Abstract
BACKGROUND. Longer‐term outcome of patients following carotid artery revascularization depends predominantly on cardiac events rather than neurological events.
AIM. To assess the longer‐term outcomes of patients with known coronary artery morphology undergoing carotid artery stenting.
METHOD. In a prospective observational study including 549 consecutive patients undergoing carotid artery stenting, a coronary angiography was performed in a single session unless a recent angiogram was available. Following the intervention, patients were followed prospectively to determine neurological events as well as major adverse coronary events (MACE) during long‐term follow‐up.
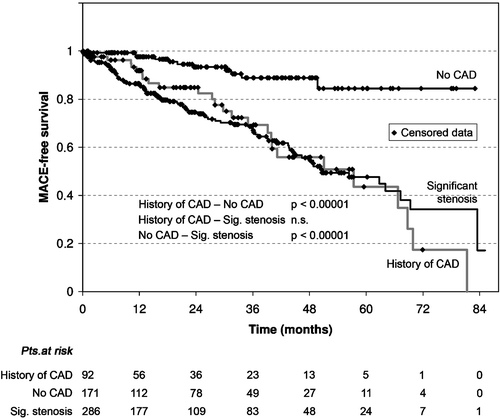
RESULTS. Coronary artery disease was present in 378 patients including 92 patients without current significant stenosis. The MACE rate was 6.4% in patients without coronary artery disease compared to 28.3% in patients with coronary artery disease (P<0.00001). Cardiac and all‐cause mortality were statistically significantly higher in patients with a significant coronary stenosis than in patients without coronary artery disease (P<0.001 and P<0.01). Cardiac mortality and all‐cause mortality were 2.3% and 7.6% in patients without coronary artery disease (patient group I), 7.6% and 13.0% in patients with coronary artery disease but no current significant stenosis (patient group II), and 10.5% and 16.1% in patients with significant coronary stenosis (patient group III). Neurological events, however, were distributed equally among the three patient groups.
CONCLUSIONS<1/emph>. In the longer term, outcomes in patients undergoing carotid artery stenting depend on concomitant coronary artery disease rather than neurological events, cardiac mortality and even all‐cause mortality depending on a significant coronary artery stenosis.
Introduction
Carotid artery stenting in patients with carotid artery stenosis has emerged as an alternative revascularization method to endarterectomy in recent years. Since atherosclerosis is a generalized disease, cardiac comorbidity plays an important role in the long‐term prognosis of patients treated with carotid artery stenting Citation1. In a recently published study we could demonstrate a high proportion of concomitant coronary artery disease in patients undergoing elective stenting of the carotid artery Citation2. However, little is known about the correlation between the presence of coronary artery disease in these patients and their long‐term prognosis. Therefore, it was the purpose of the present study to evaluate whether major adverse cardiac events (MACE) following carotid artery stenting can be predicted based on the results of a routine coronary angiography performed at the time of carotid artery stenting.
Key messages
Coronary artery disease can be expected in the majority of patients undergoing elective carotid artery stenting.
During follow‐up, cardiac and all‐cause mortality are statistically significantly higher in patients with a significant coronary stenosis than in patients without coronary artery disease.
Therefore, the mid‐term prognosis of these patients depends to a major extent on concomitant coronary artery disease rather than neurological events.
Patients and methods
The study cohort consists of 549 consecutive patients who underwent elective stenting of the internal carotid artery in our institution. Our assumption was that the complication rate of carotid artery stenting in our institution was as low as, or lower than, the complication rate of carotid endarterectomy in those studies in which surgery proved to be superior to conservative treatment. Therefore, carotid artery stenting was performed in asymptomatic patients with ⩾80% and symptomatic individuals with ⩾60% stenosis of the extracranial carotid artery, as documented in our research protocol. Every patient underwent an independent neurological examination prior to, 24 hours after, and 30 days after, carotid artery stenting. In addition, a patient's history of concomitant coronary artery disease was recorded, and all patients were thoroughly checked and questioned for symptoms of either typical angina pectoris or chest pain. Angina severity at baseline was classified according to the Canadian Cardiovascular Society classification Citation3. A positive history of coronary artery disease was defined either as a previously angiographically documented coronary artery disease according to the criteria used in the present study (see below) or as a history of myocardial infarction treated in a hospital with a discharge letter available to confirm the diagnosis. All patients without a recent coronary angiogram (<3 months) received a routine coronary angiography in a single session and immediately before carotid artery stenting. According to their clinical history and the results of the angiogram, patients were divided into three groups: patients without coronary artery disease (group I), patients with a confirmed history of coronary artery disease but no significant stenosis (group II), and patients with a significant coronary stenosis (group III). The clinical demographic data are listed in . All data were collected prospectively.
Table I. Baseline characteristics of patients without coronary artery disease, with history of coronary artery disease but absent current significant stenosis, and patients with significant stenosis.
Carotid artery angiography and stenting
Our interventional procedures followed the guidelines of good clinical practice with routine use of emboli‐protection devices since July 2002. At least two projections of the carotid artery stenosis were obtained for the calculations of the vessel diameter and the degree of the stenosis. The diameter of the stenosis was determined according to the North American Symptomatic Carotid Endarterectomy Trial (NASCET) criteria Citation4 with the distal internal carotid artery serving as the reference segment. An assessment of intracranial hemodynamics was not performed.
Coronary angiography
Coronary angiography could be performed without complications in all projected cases and was routinely performed before carotid artery stenting. All visually estimated lesions of ⩾50% were calculated after the procedure with the use of a semi‐automatic device (Hicor®, Siemens®). Only those measurements were taken for analysis in the present study. The criterion for angiographic one‐, two‐, or three‐vessel coronary artery obstruction was either a ⩾70% reduction of the internal diameter of the right or left anterior descending or left circumflex coronary artery, or a ⩾50% reduction in the internal diameter of the left main coronary artery Citation5. In patients with previous coronary artery bypass grafting a graft stenosis, just like a native vessel stenosis, was defined as ⩾70%.
Patients were usually dismissed from the hospital 2 days after a successful intervention. A follow‐up visit at the hospital was arranged 30 days after the procedure. This visit included a clinical history with respect to cardiovascular adverse events, a neurological examination performed by an independent study neurologist, electrocardiogram (ECG), and Doppler flow velocity measurement of the carotid arteries.
Definitions of peri‐interventional complications
All of the following complications occurring within 30 days of the procedure were counted as periprocedural. Neurological events were classified according to Wholey et al Citation6. A transient ischemic attack was classified as any neurological deficit that resolved within 24 hours and left no evidence of residual neurological damage. A minor stroke was classified as a new neurological event that resulted in slight impairment of neurological function that either completely resolved within 7 days or caused an increase in the National Institute of Health (NIH) stroke scale of <4 Citation7. A new neurological deficit that persisted after 7 days and increased the NIH stroke scale by ⩾4 was classified as major stroke. Myocardial infarction was defined either as the development of new Q‐waves on the ECG or as elevated Creatine Kinase‐MB levels to more than twice the normal value. The definition of a perioperative myocardial infarction after bypass surgery was based on new Q‐waves or new wall motion abnormalities detected by echocardiography but not by cardiac enzymes. Revascularization procedures were counted as adverse events if they were not performed electively in the same session as the carotid artery stenting, or if they were not done as scheduled procedures within the following 30 days.
Follow‐up
Patients were examined on a regular basis at 3, 6, 12 months, and every 12 months after the procedure. Follow‐up visits included a Doppler flow examination of both carotid arteries and a clinical neurological examination performed by an independent study neurologist. In addition, a thorough history of any major adverse cardiac event was obtained, with a MACE being defined as myocardial infarction or any coronary revascularization procedure other than the index procedure Citation8. If patients died, autopsy and clinical data as well as data from general practitioners were used to determine the cause of their death. The mode of death was classified by investigators who were unaware of the coronary morphology of each patient. Written, informed consent was obtained from each patient before carotid artery stenting, and the study, including carotid artery stenting and coronary angiography, was approved by our institutional review board.
Statistics
All variables were summarized using frequency distributions for categorical variables and mean± standard deviation for continuous variables. Comparisons between patient groups were performed using the Mann‐Whitney U‐test for continuous variables and chi‐square test for categorical variables. P‐values of ⩽0.05 were considered statistically significant. The MACE‐free survival rates were analyzed by using the Kaplan‐Meier method. Two‐sided P‐values are presented for the significance of the differences between the three groups. The statistical significance was calculated by using a Cox‐Mantel log‐rank test.
Results
The total number of attempted carotid artery interventions was 568 with 530 unilateral and 19 bilateral interventions in 549 patients. Carotid artery stenting could be performed successfully in 98% of all lesions (). An emboli‐protection device was used in 186 patients (34%). Coronary artery disease was present in 378 (69%) patients. One‐, two‐, three‐vessel disease, and left main coronary artery disease could be detected in 72 (13%), 87 (16%), 97 (18%), and 30 (5%) patients, respectively. The number of patients undergoing revascularization, success rate, and complication rate are given in . The decision of what treatment was performed was made on‐site by the operator. In summary, most symptomatic patients were treated by revascularization whereas patients without overt symptoms were generally treated conservatively unless they had a subtotal stenosis judged by the operator as unstable plaque.
Table II. Results and complications of carotid artery stenting.
Table III. Results and complications of index revascularization procedure.
Follow‐up was complete in 529 patients (96% of the study population) and not statistically different between the three patient groups. The mean time period between carotid artery stenting and last follow‐up visit was 26±21 months.
Neurological ischemic events occurred in nine patients (1.6%) and were evenly distributed among patients with and without coronary artery disease ).
Table IV. Neurological events during follow‐up.
The overall mortality rate during the follow up period was 12.9% with a statistically significant difference between group I (patients without coronary artery disease) and group III patients (patients with significant stenosis) (). A non‐fatal MACE could be observed in 77 patients. The probability of surviving without a MACE is plotted in a Kaplan‐Meier analysis in .
Table V. Major adverse cardiac events during follow‐up.
The number of MACE in patients with one‐, two‐, three‐vessel disease, and left main coronary artery disease was 18 (25%), 20 (23%), 34 (35%), and 9 (30%). Cardiac death and death of any cause occurred in 7 (10%) and 11 (15%) patients with one‐vessel disease, 9 (10%) and 12 (14%) patients with two‐vessel disease, 9 (9%) and 16 (16%) with three‐vessel disease, and 5 (17%) and 7 (23%) patients with left main coronary artery disease. The differences in clinical outcome were statistically not significant between these patient groups. In 139 patients with significant coronary artery stenosis who were treated conservatively the number of MACE was 32 (23%), compared to 49 (33%) in 147 patients who underwent a revascularization procedure. There were 17 (12%) cardiac deaths among the patients treated conservatively and 13 (9%) among the patients who had undergone revascularization. The numbers for all‐cause mortality were 27 (19%) and 19 (13%), respectively. None of the differences between the various subgroups were statistically significant.
Discussion
This is the first report demonstrating the impact of coronary artery disease on the longer‐term outcome of patients who had undergone carotid artery stenting. The coincidence of clinical manifestations of atherosclerosis in different organs is not surprising since atherosclerosis is a generalized disease Citation9. In addition, recent studies disclose a potential role of inflammation as a possible link between carotid and coronary plaque instability Citation10. Coronary artery disease is the predominant prognostic factor in patients with various clinical atherosclerotic manifestations Citation11. Data from previously published studies showed that the overall long‐term outcome of patients following stroke depends predominantly on cardiac events rather than neurological events Citation12–14. However, no systematic coronary angiographies were performed in patients treated in these studies thus leaving the question of the underlying coronary artery morphology unanswered.
Coronary angiography was chosen to define the future risk of events for two reasons: Firstly, patients undergoing carotid artery stenting have their groin already punctured. Since most complications of a routine coronary angiography result from the peripheral vascular access, the additional selective angiography of the coronary arteries itself carries very little risk if performed by an experienced cardiologist. This was successfully demonstrated in our study, as no complications whatsoever resulted from coronary angiography. Secondly, alternative diagnostic tools do not exist at this point given the fact that currently available computer tomography does not sufficiently visualize coronary arteries in the presence of severe calcification or implanted stents.
The cardiac event rate and all‐cause mortality of our patient cohort complies with previously published data given the fact that patients in our series were somewhat older and more diffusely diseased than in most other studies. For example, in a recently published meta‐analysis Katritsis et al. included 11 trials with a total of 2950 patients Citation15. The mean age of patients included in these trials was considerably lower than in our study cohort and varied between 53 and 61 years. During a mean follow‐up between 1 and 7 years, there was an all‐cause mortality of 6.6%, 5.1% suffered a nonfatal myocardial infarction, 7.2% underwent a coronary artery bypass operation, and 15.7% a percutaneous coronary intervention Citation16–23.
The results of our study show that the presence or absence of a concomitant coronary artery disease is predictive of a future MACE. The major difference in MACE is observed between patients with and without coronary artery disease regardless of the presence of a significant stenosis. The reason for this observation is probably the natural course of the disease: other than in carotid arteries, acute coronary syndromes with subsequent clinical events most often result from high‐risk coronary plaques but not from a high luminal stenosis of coronary artery plaques Citation24,25. As a second major point, 147 out of 286 patients (51%) with significant stenosis underwent a revascularization procedure at the time of carotid artery stenting. These patients were counted to the group with a significant stenosis according to their initial coronary angiogram. About half of the patients with significant stenosis were treated conservatively. These patients obviously did not influence the MACE rate. This observation fits well with the results of previously published studies showing a similar long‐term prognosis of minimally symptomatic or asymptomatic patients treated conservatively versus coronary revascularization. Data from a recently published meta‐analysis suggest no considerable difference between different treatment groups regarding mortality in predominantly asymptomatic patients with significant coronary artery stenosis Citation15. However, a percutaneous coronary intervention at the time of carotid artery stenting did not increase the MACE rate by recurrent procedures for in‐stent restenosis in our patient population. Widespread use of drug‐eluting stents might have reduced the estimated clinical restenosis rate in patients undergoing an index revascularization procedure. Whether the extended use of drug‐eluting stents could influence long‐term mortality in patients undergoing stenting for coronary artery disease remains questionable since restenosis is considered as a relatively benign disease Citation15,Citation26. The present study cannot give an answer to this point since drug‐eluting stents have not been used systematically.
The incidence of percutaneous coronary interventions during follow‐up was lower compared to patients without significant stenosis at the time of carotid artery stenting (). Most likely, patients with significant stenosis treated conservatively for various reasons were not considered candidates for a revascularization procedure during follow‐up. Whether these patients accounted for other MACEs, especially for cardiac death, can only be speculation. As most index revascularization procedures as well as interventions during follow‐up were uncomplicated, a high incidence of deaths due to pump failure or malignant arrhythmias can be assumed among patients with significant stenosis. The specific demographics of our study cohort may underestimate the percentage of otherwise symptomatic patients.
The study was not designed to decide upon the treatment of coronary artery disease. This could only have been made in a randomized study comparing conservative versus interventional treatment of coronary artery disease. Unfortunately, it would not be very realistic, in our opinion, to conduct a study that only includes patients with a specific comorbidity such as a carotid artery stenosis. In addition, it would be highly problematic from an ethical standpoint not to intervene in severely symptomatic patients with subtotal stenosis of a major coronary artery or in patients with left main coronary artery disease.
Especially in asymptomatic patients, the overall benefit of carotid artery stenting versus conservative treatment depends on the event‐free years after stenting. The results of the present study suggest that the beneficial effects of carotid artery stenting versus conservative treatment could be lower in patients with a high cardiac event rate. The study could add to current knowledge and lead to a more conservative approach with respect to the asymptomatic carotid stenosis subpopulation with severe cardiac comorbidity.
Conclusions
Patients undergoing stenting of the carotid artery have a high incidence of coronary artery disease. Since many patients are asymptomatic, a firm diagnosis can be made by routine use of coronary angiography at the time of carotid artery intervention. The long‐term prognosis of these patients depends to a major extent on cardiac events. Therefore, concomitant coronary artery disease plays an important role in the determination of the overall prognosis of these patients. Future major adverse cardiac events can be predicted by the presence of coronary artery disease with a history of treated coronary artery disease being equivalent to an angiographically significant coronary artery stenosis. However, cardiac mortality and even all‐cause mortality depend on a significant coronary artery stenosis.
References
- Yadav J. S., Wholey M. H., Kuntz R. E., Fayad P., Katzen B. T., Mishkel G. J., et al. Protected carotid‐artery stenting versus endarterectomy in high‐risk patients. N Engl J Med 2004; 351: 1493–501
- Hofmann R., Kypta A., Steinwender C., Kerschner K., Grund M., Leisch F. Coronary angiography in patients undergoing carotid artery stenting reveals a high incidence of significant coronary artery disease. Heart 2005; 91: 1438–41
- Campeau L. Grading of angina pectoris. Circulation 1976; 54: 522–3
- North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high‐grade carotid stenosis. N Engl J Med 1991; 325: 445–53
- Emond M., Mock M. B., Davis K. B., Fisher L. D., Holmes D. R., Jr., Chaitman B. R., et al. Long‐term survival of medically treated patients in the Coronary Artery Surgery Study (CASS) Registry. Circulation 1994; 90: 2645–57
- Wholey M. H., Wholey M., Mathias K., Roubin G. S., Diethrich E. B., Henry M., et al. Global experience in cervical carotid artery stent placement. Cathet Cardiovasc Interv 2000; 50: 160–7
- Brott T., Adams H. P., Olinger C. P., Marler J. R., Barsan W. G., Biller J., et al. Measurement of acute cerebral infarction: a clinical examination scale. Stroke 1989; 20: 864–70
- Schühlen H., Kastrati A., Dirschinger J., Hausleiter J., Elezi S., Wehinger A., et al. Intracoronary stenting and risk for major adverse cardiac events during the first month. Circulation 1998; 98: 104–11
- Viles‐Gonzalez J. F., Fuster V., Badimon J. J. Atherothrombosis: A widespread disease with unpredictable and life‐threatening consequences. Eur Heart J 2004; 25: 1197–207
- Lombardo A., Biasucci L. M., Lanza G. A., Coli S., Silvestri P., Cianflone D., et al. Inflammation as a possible link between coronary and carotid plaque instability. Circulation 2004; 109: 3158–63
- Fuster V. Epidemic of cardiovascular disease and stroke: the three main challenges. Circulation 1999; 99: 1132–7
- Dennis M. S., Burn J. P., Sandercock P. A., Bamford J. M., Wade D. T., Warlow C. P. Long‐term survival after first‐ever stroke: the Oxfordshire Community Stroke Project. Stroke 1993; 24: 796–800
- Hankey G. J., Jamrozik K., Broadhurst R. J., Forbes S., Burvill P. W., Anderson C. S., et al. Five‐year survival after first‐ever stroke and related prognostic factors in the Perth Community Stroke Study. Stroke 2000; 31: 2080–6
- Hartmann A., Rundek T., Mast H., Paik M. C., Boden‐Albala B., Mohr J. P., et al. Mortality and causes of death after first ischemic stroke: the Northern Manhattan Stroke Study. Neurology 2001; 57: 2000–5
- Katritsis D. G., Karvouni E., Ioannidis J. P. Meta‐analysis comparing drug‐eluting stents with bare metal stents. Am J Cardiol 2005; 95: 640–3
- Dakik H. A., Kleiman N. S., Farmer J. A., He Z. X., Wendt J. A., Pratt C. M., et al. Intensive medical therapy versus coronary angioplasty for suppression of myocardial ischemia in survivors of acute myocardial infarction: a prospective, randomized pilot study Circulation. 1998; 98: 2017–23
- Hambrecht R., Walther C., Mobius‐Winkler S., Gielen S., Linke A., Conradi K., et al. Percutaneous coronary angioplasty compared with exercise training in patients with stable coronary artery disease: a randomized trial. Circulation 2004; 109: 1371–8
- Zeymer U., Uebis R., Vogt A., Glunz H. G., Vohringer H. F., Harmjanz D., , ALKK‐Study Group, et al. Randomized comparison of percutaneous transluminal coronary angioplasty and medical therapy in stable survivors of acute myocardial infarction with single vessel disease: a study of the Arbeitsgemeinschaft Leitende Kardiologische Krankenhausaerzte. Circulation 2003; 108: 1324–8
- Hueb W., Soares P. R., Gersh B. J., Cesar L. A., Luz P. L., Puig L. B., et al. The medicine, angioplasty, or surgery study (MASS‐II): a randomized, controlled clinical trial of three therapeutic strategies for multivessel coronary artery disease: one‐year results. J Am Coll Cardiol 2004; 43: 1743–51
- Henderson R. A., Pocock S. J., Clayton T. C., Knight R., Fox K. A., Julian D. G., , Second Randomized Intervention Treatment of Angina (RITA‐2) Trial Participants, et al. Seven‐year outcome in the RITA‐2 trial: coronary angioplasty versus medical therapy. J Am Coll Cardiol 2003; 42: 1161–70
- Folland E. D., Hartigan P. M., Parisi A. F. Percutaneous transluminal coronary angioplasty versus medical therapy for stable angina pectoris: outcomes for patients with double‐vessel versus single‐vessel coronary artery disease in a Veterans Affairs Cooperative randomized trial. Veterans Affairs ACME InvestigatorS. J Am Coll Cardiol 1997; 29: 1505–11
- Pitt B., Waters D., Brown W. V., van Boven A. J., Schwartz L., Title L. M., et al. Aggressive lipid‐lowering therapy compared with angioplasty in stable coronary artery disease. Atorvastatin versus Revascularization Treatment Investigators. N Engl J Med 1999; 341: 70–6
- Rihal C. S., Raco D. L., Gersh B. J., Yusuf S. Indications for coronary artery bypass surgery and percutaneous coronary intervention in chronic stable angina: review of the evidence and methodological considerations. Circulation 2003; 108: 2439–45
- Corti R., Fuster V., Badimon J. J. Pathogenetic concepts of acute coronary syndromes. J Am Coll Cardiol 2002; 90: 7S–14S
- Van der Wal A. C., Becker A. E., Van de Loos C. M., Das P. K. Site of intimal rupture or erosion of thrombosed coronary atherosclerotic plaques is characterized by an inflammatory process irrespective of the dominant plaque morphology. Circulation 1994; 89: 36–44
- Babapulle M. N., Joseph L., Belisle P., Brophy J. M., Eisenberg M. J. A hierarchical Bayesian meta‐analysis of randomised clinical trials of drug‐eluting stents. Lancet 2004; 364: 583–91