Abstract
Background. Elevated circulating levels of pregnancy‐associated plasma protein A (PAPP‐A), a novel marker of atherosclerotic plaque instability, are associated with increased risk of future cardiac events in patients with acute coronary syndromes (ACS). However, little is known of the kinetics or clinical significance of circulating PAPP‐A after plaque rupture in acute ST‐elevation myocardial infarction (STEMI).
Aim. To evaluate the 48‐hour release of pregnancy‐associated plasma protein A (PAPP‐A) and its association with 12‐month outcome in patients with acute ST‐elevation myocardial infarction (STEMI).
Methods. Sixty‐two consecutive STEMI patients were included (40 men and 22 women, median age 67.5 years (range 34–84)), of whom 54 (87.1%) received reperfusion therapy. PAPP‐A was measured at admission and 6–12, 24 and 48 hours thereafter. In 14 patients, samples were obtained also at 1, 2 and 4 hours.
Results. There was an early peak of circulating PAPP‐A during the first 12 hours from symptom onset, followed by rapid normalization. A second, late PAPP‐A elevation was noticed in 20/62 patients (32.3%). Admission PAPP‐A >10.0 mIU/L (highest tertile) was associated (P = 0.049) with increased 12‐month risk of cardiovascular death or non‐fatal myocardial infarction. Moreover, the combination of failed early reperfusion together with late PAPP‐A elevation was strongly (7/13 versus 10/49 patients, P = 0.016) associated with adverse outcome. Admission PAPP‐A did not correlate with admission C‐reactive protein or cardiac troponin I.
Conclusions. PAPP‐A is elevated early in STEMI and then declines rapidly, a pattern consistent with release from the ruptured plaque. The variability of PAPP‐A kinetics at 48 hours reflects the success of reperfusion. This study also shows that PAPP‐A may have prognostic value in STEMI.
| Abbreviations | ||
| PAPP‐A | = | pregnancy‐associated plasma protein A |
| STEMI | = | ST‐elevation myocardial infarction |
| ACS | = | acute coronary syndromes |
| CRP | = | C‐reactive protein |
| cTnI | = | cardiac troponin I |
| PCI | = | percutaneous coronary intervention |
| ECG | = | electrocardiogram |
| proMBP | = | proform of major basic protein |
| TIMI | = | thrombolysis in myocardial infarction |
| MI | = | myocardial infarction |
Introduction
Acute plaque rupture presenting with ST‐elevation myocardial infarction (STEMI) is a frequent problem in the emergency room. Standard therapy in these patients is immediate reperfusion by thrombolysis or primary percutaneous coronary intervention (PCI). Early risk stratification of STEMI patients is traditionally based on clinical signs, presenting electrocardiogram (ECG) and, in the case of thrombolysis, follow‐up of reperfusion by ECG Citation1,2. Although elevated cardiac troponins at admission are known to indicate worse outcome Citation2, biochemical markers have not received much attention in the risk stratification of STEMI patients.
Pregnancy‐associated plasma protein A (PAPP‐A) is a zinc‐binding metzincin metalloproteinase Citation3 which is abundantly expressed in eroded and ruptured but not in stable atherosclerotic plaques Citation4. Although the exact role of PAPP‐A in atherosclerosis and its complications are still unclear, there is evidence of the association between circulating PAPP‐A level and complex atherosclerotic plaque morphology in patients with stable angina pectoris Citation5. PAPP‐A may play an important role in the inflammatory reactions of the vascular wall leading to atherosclerotic plaque disruption.
We have recently shown that elevated circulating PAPP‐A is a strong independent predictor of ischemic cardiac events and a need of revascularization in patients who present with suspected acute myocardial infarction (MI) but remain troponin‐negative Citation6. Data supporting PAPP‐A as a predictor of adverse events in acute coronary syndromes (ACS) have also been presented by other recent studies Citation7,8.
Despite increasing evidence of its specific association with vascular disease, not much is known about the origin and release kinetics of PAPP‐A in acute plaque rupture. Our preliminary experience suggested that the release of PAPP‐A in MI is highly variable Citation9 and that the overall correlation of PAPP‐A with cardiac troponin levels appears to be poor Citation6,Citation9. These questions must be addressed in detail before the potential role of PAPP‐A can be determined in the triage and care of STEMI patients. We therefore set out to investigate the release pattern circulating PAPP‐A and its association with 12‐month outcome in the acute phase of STEMI.
Key messages
Early peak of circulating pregnancy‐associated plasma protein A (PAPP‐A) followed by rapid normalization was noticed, a pattern consistent with release from the ruptured plaque.
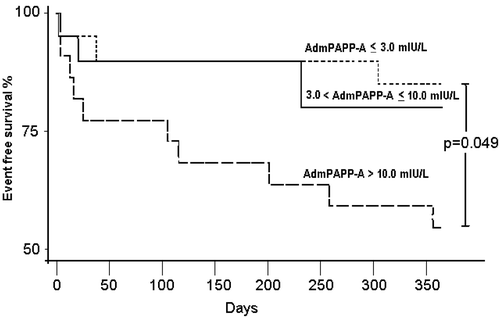
Admission PAPP‐A >10.0 mIU/L (highest tertile), and the combination of failed early reperfusion together with late PAPP‐A elevation, were strongly associated with adverse outcome.
Materials and methods
Subjects and design
We recruited 62 (40 men and 22 women, median age 67.5 years (range 34–84)) consecutive STEMI patients of whom 14 (10 men and 4 women, median age 62.5 years (range 46–81)) were selected to a subgroup with frequent early sampling. The patients presented to the emergency room of the Turku University Central Hospital between May 2000 and July 2001 for the evaluation of acute STEMI (typical chest pain >20 minutes and new >1.0 mV and >2.0 mV ST‐elevation in two consecutive limb and precordial leads, respectively). Of the 62 patients, 87.1% were treated with acute reperfusion therapy (51 with thrombolysis and 3 with primary PCI). All patients were treated according to the routine clinical protocols of the Turku University Central Hospital and followed for 12 months. Notably, all 14 patients with frequent sampling got thrombolysis with reteplase. Eight patients had contraindications to reperfusion therapy and were treated with anti‐ischemic and anti‐thrombotic medication.
A total of 27 (43.5%) patients were revascularized during follow‐up, 9 of them during the first 24 hours. The primary end‐point at 12 months was the combination of cardiovascular mortality and the first episode of non‐fatal MI. Mortality data (including cause of death) were obtained from the Statistics Finland (contains information on all deaths in Finland) and the data of non‐fatal episodes from written or telephone contacts with the patients and the records of the Turku University Central Hospital which is the only hospital providing acute cardiac and stroke care in the area. The hospital records of all patients were retrospectively reviewed by the first and third authors (JL, TI) for classification of non‐fatal end‐points. Occasional discrepancies were settled by mutual consensus of all authors. All patients gave their written informed consent to participate in the study, which had been approved by the Ethics Committee of the Turku University Central Hospital.
Biochemical measurements
Serum samples for PAPP‐A were collected immediately at admission and at the time points of 6–12, 24 and 48 hours, and in 14 patients also at 1, 2 and 4 hours (frequent early sampling group) to clarify the early PAPP‐A kinetics in STEMI. All samples were studied post hoc by an investigational point‐of‐care time‐resolved immunofluorometric assay Citation10 designed to measure total PAPP‐A, i.e. PAPP‐A whether in free form or in complex with the proform of major basic protein (proMBP). The lower limit of detection was 0.5 mIU/L and the functional sensitivity (imprecision ≈20%) 1.5 mIU/L. The between‐assay imprecision at the lowest standard (2.5 mIU/L) was 13.7%. C‐reactive protein (CRP) taken at admission was measured by an ultrasensitive Aio! assay (Innotrac Diagnostics Corp, Turku, Finland). The treating clinicians had no access to the investigational PAPP‐A and CRP information.
Cardiac troponin I (cTnI) was immediately analysed in the hospital laboratory at time points of 0, 6–12 and 24 hours, and also at 1, 2 and 4 hours in frequent sampling group, using the Bayer Immuno 1 assay (Bayer Diagnostics, Tarrytown, NY, USA) and the results were made available to the treating physicians.
ECG, coronary angiography and assessment of reperfusion
Thirteen‐lead ECG was taken at admission and in patients with thrombolysis treatment 120 +/− 30 minutes after the start of infusion. Early reperfusion was determined as >75% ST‐resolution during the first 150 minutes after the initiation of therapy, obtained in a single lead with the highest ST‐elevation on primary ECG Citation1.
During follow‐up, coronary angiography was performed in 31/62 patients (50%; coronary angiography subgroup) of which 19/31 (61.3%) were performed during the first 7 days after the index event. All angiograms were analysed off‐line by two of the authors (KN, ME) for identification of the culprit lesion, infarct‐related vessel patency (Thrombolysis In Myocardial Infarction (TIMI) flow ⩾2), and the overall extent of coronary disease.
For the purpose of this study, reperfusion was assessed by two sets of criteria. In all patients, assessment of early reperfusion was based on the presence or absence of ECG criteria (see above). In the coronary angiography subgroup late reperfusion was identified as no early reperfusion on ECG but infarct related vessel patency with TIMI flow ⩾2 being observed by angiography.
Statistical analysis
Categorical variables were compared between groups with the Chi‐square test. Continuous variables were compared with the use of Wilcoxon's rank‐sum test. Survival curves were estimated using the Kaplan‐Meier method, and differences between curves were tested with the log rank test. Correlations were tested using Spearman's correlation test. The statistical analyses were performed using SAS statistical software (Version 8.1; SAS Institute, Cary, NC). P‐values less than 0.05 were considered significant.
Results
Admission PAPP‐A
Patients were divided in tertiles according to admission PAPP‐A: <3.0, 3.0–10.0, and >10.0 mIU/L. shows the clinical characteristics of patients according to admission PAPP‐A tertiles. There were no differences between the groups in CRP, admission cTnI or maximal cTnI. When compared to patients with admission PAPP‐A <10 mUI/L, patients with admission PAPP‐A ⩾10.0 mIU/L were older and more likely to have diabetes, and the infarct localization was more often anterior. There were no statistically significant differences between the tertiles in the delay from symptom onset to the initiation of reperfusion therapy (). Admission PAPP‐A did not correlate with CRP (r = −0.05, P = 0.67) or admission cTnI (r = −0.02, P = 0.87).
Table I. Baseline characteristic of the whole and the frequent sampling group (A) and according to admPAPP‐A levels (B).
Forty‐eight‐hour release pattern of PAPP‐A
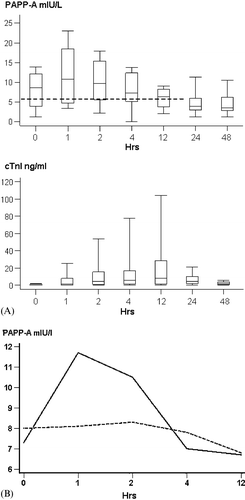
The release kinetics of PAPP‐A was investigated in detail in the frequent sampling subgroup (n = 14): 10 of the patients were men, the MI was anterior in 6 patients, and early reperfusion on ECG was noticed in 11/14 (78.6%) patients (). Median delay from the symptoms to thrombolysis was 285 [90, 360] minutes. shows the early release pattern of PAPP‐A as median [25th and 75th percentiles] values in all 14 patients. Admission PAPP‐A was 8.0 [3.7, 12.2] mIU/L. The highest value was reached at 1 hour (11.6 [4.7, 18.8]) mIU/L) and thereafter the values started to decline, reaching 6.8 [4.4, 8.7] by 12 hours. The values continued to decline after 12 hours and the lowest level of 4.7 [3.0, 6.3] mIU/L was measured at 48 hours after admission (P = 0.0058 versus 1‐hour value). There was a trend of higher median admission, but lower 1‐hour PAPP‐A values in the patients with treatment delay over the 285 minutes comparing to patients with shorter delay (8.0 [1.2, 13.9] versus 7.3 [3.8, 11.2] and 8.1 [3.9, 18.5] versus 11.7 [8.9, 18.8] respectively) (). There were no statistically significant correlations between peak PAPP‐A and peak cTnI levels (r = −0.11, P = 0.69). Comparison of the release pattern of PAPP‐A to that of cTnI revealed a distinct temporal discrimination ().
Figure 1. (A) The averaged (median 10th, 25th, 50th, 75th, 90th percentile) release pattern of PAPP‐A and cTnI of the 14 patients with frequent early blood sampling (box plots). X‐axis: Time after admission (hours). The bolded dotted line represents the upper 97.5th PAPP‐A percentile in healthy normal males (see Discussion). (B) Median release patterns of PAPP‐A during the first 12 hours in the frequent sampling group according to delay from symptom onset to thrombolysis. Solid line: short delay (<285 min), dotted line: long delay (>285 min). X‐axis: time after admission (hours).
Individual analysis of the 14 patients revealed that 2 patients showed a second, late PAPP‐A elevation at 24–48 hours. Using >9.5 mUI/L (75th percentile at 48 hours) as the cut‐off for late PAPP‐A elevation, 20/62 (32.3%) of the whole study group were identified as having a second peak. shows the clinical details of patients with (n = 20) and without (n = 42) late PAPP‐A elevation. Patients who showed a second, delayed peak had more often anterior than inferior infarctions (P = 0.028). Notably, time delays were comparable between the two groups, both from symptom onset to reperfusion therapy and from symptom onset to first PAPP‐A sampling (). However, patients with a second, delayed PAPP‐A elevation were characterized by less successful early reperfusion by ECG criteria (35% versus 88%, P<0.001).
Table II. Baseline characteristics of all patients according to the late PAPP‐A elevation status.
Biphasic PAPP‐A release pattern and late reperfusion status
The relationship of the biphasic PAPP‐A release pattern with the late reperfusion status was investigated in the coronary angiography subgroup (n = 31). All patients in this group who demonstrated early ECG reperfusion (n = 20) were found to have a patent infarct‐related coronary artery by angiography. A late PAPP‐A elevation was found in only two of them (10.0%). In contrast, it occurred in eight (72.7%) of patients with only late or no reperfusion (P<0.01 versus early reperfusion).
Of the nine patients treated with PCI during the first 24 hours, only one patient (11.1%) showed a delayed second PAPP‐A elevation. Of the 22 thrombolysed patients in the angiographic subgroup, a late PAPP‐A peak was observed in 9 cases (40.9%).
Neither the admission PAPP‐A levels nor the PAPP‐A release patterns were associated with the presence or absence of three‐vessel coronary artery disease by coronary angiography.
Clinical outcome
To determine the prognostic value of early PAPP‐A measurements, the clinical outcome as a combination of cardiovascular mortality and non‐fatal MI (primary end‐point) was studied according to admission PAPP‐A tertiles. At the end of the 12‐month follow‐up, 17/62 patients (25.7%) had met a primary end‐point. There were 11 deaths (total mortality 16.6%), of which cardiovascular causes accounted for 100%. Six patients (9.1%) suffered non‐fatal MI. shows that the cumulative risk of a primary end‐point was 15% and 20.0% if admission PAPP‐A was <3.0 mIU/L and 3.0–10.0 mIU/L respectively, but increased to 45% when the admission PAPP‐A was >10.0 mIU/L (P = 0.049).
The presence or absence of a late PAPP‐A elevation was not statistically significantly associated with outcome as single variable. However, 7/13 (53.8%) of those patients who showed late PAPP‐A elevation after having failed reperfusion by ECG criteria met the primary end‐point in 12 months (versus 20.4% out of the other 49 patients, P = 0.016).
Discussion
This is the first study to investigate the release pattern and prognostic value of circulating PAPP‐A in patients with STEMI, which represents the clinical consequences of acute plaque rupture. Although the results of this observational study are preliminary, they hold promise that PAPP‐A could be used as a prognostic tool also in STEMI patients.
Our results show that PAPP‐A elevates early during the first hours of STEMI and normalizes quite rapidly, especially in patients with early reperfusion. Our findings in the frequent sampling subgroup also suggest that in patients with short treatment delays PAPP‐A levels are increasing and in those with longer delays they are already decreasing at the time of admission (). The mechanism of the early peak is still unknown, but our data are consistent with the assumption that the release of PAPP‐A is mostly due to the plaque rupture per se. PAPP‐A probably participates in the inflammatory reactions of the vascular wall, which lead to the disruption of the atherosclerotic plaque. As a metzincin metalloproteinase Citation3, PAPP‐A could be involved in the processing of the extracellular matrix of the plaque and weakening of the fibrous cap. Notably, we could not demonstrate any correlation between either admission or maximal cTnI and PAPP‐A levels (). Thus, it is unlikely that the circulating PAPP‐A would reflect the degree of ischemic myocardial tissue damage. On the other hand, primary PCI was a rare event (n = 3) at the time of this study and our results only apply to patients treated with thrombolysis. If the circulating PAPP‐A in ACS is mostly plaque‐derived, it is possible that coronary manipulation may abruptly increase circulating PAPP‐A levels.
Patients with high PAPP‐A already at admission had worse prognosis (). The patients in the highest admission PAPP‐A tertile were older and more likely to have diabetes and a history of previous MI (), all associated with more advanced state of atherosclerosis. These findings are in line with our previous observations in cTnI‐negative ACS patients Citation6. However, we could not find any relationship between admission PAPP‐A and CRP (), early reperfusion status by ECG () or the angiographical extent of coronary disease (subgroup of 31 patients). Because the PAPP‐A levels later declined to values lower than admission PAPP‐A (), we consider it likely that the admission values measured in our study were already elevated due to the 2–3‐hour delay from symptom onset. Further studies should collect frequent blood samples as early as possible to clarify the kinetics and origin of circulating PAPP‐A after coronary occlusion.
This study was motivated by our initial observations Citation9 showing that the PAPP‐A release patterns in STEMI can be quite variable. Somewhat surprisingly, a second, although generally lower, PAPP‐A elevation occurred at 48 hours in one‐third of the STEMI patients. Using available angiographical data in a subgroup of 31 subjects, we were able to associate the absence of late PAPP‐A elevation with successful early reperfusion and patency of the infarct‐related artery. In contrast, patients with a biphasic PAPP‐A pattern were characterized by only late or no reperfusion. The possible mechanisms of delayed PAPP‐A elevations remain unknown, but it is conceivable that prolonged leakage from the ruptured plaque or rupture of another plaque could have taken place in these patients.
In a recent report we have shown that the molecular nature of the acutely released PAPP‐A is different from the form found in pregnancy and the form constituting the baseline immunoreactivity in non‐pregnant individuals Citation11. In the latter cases PAPP‐A occurs almost exclusively as a complex with proMBP whereas the ACS‐derived form lacks the proMBP part. In the present study PAPP‐A measurements were performed with total PAPP‐A assay that detects both forms. compares the total PAPP‐A levels in STEMI with the hypothetical baseline level which in a previous report Citation9 was determined to be as high as 5.68 mIU/L in healthy males (upper 97.5th percentile). It is conceivable that the measured circulating PAPP‐A levels may be affected by the variable proportion of baseline versus ACS‐derived PAPP‐A, particularly when it comes to measurements after the acute phase. A more definitive evaluation of PAPP‐A as an independent prognostic ACS marker should therefore be carried out with an assay that specifically quantifies the acutely released PAPP‐A. Such assays are currently under development in our laboratory.
In conclusion, circulating PAPP‐A is elevated early in STEMI. The variability of PAPP‐A kinetics is partly due to the success of reperfusion. The results are in line with our previous observations on PAPP‐A as a biomarker in cTnI‐negative ACS patients Citation6. They add to observations by other groups Citation7,8 by suggesting that PAPP‐A, a plaque instability marker, may be a useful prognostic tool also in STEMI. Specific assays for the atherosclerosis‐associated form of circulating PAPP‐A should be used in future studies to determine optimal sampling times and to provide reliable clinical interpretations of PAPP‐A elevations in the various phases of acute coronary syndromes.
Acknowledgements
This study was supported financially by grants from the EVO funds of the Turku and Helsinki University Hospitals and from Finnish National Technology Agency (Project 40279). Taina Lahti, RN, and Tuula Laukkanen, RN, are acknowledged for expert care of the study subjects and their blood.
References
- Zeymer U., Schroder K., Wegscheider K., Senges J., Neuhaus K. L., Schroder R. ST resolution in a single electrocardiographic lead: a simple and accurate predictor of cardiac mortality in patients with fibrinolytic therapy for acute ST‐elevation myocardial infarction. Am Heart J 2005; 149: 91–7
- Bjorklund E., Lindahl B., Johanson P., Jernberg T., Svensson A. M., Venge B., et al. Admission Troponin T and measurement of ST‐segment resolution at 60 min improve early risk stratification in ST‐elevation myocardial infarction. Eur Heart J 2004; 25: 113–20
- Boldt H. B., Overgaard M. T., Laursen L. S., Weyer K., Sottrup‐Jensen L., Oxvig C. Mutational analysis of the proteolytic domain of pregnancy‐associated plasma protein‐A (PAPP‐A): classification as a metzincin. Biochem J 2001; 358: 359–67
- Bayes‐Genis A., Conover C. A., Overgaard M. T., Bailey K. R., Christiansen M., Holmes D. R., et al. Pregnancy‐associated plasma protein A as a marker of acute coronary syndromes. N Engl J Med 2001; 345: 1022–9
- Cosin‐Sales J., Christiansen M., Kaminski P., Oxvig C., Overgaard M. T., Cole D., et al. Pregnancy‐associated plasma protein A and its endogenous inhibitor, the proform of eosinophil major basic protein (proMBP), are related to complex stenosis morphology in patients with stable angina pectoris. Circulation 2004; 109: 1724–8
- Lund J., Qin Q. P., Ilva T., Pettersson K., Voipio‐Pulkki L‐M., Porela P., et al. Circulating pregnancy‐associated plasma protein A predicts outcome in patients with acute coronary syndrome but no troponin I elevation. Circulation 2003; 108: 1924–6
- Laterza O. F., Cameron S. J., Chappell D., Sokoll L. J., Green G. B. Evaluation of pregnancy‐associated plasma protein A as a prognostic indicator in acute coronary syndrome patients. Clinica Chimica Acta 2004; 384: 163–9
- Heeschen C., Dimmeler S., Hamm C. W., Fichtlscherer S., Simoons M. L., Zeiher A. M., et al. Pregnancy‐associated plasma protein‐A levels in patients with acute coronary syndromes: comparison with markers of systemic inflammation, platelet activation, and myocardial necrosis. J Am Coll Cardiol 2005; 45: 229–37
- Qin Q. P., Laitinen P., Majamaa‐Voltti K., Eriksson S., Kumpula E‐K., Pettersson K. Release patterns of pregnancy associated plasma protein A (PAPP‐A) in patients with acute coronary syndromes. Scand Cardiovasc J 2002; 36: 358–61
- Qin Q. P., Christiansen M., Pettersson K. Point‐of‐care time‐resolved immunofluorometric assay for human pregnancy‐associated plasma protein A: use in first‐trimester screening for Down syndrome. Clin Chem 2002; 48: 473–83
- Qin Q. P., Kokkala S., Lund J., Tamm N., Voipio‐Pulkki L‐M., Pettersson K. Molecular distinction of circulating pregnancy associated plasma protein‐A in myocardial infarction and pregnancy. Clin Chem 2005; 51: 75–83