Abstract
Background
Nocturnal blood pressure (BP) is correlated with an increased risk of cardiovascular events and is an important predictor of cardiovascular death in hypertensive patients.
Objective
Nocturnal BP control is of great importance for cardiovascular risk reduction. This systematic review and meta-analysis aimed to explore the efficacy of angiotensin receptor blockers (ARBs) for nocturnal BP reduction in patients with mild to moderate hypertension.
Methods
PICOS design structure was used to formulate the data extraction. All statistical calculations and analyses were performed with R.
Results
Seventy-seven studies with 13,314 participants were included. The overall analysis indicated that nocturnal BP drop varied considerably among different ARBs. Allisartan (13.04 [95% CI (−18.41, −7.68)] mmHg), olmesartan (11.67 [95% CI (−14.12, −9.21)] mmHg), telmisartan (11.11 [95% CI (−12.12, −10.11)] mmHg) were associated with greater reduction in nocturnal systolic BP. In the aspect of the nocturnal-diurnal BP drop ratio, only allisartan was greater than 1. While, the variation tendency of last 4–6 h ambulatory BP was basically consistent with nocturnal BP. Additionally, allisartan showed improvement effect in the proportion of patients with dipping BP pattern.
Conclusions
This study demonstrates that for patients with mild to moderate hypertension, allisartan, olmesartan and telmisartan have more advantages in nocturnal BP reduction among the ARBs, while allisartan can reduce nighttime BP more than daytime BP and improve the dipping pattern.
KEY MESSAGES
This meta-analysis explores the efficacy of Angiotensin II AT1 receptor antagonists (ARBs) on nocturnal blood pressure (BP) reduction in mild to moderate hypertension.
The results demonstrate that for patients with mild to moderate hypertension, allisartan, olmesartan and telmisartan have more advantages in nocturnal BP reduction among the ARBs.
Allisartan can reduce nighttime BP more effectively than daytime BP, which also improve the dipping pattern.
Introduction
Nocturnal hypertension is defined as an elevated nighttime blood pressure (BP) in ambulatory BP monitoring (ABPM), > = 120/70 mmHg by recent Chinese and European guidelines and > =110/65 mm Hg by 2017 US guidelines [Citation1–3]. Patients with nocturnal hypertension are usually demonstrated as non-dipping pattern (< = 10% nocturnal BP decline relative to daytime BP), but they are not always the same, and cannot substitute for each other [Citation3]. Nocturnal hypertension has been recognized as a residual risk for developing cardiovascular disease, which is closely related to subclinical organ damages, myocardial infarction, stroke, heart failure, kidney injury, and cardiovascular death [Citation3]. Elevated nocturnal BP has been shown to be the most common reason for masked uncontrolled hypertension [Citation4]. Among hypertensive patients who have controlled clinic BP, the proportion of isolated uncontrolled nocturnal BP is twice that the proportion of isolated uncontrolled daytime BP which is determined by ABPM (24.3% vs. 12.9% respectively) [Citation4]. Asian people are more prone to nocturnal hypertension, which may be related to genetics, high-salt diet and high-salt sensitivity [Citation5]. Nocturnal hypertension is mostly seen in the elderly, those with sedentary lifestyle, patients with obstructive sleep apnea (OSA) or other sleep disorders, as well as those with diabetes, chronic kidney diseases (CKD) and other situation with high-salt sensitivity [Citation3]. 24-h BP control is crucial to minimize cardiovascular risks. So even after daytime BP control, nocturnal BP still needs to be addressed in clinical settings. Meanwhile, exploring which commonly used antihypertensive drugs are beneficial for nocturnal BP is meaningful.
Angiotensin II receptor blockers (ARBs) are one class of most commonly used antihypertensive agents with cardio-renal protection properties [Citation6]. It is generally believed that most ARBs function as long-acting antihypertensives. Guidelines recommend ARBs as the first-line BP-lowering agents, especially applicable to the elderly and patients with CKD or diabetes, who are more likely to develop nocturnal hypertension. Recent study has demonstrated nighttime BP reduction property of ARB in patients with mild to moderate hypertension [Citation7]. However, to the best of our knowledge, there has been no systematic review and meta-analysis evaluating the efficacy of ARBs for nocturnal BP reduction. We therefore conducted this work to explore the efficacy of ARBs in lowering nocturnal BP in patients with mild to moderate hypertension and to investigate whether the effect is consistent across the different ARBs.
Materials and methods
The current systematic review was performed according to the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA 2020) [Citation8]. We registered the protocol for this study on the website of PROSPERO (https://www.crd.york.ac.uk/prospero/#myprospero) with a registration number of CRD42023395427.
Search strategy
We conducted a systematic search in PubMed, EMBASE, Cochrane Library and Web of Science, from their inception to August 8th, 2022 without limitations in terms of the publication date and language. The details of the search strategies are presented in Supplementary Table S1. Other relevant studies or grey literature were supplemented from other sources.
Eligibility criteria and literature screening
Two reviewers independently screened the studies (with conflicts resolved by a third independent assessor) using the following pre-defined inclusion criteria:
Patients with mild to moderate hypertension treated with allisartan (allisartan isoproxil), candesartan (candesartan cilexetil), irbesartan, losartan, olmesartan (olmesartan medoxomil), telmisartan and valsartan
Outcomes included nocturnal ambulatory BP drop, last 4-6 h ambulatory BP drop, percentage of patients who exhibited a dipping pattern of BP variation (BP dipping is calculated as the percentage of nighttime BP decline relative to daytime BP. The following 4 dipping patterns are defined: extreme dipper, >20%; dipper, 10%-20%; non dipper, ≤10%; riser, ≤0%), and 24-h ambulatory BP drop by ABPM.
Duration of follow-up between 4 to 12 weeks
Randomized controlled trials (RCTs), single arm trials, and observational studies
Studies were excluded according to the following criteria:
Repeated published studies
Forced titration of drug dose during treatment
Unclear duration of follow-up or duration of follow-up less than 4 weeks or more than 12 weeks
No relevant outcomes reported
Duplicated reports
Bedtime medication studies
Morning hypertensive population
Animal studies, in vitro studies, reviews, case reports, conference papers, and dissertations
Data extraction
Data extraction was independently conducted by two assessors, using a standardized data extraction form. The senior author was responsible for resolving any discrepancies between researchers. The following information was extracted: study characteristics (first author, year of publication, country and study design); patient characteristics (sample size, gender, age, body mass index (BMI) and baseline ambulatory BP); detailed information regarding the intervention (drug, dose, and treatment duration), and the effect estimate of the interested outcomes. The primary outcomes were the nocturnal ambulatory BP drop from baseline, and nocturnal-diurnal BP drop ratio (= nighttime BP drop/daytime BP drop). The secondary outcomes were the last 4–6 h ambulatory BP drop, the percentage of patients who exhibited a dipping pattern of BP variation, and 24-h ambulatory BP drop.
Risk of bias assessment
To assess the risk of bias and the quality of the included studies, the reviewers used the Cochrane risk of bias (RoB) tool for RCTs [Citation9]. For single arm trials, the reviewers used the Quality Assessment Tool for Before-After (Pre-Post) Studies with No Control Group outlined by National Institutes of Health (NIH) to evaluate the risk of bias [Citation10]. For cohort studies, the reviewers used the Newcastle Ottawa Scale (NOS) to evaluate the risk of bias [Citation11]. Evaluations were carried out by two reviewers independently, and disagreements between individual judgements were discussed and, if necessary, a third reviewer checked the inconsistencies.
Data analysis
R Foundation for Statistical Computing (version 4.0.0) software were used to perform the statistical analysis. For the three outcomes (changes in nocturnal, last 4–6 h, 24-h mean ambulatory BP after treatment) of each intervention (allisartan, candesartan, irbesartan, losartan, olmesartan, telmisartan and valsartan), R software (meta-package commands, the R Core Team 2020) [Citation12] was used to combine data using a fixed-effects model, and its 95% confidence intervals (CI) were combined for continuous outcomes. Studies were not included in the analyses in cases of missing data, for example, missing SD values. Nocturnal-diurnal BP drop ratio after treatment was estimated by the ratio of means (ROMs) with 95% CI. Ratio with 95% CI was used to evaluate the proportion of patients with dipping BP pattern after treatment. When heterogeneity was significant (l2 > 50%), we explored the sources of heterogeneity from the following aspects: different doses of the same drug, baseline ambulatory BP, and the duration of follow-up visits. If the source of heterogeneity was not identified, we used a random-effects model to pool the results. To explore the association of baseline characteristics and changes in nighttime mean ambulatory BP, random-effects meta-regressions was used (metafor commands) [Citation13]. We conducted exploratory meta-regressions using study characteristics (baseline 24-h, daytime and nighttime mean ambulatory BP, sample size, age and BMI) as univariate explanatory variables. Sensitivity analyses were performed after excluding studies obtained from other sources.
Results
Baseline characteristics
In total, we retrieved 3,203 related studies from the 4 databases, PubMed, EMBASE, Cochrane Library and Web of Science, and 47 studies were added from other sources. Seventy-seven studies [Citation14–89] [Sun NL, 2010, unpublished data] with 13,314 participants were finally included (): 69 RCTs [Citation14,Citation16,Citation18–20,Citation22–47,Citation49–76,Citation78,Citation80,Citation82–84,Citation86–89] [Sun NL, 2010, unpublished data] and 8 before and after self-controlled studies [Citation15,Citation17,Citation21,Citation48,Citation77,Citation79,Citation81,Citation85] which were published between 1997 and 2022. The average age of most patients in the included studies was more than 50 years old. The sample size of the included studies ranged from 20 to 1,619. The baseline 24-h mean ambulatory systolic BP ranged from 127.50 ± 6.50 to 156.80 ± 10.00 mmHg, and the baseline 24-h mean ambulatory diastolic BP ranged from 77.30 ± 2.20 to 97.60 ± 8.60 mmHg. Seventeen studies evaluated losartan, 16 on valsartan, 15 on olmesartan, 11 on telmisartan, 8 on irbesartan, and 7 on candesartan. Most included studies reported a duration of follow-up from 6 to 12 weeks. The baseline characteristics of included studies are shown in .
Table 1. The basic characteristics of included studies.
Quality assessment
Among the 69 RCTs, all reported the implementation of randomized methods, 13 of which reported randomized methods in detail. Five RCTs reported allocation concealment. Sixty-five RCTs mentioned the completeness of the outcome data, and 6 RCTs reported other biases. None of the before and after self-controlled studies satisfied all NIH quality evaluation criteria. The main reasons for missing quality evaluation included failure to calculate the sample size and a poor description of outcome assessment methods (see Supplementary Figures S1-S2, Tables S2-S3).
Primary outcomes
Nocturnal BP drop
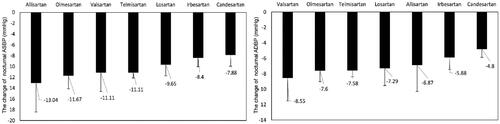
Fifty-two studies with 7190 participants were included in the analyses. The pooled nocturnal BP drop associated with ARBs differed greatly. Systolic BP reduced 13.04 [95% CI (−18.41, −7.68)] mmHg with allisartan, 11.67 [95% CI (−14.12, −9.21)] mmHg with olmesartan and 11.11 [95% CI (−12.12, −10.11)] mmHg with telmisartan, while diastolic BP reduced 8.55 [95% CI (−11.51, −5.58)] mmHg with valsartan, 7.60 [95% CI (−9.01, −6.19)] mmHg with olmesartan and 7.58 [95% CI (−8.39, −6.77)] mmHg with telmisartan ().
Nocturnal-diurnal BP drop ratio
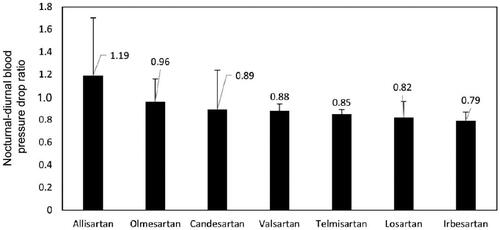
Forty-four studies with 4694 participants were included in the analyses. The pooled nocturnal-diurnal systolic BP drop ratio was 1.19 [95% CI (0.84, 1.70)] for allisartan, 0.96 [95% CI (0.80, 1.16)] for olmesartan, 0.89 [95% CI (0.63, 1.24)] for candesartan, 0.88 [95% CI (0.82, 0.94)] for valsartan, 0.85 [95% CI (0.80, 0.89)] for telmisartan, 0.82 [95% CI (0.70, 0.96)] for losartan and 0.79 [95% CI (0.72, 0.87)] for irbesartan (, see Supplementary Figure S3).
Secondary outcomes
Last 4–6 h ambulatory BP drop
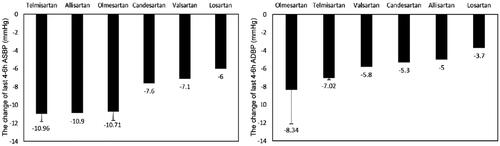
Nine studies with 2412 participants were included in the analyses. The pooled last 4–6 h ambulatory BP drop of ARBs ranged widely, systolic BP drop 10.96 [95% CI (−11.81, −10.10)] mmHg for telmisartan, 10.90 [95% CI (−13.08, −8.72)] for allisartan, and 10.71[95% CI (−11.69, −9.73)] mmHg for olmesartan, while diastolic BP drop 8.34 [95% CI (−12.10, −4.58)] mmHg for olmesartan and 7.02 [95% CI (−7.25, −6.78)] mmHg for telmisartan ().
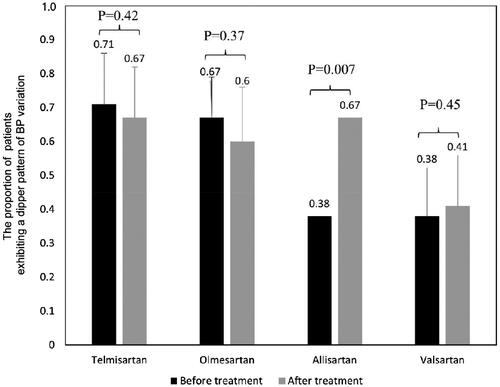
Percentage of patients exhibiting a dipping BP pattern
Six studies with 595 participants reported the percentage of patients who exhibited a dipping pattern of BP variation after treatment. The proportion was 71% before and 67% after treatment with telmisartan, 38% before and 41% after treatment with valsartan, 67% before and 60% after treatment with olmesartan. There was no significant difference between before and after treatment for each drug. However, the proportion was increased significantly from 38% to 67% after treatment with allisartan (p = 0.007) ().
24-h Ambulatory BP drop
Fifty-four studies with 6350 participants were included in the analyses. There was a large difference in the pooled 24-h ambulatory BP drop among patients treated with ARBs. The meta-analysis indicated systolic BP drop 13.20 [95% CI (−16.38, −10.02)] mmHg for olmesartan, 12.82 [95% CI (−13.94, −11.70)] mmHg for telmisartan and 12.55 [95% CI (−17.61, −7.50)] mmHg for allisartan, while diastolic BP drop 8.47 [95% CI (−9.19, −7.75)] mmHg for telmisartan and 8.39 [95% CI (−9.81, −6.97)] mmHg for olmesartan (see Supplementary Figure S4).
Subgroup analysis
Dose effect
To investigate whether the dose affected the results, we performed a subgroup analysis dividing the studies into different dose groups. Doses of candesartan, losartan and valsartan were found to be proportional to the nocturnal BP reduction within a certain range, while no such characteristic demonstrated in irbesartan, olmesartan and telmisartan. (see Supplementary Figure S5)
Baseline ambulatory BP effect
To explore the reduction of nocturnal BP in patients at different baseline BP levels, we performed a subgroup analysis stratified by baseline 24-h mean ambulatory systolic BP (130–140 mmHg, 140–150 mmHg, 150–160 mmHg). Fourteen studies did not report baseline 24-h ambulatory BP and therefore were not included in the subgroup analyses. The results showed that to the most ARBs, the higher the baseline 24-h mean ambulatory systolic BP, the greater the decrease in nighttime systolic BP (see Supplementary Figure S6).
Duration of follow up visits effect
The length of follow-up visits in the included studies was mostly from 8 to12 weeks. Hence, we divided the included studies into two groups according to the duration of follow up (8 weeks and 12 weeks). The longer the duration of losartan and valsartan administration, the more significant the decrease in nocturnal ambulatory systolic BP (see Supplementary Figure S7).
Sensitivity analysis
After removing data extracted from supplementary studies, there were no difference in the results.
Discussion
Nocturnal BP correlates with an increased risk of arterial stiffening, left ventricular hypertrophy, microalbuminuria, and adverse cardiovascular outcomes in different hypertensive populations [Citation2,Citation90,Citation91]. Studies indicate for every 5-mmHg reduction in nocturnal systolic BP, the risk of cardiovascular events decreases by 17% [Citation92]. Therefore, the management of elevated nocturnal BP should be emphasized in clinical practice. Taking long-acting antihypertensive medications is essential for nocturnal BP control, which is highly recommended in a recent consensus document on the management of nocturnal hypertension [Citation93]. To the best of our knowledge, this is the first systematic review and meta-analysis exploring the efficacy of ARBs on nocturnal BP reduction in patients with mild to moderate hypertension. Our results indicate that long-acting ARBs such as allisartan, olmesartan and telmisartan have similar efficacy in reducing nocturnal systolic BP as well as in 24-h systolic BP reduction. The efficacy of these drugs in lowering nocturnal systolic BP could be seen as the continuation of daytime antihypertensive effect.
In present study, we propose a new index for measurement of nocturnal BP reduction, that is, nocturnal-diurnal BP drop ratio. This outcome measure is intended to show the advantage of drugs in lowering nocturnal ambulatory BP, in those with BP drop ratio greater than 1 indicating BP lowering more in nighttime than in daytime. Of note, allisartan, a novel long-acting (half-life is about 10 h) nonpeptide ARB precursor drug that is used to treat hypertension, is the only ARB with a nocturnal-diurnal systolic BP drop ratio greater than 1 and tends to reduce elevated nighttime systolic BP more than daytime systolic BP. Moreover, allisartan also improves the proportion of patients with dipping BP pattern after treatment. These results indicate that, in addition to blocking the activity of renin-angiotensin-aldosterone system (RAAS), allisartan may also exert these effects via other auxiliary mechanisms. Clinical studies have demonstrated that allisartan could effectively reduce the serum uric acid (UA) level in hypertensive patients with hyperuricemia [Citation94,Citation95]. Animal studies in zebrafish hyperuricemia model have shown that allisartan could significantly upregulate intestinal urate transporters such as ABCG2, PDZK1, and SLC2A9, which might be the possible mechanism of UA reduction [Citation96]. EXP3174, an active metabolite of losartan and allisartan, has been shown to inhibit urate transporter 1 (URAT1) in the proximal convoluted tubule, thereby reducing UA reabsorption and promoting its excretion [Citation97]. Previous studies have shown serum UA level correlates with nighttime rather than daytime BP [Citation98], and it can be an independent predictor of non-dipping BP pattern [Citation99]. Moreover, UA can increase BP by activating RAAS, inducing oxidative stress and other pathways [Citation100], and high concentration of UA may cause elevated nocturnal BP, which due to the circadian rhythm of UA itself [Citation101]. It has also been reported urate-lowering therapy can reduce nocturnal BP and increase the percentage of dipping pattern [Citation102]. Allisartan may therefore be more advantageous to nocturnal BP reduction and dipping BP rhythm restoration.
Telmisartan, allisartan and olmesartan decreased systolic BP significantly in the last 4–6 h after daily dosing, which may be related to the longer half-lives [Citation90]. Due to the short half-lives (< 8 h) of valsartan and other ARBs, their blood concentration decreases significantly at night after routine morning administration, resulting in poor control of the last 4–6 h ambulatory BP [Citation90]. The results of this study suggest half-life may be one of the key factors affecting the nocturnal BP drop seen with ARBs.
Previous studies have shown long-acting ARBs exhibit a flat dose response curve, and although increasing the dose would appropriately prolong the drug action time, it does not enhance the antihypertensive effect [Citation103]. This observation is consistent with our subgroup finding that the efficacy of real long-acting ARBs, such as telmisartan, olmesartan and irbesartan are not affected by the dose. On the other hand, increasing the dose of valsartan and other drugs compensate for their relatively short half-lives and may increase the efficacy on nocturnal BP control.
Our study has several limitations. First, despite that we have included studies of different ARBs with 24-h ambulatory BP measurement reported in English in the aforementioned 4 databases, some studies not included, such as those reported in Chinese, Japanese or other than English, or those without key parameters reported in English in the abstract, might be omitted. Next, there have been relatively less clinical studies included with allisartan, a novel ARB, than those with losartan or valsartan, which might bring bias in this analysis. While the nocturnal BP-lowering effects of allisartan has been consistently demonstrated in large sample phase IV clinical study and a randomized controlled trial involved masked hypertension [Citation104], indicating the efficacy of allisartan for nocturnal BP reduction. Additionally, although we performed subgroup analyses stratified on the basis of baseline 24-h mean ambulatory systolic BP (130–140 mmHg, 140–150 mmHg, 150–160 mmHg) to reduce the impact of baseline BP on study outcomes, a few studies did not report baseline BP. Additionally, the current work only included trials and studies of ARBs, BP-lowering medications other than ARBs, such as ARNI (angiotensin receptor neprilysin inhibitors, including sacubitril/valsartan and sacubitril/allisartan), novel non-steroidal selective mineralocorticoid receptor antagonists (MRAs) esaxerenone, dual endothelin receptor antagonist aprocitentan, have been demonstrated with nocturnal BP reduction advantages in different studies [Citation47,Citation93,Citation105–107]. The exact efficacy of different medications on nocturnal BP reduction is to be elucidated in further researches [Citation93]. Finally, our study included only patients with mild to moderate hypertension and the results cannot be simply extrapolated to those with severe or resistant hypertension, in which more complex pathophysiological mechanisms are involved and multiple drugs combination rather than single BP-lowering medication is needed.
Conclusion
This meta-analysis demonstrates that for patients with mild to moderate hypertension, allisartan, olmesartan and telmisartan have more advantages in nocturnal BP reduction among the ARBs, while allisartan reduces nighttime BP more than daytime BP and improves the dipping pattern. Further large-scale, head-to-head comparative studies are warranted to confirm these findings.
Author contributions
Jing Liu was involved in the conception and design of the work. Wei Chen, Shihuan Shao, Yuanyuan Chen, Hongyi Wang, Yang Xi and Luyan Wang were involved in the analysis and interpretation of the data. Jing Liu drafted the paper and revised it critically for intellectual content. All authors approved the final version to be published and agreed to be accountable for all aspects of the work.
Supplemental Material
Download MS Word (234.1 KB)Acknowledgments
We gratefully acknowledge Fang Wu for editorial assistance. Data collection, assembly and statistical expertise were provided by Yang Zhang, Sai Zhao and Wenjie Zhang from Systematic Review Solutions, Ltd.
Disclosure statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Data availability statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Additional information
Funding
References
- Liu J. Highlights of the 2018 chinese hypertension guidelines. Clin Hypertens. 2020;26(1):1. doi:10.1186/s40885-020-00141-3.
- Whelton PK, Carey RM, Aronow WS, et al. 2017ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Hypertension. 2018;71(6):1269–16. doi:10.1161/HYP.0000000000000066.
- Kario K. Nocturnal hypertension: new technology and evidence. Hypertension. 2018;71(6):997–1009. doi:10.1161/HYPERTENSIONAHA.118.10971.
- Banegas JR, Ruilope LM, de la Sierra A, et al. High prevalence of masked uncontrolled hypertension in people with treated hypertension. Eur Heart J. 2014;35(46):3304–3312. doi:10.1093/eurheartj/ehu016.
- Chen J. Sodium sensitivity of blood pressure in chinese populations. Curr Hypertens Rep. 2010;12(2):127–134. doi:10.1007/s11906-009-0088-4.
- Dézsi CA. The different therapeutic choices with ARBs. Which one to give? When? Why? Am J Cardiovasc Drugs. 2016;16(4):255–266. doi:10.1007/s40256-016-0165-4.
- Li J, Jiang H, Qin TL, et al. Evaluation of antihypertensive effects of domestic olmesartan tablets on morning blood pressure surge by ambulatory blood pressure monitoring in patients with mild to moderate essential hypertension. Chin Pharm J. 2015;50:993–996. doi:10.11669/cpj.2015.11.018.
- Parums DV. Editorial: review articles, systematic reviews, meta-analysis, and the updated preferred reporting items for systematic reviews and meta-analyses (PRISMA) 2020 guidelines. Med Sci Monit. 2021;27:e934475. doi:10.12659/MSM.934475.
- Higgins JP, Altman DG, Gøtzsche PC, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343(oct18 2):d5928–d5928. doi:10.1136/bmj.d5928.
- NIH. Quality assessment tool for before-after (Pre-Post) studies with no control group. 2013. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools.
- Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2014. https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evidence-Based Mental Health. 2019;22:153–160.
- Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Soft. 2010;36(3):1–48. doi:10.18637/jss.v036.i03.
- Pechère-Bertschi A, Nussberger J, Decosterd L, et al. Renal response to the angiotensin II receptor subtype 1 antagonist irbesartan versus enalapril in hypertensive patients. J Hypertens. 1998;16(3):385–393. doi:10.1097/00004872-199816030-00016.
- White WB, Weber MA, Davidai G, et al. Ambulatory blood pressure monitoring in the primary care setting: assessment of therapy on the circadian variation of blood pressure from the MICCAT-2 trial. Blood Press Monit. 2005;10(3):157–163. doi:10.1097/00126097-200506000-00008.
- Baguet JP, Nisse-Durgeat S, Mouret S, et al. A placebo-controlled comparison of the efficacy and tolerability of candesartan cilexetil, 8 mg, and losartan, 50 mg, as monotherapy in patients with essential hypertension, using 36-h ambulatory blood pressure monitoring. Int J Clin Pract. 2006;60(4):391–398. doi:10.1111/j.1368-5031.2006.00903.x.
- Bai J, Zhu JR, Cai S, et al. Clinical efficacy and safety of telmisartan in patients with mild to moderatehypertension: a multi-center randomized controlled clinical trial. Chin J Cardiol. 2002;30:738–742. doi:10.3760/j:issn:0253-3758.2002.12.008.
- Brunner HR, Stumpe KO, Januszewicz A. Antihypertensive efficacy of olmesartan medoxomil and candesartan cilexetil assessed by 24-hour ambulatory blood pressure monitoring in patients with essential hypertension. Clin Drug Investig. 2003;23(7):419–430. doi:10.2165/00044011-200323070-00001.
- Cai SY, Zhang XH, Hu XS, et al. Irbesartan in treatment of mild to moderate essential hypertension. Chin J New Drugs Clin Rem. 2002;21:277–280. doi:10.3969/j.issn.1007-7669.2002.05.007.
- Cao YA, Peng CS, Long NZ, et al. The clinical observation of combined treatment with losartan potassium and indapamide for essential hypertension. Prog Mater Sci. 2001;25:303–307. doi:10.3969/j.issn.1001-5094.2001.05.012.
- Chen SX, Guo YZ, Wu ST, et al. Antihypertensive effects of irbesartan single and combined treatments on 60 patients with mild to moderate essential hypertension. Chin J New Drugs Clin Rem. 2004;23:651–654. doi:10.3969/j.issn.1007-7669.2004.10.001.
- Cheung DG, Aizenberg D, Gorbunov V, et al. Efficacy and safety of sacubitril/valsartan in patients with essential hypertension uncontrolled by olmesartan: a randomized, double-blind, 8-week study. J Clin Hypertens. 2018;20(1):150–158. doi:10.1111/jch.13153.
- Chrysant SG, Marbury TC, Robinson TD. Antihypertensive efficacy and safety of olmesartan medoxomil compared with amlodipine for mild-to-moderate hypertension. J Hum Hypertens. 2003;17(6):425–432. doi:10.1038/sj.jhh.1001577.
- Chung O, Hinder M, Sharma AM, et al. Comparison of the efficacy and safety of losartan (50-100 mg) with the T-type calcium channel blocker mibefradil (50-100 mg) in mild to moderate hypertension. Fund Clin Pharmacol. 2000;14:31–41. doi:10.1111/j.1472-8206.2000.tb00391.x.
- Chung WB, Ihm SH, Jang SW, et al. Effect of fimasartan versus valsartan and olmesartan on office and ambulatory blood pressure in korean patients with mild-to-moderate essential hypertension: a randomized, double-blind, active control, three-parallel group, forced titration, multicenter, phase IV study (fimasartan achieving systolic blood pressure target (FAST) study). Drug Des Devel Ther. 2020;14:347–360. doi:10.2147/DDDT.S231293.
- Coca A, Calvo C, García-Puig J, et al. A multicenter, randomized, double-blind comparison of the efficacy and safety of irbesartan and enalapril in adults with mild to moderate essential hypertension, as assessed by ambulatory blood pressure monitoring: the MAPAVEL study (monitorización ambulatoria presión arterial APROVEL). Clin Ther. 2002;24(1):126–138. doi:10.1016/s0149-2918(02)85010-x.
- Düsing R, Brunel P, Baek I, et al. Sustained decrease in blood pressure following missed doses of aliskiren or telmisartan: the ASSERTIVE double-blind, randomized study. J Hypertens. 2012;30(5):1029–1040. doi:10.1097/HJH.0b013e328351c263.
- Fan YM, Zeng QY, Mai WY, et al. Clinical study on treating mild to moderate essential hypertension with combination of AT1 antagonist and ACEI. Front Cardiovasc Med. 2002;02:112–115. doi:10.3969/j.issn.1673-6583.2002.02.017.
- Fang GQ, Zhang LT, Chen YH, et al. Efficacy and safety evaluation of levamlodipine in the treatment of primary mild to moderate hypertension. Clin Med Chin. 2003;19:36–37. doi:10.3760/cma.j.issn.1008-6315.2003.10.019.
- Fogari R, Zoppi A, Mugellini A, et al. Hydrochlorothiazide added to valsartan is more effective than when added to olmesartan in reducing blood pressure in moderately hypertensive patients inadequately controlled by monotherapy. Adv Ther. 2006;23(5):680–695. doi:10.1007/BF02850307.
- Fogari R, Zoppi A, Mugellini A, et al. Effectiveness of hydrochlorothiazide in combination with telmisartan and olmesartan in adults with moderate hypertension not controlled with monotherapy: a prospective, randomized, open-label, blinded end point (PROBE), parallel-arm study. Curr Ther Res Clin Exp. 2008;69(1):1–15. doi:10.1016/j.curtheres.2008.02.003.
- Gao CY, Chen LM, Ying JQ, et al. Efficacy of combination of three drugs (telmisartan, hydrochlorothiazide, levo-amlodipine) in small doses on Middle-aged and elderly non-dipper hypertension. Chin J Biochem Pharm. 2014;34:143–145.
- Germino FW, Neutel JM, Dubiel R, et al. Efficacy of olmesartan medoxomil and hydrochlorothiazide fixed-dose combination therapy in patients aged 65 years and older with stage 1 and 2 hypertension or isolated systolic hypertension. Am J Cardiovasc Drugs. 2012;12(5):325–333. doi:10.1007/BF03261841.
- Giles TD, Alessi T, Purkayastha D, et al. Comparative efficacy of aliskiren/valsartan vs valsartan in nocturnal dipper and non-dipper hypertensive patients: a pooled analysis. J Clin Hypertens (Greenwich). 2012;14(5):299–306. doi:10.1111/j.1751-7176.2012.00608.x.
- Guo YZ, Qian YS, Ma AQ, et al. Efficacy and safety of irbesartan in treating essential hypertension. Chin J New Drugs Clin Rem. 2002;11:645–648. doi:10.3969/j.issn.1007-7669.2002.11.002.
- Guo YZ, Qian YS, Tao B, et al. Comparison of efficacy and safety of levoamlodipine besylate and losartanin treating 140 patients with essential hypertension. Chin J New Drugs Clin Rem. 2003;22:766–769. doi:10.3969/j.issn.1007-7669.2003.12.008.
- Hermida RC, Calvo C, Ayala DE, et al. Treatment of non-dipper hypertension with bedtime administration of valsartan. J Hypertens. 2005;23(10):1913–1922. doi:10.1097/01.hjh.0000182522.21569.c5.
- Hermida RC, Calvo C, Ayala DE, et al. Decrease in urinary albumin excretion associated with the normalization of nocturnal blood pressure in hypertensive subjects. Hypertension. 2005;46(4):960–968. doi:10.1161/01.HYP.0000174616.36290.fa.
- Hermida RC, Calvo C, Ayala DE, et al. Administration time-dependent effects of valsartan on ambulatory blood pressure in elderly hypertensive subjects. Chronobiol Int. 2005;22(4):755–776. doi:10.1080/07420520500180488.
- Hermida RC, Ayala DE, Fernández JR, et al. Comparison of the efficacy of morning versus evening administration of telmisartan in essential hypertension. Hypertension. 2007;50(4):715–722. doi:10.1161/HYPERTENSIONAHA.107.094235.
- Hermida RC, Ayala DE, Chayan L, et al. Administration-time-dependent effects of olmesartan on the ambulatory blood pressure of essential hypertension patients. Chronobiol Int. 2009;26(1):61–79. doi:10.1080/07420520802548135.
- Houlihan CA, Allen TJ, Baxter AL, et al. A low-sodium diet potentiates the effects of losartan in type 2 diabetes. Diabetes Care. 2002;25(4):663–671. doi:10.2337/diacare.25.4.663.
- Hu YR, Chen SX, Zhang J, et al. Efficacy and safety of domestic olmesartan in treatment of mild to moderate essential hypertension. J Shanghai Jiaotong Univ. 2009;29:1359–1362.
- Hua CX, Hua L, Li N, et al. Efficacy of monotherapy with 15 antihypertensive agents in treating essential hypertension assessed by 24-hour ambulatory blood pressure monitoring. Acta Acad Med Sin. 2007;06:792–796.
- Huang J, Guo JZ, Tao P, et al. Efficacy and safety evaluation of losartan in the treatment of mild and moderate hypertension. Chin J Cardiol. 1999;27:36–39. doi:10.3760/j:issn:0253-3758.1999.03.010.
- Huang RJ, Guo YB, Zheng YX. The effect of eplerenone and losartan on arterial stiffness and centraarterial pressure in patients with mild to moderate essential hypertension. Chin J Hyperten. 2012;20:776–780. doi:10.16439/j.cnki.1673-7245.2012.08.021.
- Huo Y, Li W, Webb R, et al. Efficacy and safety of sacubitril/valsartan compared with olmesartan in asian patients with essential hypertension: a randomized, double-blind, 8-week study. J Clin Hypertens (Greenwich). 2019;21(1):67–76. doi:10.1111/jch.13437.
- Izzo JL, Jr, Jia Y, Zappe DH. Influence of age and race on 24-hour ambulatory blood pressure responses to valsartan, hydrochlorothiazide, and their combination: implications for clinical practice. J Clin Hypertens. 2017;19(2):143–150. doi:10.1111/jch.12891.
- Kuschnir E, Bendersky M, Resk J, et al. Effects of the combination of low-dose nifedipine GITS 20 mg and losartan 50 mg in patients with mild to moderate hypertension. J Cardiovasc Pharmacol. 2004;43(2):300–305. doi:10.1097/00005344-200402000-00021.
- Lacourcière Y, Asmar R. A comparison of the efficacy and duration of action of candesartan cilexetil and losartan as assessed by clinic and ambulatory blood pressure after a missed dose, in truly hypertensive patients: a placebo-controlled, forced titration study. Candesartan/losartan study investigators. Am J Hypertens. 1999;12(12 Pt 1-2):1181–1187. doi:10.1016/s0895-7061(99)00142-9.
- Lacourcière Y, Bélanger A, Godin C, et al. Long-term comparison of losartan and enalapril on kidney function in hypertensive type 2 diabetics with early nephropathy. Kidney Int. 2000;58(2):762–769. doi:10.1046/j.1523-1755.2000.00224.x.
- Lacourcière Y, Krzesinski JM, White WB, et al. Sustained antihypertensive activity of telmisartan compared with valsartan. Blood Press Monit. 2004;9(4):203–210. doi:10.1097/00126097-200408000-00005.
- Lee H, Kim KS, Chae SC, et al. Ambulatory blood pressure response to once-daily fimasartan: an 8-week, multicenter, randomized, double-blind, active-comparator, parallel-group study in korean patients with mild to moderate essential hypertension. Clin Ther. 2013;35(9):1337–1349. doi:10.1016/j.clinthera.2013.06.021.
- Lee HY, Kim CH, Song JK, et al. 24-Hour blood pressure response to lower dose (30 mg) fimasartan in korean patients with mild to moderate essential hypertension. Korean J Intern Med. 2017;32(6):1025–1036. doi:10.3904/kjim.2016.094.
- Leonetti, G., Rappelli, A., Omboni, S., on Behalf of the Study Group, on Behalf of the Study Group. A similar 24-h blood pressure control is obtained by zofenopril and candesartan in primary hypertensive patients. Blood Press, sup1. 2006;15:18–26. doi:10.1080/08038020510046689.
- Li J, Qin TL, Jiang H, et al. The efects of olmesartan on ambulatory blood pressures and blood pressure variability in patients with mild to moderate essential hypertension. Chin J Intern Med. 2014;53:788–792. doi:10.3760/cma.j.issn.0578-1426.2014.10.007.
- Li J, Qin TL, Zhao LS, et al. Effecacy and safety of domestic and foreign olmesartan medoxomil in mild-moderate essential hypertension patients. Chin J Geriatr Heart Brain Ves Dis. 2014;16:1275–1278. doi:10.3969/j.issn.1009-0126.2014.12.013.
- Lian FM, Tong XL, Xu LP, et al. Clinical study on jiangturuqinggan prescription for treating hypertension with metabolic syndrome. J Basic Chin Med. 2011;17:649–652. J doi:10.19945/j.cnki.issn.1006-3250.2011.06.029.
- Mai WY, Ceng QY, Li YJ, et al. Effect of at_1 antagonist losartan on prostacycline and endothelin in patients with essential hypertension. Front Cardiovasc Med. 2000;27:239–242.
- Malacco E, Piazza S, Meroni R, et al. Comparison of valsartan and irbesartan in the treatment of mild to moderate hypertension: a randomized, open-label, crossover study. Curr Ther Res Clin Exp. 2000;61(11):789–797. doi:10.1016/S0011-393X(00)90005-5.
- Malacco E, Omboni S, Volpe M, et al. Antihypertensive efficacy and safety of olmesartan medoxomil and ramipril in elderly patients with mild to moderate essential hypertension: the ESPORT study. J Hypertens. 2010;28(11):2342–2350. doi:10.1097/HJH.0b013e32833e116b.
- Mallion J, Siche J, Lacourcière Y. ABPM comparison of the antihypertensive profiles of the selective angiotensin II receptor antagonists telmisartan and losartan in patients with mild-to-moderate hypertension. J Hum Hypertens. 1999;13(10):657–664. doi:10.1038/sj.jhh.1000925.
- Mallion JM, Omboni S, Barton J, et al. Antihypertensive efficacy and safety of olmesartan and ramipril in elderly patients with mild to moderate systolic and diastolic essential hypertension. Blood Press Suppl. 2011;1:3–11. doi:10.3109/08037051.2010.532332.
- Mancia G, Korlipara K, van Rossum P, et al. An ambulatory blood pressure monitoring study of the comparative antihypertensive efficacy of two angiotensin II receptor antagonists, irbesartan and valsartan. Blood Press Monit. 2002;7(2):135–142. doi:10.1097/00126097-200204000-00008.
- Manzur F, Rico A, Romero JD, et al. Efficacy and safety of valsartan or chlorthalidone vs. Combined valsartan and chlorthalidone in patients with mild to moderate hypertension: the VACLOR study. Clin Med Insights Cardiol. 2018;12:1179546818796482. doi:10.1177/1179546818796482.
- Matsumoto S, Shimodozono M, Miyata R, et al. Benefits of the angiotensin II receptor antagonist olmesartan in controlling hypertension and cerebral hemodynamics after stroke. Hypertens Res. 2009;32(11):1015–1021. doi:10.1038/hr.2009.143.
- Moltzer E, Mattace Raso FU, Karamermer Y, et al. Comparison of candesartan versus metoprolol for treatment of systemic hypertension after repaired aortic coarctation. Am J Cardiol. 2010;105(2):217–222. doi:10.1016/j.amjcard.2009.08.674.
- Munakata M, Nagasaki A, Nunokawa T, et al. Effects of valsartan and nifedipine coat-core on systemic arterial stiffness in hypertensive patients. Am J Hypertens. 2004;17(11 Pt 1):1050–1055. doi:10.1016/j.amjhyper.2004.06.028.
- Neutel J, Weber M, Pool J, et al. Valsartan, a new angiotensin II antagonist: antihypertensive effects over 24 hours. Clin Ther. 1997;19(3):447–458. doi:10.1016/s0149-2918(97)80129-4.
- Neutel JM, Kolloch RE, Plouin PF, et al. Telmisartan vs losartan plus hydrochlorothiazide in the treatment of mild-to-moderate essential hypertension–a randomised ABPM study. J Hum Hypertens. 2003;17(8):569–575. doi:10.1038/sj.jhh.1001592.
- Palatini P, Malacco E, Di SS, et al. Trough:peak ratio and smoothness index in the evaluation of 24-h blood pressure control in hypertension: a comparative study between valsartan/hydrochlorothiazide combination and amlodipine. Eur J Clin Pharmacol. 2002;57(11):765–770. doi:10.1007/s00228-001-0393-6.
- Palatini P, Jung W, Shlyakhto E, et al. Maintenance of blood-pressure-lowering effect following a missed dose of aliskiren, irbesartan or ramipril: results of a randomized, double-blind study. J Hum Hypertens. 2010;24(2):93–103. doi:10.1038/jhh.2009.38.
- Perez A, Cao C. The impact of azilsartan medoxomil treatment (capsule formulation) at doses ranging from 10 to 80 mg: significant, rapid reductions in clinic diastolic and systolic blood pressure. J Clin Hypertens. 2017;19:312–321. doi:10.1111/jch.12895.
- Qian YS, Wang XY, Huang GZ, et al. Treatment of 118 cases of essential hypertension with candesartan ester tablets. Chin J New Drugs Clin Rem. 2005;17:849–853. doi:10.3969/j.issn.1007-7669.2005.11.003.
- Qin YP, Wang ZP, Li L. Comparative study on the efficacy of domestic felodipine and losartan intreatment of hypertension. Chin J New Drug. 2000;9:837–839. doi:10.3321/j.issn:1003-3734.2000.12.011.
- Ribeiro AB, Mion D, Jr, Marin MJ, et al. Antihypertensive efficacy of amlodipine and losartan after two ‘missed’ doses in patients with mild to moderate essential hypertension. J Int Med Res. 2007;35(6):762–772. doi:10.1177/147323000703500604.
- Smolensky MH, Hermida RC, Portaluppi F. Comparison of the efficacy of morning versus evening administration of olmesartan in uncomplicated essential hypertension. Chronobiol Int. 2007;24(1):171–181. doi:10.1080/07420520600969277.
- Stanton A, Jensen C, Nussberger J, et al. Blood pressure lowering in essential hypertension with an oral renin inhibitor, aliskiren. Hypertension. 2003;42(6):1137–1143. doi:10.1161/01.HYP.0000101688.17370.87.
- Sun N, Feng Y, Gao P, et al. Efficacy and tolerability of once-daily 160 mg valsartan in chinese patients with mild to moderate hypertension. Exp Ther Med. 2017;13(3):1109–1116. doi:10.3892/etm.2017.4051.
- Tong XL, Lian FM, Zhou Q, et al. Prospective multicenter clinical trial of chinese herbal formula JZQG (jiang zhuo qing gan) for hypertension. Am J Chin Med. 2013;41(1):33–42. doi:10.1142/S0192415X13500031.
- Wang F, Hua L, Wang L, et al. To evaluate the efficacy and safety of domestic candesartan ester in treating hypertension by ambulatome blood pressure monitoring. Clin Cardiol. 2004;20:722–724. doi:10.3969/j.issn.1001-1439.2004.12.008.
- Wang F, Wang L, Fan CM, et al. Randomized double blind observation of domestic candesartan ester/enalapril dynamic antihypertensive effect. Chin J Med. 2006;41:39–41. doi:10.3969/j.issn.1008-1070.2006.09.020.
- Wang YM, Liu GC. Chronotherapy of telmisartan in the treatment of essential hypertension. Chin J Gerontol. 2011;31:756–758. doi:10.3969/j.issn.1005-9202.2011.05.012.
- Wang W, Feng H, Gao W. Effect of low dose combination of common antihypertensive drugs on senile non-arytenoid hypertension. Chin J Gerontol. 2015;35:3281–3282. doi:10.3969/j.issn.1005-9202.2015.12.041.
- Wang HY, Guo L, Wang JH, et al. Clinical trial of allisartan isoproxil tablets in the treatment of patients with essential hypertension. Chin J Clin Pharmacol. 2022;38:755–759. doi:10.13699/j.cnki.1001-6821.2022.08.001.
- Williams B, Lacourcière Y, Schumacher H, et al. Antihypertensive efficacy of telmisartan vs ramipril over the 24-h dosing period, including the critical early morning hours: a pooled analysis of the PRISMA I and II randomized trials. J Hum Hypertens. 2009;23(9):610–619. doi:10.1038/jhh.2009.4.
- Wu SL, Wang RT, Xiang YJ, et al. Efficacy observation of telmisartan at different administration time in theTreatment of non-dipper hypertension. China Pharmacy. 2011;22:1294–1296.
- Kawano Y, Sato Y, Yoshinaga K. A randomized trial of the effect of an angiotensin II receptor blocker SR47436 (irbesartan) on 24-hour blood pressure in patients with essential hypertension. Hypertens Res. 2008;31(9):1753–1763. doi:10.1291/hypres.31.1753.
- Tanigawara Y, Yoshihara K, Kuramoto K, et al. Comparative pharmacodynamics of olmesartan and azelnidipine in patients with hypertension: a population pharmacokinetic/pharmacodynamic analysis. Drug Metab Pharmacokinet. 2009;24(4):376–388. doi:10.2133/dmpk.24.376.
- Stergiou G, Brunström M, MacDonald T, et al. Bedtime dosing of antihypertensive medications: systematic review and consensus statement: international society of hypertension position paper endorsed by world hypertension league and european society of hypertension. J Hypertens. 2022;40(10):1847–1858. doi:10.1097/HJH.0000000000003240.
- Liu J, Su X, Nie Y, et al. Nocturnal blood pressure rather than night-to-day blood pressure ratio is related to arterial stiffening in untreated young and Middle-aged adults with non-dipper hypertension. J Clin Hypertens. 2022;24(8):1044–1050. doi:10.1111/jch.14546.
- Hermida RC, Ayala DE, Mojón A, et al. Decreasing sleep-time blood pressure determined by ambulatory monitoring reduces cardiovascular risk. J Am Coll Cardiol. 2011;58(11):1165–1173. doi:10.1016/j.jacc.2011.04.043.
- Liu J, Li Y, Zhang X, et al. Chinese hypertension league expert consensus committee on the management of nocturnal hypertension. Management of nocturnal hypertension: an expert consensus document from chinese hypertension league. J Clin Hypertens. 2024;26(1):71–83. doi:10.1111/jch.14757.
- Wang HY, Xi Y, Ma QC, et al. Clinical study on the effects of allisartan isoproxil in the treatment of mild tomoderate essential hypertension with hyperuricemia. Chin J Pract Intern Med. 2022;42:930–935. doi:10.19538/j.nk2022110112.
- Jiang L, Du GS, Chen RJ, et al. Therapeutic effect of allisartan isoproxil on mild to moderate hypertension with hyperuricemia. Lingnan J Emerg Med. 2019;24(5):485–486. doi:10.3969/j.issn.1671-301X.2019.05.025.
- Xiao Y, Miao ZY, Sun JG, et al. Allisartan isoproxil promotes uric acid excretion by interacting with intestinal urate transporters in hyperuricemic zebrafish (Danio rerio). Bull Exp Biol Med. 2023;175(5):638–643. doi:10.1007/s10517-023-05917-9.
- Roch-Ramel F, Guisan B, Diezi J. Effects of uricosuric and antiuricosuric agents on urate transport in human brush-border membrane vesicles. J Pharmacol Exp Ther. 1997;280(2):839–845.
- Çağlı K, Turak O, Canpolat U, et al. Association of serum uric acid level with blood pressure variability in newly diagnosed essential hypertension. J Clin Hypertens (Greenwich). 2015;17(12):929–935. doi:10.1111/jch.12641.
- Turak O, Ozcan F, Tok D, et al. Serum uric acid, inflammation, and non-dipping circadian pattern in essential hypertension. J Clin Hypertens. 2013;15(1):7–13. doi:10.1111/jch.12026.
- Sanchez-Lozada LG, Rodriguez-Iturbe B, Kelley EE, et al. Uric acid and hypertension: an update with recommendations. Am J Hypertens. 2020;33(7):583–594. doi:10.1093/ajh/hpaa044.
- Kanabrocki EL, Ryan MD, Hermida RC, et al. Altered circadian relationship between serum nitric oxide, carbon dioxide, and uric acid in multiple sclerosis. Chronobiol Int. 2004;21(4-5):739–758. doi:10.1081/cbi-200025981.
- Madero M, Rodríguez Castellanos FE, Jalal D, et al. A pilot study on the impact of a low fructose diet and allopurinol on clinic blood pressure among overweight and prehypertensive subjects: a randomized placebo-controlled trial. J Am Soc Hypertens. 2015;9(11):837–844. doi:10.1016/j.jash.2015.07.008.
- Taddei S. RAS inhibitors’ dose-dependent efficacy: myth or reality? Curr Med Res Opin. 2015;31(7):1245–1256. doi:10.1185/03007995.2015.1053047.
- Huang JF, Li MX, Zhang DY, et al. Antihypertensive treatment in masked hypertension for target organ protection (anti-MASK): a randomized, double-blind, placebo-controlled trial. Blood Press Monit. 2022;27(Suppl 1):e305. doi:10.1097/01.mbp.0000905172.14581.d1.
- Wang J. Efficacy and safety of sacubitril/allisartan for the treatment of primary hypertension: a phase 3 randomized, double-blind study. J Am Coll Cardiol. 2024;83(13):1705. doi:10.1016/S0735-1097(24)03695-7.
- Kario K, Ito S, Itoh H, et al. Effect of the nonsteroidal mineralocorticoid receptor blocker, esaxerenone, on nocturnal hypertension: a post hoc analysis of the ESAX-HTN study. Am J Hypertens. 2021;34(5):540–551. doi:10.1093/ajh/hpaa155.
- Schlaich MP, Bellet M, Weber MA, et al. Dual endothelin antagonist aprocitentan for resistant hypertension (PRECISION): a multicentre, blinded, randomised, parallel-group, phase 3 trial. Lancet. 2022;400(10367):1927–1937. doi:10.1016/S0140-6736(22)02034-7.