152):190054. doi: 10.1183/16000617.0054-2019.
- Guilleminault L, Conde E, Reber LL. Pharmacological approaches to target type 2 cytokines in asthma. Pharmacol Ther. 2022;237:108167. doi: 10.1016/j.pharmthera.2022.108167.
- P C, H E, C C, et al. Interleukins 4 and 13 in asthma: key pathophysiologic cytokines and druggable molecular targets. Front Pharmacol. 2022;13:851940. [cited 2024 Jan 7]. https://pubmed.ncbi.nlm.nih.gov/35350765/.
- Bachert C, Han JK, Desrosiers M, et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet. 2019;394(10209):1638–1650. doi: 10.1016/S0140-6736(19)31881-1.
- Castro M, Corren J, Pavord ID, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med. 2018;378(26):2486–2496. doi: 10.1056/NEJMoa1804092.
- Chong LY, Piromchai P, Sharp S, et al. Biologics for chronic rhinosinusitis. Cochrane Database Syst Rev. 2021;3(3):CD013513.
- P C, B A, B M, et al. Real-life effects of dupilumab in patients with severe type 2 asthma, according to atopic trait and presence of chronic rhinosinusitis with nasal polyps. Front Immunol. 2023;14:1121237.
- Simpson EL, Akinlade B, Ardeleanu M. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2017;376(11):1090–1091.
- Calugareanu A, Jachiet M, Tauber M, et al. Effectiveness and safety of dupilumab for the treatment of prurigo nodularis in a French multicenter adult cohort of 16 patients. J Eur Acad Dermatol Venereol. 2020;34(2):e74–e76. doi: 10.1111/jdv.15957.
- Hirano I, Dellon ES, Hamilton JD, et al. Efficacy of dupilumab in a phase 2 randomized trial of adults with active eosinophilic esophagitis. Gastroenterology. 2020;158(1):111–122.e10. doi: 10.1053/j.gastro.2019.09.042.
- Rabe KF, FitzGerald JM, Bateman ED, et al. Dupilumab is effective in patients with moderate-to-severe uncontrolled GINA-Defined type 2 asthma irrespective of an allergic asthma phenotype. J Allergy Clin Immunol Pract. 2022;10(11):2916–2924.e4. doi: 10.1016/j.jaip.2022.06.036.
- Olaguibel JM, Sastre J, Rodríguez JM, et al. Eosinophilia induced by blocking the IL-4/IL-13 pathway: potential mechanisms and clinical outcomes. J Investig Allergol Clin Immunol. 2022;32(3):165–180. doi: 10.18176/jiaci.0823.
- Menzella F, Montanari G, Patricelli G, et al. A case of chronic eosinophilic pneumonia in a patient treated with dupilumab. Ther Clin Risk Manag. 2019;15:869–875. doi: 10.2147/TCRM.S207402.
- Suzaki I, Tanaka A, Yanai R, et al. Eosinophilic granulomatosis with polyangiitis developed after dupilumab administration in patients with eosinophilic chronic rhinosinusitis and asthma: a case report. BMC Pulm Med. 2023;23(1):130. doi: 10.1186/s12890-023-02415-6.
- Kai Y, Yoshikawa M, Matsuda M, et al. Successful management of recurrent allergic bronchopulmonary aspergillosis after changing from mepolizumab to dupilumab: a case report. Respir Med Case Rep. 2022;39:101723. doi: 10.1016/j.rmcr.2022.101723.
- Wenzel S, Ford L, Pearlman D, et al. Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med. 2013;368(26):2455–2466. doi: 10.1056/NEJMoa1304048.
- Rabe KF, Pavord ID, Castro M, et al. Dupilumab efficacy and safety in patients with asthma and blood eosinophils ≥500 cells·µL-1. Eur Respir J. 2022;59(6):2102577. doi: 10.1183/13993003.02577-2021.
- Tosuji E, Inaba Y, Muraoka K, et al. The clinical significance of dupilumab-induced blood eosinophil elevation in Japanese patients with atopic dermatitis. Drug Discov Ther. 2022;16(4):164–168. doi: 10.5582/ddt.2022.01046.
- Ferrucci S, Angileri L, Tavecchio S, et al. Elevation of peripheral blood eosinophils during dupilumab treatment for atopic dermatitis is associated with baseline comorbidities and development of facial redness dermatitis and ocular surface disease. J Dermatolog Treat. 2022;33(5):2587–2592. doi: 10.1080/09546634.2022.2049588.
- Wechsler M, Klion A, Paggiaro P, et al. Effect of dupilumab on blood eosinophil counts in patients with asthma, chronic rhinosinusitis with nasal polyps, atopic dermatitis, or eosinophilic esophagitis. J Allergy Clin Immunol Pract. 2022;10(10):2695–2709. https://pubmed.ncbi.nlm.nih.gov/35636689/
- Caminati M, Olivieri B, Dama A, et al. Dupilumab-induced hypereosinophilia: review of the literature and algorithm proposal for clinical management. Expert Rev Respir Med. 2022;16(7):713–721. doi: 10.1080/17476348.2022.2090342.
- Rabe KF, Nair P, Brusselle G, et al. Efficacy and safety of dupilumab in glucocorticoid-dependent severe asthma. N Engl J Med. 2018;378(26):2475–2485. doi: 10.1056/NEJMoa1804093.
- Blauvelt A, de Bruin-Weller M, Gooderham M, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389(10086):2287–2303. doi: 10.1016/S0140-6736(17)31191-1.
- Iino Y, Sekine Y, Yoshida S, et al. Dupilumab therapy for patients with refractory eosinophilic otitis media associated with bronchial asthma. Auris Nasus Larynx. 2021;48(3):353–360. doi: 10.1016/j.anl.2020.09.001.
- Shimizu H, Hayashi M, Kato H, et al. IL13 may play an important role in developing eosinophilic chronic rhinosinusitis and eosinophilic otitis media with severe asthma. Int J Mol Sci. 2021;22(20):11209. doi: 10.3390/ijms222011209.