Abstract
Background
Although normal acute phase reactants (APRs) play an important role in assessing disease activity of rheumatoid arthritis (RA), some studies pointed out the discordance between disease activity and APR level. Neutrophil-to-lymphocyte ratios (NLRs), platelet-to-lymphocyte ratios (PLRs) and lymphocyte-to-monocyte ratios (LMRs) have been reported to be sensitive measures of inflammatory reaction. This study aims to explore the value of these haematological makers in assessment of APR-negative RA patients.
Methods
Out of a cohort of 418 consecutive patients with RA, we enrolled 135 patients with normal APR for this study. We performed ultrasound assessments to evaluate synovitis and bone erosion in the affected joints. Synovitis was evaluated by ultrasound grey scale (GS) and power Doppler (PD) with semi-quantitative scoring (0–3). Demographic, clinical and laboratory data were collected from the patients. Disease Activity Score-28 joints (DAS28), NLR, MLR and PLR were calculated.
Results
In RA patients with normal APR, PLR exhibited a positive correlation with ultrasound-detected synovitis and bone erosion, whereas NLR, MLR showed no significant correlation with ultrasonography parameters. The area under the ROC curve (AUC) for identifying synovitis with a GS grade ≥2 based on a PLR cutoff value of ≥159.6 was 0.7868 (sensitivity: 80.95%, specificity: 74.24%). For synovitis with a PD grade ≥2, the AUC was 0.7690, using a PLR cutoff value of ≥166.1 (sensitivity: 68.0%, specificity: 83.87%).
Conclusions
Our findings suggested that PLR might be a reliable and cost-effective marker for identifying moderate-to-severe synovitis in RA patients with normal APR.
1. Introduction
Rheumatoid arthritis (RA) is a chronic systemic autoimmune disease, primarily targeting the joints and culminating in incapacitation [Citation1]. The acute phase reactant (APR) increased dramatically in response to tissue damage and inflammatory processes. Hence, characterization of APR responses in RA is essential to gain insights into the activity of this disease [Citation2,Citation3]. It is well-established that both erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) exhibit a positive correlation with disease activity and radiological deterioration [Citation4–7]. However, several studies have already pointed out the discordance between disease activity and APR levels. One prior study revealed histological inflammation in the synovium in 49.4% of patients who maintained normal CRP levels [Citation8]. Moreover, it was observed that over half of patients with clinically unremitting RA displayed normal ESR and CRP values [Citation7].
Due to the heterogeneity of RA, the markers such as CRP and ESR exhibit certain limitations in their capacity to assess disease activity. There arises a need to find new biomarkers capable of assessing inflammation. Neutrophils (NEUTs), lymphocytes (LYs) and platelets (PLTs) have been recognized as pivotal components in the inflammatory process [Citation9]. Recent years have witnessed the emergence of novel indices, the neutrophil-to-lymphocyte ratio (NLR), lymphocyte-to-monocyte ratio (LMR) and platelet-to-lymphocyte ratio (PLR), all of which are derived from routine haematological tests. These inflammatory markers promised fresh insights into the disease dynamics [Citation10–14]. In this study, we have unveiled an association between PLR and ultrasound-detected synovitis in RA patients with normal APR levels.
2. Methods
2.1. Study population
We performed the retrospective study by collecting data from 418 consecutive patients with RA who hospitalized in Peking University Peoples Hospital between 2016 and 2020. All the patients met the 1987 revised American College of Rheumatology (ACR) or 2010 ACR/European Alliance of Associations for Rheumatology (EULAR) classification criteria. Of these patients, 135 had normal APR (ESR <15 mm/h for male, ESR <20 mm/h for female and CRP < 1.0 mg/dl). This study adhered the principles of the Declaration of Helsinki. Ethics approval was obtained from the Peking University People’s Hospital Ethics Committee (2020PHB042-01). This study used anonymous clinical data from treatment which patient agreed by written consent, the patients were not required to provide informed consent to the retrospective study.
2.2. Clinical and laboratory assessments
Demographics and clinical characteristics were collected, including age, gender, disease duration, tender and swollen joint counts, as well as the presence of extra-articular manifestations (EAMs) and comorbidities. Extra-articular manifestations included but did not limit to rheumatoid nodules, pleurisy/pericarditis and interstitial lung disease. Comorbidities included osteoporosis, hypothyroidism, hyperthyroidism, diabetes, coronary heart disease, hypertension, hyperlipidaemia, peripheral atherosclerosis, hyperuricaemia, cataracts, cerebral haemorrhage, cerebral infarction, renal insufficiency, hepatic insufficiency, chronic gastrointestinal diseases, thrombosis and malignant tumour. A comprehensive blood analysis was conducted, involving the assessment of NEUTs, LYs, monocytes (MOs) and PLTs, which were meticulously recorded to enable the subsequent calculation of indices such as NLR, LMR and PLR.
2.3. Ultrasound imaging of joints
Detailed physical examination was made by rheumatologist to determine the affected joints for ultrasound detection. One rheumatologist experienced in ultrasonography performed the ultrasonographic evaluations according to semi-quantitative scoring system [Citation15]. Finally, 88 patients, constituting approximately 65% of the total study population, underwent the ultrasound examinations, and all of them completed the ultrasound within a week of haematology collection.
Grey scale (GS) for grading synovial hypertrophy (0–3) in each joint:
Power Doppler (PD) for grading blood flow signal (0–3) in each joint:
2.4. Statistics analysis
Statistical analyses were conducted with SPSS Statistics 24 (SPSS Inc., Chicago, IL). Continuous numeric variables were expressed as medians along with the corresponding interquartile ranges (IQRs), while categorical variables were represented as absolute counts along with the respective percentages.
The correlations between haematological markers and the ultrasound-detected synovitis were examined using Spearman’s correlation coefficient and ordinal logistic regression analysis. The odds ratios (ORs), along with 95% confidence intervals (CIs), were utilized to quantify the strengths of associations between variables. GraphPad Prism 9 software (La Jolla, CA) was used to construct receiver operating characteristic (ROC) curves, assessing the performance of PLR in relation to synovitis. This analysis enabled the identification of an optimal cut-off value for utilizing PLR as a predictive tool for synovitis in RA patients. Comparisons of clinical characteristics were undertaken using the Mann–Whitney U-test for continuous numeric variables, while categorical variables were meticulously scrutinized via the Chi-square test. p ≤ .05 was considered statistically significant in our analyses.
3. Results
3.1. Demographic and clinical characteristics of the patients
The demographic and clinical characteristics of the patient cohort are summarized in . Within the 135 patients with RA, the median age was 58 (IQR, 51–66), and the median DAS28-ESR was 2.89 (IQR, 2.18–4.30). Ultrasonography was performed on the affected joints in 88 (65.2%) patients. Among this subset, 67 patients (53.3%) had GS synovitis, while 57 patients (42.2%) exhibited positive PD signals within the synovium. Notably, 56 patients (41.5%) presented with the evidence of bone erosions, and 72 individuals (71.1%) were found to have joint effusion.
Table 1. Demographic and clinical characteristics in RA patients.
We also observed the haematological markers, including RDW, NLR, LMR and PLR. The median values for the patient cohort were as follows: RDW, 13.8 (IQR, 13.2–14.5); NLR, 2.0 (IQR, 1.42–3.00); LMR, 3.0 (IQR, 2.16–4.00); PLR, 138.26 (IQR, 107.40–182.32).
3.2. Correlations between PLR and ultrasound parameters
Correlations between NLR, PLR, LMR and ultrasound parameters were analysed in the study. Our findings unveil a noteworthy pattern, indicating that PLR, in particular, exhibited a positive association with ultrasound-detected GS synovitis (r = 0.419, p = .001), PD signals (r = 0.363, p = .001) and the presence of bone erosions (r = 0.252, p = .015). The result posits PLR as a promising candidate for predicting synovitis in RA patients, especially those with normal APR levels. Remarkably, our study did not unearth any statistically significant correlations between DAS28 and these haematological markers, as detailed in . Additionally, logistic regression analysis was conducted to verify the correlation of haematological indices with GS synovitis and PD signals. The analysis revealed that PLR was significantly correlated with GS synovitis (p = .024) and PD signals (p = .001), as described in and .
Table 2. Correlations of haematological makers with ultrasound parameters and DAS-28 scores.
Table 3. Results of ordinal logistic regression analysis of haematological makers with GS grades.
Table 4. Results of ordinal logistic regression analysis of haematological makers with PD grades.
3.3. The efficacy of PLR to identify synovitis
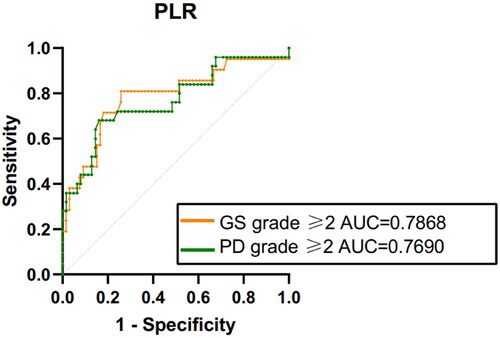
To assess the utility of PLR as a means of identifying synovitis in RA patients with normal APR, we constructed the ROC curves. The results showed that area under the ROC curve (AUC), utilizing a cut-off PLR value of ≥159.6 to identify synovitis with ultrasound GS grade of ≥2, was 0.7868 (sensitivity: 80.95%, the specificity: 74.24%).
Moreover, for the detection of synovitis distinguished by PD grade of ≥2, the AUC was 0.7690, with the cut-off PLR value being ≥166.1. In this context, the sensitivity was 68.0% and specificity 83.87%. The results, graphically depicted in , underscore the potential of PLR as a valuable tool in the identification of synovitis in RA patients who maintain normal APR levels.
3.4. Comparison of patients with high and low PLR
The patient cohort was stratified into two groups based on whether their PLR exceeded or equalled 166.1. Subsequent evaluation of the clinical characteristics within these two groups unveiled noteworthy disparities. Notably, individuals with higher PLR levels were found to be of a significantly younger age when compared to those in the lower PLR group (p = .015). Furthermore, it was discerned that the high PLR group exhibited significantly elevated rates of patients presenting with both bone erosions and rheumatoid nodules (both p < .05). Conversely, patients within the low PLR group were more predisposed to osteoporosis, as suggested by the observed difference (p = .04). Moreover, the low PLR group exhibited a higher prevalence of comorbidities when contrasted with the high PLR group (p = .001), as expounded upon in . Furthermore, patients within the high PLR group consistently displayed a higher likelihood of exhibiting abnormal ultrasound parameters indicative of synovitis.
Table 5. Clinical characteristics of RA patients in different groups.
4. Discussion
For patients with RA, the presence of active disease signifies the potential for unfavourable outcomes in terms of functional disability, radiological joint damage and comorbidities. Previous investigations and clinical guidelines have traditionally relied on combinations of parameters such as tender joint counts, swollen joint counts, patient global assessment scores on a 10-point scale, levels of CRP and ESR to evaluate disease activity [Citation16]. However, there are still active RA patients who exhibit normal CRP and ESR levels. Given the multifaceted nature of RA, there is a pressing need for more robust biomarkers to enable early identification of active disease and facilitate long-term patient management.
Our study revealed a moderate correlation between PLR and ultrasound-detected synovitis and bone erosion in RA patients, in line with previous research findings [Citation17]. However, NLR and LMR did not exhibit correlations with ultrasound-detected and clinical disease activities in our study, despite previous reports suggesting associations between NLR and LMR with clinical activities [Citation18–21]. The underlying mechanism for this discrepancy remains unclear; one plausible explanation could be variations between patients with elevated APR and those with normal APR levels. Notably, patients with higher PLR levels were reported to be younger, consistent with earlier studies [Citation22]. This observation may be linked to age-related reductions in PLT counts [Citation23]. Another potential mechanism could involve a bias in hematopoietic stem cell populations toward megakaryocytes, leading to increased interactions with other cell types, resulting in heightened cytokine production during aging [Citation24]. Additionally, our study indicated that higher PLR levels were associated with an increased risk of bone erosion and rheumatoid nodules, suggesting worse outcomes in patients with normal APR.
There is substantial evidence supporting elevated PLR in RA patients with active disease. Prior studies have reported higher PLT counts in the synovial fluid of RA patients compared to those with osteoarthritis, with PLT count demonstrating a significant positive correlation with total white cell count and disease activity [Citation25]. Furthermore, the presence of abundant PLT microparticles in the synovial fluid of RA patients has been reported, facilitating the release of pro-inflammatory cytokines such as IL-6 and IL-8 from fibroblast-like synovial cells [Citation26]. Concurrently, RA patients with active disease often exhibit lower LY counts, though the exact mechanisms remain elusive [Citation27]. Recent clinical investigations have confirmed a positive correlation between high PLR and disease activity in RA patients, indicative of an adverse clinical prognosis. It is worth emphasizing that both PLR and NLR possess independent diagnostic value for RA [Citation28]. In our study, we found that PLR was not significantly associated with DAS28. This finding underscores the potential complementary role of PLR alongside existing markers for evaluating disease activity.
In the realm of clinical practice, a considerable number of RA patients with normal APR levels exhibit active disease, yet few studies have explored the diagnostic performance of PLR in this subgroup. Our results suggest that PLR serves as a valuable haematological indicator for assessing ultrasound-detected disease activity in RA patients when APR levels fall within the normal range. We recommended a PLR cutoff value of >166.1 for identifying synovitis in RA patients with normal APR levels.
Several limitations merit consideration in our study. First, the retrospective design introduces the possibility of information bias. Second, inherent selection bias arises due to the limited number of cases involved. Future research endeavours should aim to address these limitations through larger prospective multicentre studies.
5. Conclusions
Platelet-to-lymphocyte ratio, as a cellular marker derived from routine haematological parameters, has the potential to serve as a cost-effective and dependable indicator for detecting moderate-to-severe synovitis within the subset of RA patients presenting with normal APRs.
Author contributions
JHC and WXC performed data collection, analysis and drafted the manuscript. XHX, MYZ, XS and HY participated in data collection. RL conceived the study and revised the manuscript. All authors approved the submitted version.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The authors confirm that data supporting the findings of this study are available within the article.
Additional information
Funding
References
- Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388(10055):2023–2038. doi: 10.1016/S0140-6736(16)30173-8.
- Pedrazzi AHP. Acute phase proteins: clinical and laboratory diagnosis. A review. Ann Pharm Fr. 1998;56(3):108–114.
- Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340(6):448–454. doi: 10.1056/NEJM199902113400607.
- Jansen LM, van der Horst-Bruinsma IE, van Schaardenburg D, et al. Predictors of radiographic joint damage in patients with early rheumatoid arthritis. Ann Rheum Dis. 2001;60(10):924–927. doi: 10.1136/ard.60.10.924.
- Viatte S, Plant D, Lunt M, et al. Is it possible to predict radiological damage in early rheumatoid arthritis (RA)? A report on the occurrence, progression, and prognostic factors of radiological erosions over the first 3 years in 866 patients from the early RA study (ERAS). J Rheumatol. 2004;40(2):144–156. doi: 10.3899/jrheum.121034.
- Crowson CS, Rahman MU, Matteson EL. Which measure of inflammation to use? A comparison of erythrocyte sedimentation rate and C-reactive protein measurements from randomized clinical trials of golimumab in rheumatoid arthritis. J Rheumatol. 2009;36(8):1606–1610. doi: 10.3899/jrheum.081188.
- Kay J, Morgacheva O, Messing SP, et al. Clinical disease activity and acute phase reactant levels are discordant among patients with active rheumatoid arthritis: acute phase reactant levels contribute separately to predicting outcome at one year. Arthritis Res Ther. 2014;16(1):R40. doi: 10.1186/ar4469.
- Orr CK, Najm A, Young F, et al. The utility and limitations of CRP, ESR and DAS28-CRP in appraising disease activity in rheumatoid arthritis. Front Med. 2018;5:185. doi: 10.3389/fmed.2018.00185.
- Rossaint J, Margraf A, Zarbock A. Role of platelets in leukocyte recruitment and resolution of inflammation. Front Immunol. 2018;9:2712. doi: 10.3389/fimmu.2018.02712.
- Naszai M, Kurjan A, Maughan TS. The prognostic utility of pre-treatment neutrophil-to-lymphocyte-ratio (NLR) in colorectal cancer: a systematic review and meta-analysis. Cancer Med. 2021;10(17):5983–5997. doi: 10.1002/cam4.4143.
- Jin Z, Cai G, Zhang P, et al. The value of the neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio as complementary diagnostic tools in the diagnosis of rheumatoid arthritis: a multicenter retrospective study. J Clin Lab Anal. 2021;35:e23569.
- Wu YL, Fulgenzi CAM, D'Alessio A, et al. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios as prognostic biomarkers in unresectable hepatocellular carcinoma treated with atezolizumab plus bevacizumab. Cancers. 2022;14(23):5834.
- Fu W, Ye W, Liu X, et al. Meta-analysis of the neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios in Henoch-Schonlein purpura and its complications. Int Immunopharmacol. 2021:94:107454.
- Ceylan OM, Yılmaz M, Yilmaz H, et al. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios as inflammation markers in patients with papilledema due to idiopathic intracranial hypertension. Indian J Ophthalmol. 2021;69(6):1499–1505. doi: 10.4103/ijo.IJO_2030_20.
- D’Agostino M-A, Terslev L, Aegerter P, et al. Scoring ultrasound synovitis in rheumatoid arthritis: a EULAR-OMERACT ultrasound taskforce—part 1: definition and development of a standardised, consensus-based scoring system. RMD Open. 2017;3(1):e000428. doi: 10.1136/rmdopen-2016-000428.
- Felson DT, Smolen JS, Wells G, et al. American College of Rheumatology/European League against rheumatism provisional definition of remission in rheumatoid arthritis for clinical trials. Ann Rheum Dis. 2011;70(3):404–413. doi: 10.1136/ard.2011.149765.
- Targońska-Stępniak B, Zwolak R, Piotrowski M, et al. The relationship between hematological markers of systemic inflammation (neutrophil-to-lymphocyte, platelet-to-lymphocyte, lymphocyte-to-monocyte ratios) and ultrasound disease activity parameters in patients with rheumatoid arthritis. J Clin Med. 2020;9(9):2760. doi: 10.3390/jcm9092760.
- Lee H-N, Kim Y-K, Kim G-T, et al. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratio as predictors of 12-week treatment response and drug persistence of anti-tumor necrosis factor-α agents in patients with rheumatoid arthritis: a retrospective chart review analysis. Rheumatol Int. 2019;39(5):859–868. doi: 10.1007/s00296-019-04276-x.
- Boulos D, Proudman SM, Metcalf RG, et al. The neutrophil–lymphocyte ratio in early rheumatoid arthritis and its ability to predict subsequent failure of triple therapy. Semin Arthritis Rheum. 2019;49(3):373–376. doi: 10.1016/j.semarthrit.2019.05.008.
- Wang Z, Kong L, Zhang H, et al. Tumor necrosis factor alpha -308G/a gene polymorphisms combined with neutrophil-to-lymphocyte and platelet-to-lymphocyte ratio predicts the efficacy and safety of anti-TNF-α therapy in patients with ankylosing spondylitis, rheumatoid arthritis, and psoriasis arthritis. Front Pharmacol. 2021;12:811719. doi: 10.3389/fphar.2021.811719.
- Du J, Chen S, Shi J, et al. The association between the lymphocyte-monocyte ratio and disease activity in rheumatoid arthritis. Clin Rheumatol. 2017;36(12):2689–2695. doi: 10.1007/s10067-017-3815-2.
- Targońska-Stępniak B, Grzechnik K, Kolarz K, et al. Systemic inflammatory parameters in patients with elderly-onset rheumatoid arthritis (EORA) and young-onset rheumatoid arthritis (YORA)—an observational study. J Clin Med. 2021;10(6):1204. doi: 10.3390/jcm10061204.
- Montenont E, Rondina MT, Campbell RA. Altered functions of platelets during aging. Curr Opin Hematol. 2019;26(5):336–342. doi: 10.1097/MOH.0000000000000526.
- Rundberg Nilsson A, Soneji S, Adolfsson S, et al. Human and murine hematopoietic stem cell aging is associated with functional impairments and intrinsic megakaryocytic/erythroid bias. PLOS One. 2016;11(7):e0158369. doi: 10.1371/journal.pone.0158369.
- Farr M, Wainwright A, Salmon M, et al. Platelets in the synovial fluid of patients with rheumatoid arthritis. Rheumatol Int. 1984;4(1):13–17. doi: 10.1007/BF00683878.
- Boilard E, Nigrovic PA, Larabee K, et al. Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science. 2010;327(5965):580–583. doi: 10.1126/science.1181928.
- Symmons DP, Farr M, Salmon M, et al. Lymphopenia in rheumatoid arthritis. J R Soc Med. 1989;82(8):462–463.
- Zinellu A, Mangoni AA. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratio and disease activity in rheumatoid arthritis: a systematic review and meta-analysis. Eur J Clin Invest. 2023;53(2):e13877. doi: 10.1111/eci.13877.